Abstract
Posaconazole (PSC) is an antifungal drug recommended as an alternative for the treatment of invasive aspergillosis in patients who are refractory or intolerant to primary antifungal therapy. We have evaluated the in vitro activity of PSC against 21 strains of the Aspergillus terreus complex using both broth microdilution and disk diffusion (Neo Sensitabs) methods. PSC showed the same high level of activity against all the strains with the two in vitro methods used. We developed a murine model of disseminated infection to evaluate the efficacy of PSC at 5, 10, or 20 mg/kg of body weight twice a day by using 6 different strains chosen randomly. PSC showed good efficacy, especially at 20 mg/kg, as measured by prolonged survival, tissue burden reduction, histopathology, and lowered galactomannan levels. The PSC levels in serum on the fourth day of treatment were higher than the MICs for the strains tested.
Invasive aspergillosis remains a major cause of morbidity and mortality in immunocompromised hosts and has become more common over the last few decades (2). The most common species causing invasive infections are Aspergillus fumigatus and Aspergillus flavus. However, Aspergillus terreus has also become an increasingly significant pathogen in recent years (1). Despite its toxicity, amphotericin B (AMB) has been the first choice for the treatment of invasive aspergillosis for many years. However, the intrinsic resistance of A. terreus to polyenes has been reported (7, 8, 15), and different data from previous in vitro and animal studies and clinical practice suggest that AMB is not an effective option for invasive A. terreus infections (7, 15, 16, 19). New treatment strategies, such as the use of expanded-spectrum triazoles like voriconazole (VRC) as a first-line treatment and posaconazole (PSC) as prophylaxis and salvage therapy, are recommended in clinical practice guidelines for invasive aspergillosis (18). There are a few reports of experiments on the efficacy of PSC in disseminated infections caused by A. terreus (7, 19). However, in such studies only one strain was tested, and as it is well known that the antifungal susceptibility of a given species can be strain dependent, the results of such studies cannot be considered conclusive. In this study we have tested a significant number of strains in order to determine if the putative susceptibility of A. terreus complex isolates to PSC can be generalized to most of the strains of this group. We have tested the in vitro activity of PSC using both broth microdilution and disk diffusion methods against a set of strains of the A. terreus complex, and we have evaluated the efficacy of such a drug against six randomly chosen strains in a neutropenic murine model of disseminated infection in order to evaluate if the in vitro data are useful for predicting clinical outcomes.
MATERIALS AND METHODS
Twenty-one clinical strains of the Aspergillus terreus complex were used in the in vitro study (Table 1). Their susceptibilities to PSC were evaluated by using two methods: a broth microdilution method, performed according to CLSI guidelines for filamentous fungi (3), and a disk diffusion method, using nonsupplemented Mueller-Hinton agar and tablets (Neo Sensitabs; Rosco Diagnostica, Taastrup, Denmark) containing 5 μg of PSC (4). The MIC (μg/ml) and inhibition zone diameters (IZDs) (mm) were read at 24 and 48 h, respectively (3, 6). The suggested breakpoints for PSC against molds are as follows: susceptible, ≤1 μg/ml and ≥17 mm; intermediate, 2 μg/ml and 14 to 16 mm; and resistant, ≥4 μg/ml and ≤13 mm for MICs and IZDs, respectively (3, 5).
TABLE 1.
In vitro activities of PSC against 21 isolates of the Aspergillus terreus complex
| Strain | MIC (μg/ml) | IZD (mm) |
|---|---|---|
| FMR 8751 | 0.25 | 34.2 |
| FMR 8752 | 0.25 | 29.3 |
| FMR 8759 | 0.12 | 33.6 |
| FMR 8805 | 0.25 | 33.6 |
| FMR 8806 | 0.25 | 32.3 |
| FMR 8300 | 0.25 | 32.7 |
| FMR 8301 | 0.25 | 30 |
| FMR 6581 | 0.25 | 29.3 |
| FMR 8195 | 0.25 | 26 |
| FMR 8380 | 0.25 | 27 |
| FMR 8387 | 0.25 | 30.5 |
| FMR 8753 | 0.12 | 28.7 |
| FMR 8754 | 0.12 | 30.7 |
| FMR 8755 | 0.25 | 30.2 |
| FMR 8758 | 0.25 | 29 |
| FMR 8761 | 0.25 | 28 |
| FMR 8762 | 0.12 | 27 |
| FMR 11214 | 0.12 | 26 |
| FMR 10937 | 0.25 | 24 |
| FMR 11215 | 0.25 | 29 |
| FMR 11216 | 0.12 | 29.5 |
| Paecilomyces variotti (ATCC 36257) | 0.25 | 39 |
For in vivo studies, six randomly chosen strains of the A. terreus complex previously tested in vitro were used. They were FMR 8806, FMR 8754, FMR 8753, FMR 8759, FMR 8752, and FMR 10937.
The isolates were stored at −80°C, and prior to testing they were subcultured on potato dextrose agar (PDA) at 35°C. On the day of infection, cultures on PDA were suspended in sterile saline and filtered through sterile gauze to remove clumps of spores or hyphae. The resulting suspensions were adjusted to the desired inoculum based on hemocytometer counts and by serial plating onto PDA to confirm viability.
Male OF1 mice (Charles River, Criffa S.A., Barcelona, Spain) weighing 30 g were used in this study. Animals were housed under standard conditions. All animal care procedures were supervised and approved by the Universitat Rovira i Virgili Animal Welfare and Ethics Committee. Animals were immunosuppressed 1 day prior to infection by a single intraperitoneal (i.p.) injection of 200 mg/kg of body weight of cyclophosphamide (Genoxal; Laboratories Funk S.A., Barcelona, Spain), plus a single intravenous (i.v.) injection of 150 mg/kg of body weight of 5-fluorouracyl (Fluorouracilo; Ferrer Farma S.A., Barcelona, Spain). This immunosuppression has demonstrated peripheral blood polymorphonuclear leukocyte (PMN) counts of <100 PMNs/ml from days 3 to 9 or later. Mice were challenged with 2 × 105 CFU in 0.2 ml of sterile saline, injected via the lateral tail vein. Preliminary experiments testing several strains demonstrated that this inoculum was appropriate for producing an acute infection, with 100% of the animals dying within 10 days (data not shown).
The drug assayed was PSC (Noxafil; Schering-Plough Ltd., Hertfordshire, United Kingdom), administered at 5, 10, or 20 mg/kg of body weight/dose twice a day (BID) by gavage. The efficacy of PSC was evaluated as prolonging survival in comparison to controls, by tissue burden reduction, by histopathological studies, and by determinations of galactomannan serum levels by an enzyme immunoassay. Treatments began 1 day after infection and lasted for 7 days. For survival studies, groups of 10 mice were randomly established for each strain and each treatment and checked daily for 30 days after challenge. Controls received no treatment. For tissue burden studies, groups of 10 mice were also established, and the animals were sacrificed on day 4 postinfection in order to compare the results with those for the controls. Lungs, kidneys, and brain were aseptically removed, and approximately half of each organ was weighed and homogenized in 1 ml of sterile saline. Serial 10-fold dilutions of the homogenates were plated onto PDA and incubated for 48 h at 35°C. The numbers of CFU/g of tissue were calculated. For the histopathology study, half of each organ was fixed with 10% buffered formalin. Samples were dehydrated, paraffin embedded, and sliced into 2-μm sections, which were stained with hematoxylin-eosin (H-E), periodic acid-Schiff (PAS) stain, or Grocott methamine silver and examined in blinded fashion by light microscopy.
Groups of 5 immunosuppressed mice were challenged with strain FMR 8752 to determine the galactomannan serum levels (Platelia Aspergillus; Bio-Rad, Marnes la Coquette, France) on the seventh day of treatment. Galactomannan values were expressed as a galactomannan index (GMI), defined as the optical density of a sample divided by the optical density of a threshold serum provided in the test kit (14). Additionally, groups of 5 immunosuppressed mice were challenged with the same strain and treated with PSC at 5, 10, or 20 mg/kg BID to determine levels of this drug in serum by bioassay 4 h after drug administration on day 4 of therapy (13).
The mean survival time was estimated by the Kaplan-Meier method and compared among groups by using the log rank test. Colony counts from tissue burden studies were analyzed by using the Mann-Whitney U test.
RESULTS
The results for antifungal in vitro susceptibility are shown in Table 1. PSC showed a high level of activity against all strains of the A. terreus complex tested, with both MIC and IZD values being included in the suggested ranges of susceptibility, i.e., all the strains showed MICs of ≤1 μg/ml and IZDs of ≥17 mm.
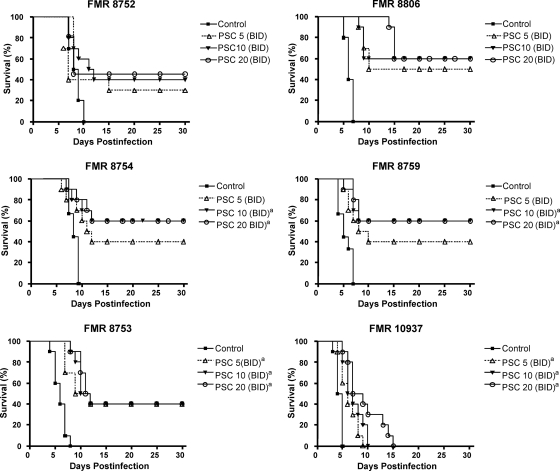
Figure 1 shows the efficacy of PSC in prolonging the survival of mice. For all strains, PSC at 10 or 20 mg/kg significantly prolonged survival with respect to the control group. Moreover, PSC at 5 mg/kg also significantly prolonged survival with respect to the untreated group for two of the strains assayed. For all the strains the rate of survival ranged between 40% and 60% at the end of assay, with the exception of strain FMR 10937, which produced 100% mortality.
FIG. 1.
Cumulative mortality of mice infected with 2 × 105 CFU of A. terreus complex isolates (n = 10 animals per group). (Top left) FMR 8752; (top right) FMR 8806; (middle left) FMR 8754; (middle right) FMR 8759; (bottom left) FMR 8753; (bottom right) FMR 10937. PSC 5, posaconazole at 5 mg/kg BID; PSC 10, posaconazole at 10 mg/kg BID; PSC 20, posaconazole at 20 mg/kg BID. a, P < 0.05 versus the control.
In general, PSC at either 10 or 20 mg/kg BID was able to significantly reduce the fungal load in all organs tested (Table 2). For all strains, PSC at 20 mg/kg was the most effective treatment in reducing the fungal load in kidneys, lungs, and brain with respect to the control group, and in some cases, with respect to the other PSC regimens, PSC at 5 mg/kg BID was clearly less effective.
TABLE 2.
Effects of PSC on colony counts of A. terreus in kidney, brain, and lung of micea
| Strain and treatment | Mean log10 CFU/g of tissue (95% CI) |
||
|---|---|---|---|
| Kidney | Brain | Lung | |
| FMR 8752 | |||
| None | 4.49 (4.18-4.81) | 3.48 (3.29-3.65) | 3.39 (2.93-3.84) |
| PSC 5 | 2.63 (2.55-2.70)b | 2.88 (2.33-3.43) | 2.90 (2.45-3.35) |
| PSC 10 | 2.38 (2.17-2.60)b | 2.53 (2.29-2.78)b | 2.76 (2.60-2.92) |
| PSC 20 | 2.11 (1.99-2.23)b,c | 1.82 (1.32-2.33)b,c | 2.61 (2.35-2.87)b |
| FMR 8806 | |||
| None | 2.62 (2.45-2.80) | 2.58 (1.53-3.63) | 2.88 (1.78-3.98) |
| PSC 5 | 2.32 (2.14-2.50)b | 2.45 (1.49-3.40) | 2.65 (1.98-3.32) |
| PSC 10 | 2.31 (2.10-2.52)b | 1.58 (0.74-2.42) | 2.22 (1.51-2.93) |
| PSC 20 | 1.89 (1.62-2.16)b,c,d | 1.27 (0.47-2.06)b,c | 1.74 (1.22-2.26)b |
| FMR 8754 | |||
| None | 4.13 (3.91-4.35) | 3.63 (3.14-4.12) | 2.42 (1.70-3.15) |
| PSC 5 | 2.73 (2.15-3.31)b | 2.65 (2.07-3.23)b | 1.33 (0.78-1.89)b |
| PSC 10 | 1.76 (1.22-2.30)b | 0.61 (0.11-1.11)b,c | 0.83 (0.24-1.42)b |
| PSC 20 | 1.29 (0.71-1.88)b,c | 0.40 (0.30-0.76)b,c | 0.38 (0.07-0.68)b,c |
| FMR 8759 | |||
| None | 4.21 (4.02-4.40) | 3.98 (3.53-4.43) | 3.23 (2.43-4.03) |
| PSC 5 | 3.16 (2.16-3.71) | 3.18 (2.60-3.75)b | 2.39 (1.70-3.08) |
| PSC 10 | 1.90 (1.10-2.71)b | 1.01 (0.33-1.69)b,c | 1.25 (0.34-2.16)b,c |
| PSC 20 | 1.97 (1.37-2.56)b,c | 0.40 (0.30-0.76)b,c | 1.11 (0.32-1.89)b,c |
| FMR 8753 | |||
| None | 2.99 (1.77-4.20) | 3.98 (3.72-4.23) | 3.21 (2.25-4.16) |
| PSC 5 | 2.27 (1.83-2.71) | 3.07 (2.29-3.86) | 2.48 (1.68-3.29) |
| PSC 10 | 1.76 (1.20-2.32) | 0.80 (0.06-1.54)b,c | 1.18 (0.51-1.85)b,c |
| PSC 20 | 1.63 (1.14-2.12) | 0.62 (0.02-1.22)b,c | 1.23 (0.53-1.94)b,c |
| FMR 10937 | |||
| None | 3.39 (2.93-3.85) | 4.08 (3.99-4.17) | 3.41 (2.92-3.90) |
| PSC 5 | 2.88 (2.45-3.31) | 4.00 (3.87-4,13) | 2.82 (2.48-3.17) |
| PSC 10 | 2.56 (2.31-2.86)b | 3.24 (2.82-3.67)b,c | 1.11 (0.53-1.68)b,c |
| PSC 20 | 2.23 (1.65-2.80)b,c | 2.57 (1.90-3.24)b,c,d | 0.81 (0.28-1.35)b,c |
n = 10 animals per group. PSC 5, posaconazole at 5 mg/kg BID; PSC 10, posaconazole at 10 mg/kg BID; PSC 20, posaconazole at 20 mg/kg BID; CI, confidence interval.
P < 0.05 versus control.
P < 0.05 versus PSC at 5 mg/kg BID.
P < 0.05 versus PSC at 10 mg/kg BID.
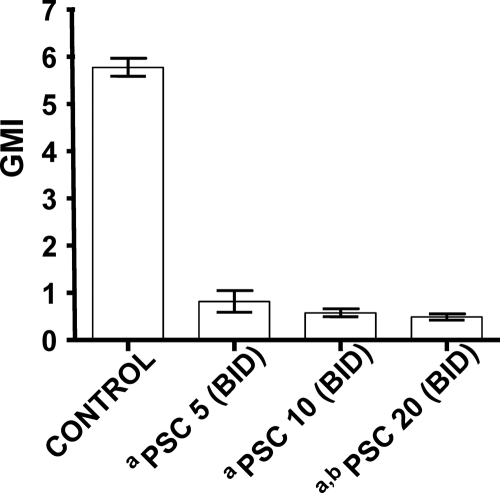
Galactomannan serum levels were significantly lower in mice treated with any regimen of PSC than in controls animals (Fig. 2). The galactomannan concentration in serum at day 7 of the experiment was significantly lower in animals treated with 20 mg/kg PSC BID than in those treated with 5 mg/kg PSC BID.
FIG. 2.
Galactomannan serum levels in A. terreus-infected mice measured on day 7 (final treatment) (n = 5 animals per group). PSC 5, posaconazole at 5 mg/kg BID; PSC 10, posaconazole at 10 mg/kg BID; PSC 20, posaconazole at 20 mg/kg BID. a, P < 0.05 versus control; b, P < 0.05 versus PSC 5. GMI, galactomannan index.
PSC serum levels were as follows: the drug concentration in serum for treatment with 5 mg/kg PSC BID was 2.56 ± 0.93 μg/ml, that for treatment with 10 mg/kg PSC BID was 5.65 ± 0.62 μg/ml (P < 0.05 versus PSC at 5 mg/kg BID), and that for treatment with 20 mg/kg PSC BID was 6.45 ± 0.28 μg/ml (P < 0.05 versus PSC at 5 mg/kg BID; n = 5 animals per group). All serum concentrations were higher than the PSC MIC for the strain tested. No statistically significant differences were found between the serum levels obtained with PSC at 10 mg/kg and those obtained with PSC at 20 mg/kg, with these levels being significantly higher than those obtained with PSC at 5 mg/kg.
In untreated controls and in mice treated with PSC at 5 mg/kg BID, the histological study showed kidney, lung, and brain fungal invasion, with signs of necrosis in kidney and brain and the presence of interstitial edema in lung tissue. Neither tissue lesions nor fungal invasion was observed for sections of organs from mice treated with PSC at 10 or 20 mg/kg BID (Fig. 3).
FIG. 3.
Histopathology of brain, lungs, and kidneys of neutropenic mice infected by A. terreus and treated with PSC. (A to C) Brain (PAS stain) (magnification, ×300) (A), kidney (Grocott stain) (magnification, ×300) (B), and lung (Grocott stain) (magnification, ×450) (C) sections from untreated mice or mice treated with 5 mg/kg BID showing tissue invasion by hyphae. (D to F) Brain (PAS stain) (magnification, ×300) (D), kidney (PAS stain) (magnification, ×300) (E), and lung (PAS stain) (magnification, ×300) (F) of mice treated with 20 mg/kg BID showing the absence of fungal elements and the absence of an inflammatory response.
DISCUSSION
At present, PSC is considered an alternative for the treatment of patients with invasive aspergillosis that is refractory or intolerant to primary antifungal therapy (18). We have evaluated the effectiveness of PSC against a relative large number of strains of the A. terreus complex, which is unusual in this type of study, where only a single strain is generally tested.
In previous studies, a good in vitro activity of PSC against A. terreus was demonstrated (9, 12). In this study, excellent correlation was found between the in vitro results obtained with microdilution and disk diffusion methods, suggesting that the disk diffusion method could be a good alternative for determining the susceptibility of PSC, due to its simplicity, low cost, and high reproducibility.
So far, the efficacy of PSC for the treatment of experimental infections by A. terreus has been evaluated by only two studies (7, 19). Using a rabbit model, Walsh et al. (19) demonstrated the efficacy of PSC at 20 mg/kg. Graybill et al. (7), using a murine model similar to the one used in the current study, demonstrated the efficacy of PSC at 40 mg/kg in prolonging survival and reducing the fungal load in spleen and lungs. However, those authors tested only one strain and did not evaluate other PSC dosages. We have demonstrated the efficacy of PSC at 10 and 20 mg/kg BID in prolonging mouse survival and in reducing fungal burden in brain, kidneys, and lungs against infections by six strains, which indicated a generally good response of the members of the A. terreus complex to PSC. An interesting finding of the present study was the relationship between the in vivo results, the PSC serum levels, and the dose-related reduction of serum galactomannan levels obtained. The latter test is a useful criterion of aspergillosis morbidity and a marker of clinical antifungal efficacy and prognosis (10, 11, 14). We have confirmed that this test is a good marker of fungal tissue burden in experimental invasive aspergillosis infections (17).
In summary, our study has shown the high level of in vitro activity and good efficacy of PSC against experimental invasive infections by different isolates of the A. terreus complex. Further studies are warranted to assess the potential use of PSC as a primary option for the treatment of human A. terreus infections.
Footnotes
Published ahead of print on 29 November 2010.
REFERENCES
- 1.Blum, G., et al. 2008. A 1-year Aspergillus terreus surveillance study at the University Hospital of Innsbruck: molecular typing of environmental and clinical isolates. Clin. Microbiol. Infect. 12:1146-1151. [DOI] [PubMed] [Google Scholar]
- 2.Chandrasekar, P. 2009. Invasive mold infections: recent advances in management approaches. Leuk. Lymphoma 50:703-715. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, 2nd ed. Document M38-A2. CLSI, Wayne, PA.
- 4.Clinical and Laboratory Standards Institute. 2009. Method for antifungal disk diffusion susceptibility testing of filamentous fungi; proposed guideline. Document M51-P. CLSI, Wayne, PA.
- 5.Espinel-Ingroff, A., et al. 2007. Multicenter evaluation of a new disk agar diffusion method for susceptibility testing of filamentous fungi with voriconalzole, posaconazole, itraconazole, amphotericin B, and caspofungin. J. Clin. Microbiol. 45:1811-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A., and E. Canton. 2008. Comparison of Neo-Sensitabs tablet diffusion assay with CLSI broth microdilution M38-A and disk diffusion methods for testing susceptibility of filamentous fungi with amphotericin B, caspofungin, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 46:1793-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graybill, J. R., S. Hernandez, R. Bocanegra, and L. K. Najvar. 2004. Antifungal therapy of murine Aspergillus terreus infection. Antimicrob. Agents Chemother. 48:3715-3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachem, R. Y., et al. 2004. Aspergillus terreus: an emerging amphotericin B-resistant opportunistic mold in patients with hematologic malignancies. Cancer 101:1594-1600. [DOI] [PubMed] [Google Scholar]
- 9.Lass-Flörl, C., A. Alastruey-Izquierdo, M. Cuenca-Estrella, S. Perkhofer, and J. L. Rodríguez-Tudela. 2009. In vitro activities of various antifungal drugs against Aspergillus terreus: global assessment using the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 53:794-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marr, K. A., et al. 2004. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J. Infect. Dis. 190:641-649. [DOI] [PubMed] [Google Scholar]
- 11.Petraitiene, R., et al. 2001. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob. Agents Chemother. 45:857-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., et al. 2008. In vitro survey of triazole cross-resistance among more than 700 clinical isolates of Aspergillus species. J. Clin. Microbiol. 46:2568-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez, M. M., et al. 2009. Effects of double and triple combinations of antifungal drugs in a murine model of disseminated infection by Scedosporium prolificans. Antimicrob. Agents Chemother. 53:2153-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sionov, E., S. Mendlovic, and E. Segal. 2006. Efficacy of amphotericin B-intralipid in combination with caspofungin against experimental aspergillosis. J. Infect. 53:131-139. [DOI] [PubMed] [Google Scholar]
- 15.Steinbach, W. J., et al. 2004. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin. Infect. Dis. 39:192-198. [DOI] [PubMed] [Google Scholar]
- 16.Steinbach, W. J., J. R. Perfect, W. A. Schell, T. J. Walsh, and D. K. Benjamin, Jr. 2004. In vitro analyses, animal models, and 60 clinical cases of invasive Aspergillus terreus infection. Antimicrob. Agents Chemother. 48:3217-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallor, A. C., et al. 2008. Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob. Agents Chemother. 52:2593-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh, T. J., et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327-360. [DOI] [PubMed] [Google Scholar]
- 19.Walsh, T. J., et al. 2003. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J. Infect. Dis. 188:305-331. [DOI] [PubMed] [Google Scholar]