Abstract
In this work, we sought to understand how glycolipid production and the availability of nutrients could explain the ecology of Pseudozyma flocculosa and its biocontrol activity. For this purpose, we compared the development of P. flocculosa to that of a close relative, the plant pathogen Ustilago maydis, under different environmental conditions. This approach was further supported by measuring the expression of cyp1, a pivotal gene in the synthesis of unique antifungal cellobiose lipids of both fungi. On healthy cucumber and tomato plants, the expression of cyp1 remained unchanged over time in P. flocculosa and was undetected in U. maydis. At the same time, green fluorescent protein (GFP) strains of both fungi showed only limited green fluorescence on control leaves. On powdery mildew-infected cucumber leaves, P. flocculosa induced a complete collapse of the pathogen colonies, but glycolipid production, as studied by cyp1 expression, was still comparable to that of controls. In complete contrast, cyp1 was upregulated nine times when P. flocculosa was applied to Botrytis cinerea-infected leaves, but the biocontrol fungus did not develop very well on the pathogen. Analysis of the possible nutrients that could stimulate the growth of P. flocculosa on powdery mildew structures revealed that the complex Zn/Mn played a key role in the interaction. Other related fungi such as U. maydis do not appear to have the same nutritional requirements and hence lack the ability to colonize powdery mildews. Whether production of antifungal glycolipids contributes to the release of nutrients from powdery mildew colonies is unclear, but the specificity of the biocontrol activity of P. flocculosa toward Erysiphales does appear to be more complex than simple antibiosis.
The anamorph genus Pseudozyma encompasses a small group of basidiomycetous yeasts that have been recently assembled from nuclear large subunit (LSU) ribosomal DNA (rDNA) sequences (1). Although most species within the genus have drawn little interest in the literature, Pseudozyma flocculosa (Traquair, Shaw & Jarvis) Boekhout & Traquair (syn: Sporothrix flocculosa) has received the most attention because of its antagonistic properties against powdery mildew fungi. These observations have led to the exploitation of this fungus as a potential biocontrol agent (BCA) of Erysiphales. Using green fluorescent protein (GFP)-expressing strains of P. flocculosa, Neveu et al. (18) demonstrated that the fungus grew specifically in the presence of powdery mildew colonies. This activity was believed to be initiated by the release of a glycolipid, flocculosin, which displayed strong antifungal and antibacterial properties in vitro (2, 15, 16). However, this hypothesis was questioned upon the discovery that P. flocculosa was phylogenetically related to the fungal pathogen Ustilago maydis (de Candolle) Corda, which produced a nearly identical glycolipid, ustilagic acid (16). Hewald et al. (9) suggested that ustilagic acid acted as a pheromone carrier, thus facilitating the recognition of compatible mating types on the leaf, but Teichmann et al. (21) speculated that Ustilago maydis could have biocontrol potential against Botrytis cinerea because of the antifungal activity of ustilagic acid toward the pathogen's conidia.
The previous results raised the obvious question about the ecological roles of flocculosin and ustilagic acid, as their production appeared to benefit both a plant pathogen, U. maydis, and a beneficial fungus, P. flocculosa. In vitro studies report that nitrogen supply interferes with ustilagic acid production, although no ecological link has been established with these data. In the case of flocculosin, removal of yeast extract or the synthetic ingredient yeast nitrogen base (YNB) from a complex culture medium stimulates its synthesis (7, 8). The presence of the same factors will stimulate growth of the fungus to the detriment of flocculosin production.
In spite of the possible different roles of flocculosin and ustilagic acid, the similarity of their chemical structures and the phylogenetic relatedness between the producing organisms suggested a common genetic origin. Indeed, Marchand et al. (13) identified from P. flocculosa the homolog of the U. maydis cyp1 gene, coding for a monooxygenase, involved in the biosynthesis of ustilagic acid (9). They further showed a strong correlation between flocculosin production and cyp1 gene expression in vitro. However, they did not observe an upregulation of cyp1 when P. flocculosa was sprayed on powdery mildew-infected leaves, thereby suggesting that other factors contributed to the biocontrol activity of the fungus.
In this work, we sought to understand how glycolipid production and the availability of nutrients in situ could explain the ecology and biocontrol activity of P. flocculosa. To this end, we compared the behaviors of P. flocculosa and U. maydis on cucumber and tomato plants infected by Podosphaera xanthii and B. cinerea, respectively, and we analyzed (i) the expression of cyp1 under different conditions, as a measure of glycolipid synthesis by both fungi; (ii) the development of both fungi under the same conditions using GFP-expressing strains; and (iii) the specific constituents in YNB that influence the growth of P. flocculosa, in an attempt to identify the factors that triggered its development on powdery mildewed plants.
MATERIALS AND METHODS
Fungal material.
P. flocculosa (DAOM 196992) was used throughout this study. Stock cultures were sporidia lyophilized in maltose (20%) and kept at −80°C as aliquots of ca. 1 × 106 cells. Mother cultures were obtained by inoculating 100 ml YMPD medium (yeast extract, 3 g liter−1; malt extract, 3 g liter−1; peptone water, 5 g liter−1; and dextrose, 10 g liter−1) in a 500-ml baffled flask with one bottle of lyophilized culture previously hydrated with 3 ml of sterile water. Seed cultures were prepared by inoculating 100 ml of YMPD medium with 5 ml of a 3-day-old mother culture.
U. maydis strain MB215 (DSM 17144) is a wild isolate and was collected in northern Germany (9). It was grown at 28°C in liquid YEPS medium (yeast extract, 10 g liter−1; peptone, 20 g liter−1; and dextrose, 20 g liter−1).
For fluorescence microscopy observations, GFP transformants of P. flocculosa strain Act-4 (18) and U. maydis (strain 312; graciously provided by M. Bölker, Philipps-Universität Marburg, Germany) were used.
All culture medium ingredients were supplied by BD Biosciences (Mississauga, Ontario, Canada).
Infection.
Podosphaera xanthii ([Castagne] Braun & Shishkoff) was used to infect cucumber plants. Conidia were collected from already-infected plants by gently shaking the leaves over an empty petri dish. To infect cucumber plants, freshly collected P. xanthii conidia in petri dishes were scattered on leaves and allowed to develop.
Botrytis cinerea (Persoon; anamorph) was used to infect tomato plants. B. cinerea inoculum was obtained by flooding 3-day-old cultures grown on potato dextrose agar. Plants were then sprayed with B. cinerea conidia adjusted to ca. 1 ×106 cells ml−1 until runoff.
Experimental plants were grown in growth chambers under a 16-h photoperiod at 24°C during the day and 18°C at night. For B. cinerea experiments, relative humidity was kept over 90% night and day. With both pathogens, the first symptoms of disease appeared 1 week later.
In situ bioassays with P. flocculosa and U. maydis.
Ten days after inoculation, healthy or diseased leaves (ca. 20% disease area) were inoculated with a solution of 1 × 106 cells ml−1 of either P. flocculosa or U. maydis by spraying until runoff. Controls consisted of diseased leaves or healthy leaves sprayed with a saline solution. At 0, 12, 24, and 48 h after application, leaf discs, 2 cm in diameter, were excised from leaves of the different treatments. Each replicate consisted of three leaf samples, and for every treatment, three replicates were immediately frozen in liquid nitrogen and kept at −80°C for subsequent quantitative reverse transcription-PCR (qRT-PCR) analyses.
qRT-PCR analysis of cyp1 expression.
Differential expression of the P. flocculosa and U. maydis monooxygenase cyp1 genes (GenBank accession no. EU556541 and EAK87170, respectively) was assessed by qRT-PCR using a Mastercycler EP Realplex 2S (Eppendorf, Mississauga, ON, Canada). Total RNA was extracted from fungal biomass from leaf discs using 2 ml of Trizol reagent according to the supplier's directives (Invitrogen, Carlsbad, CA) and then quantified using a Nanodrop spectrophotometer (Nanodrop, Wilmington, DE). For each of the three replicates, 1 μg of total RNA was first treated with RQ1 RNase-free DNase (Promega Corp., Madison, WI) and then reverse transcribed into first-strand cDNA using oligo(dT) (18) and Superscript II reverse transcriptase (Invitrogen) in a 20-μl reaction volume. Primer pairs for selected genes were designed with Oligo Explorer software (Genelink) and were as follows: for the P. flocculosa actin gene (act) (17), 5′-TCTGCTTCGAGACCTTCAAC-3′ and 5′-GAGTAGCCCTCGTAGATGG-3′; for P. flocculosa cyp1, 5′-TGTCAAGATCGCCTTTAGCC-3′ and 5′-CGGTCTCGTTGTACTTTTCG-3′; for U maydis tef1, 5′-TGGTAAGACCCTCCTTGACG-3′ and 5′-ACCGCCGATCTTGTAGACAT-3′; and for U. maydis cyp1, 5′-TGGTAAGACCCTCCTTGACG-3′ and 5′-ACCGCCGATCTTGTAGACAT-3′.
PCR amplifications were performed in a 20-μl reaction volume using the QuantiTect SYBR green PCR kit (Qiagen, Mississauga, ON, Canada) with 1 μl of sample cDNA. PCR was performed as follows: 15 min at 95°C, 50 cycles of 20 s at 95°C, 20 s at 58°C, and 20 s at 72°C. Each sample from each replicate was analyzed twice. The specificity of primer pairs was verified by both 2% agarose gel and melting curve analyses. The P. flocculosa actin gene (act) and the U. maydis elongation factor gene (tef1) were used for normalization as a proportion of cyp1/act or tef1 expression. Efficiencies of each PCR amplification were determined by the Realplex software version 1.5 (Eppendorf), and the quantification of the relative changes in cyp1 gene expression was performed using the Pfaffl method (19). Data were processed through a Dunnett's test, with 1 at time zero representing the control group.
As control experiments to assess the reliability of the cyp1 primer pairs for U. maydis, the fungus was grown in axenic culture media conducive (YNB) or repressive (YEPS) to glycolipid production (9), and cyp1 expression was measured after 24 h. A similar approach was used by Marchand et al. (13) for measuring flocculosin production.
Biocontrol activity and fluorescence microscopy observations.
GFP transformants of P. flocculosa and U. maydis strains were used to visualize, in situ, their respective colonization of two foliar pathogens. Leaves (three replicates for each treatment) were subjected to the following treatments: (i) control (cucumber leaves sprayed with water), (ii) gray mold (tomato leaves infected by B. cinerea), (iii) powdery mildew (cucumber leaves infected by P. xanthii), and (iv) yeast nitrogen base (YNB) (cucumber leaves sprayed with YNB [3 g liter−1]). P. flocculosa or U. maydis sporidium suspensions (ca. 1 × 106 cells ml−1) were sprayed uniformly on leaf samples until runoff. Leaf discs were left to dry for 5 min and were placed on wet Whatman filter paper covering the bottoms of 9-cm petri dishes. The petri dishes were then sealed with Parafilm and placed in a growth chamber with a 16-h photoperiod at 22/20°C as described by Hajlaoui and Bélanger (6). After 24 h, the optimal time to visualize the antagonistic process of P. flocculosa (3), the biocontrol activity of P. flocculosa and U. maydis on treatments 2 and 3 was assessed using a quantitative scale for conidial chain collapse prior to fluorescence microscopy observations. Each leaf sample was observed with a stereomicroscope (Olympus SZ61; Olympus Optical Company Ltd., Shinjuku-ku, Japan) and rated according to the proportion of collapsed conidial chains, as described by Jarvis et al. (10): 0, no collapse of conidial chains; 1, 1 to 25%; 2, 26 to 50%; 3, 51 to 75%; and 4, 76 to 100%. The experiment was repeated three times.
Subsequently, zones of interaction were observed under fluorescence microscopy. All observations were made with an Olympus microscope (model BH2-RFCA; Olympus, Tokyo, Japan) equipped with a BH2-RFL-T3 mercury lamp. The observations were carried out under blue light (488 nm). Images were recorded using a Coolsnap-Pro (MediaCybernetics, Silver Spring, MD) color camera and analyzed with Image Pro Plus software (MediaCybernetics). Observations were made at ×40 and/or ×100.
Analysis of YNB fractions on P. flocculosa growth.
YNB was used throughout this experiment. To evaluate the effect of its constituents on P. flocculosa growth, i.e., production of cell biomass, a minimal culture medium (called control medium [CM]) was used in combination with YNB or its different fractions. CM was previously developed for flocculosin production (7). It is composed of sucrose (35 g liter−1), (NH4)2SO4 (1 g liter−1), KH2PO4 (1 g liter−1), MgSO4·7H2O (0.5 g liter−1), and FeSO4·7H2O (0.01 g liter−1), added to 50 mM citrate buffer consisting of sodium citrate dihydrate (12.85 g liter−1) and citric acid (1.21 g liter−1), to maintain the pH at 6.0. One hundred milliliters of CM was poured into 500-ml baffled flasks prior to sterilization at 121°C for 20 min.
YNB, or its fractions, was filter sterilized and added to flasks containing CM before inoculation. All fractions were equivalent to 3 g liter−1 of YNB when added to CM. All tested fractions were compared to CM (where flocculosin was produced, as evidenced by the presence of needle-shaped crystals) and to CM plus YNB (where sporidia and mycelium were produced). Flasks were inoculated with 5 ml of 3-day-old seed cultures of P. flocculosa and were left at 28°C for 48 h on a rotary shaker set at 150 rpm.
The organic fraction of YNB (vitamins) was first isolated using reverse-phase (C18) silica gel cartridges (SepPak; Waters, Montreal, Canada) according to the supplier's protocol. Briefly, 1 g YNB diluted in 10 ml water was passed through a C18 cartridge previously conditioned with 5 volumes of water slightly acidified with 0.5% acetic acid. The flowthrough was kept for subsequent bioassays, and the retained organic fraction was eluted with 5 volumes of methanol. These two fractions, aqueous and organic, were roto-evaporated under vacuum to dryness and rehydrated with water prior to being tested for their activity on P. flocculosa growth.
The inorganic products from the aqueous fraction were obtained from the hydrolysis of the C18 flowthrough (see above) with 4 N nitric acid at 100°C for 1 h. The mixture was then burned in a furnace at 505°C to obtain ashes. The ashes were diluted in water, filter sterilized, and added to CM.
To identify the growth-promoting elements within the inorganic fraction of YNB, individual constituents (Voigt Global Distribution LLC, Kansas City, MO) were tested separately or in combination. For each trace element or tested combination, the equivalent of 3 g liter−1 YNB was added to CM for each of the following components at the indicated concentrations: KI, 100 μg liter−1; ZnSO4, 400 μg liter−1; MnSO4, 400 μg liter−1; CuSO4, 400 μg liter−1; Na2MoO4, 200 μg liter−1; and H3BO3, 500 μg liter−1.
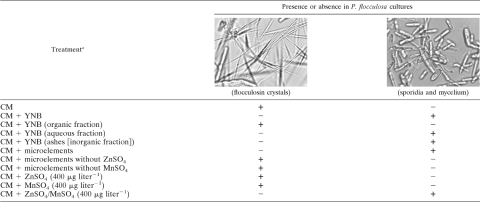
Results of the bioassays were analyzed by microscopic observations of the cultures and were scored based on either the presence of sporidia and mycelium or the presence of flocculosin crystals (7) (Table 1).
TABLE 1.
Effect of yeast nitrogen base (YNB), its different fractions, and microelements on Pseudozyma flocculosa growth
a CM, control medium. Microelements include CuSO4(400 μg liter−1), Na2MoO4(200 μg liter−1), MnSO4(400 μg liter−1), ZnSO4(400 μg liter−1), H3BO3 (500 μg liter−1), and KI (100 μg liter−1).
Development of P. flocculosa on cucumber leaves treated with zinc and manganese solution.
Leaves of cucumber plants were sprayed until runoff with a solution containing 400 μg liter−1 of MnSO4 and 400 μg liter−1 of ZnSO4 or water (control). The leaves were then air dried. Cucumber leaves were then sprayed with a solution of P. flocculosa spores (1 × 106 cells ml−1) until runoff. Plastic bags were placed around treated leaves after 10 min and were loosely clipped to maintain a constant relative humidity. Microscope observations were made 24 h later using a JEOL JSM6360LV microscope (JEOL, Peabody, MA). No fixation was made on samples.
RESULTS
qRT-PCR analysis of cyp1 expression.
In control experiments, the expression of cyp1 in U. maydis was over 50-fold higher after 24 h of culture in YNB medium, known to stimulate the production of ustilagic acid, than in YEPS medium that inhibits its production (data not shown). An increase of a similar order of magnitude (50- to 125-fold) was obtained with P. flocculosa (13).
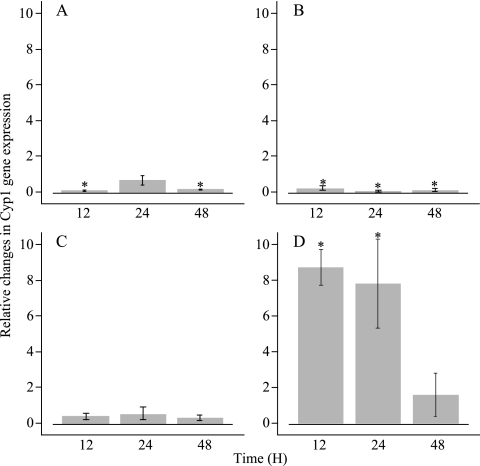
The expression of cypl was assessed in situ through qRT-PCR after 0, 12, 24, and 48 h. On both cucumber and tomato control plants, cyp1 expression of P. flocculosa was fairly constant over the experimental period (Fig. 1A and C). A significant downregulation was observed at 12 and 48 h on cucumber plants. On powdery mildew-infected cucumber plants, the pattern of cyp1 expression was nearly similar to the one in the absence of the pathogen (Fig. 1B). On the other hand, a significant upregulation was noted when P. flocculosa was sprayed on tomato leaves infected with B. cinerea (Fig. 1D). This trend was maintained over the first 24 h of the sampling period, reaching a maximum of a 9-fold upregulation at 12 h, and subsided to a basal level at 48 h. In the case of U. maydis, no significant accumulation of cyp1 transcripts was measured at any time in any of the four treatments (results not shown).
FIG. 1.
Transcript accumulation of Pseudozyma flocculosa cyp1 gene after inundating applications of conidia on healthy cucumber plant (A), powdery mildew-infected cucumber plant (B), healthy tomato plant (C), and Botrytis cinerea-infected tomato plant (D). Relative levels of cyp1 expression were measured by quantitative reverse transcription-PCR (qRT-PCR) methodology. Expression levels were normalized to that of the actin control gene. The indicated values correspond to the means of three replicates. Error bars represent the standard error for each treatment. Values significantly different from the ratio of 1 at time zero (P < 0.05) are represented with an asterisk.
Biocontrol activity and fluorescence microscopy observations.
Assessment of biocontrol activity confirmed the strong deleterious effect of P. flocculosa against powdery mildew. A maximum score of 5 was recorded for all samples observed, indicating a total collapse of the pathogen's conidial chains. When applied to B. cinerea, P. flocculosa induced only a very limited collapse of conidial chains that never exceeded 10% (score of 1 ± 0). For its part, U. maydis never caused conidial chain collapse, whether applied to P. xanthii or B. cinerea, and all observed samples received a score of 0.
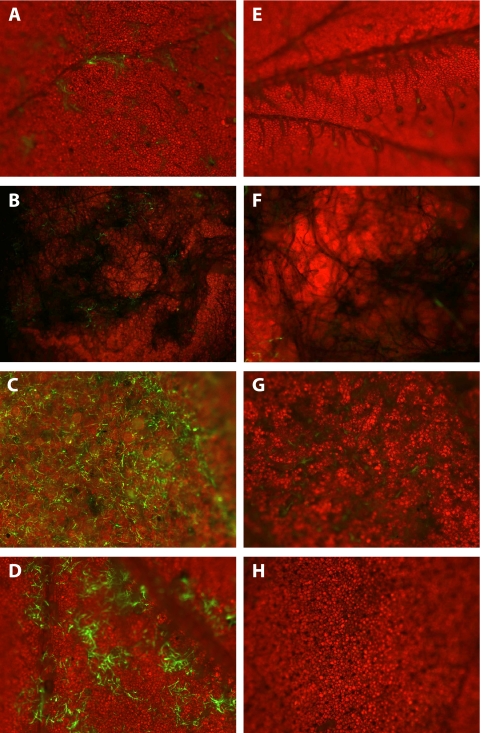
Following inundating applications of a GFP-expressing transformant of P. flocculosa on healthy cucumber leaves, very little green fluorescence was observed 24 h after treatment (Fig. 2A) and none at later times (not shown). In the case of applications to B. cinerea-infected tomato leaves, only sparse fluorescent filaments were noted, and a close-up of them is shown in Fig. 2B. In contrast, green fluorescence emitted on powdery mildew-infected leaves was indicative of a dense and uniform presence of P. flocculosa after 24 h (Fig. 2C). This fluorescence was systematically present wherever there was presence of powdery mildew colonies. Similarly, the spraying of a YNB solution on cucumber leaves appeared to stimulate the growth of P. flocculosa, based on the green fluorescence (Fig. 2D). In the case of U. maydis, no or very little fluorescence was detected after 24 h whether it was applied to infected or healthy leaves, indicating a restricted ability to develop on the tested substrates (Fig. 2E to H).
FIG. 2.
Fluorescence microscopy observations of cucumber and tomato leaves 24 h after being sprayed with a spore suspension of GFP-transformed Pseudozyma flocculosa (A to D) or Ustilago maydis (E to H) strains. (A and E) Control cucumber leaves show no fluorescence in either treatment; (B and F) Botrytis cinerea-infected tomato leaves show a few fluorescent mycelial fragments in the case of P. flocculosa and very faint fluorescence with U. maydis; (C and G) powdery mildew-infected cucumber leaves show extensive fluorescence following P. flocculosa application compared to very little with U. maydis; and (D and H) control cucumber leaves sprayed with yeast nitrogen base solution stimulate the development of P. flocculosa but not of U. maydis. Magnifications: ×40 (A to E, G, and H); ×100 (F). Blue light, 433 nm. Each panel is representative of observations made from three replicates.
Analysis of YNB fractions on P. flocculosa metabolism.
Following the first fractionation step, addition of the organic fraction to CM had no effect on flocculosin production (Table 1) (Fig. 3A). The aqueous fraction (flowthrough) yielded the same growth pattern of P. flocculosa (i.e., sporidia/mycelium and absence of flocculosin) as YNB (Table 1) (Fig. 3B). For its part, the inorganic fraction, obtained from the ashes of the aqueous fraction, triggered growth of the fungus (Table 1).
FIG. 3.
Light microscopy observations of 48-h-old Pseudozyma flocculosa showing the production of flocculosin in cultures grown in control medium (CM) (A) and the production of sporidia and mycelium in cultures grown in CM amended with yeast nitrogen base (3g liter−1) (B), CM amended with microelements (CuSO4, 400 μg liter−1; Na2MoO4, 200 μg liter−1; MnSO4, 400 μg liter−1; ZnSO4, 400 μg liter−1; H3BO3, 500 μg liter−1; and KI, 100 μg liter−1) (C), and CM amended with ZnSO4 (400 μg liter−1) and MnSO4 (400 μg liter−1) (D). Black arrows, flocculosin crystals; white arrows, sporidia; double black arrows, mycelium.
When a complete solution of microelements was added to CM, P. flocculosa growth was stimulated in the same manner as observed in CM plus YNB (Table 1) (Fig. 3C). However, the removal of Zn or Mn from the microelement solution favored the production of flocculosin to the detriment of sporidia and mycelium (Table 1). Furthermore, the addition of Zn or Mn alone to CM maintained flocculosin production. On the other hand, the combined addition of Zn and Mn to CM restored the production of sporidia/mycelium (Table 1) (Fig. 3D), as observed with the medium containing all microelements, thus suggesting that the complex ZnSO4/MnSO4 accounted for a metabolic switch from flocculosin synthesis to biomass production in P. flocculosa cultures.
Development of P. flocculosa on cucumber leaves treated with zinc and manganese solution.
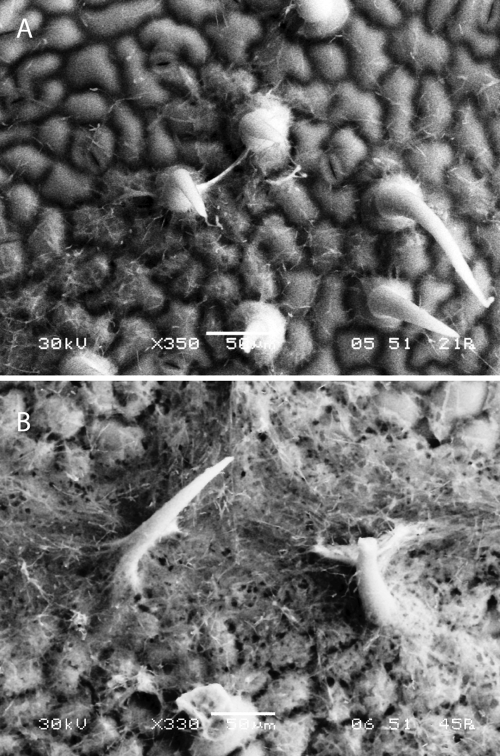
Based on the observation that the complex Zn/Mn appeared to trigger the growth of P. flocculosa, we were interested in determining if a solution of both microelements would stimulate development of the fungus on a leaf surface, as observed in the presence of powdery mildew colonies. For this purpose, we sprayed a spore suspension of P. flocculosa on cucumber leaves treated or not with the microelements. Scanning electron microscopy (SEM) observations of P. flocculosa growth on control cucumber leaves indicated a limited development of the fungus concentrated around the trichomes of the leaves (Fig. 4A). On the other hand, P. flocculosa developed abundantly on cucumber leaves that had been sprayed with a Zn/Mn solution (Fig. 4B).
FIG. 4.
SEM observations of Pseudozyma flocculosa showing limited development around the trichome areas of cucumber leaves sprayed with water (control) (A) and extensive growth resulting in a dense mycelial mat on leaves sprayed with a ZnSO4 (400 μg liter−1) and MnSO4 (400 μg liter−1) solution (B).
DISCUSSION
In this study, our goal was to determine some of the factors that influenced the biocontrol activity of the basidiomycetous yeast P. flocculosa in the phyllosphere. As a first approach, we followed the in situ expression of cyp1, a pivotal gene in the synthesis of flocculosin and ustilagic acid, under different environmental conditions. We further tried to comprehend those results by following the development of GFP-expressing strains of P. flocculosa and U. maydis under the same conditions. On healthy cucumber and tomato leaves, the cyp1 gene never appeared to be stimulated over the course of the experiments (48 h). This would indicate that nutrient availability on the leaf is not sufficient to trigger the production of flocculosin. Incidentally, this lack of nutrients also appeared to inhibit the development of P. flocculosa on control leaves, given that limited fluorescence was observed for that treatment. On the other hand, it was rather surprising to observe a similar low level of cyp1 expression on powdery mildew-infected cucumber leaves, suggesting that the presence of the pathogen did not induce the production of the glycolipid. This low expression of cyp1 did not likely result from nutrient shortage but rather from the activation of the metabolic pathways leading to biomass production. Indeed, it has been shown previously by Clément-Mathieu et al. (3) that quantitative development of P. flocculosa is directly linked with its biocontrol activity and that green fluorescence emission of GFP strains is also a direct indicator of both phenomena. In this study, our GFP observations revealed an abundant and specific colonization of powdery mildew structures by P. flocculosa within 24 h following its application. This strongly suggests that the BCA derives nutrients from powdery mildew cells, which in turn favors its vegetative development over those cells. Indeed, if leakage from infected leaf cells served as a source of nutrients for P. flocculosa, one would expect similar results on Botrytis-infected plants, which was clearly not the case in this work. Whether flocculosin has contributed to this process by damaging the powdery mildew cells is suspected but unclear from our results.
In complete contrast to the P. flocculosa-P. xanthii interaction, the expression of cyp1 was upregulated by as much as 9-fold during the first 24 h when P. flocculosa was applied to B. cinerea colonies. These data were unexpected given that the BCA is not known to have biocontrol activity against this pathogen, a condition confirmed by its limited development on B. cinerea based on our biocontrol data and the fluorescent activity of the GFP-expressing strain. These results can only be explained by the fact that the interaction between B. cinerea and the leaf provided factors that induced the production of flocculosin. In a previous study, Hammami et al. (7) showed that flocculosin synthesis was dependent on the availability of a carbon source but a limitation of other nutrients. B. cinerea is well known to secrete a number of hydrolytic enzymes that degrade cutin, pectin, and cellulose (4). Hence, it seems that tissue maceration caused by the presence of B. cinerea on tomato leaves provided a carbon source for flocculosin synthesis and thus upregulation of cyp1 in the early events of the interaction. Once this carbon source was exhausted, the metabolism of flocculosin production was downregulated, as illustrated by the level of cyp1 expression at 48 h.
Given that U. maydis shares many similarities with P. flocculosa, including a cyp1 gene specifically involved in the production of an antifungal glycolipid (13), it sparked our interest to determine if this plant pathogen could theoretically display biocontrol activity under natural conditions on the basis of this property. As a matter of fact, Teichmann et al. (21) did report that ustilagic acid was fungitoxic toward B. cinerea spores, which led the group to suggest a potential biocontrol role for U. maydis. Our results showed that cyp1 was not expressed by U. maydis under any of the conditions tested, including B. cinerea-infected plants. Furthermore, a GFP-transformed strain of the pathogen showed hardly discernible fluorescent structures when applied to healthy or infected leaves. This conclusion supports results obtained by Clément-Mathieu et al. (3), who compared many related Pseudozyma species that had the ability to produce glycolipids. They were able to link biocontrol activity with biomass development and found that only P. flocculosa could display biocontrol activity, a result suggesting that factors other than glycolipid production were involved in its antagonistic properties.
In order to identify some of those factors that could stimulate biocontrol activity/growth of P. flocculosa, we proceeded to fractionate YNB, a synthetic yeast extract, known to promote cell growth over flocculosin production (7). This approach led us to the conclusion that, of all the constituents present in YNB, the complex Zn/Mn alone was sufficient to initiate the growth transition. Given their relatively recent identification, very little is known about the physiology of Pseudozyma spp. in general and P. flocculosa in particular. From previous observations, P. flocculosa cells in a resting stage will produce flocculosin as long as a source of sugar is available (7). Transition to filamentous growth, typical of P. flocculosa development on powdery mildew colonies, can be obtained by the simple addition of trace elements in the culture medium. What remains unclear is the relative importance of flocculosin release and filamentous growth in the overall process of antagonism that seems specifically directed toward powdery mildews. In this work, the observation that a simple application of a Zn/Mn solution at the surface of the leaf stimulated the growth of the fungus to levels approaching that of the presence of powdery mildew indicates a role of these elements in the biocontrol activity of P. flocculosa. Both Mn and Zn together with Fe have been reported to be the only trace elements essential for growth of certain fungi (20). While iron has been found to be a limiting element in the biocontrol activity of Pseudomonas fluorescens and its release of antibiotics (22), it does not appear to play a role in flocculosin production. For its part, Mn is known to be specifically involved in many cellular processes, such as cell wall synthesis, sporulation, and carbon metabolism (12, 23). The omission of Mn2+ from the nutrient medium of Aspergillus niger resulted in abnormal morphological development characterized by increased spore swelling and squat bulbous hyphae (11). From a biological point of view, Zn is one of the most important elements, is involved in every form of life (14), and has long been known to be essential for the growth of many fungi and important in the structure and function of enzymes involved in nucleic acid metabolism and cell division (5). Although the importance of Zn and Mn in the life cycle of most microorganisms is undeniable, the literature defining a role for the combined action of Zn and Mn is much more scarce. It thus appears that P. flocculosa has developed a rather unique metabolism that is dependent on the complex Zn/Mn to antagonize powdery mildew cells. Exploitation of this property could lead to a more efficient use of the BCA in controlling the disease in natural systems.
U. maydis, in spite of its close genetic link to P. flocculosa and ability to produce antifungal glycolipids, did not respond to the same plant, pathogen, or nutrient stimuli used in this study. Conversely, other fungi, such as B. cinerea, did not seem to provide the same favorable conditions for P. flocculosa to grow to an antagonistic level. From these observations, we can speculate that nutrients extracted from the plant by the powdery mildew haustoria contribute to the development of P. flocculosa, which in turn causes the collapse of the pathogen (6). This is further confirmed by the observation that P. flocculosa will develop on living powdery mildew cells only (3). What is unclear is whether flocculosin participates in this complex process by causing the release of nutrients from powdery mildew structures. Admittedly, the creation of a P. flocculosa cyp1 mutant defective in flocculosin production would represent the best tool to determine with exactitude the role of this glycolipid in the ecology of the fungus. However, our numerous efforts to obtain such a mutant have met with frustration. Bölker's group (9) was able to obtain a cyp1 mutant with U. maydis that was deficient in ustilagic acid production. This mutation did not appear to interfere with the mating ability or the pathogenesis of the fungus. The authors suggested that it may act as a pheromone carrier for mating recognition, a seemingly vestigial trait in P. flocculosa, given its inability to mate. The recent sequencing of the P. flocculosa genome (8) will prove useful in trying to establish how a similar trait conserved by two organisms with different lifestyles can affect their fitness.
Acknowledgments
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Research Chairs Program to R. R. Bélanger.
Footnotes
Published ahead of print on 29 November 2010.
REFERENCES
- 1.Begerow, D., R. Bauer, and T. Boekhout. 2000. Phylogenetic placements of ustilaginomycetous anamorphs as deduced from nuclear LSU rDNA sequences. Mycol. Res. 104:53-60. [Google Scholar]
- 2.Cheng, Y. L., et al. 2003. Insertional mutagenesis of a fungal biocontrol agent led to discovery of a rare cellobiose lipid with antifungal activity. Appl. Environ. Microbiol. 69:2595-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clément-Mathieu, G., F. Chain, G. Marchand, and R. R. Bélanger. 2008. Leaf and powdery mildew colonization by glycolipid-producing Pseudozyma species. Fungal Ecol. 1:69-77. [Google Scholar]
- 4.Elad, Y., and K. Evensen. 1995. Physiological aspects of resistance to B. cinerea. Phytopathology 85:637-643. [Google Scholar]
- 5.Failla, M. L. 1977. Cyclic accumulation of zinc by Candida utilis during growth in batch culture. J. Gen. Microbiol. 99:85-97. [DOI] [PubMed] [Google Scholar]
- 6.Hajlaoui, M. R., and R. R. Bélanger. 1993. Antagonism of the yeast-like phylloplane fungus Sporothrix flocculosa against Erysiphe graminis var tritici. Biocontrol Sci. Technol. 3:427-434. [Google Scholar]
- 7.Hammami, W., C. Labbé, F. Chain, B. Mimee, and R. R. Bélanger. 2008. Nutritional regulation and kinetics of flocculosin synthesis by Pseudozyma flocculosa. Appl. Microbiol. Biotechnol. 80:307-315. [DOI] [PubMed] [Google Scholar]
- 8.Hammami, W., F. Chain, D. Michaud, and R. R. Bélanger. 2010. Proteomic analysis of the metabolic adaptation of the biocontrol agent Pseudozyma flocculosa leading to glycolipid production. Proteome Sci. 8:7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewald, S., K. Josephs, and M. Bölker. 2005. Genetic analysis of biosurfactant production in Ustilago maydis. Appl. Environ. Microbiol. 71:3033-3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarvis, W. R., L. A. Shaw, and J. A. Traquair. 1989. Factors affecting antagonism of cucumber powdery mildew by Stephanoascus flocculosus and Stephanoascus rugulosus. Mycol. Res. 92:162-165. [Google Scholar]
- 11.Kisser, M., C. P. Kubicek, and M. Röhr. 1980. Influence of manganese on morphology and cell wall composition of Aspergilllus niger during citric acid fermentation. Arch. Microbiol. 128:26-33. [DOI] [PubMed] [Google Scholar]
- 12.Kubicek, C. P., and M. Röhr. 1977. Influence of manganese on enzyme synthesis and citric acid accumulation in Aspergillus niger. Eur. J. Appl. Microbiol. 4:167-175. [Google Scholar]
- 13.Marchand, G., et al. 2009. Identification of genes potentially involved in the biocontrol activity of Pseudozyma flocculosa. Phytopathology 99:1142-1149. [DOI] [PubMed] [Google Scholar]
- 14.Mestek, O., J. Komínková, R. Koplík, and M. Suchánek. 2001. Determination of zinc in plant samples by isotope dilution inductively coupled plasma mass spectrometry. Talanta 54:927-934. [DOI] [PubMed] [Google Scholar]
- 15.Mimee, B., C. Labbé, and R. R. Bélanger. 2009. Catabolism of flocculosin, an antimicrobial metabolite produced by Pseudozyma flocculosa. Glycobiology 19:995-1001. [DOI] [PubMed] [Google Scholar]
- 16.Mimee, B., C. Labbé, R. Pelletier, and R. R. Bélanger. 2005. Antifungal activity of flocculosin, a novel glycolipid isolated from Pseudozyma flocculosa. Antimicrob. Agents Chemother. 49:1597-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neveu, B., M. Michaud, F. Belzile, and R. R. Bélanger. 2007. The Pseudozyma flocculosa actin promoter allows the strong expression of a recombinant protein in the Pseudozyma species. Appl. Microbiol. Biotechnol. 74:1300-1307. [DOI] [PubMed] [Google Scholar]
- 18.Neveu, B., C. Labbé, and R. R. Bélanger. 2007. GFP technology for the study of biocontrol agents in tritrophic interactions: a case study with Pseudozyma flocculosa. J. Microbiol. Methods 68:275-281. [DOI] [PubMed] [Google Scholar]
- 19.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST227) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawla, G. S. 1969. A note on trace elements for the growth of Nigrospora oryzae (B. and Br.) Petch. New Phytol. 68:941-943. [Google Scholar]
- 21.Teichmann, B., U. Linne, S. Hewald, M. A. Marahiel, and M. Bölker. 2007. A biosynthetic gene cluster for a secreted cellobiose lipid with antifungal activity from Ustilago maydis. Mol. Microbiol. 66:525-533. [DOI] [PubMed] [Google Scholar]
- 22.Temple, T. N., V. O. Stockwell, J. E. Loper, and K. B. Johnson. 2004. Bioavailability of iron to Pseudomonas fluorescens strain A506 on flowers of pear and apple. Phytopathology 94:1286-1294. [DOI] [PubMed] [Google Scholar]
- 23.Zonneveld, B. J. M. 1975. Sexual differentiation in Aspergillus nidulans. The requirement for manganese and its effect on α-1,3 glucan synthesis and degradation. Arch. Microbiol. 105:101-104. [DOI] [PubMed] [Google Scholar]