Abstract
Catheters and other indwelling devices placed inside human body are prone to bacterial infection, causing serious risk to patients. Infections associated with implants are difficult to resolve, and hence the prevention of bacterial colonization of such surfaces is quite appropriate. In this context, the development of novel antimicrobial biomaterials is currently gaining momentum. We describe here the preparation and antibacterial properties of an enzyme-embedded polycaprolactone (PCL)-based coating, coimpregnated with the antibiotic gentamicin sulfate (GS). The enzyme uses PCL itself as substrate; as a result, the antibiotic gets released at a rate controlled by the degradation of the PCL base. In vitro drug release studies demonstrated sustained release of GS from the PCL film throughout its lifetime. By modulating the enzyme concentration in the PCL film, we were able to vary the lifetime of the coating from 33 h to 16 days. In the end, the polymer is completely degraded, delivering the entire load of the antibiotic. The polymer exhibited antibacterial properties against three test isolates: Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Foley urinary catheters coated with the modified polymer exhibited sustained in vitro release of GS over a 60-h period. The results suggest that the antibiotic-plus-enzyme-loaded polymer can be used as tunable self-degrading antimicrobial biomaterial coating on catheters.
Hospital-acquired infections are responsible for considerable mortality and morbidity in humans (19). Nosocomial infections are predominantly associated with microbial biofilm formation on implants or indwelling medical devices. Implant-related infections are considered serious complications of surgery and are often very difficult to treat. Medical implants (catheters, stents, intrauterine devices, etc.) placed inside the human body provide ideal surfaces for bacterial colonization (2). Urinary tract infections (UTIs) are the most prevalent among hospital-acquired infection, 80% of which is associated with the risk of catheter-associated urinary tract infection (CAUTI) (26). Microbes colonize the catheter surfaces, leading to formation of a biofilm (7, 16). Two types of bacteria, planktonic (free floating) and sessile (adherent), may exist in the infected bladder (22, 26), eliciting an immune response that results in acute and chronic cystitis with pyuria (4). With time, microbes may move up to the kidneys, and a similar biofilm may develop within the pelvis and tubular systems; the subsequent inflammatory response is recognized as acute or chronic pyelonephritis (4). As a result, there has been a great deal of interest in devising strategies to minimize biofilm formation to prevent CAUTI (26).
The most widely used materials for urological devices are silicone, latex, or polyurethane-based polymers (13). Various coating approaches have been proposed to prevent CAUTI, including the use of silver or silver containing compounds (1, 11), some of which gave encouraging results in clinical trials (4). The clinical effectiveness of silver appears to be dependent on the silver matrix used (26). Soft hydrogel- or silver hydrogel-coated catheters are proposed to provide an antiseptic effect by creating a physical barrier to resist infection (12, 23). Medical devices coated with antibiotics such as gentamicin, norfloxacin, nitrofurazone, minocycline, and rifampin have been developed for biofilm prevention (6, 24, 26, 28). However, most of these coatings suffer from short-lived antibiotic release profile (12), making them inappropriate for relatively long-term use.
One plausible approach to overcome biofilm-mediated infection involves the use of biodegradable polymeric coating impregnated with antibiotics. The degradation of the polymer will lead to release of the antibiotic and thereby the prevention of microbial growth. A guiding principle in the development of such a polymer-based coating is the need for sustained release of the antimicrobial agent during the entire course of catheterization. In order to accomplish this, it should be ensured that (i) the antimicrobial agent is uniformly distributed within the polymer and (ii) the degradation of the polymer is uniform. Another important aspect is that since the duration of catheterization is dependent on the clinical condition, the duration of the antibiotic release should be tunable from a few days to a few months (13). Therefore, one should ideally be able to modulate the antimicrobial agent release rate and also control the lifetime of the polymer film. In this direction, we developed a novel polycaprolactone (PCL)-based biocatalytic polymer as a potential coating for urinary medical devices. In in vitro tests, the polymer based antimicrobial coating exhibited sustained release of gentamicin sulfate (commonly used as an antibiotic agent for the treatment of UTIs) (4) at inhibitory concentrations. PCL is an extensively studied polymer; it is nontoxic, biocompatible, biodegradable, and compatible with many drugs (27).
PCL being hydrophobic, our first challenge was to uniformly distribute the water soluble antibiotic in the polymer phase. We used hydrophobic ion pairing (HIP), a technique by which one can solubilize hydrophilic molecules such as an active pharmaceutical ingredient or proteins in organic solvents (20). In this method, the charged moiety of target molecule can be ion paired with oppositely charged surfactant, which would provide partial hydrophobicity to the target molecule, substantially increasing its solubility in low-dielectric organic solvent (9). Furthermore, the surfactant would act as a shield against the destructive effect of the organic solvent, thereby preventing the loss of activity of the target molecule (9). In order to ensure uniform PCL degradation and to be able to modulate the lifetime and gentamicin sulfate (GS) release rate, we incorporated the PCL-degrading enzyme lipase B from Candida antarctica (CALB) in the PCL matrix. The water-soluble enzyme was rendered hydrophobic by coating with a surfactant to ensure uniform distribution within the PCL matrix. It was anticipated that embedding the enzyme in the polymer would make the PCL base bio-absorbable and, as a consequence of lipase action, the PCL would degrade at uniform rate, thereby leading to the continuous release of GS. It was also postulated that by varying the concentration of the lipase, it would be possible to modulate the lifetime of the polymer film.
The modified polymer film, coimpregnated with hydrophobic ion-paired GS and surfactant-coated enzyme (CALB) film was termed the “lipase-gentamicin-impregnated polymer” (LGIP). We describe here the preparation of the polymer film and demonstrate uniformity of its antibiotic release profile. Its performance is compared to that of two control polymer preparations, namely, (i) PCL-alone film and (ii) PCL film impregnated with GS but not with lipase. We also show that GS release from LGIP is able to prevent the planktonic and biofilm modes of growth of three test organisms, viz. Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Finally, the utility of LGIP as coating material on Foley catheter is demonstrated under in situ conditions.
MATERIALS AND METHODS
Materials.
Gentamicin (80 mg/2 ml) was obtained from Ranbaxy Laboratories, New Delhi, India. Lipase from Candida antarctica (CALB) was purchased from Fluka, Germany. Sodium bis(2-ethylhexyl)sulfosuccinate (AOT) and polycaprolactone (PCL, average Mr = ∼65,000) were obtained from Sigma-Aldrich, St. Louis, MO. All organic solvents (AR grade) were from Merck, Germany. Ortho phthalic carboxyaldehydye (OPA), isopropyl alcohol (IPA), sodium borate, methanol, and 2-mercaptoethanol were obtained from Merck, Germany.
Bacterial strains and their MICs.
P. aeruginosa PAO1 (wild type) (14), Escherichia coli (14), and Staphylococcus aureus V329 strain (29) were chosen as test organisms to study the antimicrobial activity of the PCL film in the present study. Cultures were grown overnight in LB at 30°C. Later, the cultures were washed twice with normal saline and further diluted to 105 CFU/ml for use as an inoculum for the growth experiments. The MIC of gentamicin was determined according to the National Committee for Clinical Laboratory Standards guidelines by the agar dilution technique (21). The MIC was recorded as the lowest concentration of the antibiotic allowing no visible growth.
Hydrophobic ion-pairing GS.
To extract GS in organic solvent, the method of Mader et al. (18) was used with some modification. GS from the injection vials was lyophilized (Heto Holten A/S, Denmark), and the lyophilized GS powder (20 mg) was dissolved in buffer containing 10 mM potassium chloride, 10 mM sodium acetate, and 10 mM calcium chloride (pH 5.0). To this, an equal volume of 2 mM AOT/dichloromethane (DCM) was added. The biphasic mixture was vortexed for 1 min, followed by centrifugation at 5,000 rpm for 5 min to obtain clear phases. The organic phase was separated and dried by nitrogen purging to obtain GS-AOT complex. The complex weighed 10 mg.
Surfactant coating lipase.
Two concentrations of CALB (7.55 and 28 U) were used for the coating procedure. The enzyme was dissolved in 20 mM sodium phosphate buffer (pH 7.5). To each, an equal volume of 10 mM AOT dissolved in 20 mM sodium phosphate buffer (pH 7.5) was added. The resulting solution was incubated at 4°C for 8 h, followed by lyophilization (Heto Holten A/S, Denmark) to get completely dried AOT-coated CALB weighing 8.0 and 20 mg (for 7.55 and 28 U of CALB, respectively).
Modified polymer preparation. (i) Lipase-gentamicin-impregnated polymer (LGIP) film.
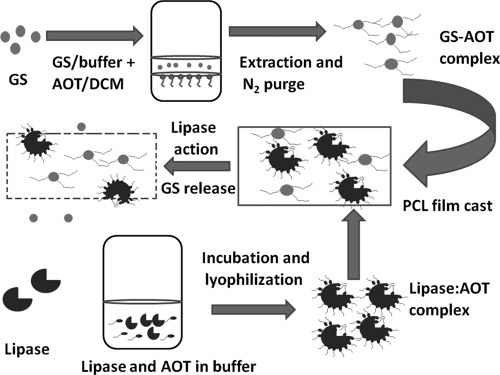
GS-AOT complex (10 mg) and lipase-AOT conjugate (8 mg) were separately dissolved in 400 μl of a 10% (wt/vol) PCL-toluene mix. Just before casting, the two were mixed thoroughly by vortexing at 100 rpm. The resulting polymer solution was clear and devoid of any suspended particles. It was cast on glass slides (5.5 by 2.5 cm), and the film was air dried overnight at 25°C. The film weighed 115 mg and contained 11% (wt/wt) GS-AOT complex and 7.55 U of AOT-coated CALB. All of the experiments were carried out using the above composition unless stated otherwise. The preparation of the LGIP is shown as a schematic diagram in Fig. 1.
FIG. 1.
Schematic diagram of LGIP preparation.
(ii) Control films.
Two types of films were used as controls in the experiments. The first, referred to here as the Control film, contained only PCL. It was made by film casting 800 μl of 10% PCL-toluene mix. The second, referred to here as the Expt-Control film, consisted of PCL film containing GS-AOT complex. GS-AOT complex (10 mg) was dissolved in 800 μl of 10% PCL-toluene mix and film cast as described above. The films weighed 100 and 108 mg, respectively.
In vitro GS release studies and protein measurements.
In vitro release studies were performed by incubating the dried polymer films in petri dishes containing 20 ml of phosphate-buffered saline (PBS; pH 7.5) at 30°C. After each time interval, the entire spent buffer was removed (and used for GS and lipase estimation) and replaced with fresh 20 ml of PBS.
GS and CALB estimation.
Estimation of the GS released into the leachate was carried out by the OPA method (9). Briefly, the derivatization reagent was prepared by dissolving 0.5 g of OPA in 12.5 ml of methanol after stirring at room temperature for 30 min. To this solution, 112.5 ml of 0.04 M sodium borate buffer (pH 9.4) was added, along with 0.6 ml of 2-mercaptoethanol. The resulting solution was stored in an amber bottle at 4°C. For GS analysis, 0.5 ml of sample, 0.5 ml of IPA, and 0.5 ml of OPA reagent were mixed and incubated in dark at room temperature for 45 min. Absorbance at 333 nm was measured spectrophotometrically (Shimadzu, Japan). The lipase released from the film into the leachate was estimated as protein by using a BCA protein assay kit (Pierce, Rockford, IL).
Planktonic antimicrobial activity.
The antibacterial activity of the polymer films was tested against three bacterial isolates viz., E. coli, P. aeruginosa, and S. aureus at 30°C. The polymer films (the LGIP, Expt-Control, and Control films) were incubated in petri plates containing 20 ml of Tris medium (pH 7.5) with 1% glucose. Inocula containing 105 E. coli, P. aeruginosa, and S. aureus cells were separately added to each petri plate. After each time interval, the entire spent medium was replaced with 20 ml of fresh growth medium containing fresh inoculum (105 cells). The spent medium was serially diluted and plated on LB agar, followed by incubation at 30°C for 24 h for bacterial growth monitoring.
Different media were used for GS leaching and planktonic growth studies. OPA (see above) was found to react with Tris, and this interfered with the GS estimation procedure. Therefore, a GS release study was carried out in PBS, and growth measurements were made with Tris minimal medium containing 1% glucose.
Antibiofilm activity.
A biofilm assay was carried out to study the ability of released GS to inhibit colonization by P. aeruginosa. LGIP (containing 11% GS-AOT and 7.55 U of AOT-modified CALB) and the Expt-Control film (containing 11% GS-AOT) were incubated in 20 ml of Tris medium (pH 7.5) containing 1% glucose. Into this, 105 P. aeruginosa cells were inoculated and, at the end of every 24 h, the entire spent medium was replaced with 20 ml of fresh growth medium. During each buffer exchange, fresh inoculum (105 cells) was also added. The bacterial biofilm formation on the PCL films (LGIP and Expt-Control) was monitored by confocal microscopy after 3, 7, and 15 days of incubation.
Technique for catheter coating.
A 5.0-mm-diameter Foley catheter (catalog no. 180630; Willy Rusch, Germany) was obtained and aseptically cut into 1-cm sections. The sections were dipped in LGIP mix (containing 11% [wt/wt] GS-AOT complex and 7.55 U of AOT-coated CALB dissolved in 800 μl of 10% PCL-toluene) for 2 min and air dried overnight at 25°C. The polymer loading on the catheter was 6.46 mg/cm2.
In vitro release of GS from catheter.
Catheter segments coated with LGIP were individually placed in vials containing 2.0 ml of PBS. The catheters were incubated at 30°C with shaking at 100 rpm. At 30 min and 1, 3, 5, 12, 18, 24, 36, 48, and 60 h, 2.0-ml portions of spent buffer were removed for GS estimation and replaced with fresh buffer.
Confocal laser scanning microscopy and image analysis.
The polymer films (LGIP and Expt-Control) were incubated in 20 ml of Tris medium (pH 7.5) containing 1% glucose. About 105 cells of P. aeruginosa were inoculated. After each time interval, the polymer samples were withdrawn for microscopic analysis. The films were stained with 0.01% acridine orange. All microscopic observations and image analyses were performed with a Leica TCS SP2 AOBS confocal laser scanning system (Leica Microsystems, Germany) attached to an inverted microscope (Leica DMIRE2). A 63× 1.2 numerical aperture water immersion objective lens was used to acquire all images.
SEM measurements.
Polymer samples were applied to carbon-coated specimen stubs, and images were obtained by field-emission scanning electron microscopy (SEM; ESEM XL30; Philips, Netherlands) at an accelerating potential of 15 kV and a working distance of 15 mm. The diameters of the pores were measured by using ImageJ freeware (National Institutes of Health).
Statistics.
All experiments were done in triplicate. Therefore, the data are representative of at least three independent experiments and are reported as means ± the standard deviations (SD).
RESULTS
Microorganisms and their sensitivities.
The susceptibilities of the test bacteria used in the present study toward GS were evaluated. The GS MICs against the three test cultures—P. aeruginosa PAO1, S. aureus V329, and E. coli JM101—were determined to be 4, 1, and 1 μg/ml, respectively.
GS leaching rate from LGIP and Expt-Control films.
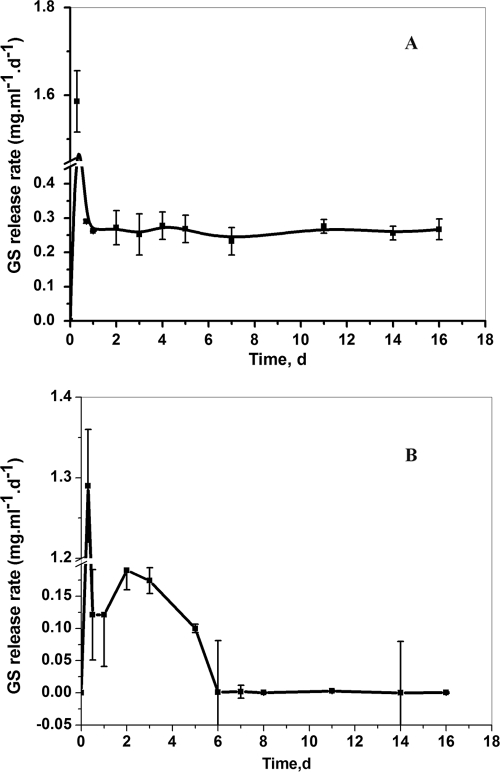
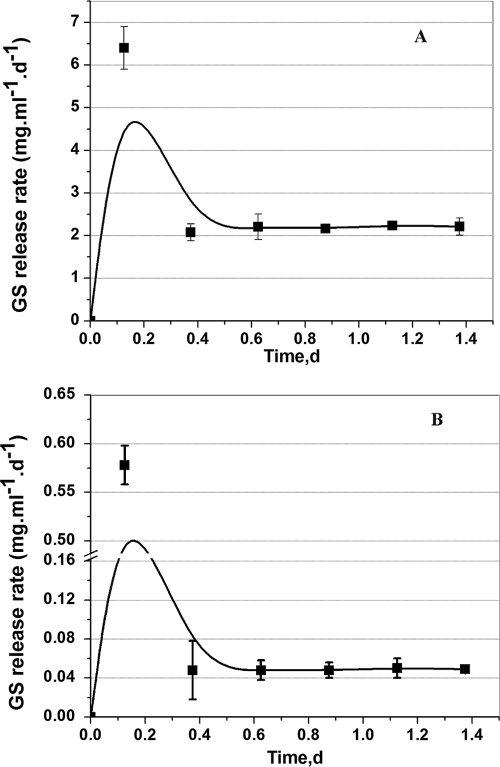
Figure 2A displays the release rates of GS from the LGIP (PCL film embedded with 11% GS-AOT complex and 7.55 U of AOT-coated lipase). During the initial 3 h after incubation, a burst release of GS (∼1.6 mg/ml/day) was observed; thereafter, the release rate sharply declined and stabilized at about ∼0.25 mg/ml/day from 24 h to 16 days of incubation. During the course of incubation, the film also underwent self-degradation and weight loss. By day 14, the film underwent 85% weight reduction (gravimetric data not shown). The entire film was completely degraded by the day 16 of incubation.
FIG. 2.
(A) GS release rate from the LGIP (PCL film embedded with 7.55 U of AOT-coated lipase and 11% [wt/wt] GS-AOT complex). On day 16 the LGIP film is completely degraded. (B) GS release rate from the Expt-Control film (PCL film embedded with 11% [wt/wt] GS-AOT complex). Each point represents the mean ± the SD of three experiments.
PCL being biodegradable, the antimicrobial agent incorporated in the polymer can be expected to be released as the polymer gets degraded. The added advantage of enzyme-triggered release was ascertained by studying the drug release profile from enzyme-laden film vis-à-vis that from a polymer film containing only GS but not the enzyme. The results showed that GS release from naturally degrading PCL film (Expt-Control film embedded with only 11% GS-AOT complex, but no lipase) was sporadic and not suitable for controlled release applications (Fig. 2B). The release rate was nonuniform and started declining rapidly from day 5 of incubation. The large error bars (Fig. 2B) in the GS release rate indicate that the antibiotic released by natural degradation of the polymer is highly unpredictable and therefore undesirable for controlled delivery systems.
LGIP undergoes degradation by lipase leading to release of CALB in the bulk medium. Figure 3 shows the protein (CALB) leaching rate from LGIP. Burst release of protein is seen during initial 3 h, followed by uniform release at the rate of 0.23 to 0.24 mg/ml/day from 24 h to 16 days of incubation.
FIG. 3.
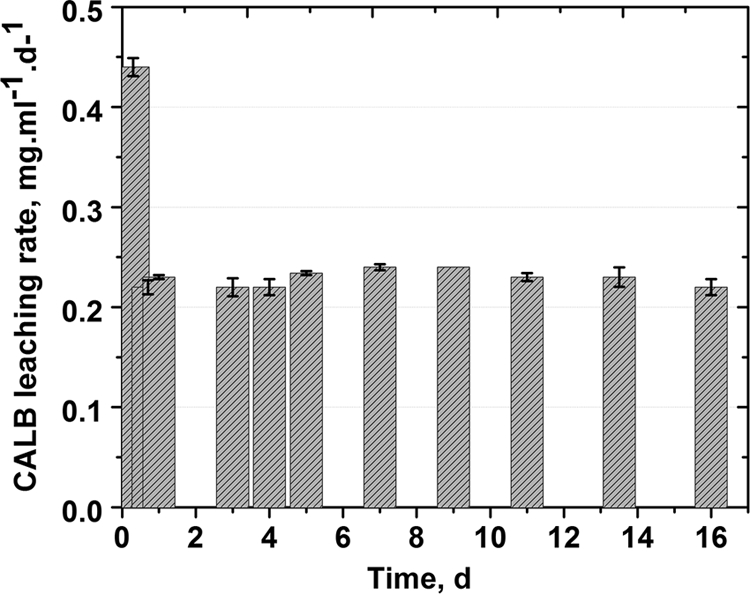
Protein release profile from the LGIP (PCL film embedded with 7.55 U of AOT-modified lipase and 11% [wt/wt] GS-AOT complex). Each point represents the mean ± the SD of three experiments.
Planktonic antibacterial activity.
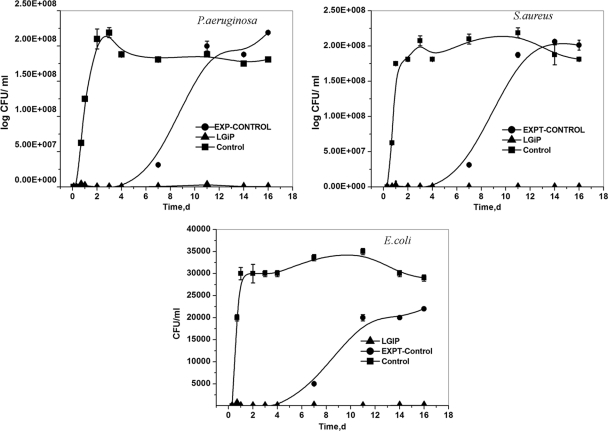
The antibacterial activity of the LGIP, the Expt-Control, and Control films was studied by measuring the viable counts of the test microorganisms. Figure 4 displays the growth curves of the three bacterial isolates when incubated in medium containing LGIP, Expt-Control, or Control films. No growth was recorded throughout the incubation period when the test organisms were grown in the presence of LGIP, whereas the Expt-Control films exhibited growth inhibition until day 5 of incubation. Upon further incubation in the presence of Expt-Control films, the turbidity increased, clearly indicating that the release of GS was insufficient to inhibit the bacterial growth. After 6 days, a rapid increase in the growth could be seen, presumably as a result of GS release below MIC levels (Fig. 2B).
FIG. 4.
Antimicrobial activities of LGIP (PCL film embedded with 7.55 U of AOT-coated lipase and 11% [wt/wt] GS-AOT complex), Expt-Control (PCL film embedded with 11% [wt/wt] GS-AOT complex), and Control (PCL film only) films. The films were exposed to 20 ml of Tris-glucose medium (pH 7.5) containing 105 cells as an inoculum at 30°C. At each time interval, the spent buffer was withdrawn to measure the viable counts. The spent medium was then replaced with a fresh growth medium containing 105 cells as an inoculum and reincubated. LGIP (•), Expt-Control (▴), and Control (▪) data are indicated. Each point represents the mean ± the SD of three experiments.
The anti-biofilm capability of the LGIP was also investigated by studying the biofilm formation by PAO1 on the polymeric surface by using confocal laser scanning microscopy. Figure 5A shows the initiation of PAO1 adhesion on Expt-Control films on day 3, which increased on day 7 of incubation (Fig. 5B). By day 15, a well-formed PAO1biofilm with several microcolonies was established on the Control film surface (Fig. 5C). However, on the LGIP film (Fig. 5D to F), significant inhibition of biofilm formation was observed throughout the incubation period. Although some cells adhered on the polymer surface, no microcolony formation was observed even after 15 days of incubation, clearly demonstrating an anti-biofilm property of the LGIP (Fig. 5F).
FIG. 5.
Confocal microscopic images of LGIP and Expt-Control films (embedded with 11% [wt/wt] GS-AOT complex). The films were exposed to 20 ml of Tris-glucose medium containing 105 cells as an inoculum at pH 7.5 at 30°C. At various time intervals, the polymer films were used for microscopic analysis. (A to C) Expt-Control film after 3, 7, and 15 days of incubation, respectively; (D to F) LGIP film after 3, 8, and 15 days of incubation, respectively.
PCL morphology during degradation.
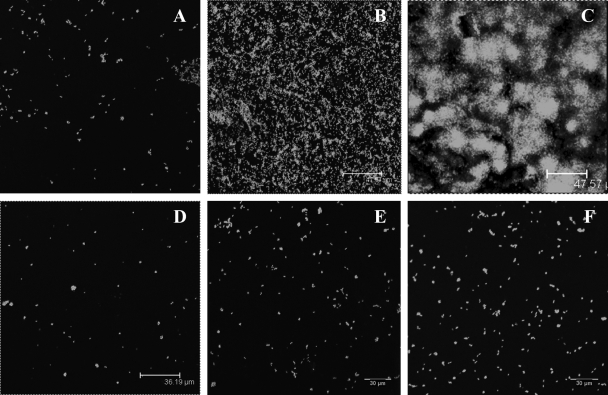
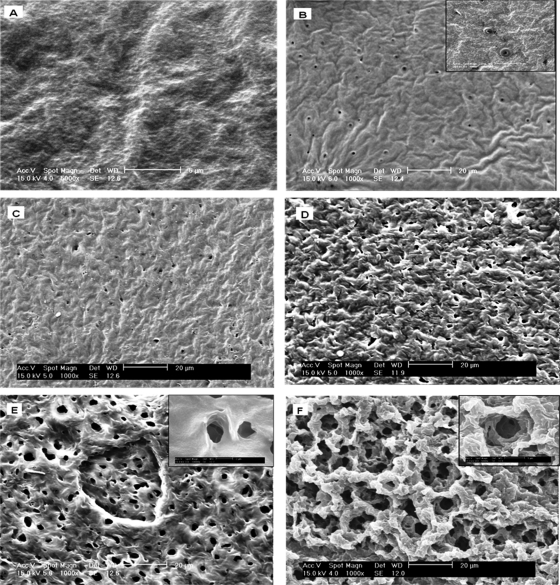
SEM was used to monitor the degradation of the LGIP and Control films. The SEM images recorded at 0 h and after 7 h of incubation of the Expt-Control films are shown in Fig. 6A and B, respectively. Prior to incubation in buffer (0 h) the surface of film appears uniform (Fig. 6A). However, after 24 h, the SEM image shows that the film developed pores (with an average diameter of 10 μm) distributed over a smooth surface (Fig. 6B). Further incubation of the film for 11 days did not lead to any significant change in its topology, except that the film became slightly rippled (Fig. 6C). On the contrary, LGIP displays initiation of the pores on the surface on the film at 3 h (Fig. 6D), with the pores (ca. 15 μm in diameter) being larger than the ones present in PCL film embedded with GS alone (10 μm, Fig. 7B). On further incubation, the film developed an extensive network of pores. The average diameter of the pores increased to 37 μm by day 24 (Fig. 6E). As can be seen from Fig. 6C and F, the morphology of the pores in the control film on day 11 is completely different from that seen in the LGIP film on day 11. The inset images clearly show the difference in the morphology of the pores formed due to natural degradation (Fig. 6B) and through enzymatic degradation (Fig. 6E and F). The pores formed in the former are superficial, whereas the ones formed in the latter show the surface beneath the topical layer, which also has undergone degradation. On day 11, the LGIP is seen to be highly eroded, displaying significant degradation of the film. From the results of the SEM study, analyzed in conjunction with GS and lipase release, it is obvious that when the lipase enzyme is embedded in the PCL film, incubation results in the formation of a network of pores, leading to the release of GS into the media, effectively inhibiting bacterial growth.
FIG. 6.
SEM images of LGIP and Expt-Control PCL films incubated in sterile PBS buffer (pH 7.5) at 30°C. (A) PCL film at 0 h (before exposure to the medium); (B) Expt-Control film at 3 h (the inset shows the topology of pores taken at a higher resolution [scale bar, 5 μm]); (C) Expt-Control film after 11 days of incubation; (D) LGIP film at 3 h; (E) LGIP film at 24 h (the inset shows the topology of pores taken at a higher resolution [scale bar, 2 μm]); (F) LGIP film on day 11 of incubation (the inset shows the topology of pores taken at a higher resolution [scale bar, 5 μm]).
FIG. 7.
(A) GS release profile from PCL film embedded with 28 U of AOT-modified lipase and 11% (wt/wt) GS-AOT complex immersed in PBS buffer (pH 7.5) at 30°C. (B). GS release profile from PCL film embedded with 28 U of AOT-modified lipase and 3% (wt/wt) GS-AOT complex immersed in PBS buffer (pH 7.5) at 30°C. Each point represents the mean ± the SD of three experiments.
Modulating the coating lifetime and GS release.
The PCL film embedded with 7.55 U of AOT-coated lipase completely disintegrates on day 16 of incubation (Fig. 2A). We attempted to design LGIP for a shorter lifetime, displaying both higher and lower GS release rates. Figure 7 shows that by increasing the initial concentration of AOT-coated lipase from 7.55 U (Fig. 2) to 28 U (Fig. 8), the lifetime of the coating was shortened from 16 days to 33 h (1.37 days). We postulated that a higher concentration of lipase would increase the degradation rate, thereby decreasing the polymer lifetime. While keeping the concentration of GS-AOT complex constant (11% [wt/wt] initial loading), we observed an increase in the GS release rate as the lipase concentration was increased from 7.55 to 28 U, as shown in Fig. 2A and 7A. The GS release rate increased from ∼0.25 mg ml−1 day−1 (11% [wt/wt] initial loading, 7.55 U of AOT modified lipase, Fig. 2A) to ∼2.18 mg ml−1 day−1 (11% [wt/wt] initial loading, 28 U of AOT modified lipase, Fig. 7A). Similarly, decreasing the initial loading of GS from 11% (wt/wt), as shown in Fig. 2A, to 3%, as shown in Fig. 7B, also decreased the GS release rate from ∼ 0.25 to ∼0.05 mg ml−1 day−1.
FIG. 8.
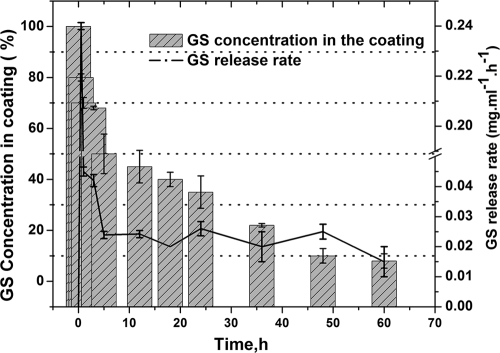
GS release profile from LGIP (PCL film embedded with 7.55 U of AOT-modified lipase and 11% [wt/wt] GS-AOT complex) coated on a urinary catheter immersed in PBS buffer (pH 7.5) at 30°C and 100 rpm. Each point represents the mean ± the SD of three experiments.
In vitro GS release from catheter.
As shown in Fig. 8, GS was released with an initial burst. After 5 h, 50% of the incorporated antibiotic was released from the coating into the medium. This was followed by a steady-state release. After 24 h an additional 15% was released, and after 48 h 90% of the antibiotic was released in the buffer.
DISCUSSION
In the last two decades, a number of antimicrobial-coated catheter designs have been developed and studied for their ability to control bacterial adhesion and biofilm formation (5, 25). However, most of the coatings suffer from various limitations, especially short-term or nonuniform release of the antimicrobial from the catheter (5, 17). The nonuniform release can often be attributed to the method of incorporation, such as mechanical mixing to embed the antibiotic in the coating matrix. In order to circumvent this limitation, it is necessary to adopt a procedure that ensures uniform distribution of the antimicrobial agent. Moreover, when the antimicrobial agent possesses a high degree of hydrophilicity, solubilizing it in a hydrophobic matrix becomes difficult, which may result in nonuniform release. Considering this fact, we adopted hydrophobic-ion-pairing (HIP) approach for uniform distribution of the hydrophilic antibiotic in the hydrophobic polymer base. Using the HIP method, we could incorporate the aminoglycoside antibiotic GS into a PCL base. GS was extracted from its aqueous solution into dichloromethane by forming an ion-paired complex with AOT. Toluene was used as a solvent for PCL solubilization and for casting PCL films. The ion-paired complex was completely solubilized in the polymer solution, visually displaying no aggregates in the cast polymer, compared to the unmodified GS powder, which aggregated in the polymer solution (data not shown).
Our results affirm the fact that the Expt-Control film (PCL film embedded with 11% [wt/wt] GS-AOT complex, Fig. 2B) only released GS at an inhibitory concentration during the initial period of incubation (5 days). After 5 days, there was a sharp decrease in GS release, the concentration reaching below inhibitory concentrations for all of the isolates, resulting in growth resumption (Fig. 4). We hypothesize that the main reason for nonuniform release profile from the Expt-Control film is the uncontrolled manner of polymer degradation.
To obtain uniform release of GS from the PCL, we incorporated lipase enzyme (CALB) into the polymer after treating it with AOT surfactant. This rendered the water-soluble enzyme completely soluble in toluene, the organic solvent used for casting. The resultant LGIP film exhibited a release profile that was far superior to unmodified PCL. Immediately after incubation (3 h), it released GS at a higher rate (1.6 mg/ml/day), followed by a steady-state release rate (∼0.25 mg/ml/day) during the rest of its entire lifetime (Fig. 2A). Such an initial burst release is desirable in infection control since it will be able to tackle the elevated risk of infection from bacteria introduced during the initial period (30). The effectiveness of antimicrobial coatings not only depends on the sustained release rates of the antibiotic but also on its concentration. The release of an antibiotic below its MIC may lead to development of bacterial resistance to the antibiotic, thus making it difficult to manage the infection (30). The MICs of GS against three test cultures (P. aeruginosa PAO1, S. aureus V329, and E. coli JM101) were determined (see Materials and Methods) in order to find out whether the concentration resulting from GS release from the polymer would be sufficient to suppress microbial growth. It was confirmed that the concentrations obtained from the LGIP film were in excess of the MIC for the three test cultures. In addition, when the release rates obtained from the LGIP and Expt-Control films were corroborated with the planktonic antimicrobial data shown in Fig. 4, it was clear that the former has a definite edge over the latter in controlling bacterial growth during the entire period. In the Expt-Control film, the antibacterial activity was seen only until day 5 of incubation. LGIP displayed a uniform antibiotic release invariably higher than the MIC of the test microorganisms. Based on these results, it is safe to conclude that LGIP is unlikely to lead to resistance development in the bacteria concerned. However, this requires further experimental validation.
Our study suggests that LGIP achieved the two objectives that we set out to achieve: (i) steady and consistent release of GS at inhibitory levels, rendering the polymer antibacterial, and (ii) complete removal of the PCL base at the end. The latter would ensure that no polymer substratum (that could serve as base for microbial adherence) remains after cessation of the GS release.
Biodegradation of polymers such as PCL occurs through enzyme and/or chemical hydrolysis (8). Therefore, the availability of water in the film would act as the limiting factor in the degradation process. Investigations using SEM allowed us to ascertain the difference in degradation mechanism occurring in LGIP and control films (Fig. 6). Based on the SEM results, we propose the following mechanism to explain the release of GS from the LGIP. (i) The polymer comes into contact with aqueous medium. (ii) Degradation starts at the water-polymer interface, presumably by both natural degradation and enzymatic processes, leading to the formation of pores (Fig. 6D), accompanied by the release of the GS into the bulk. (iii) Upon further incubation, water enters the pores through capillary action and reaches the interior of the polymer through the network of pores. This kind of degradation process would ensure GS release from within the film rather than from the surface alone. This is evident from the SEM images (Fig. 6E and F) and the GS release data (Fig. 2A). SEM data show that incubation of LGIP results in the formation of network of pores, which are ∼15 μm in diameter within 3 h, increasing to 37 μm by 24 h. On day 11, the LGIP has an extensive network of pores (the degraded polymer layer beneath the topical layer can be seen in the insets in Fig. 6E and F). It is presumed that the formation of such a network facilitates the percolation of water to the interior of the film. The control film, in contrast, forms pores of 10 μm after 24 h of incubation (Fig. 6B). The porosity does not increase on further incubation (Fig. 6C). The GS release in this case is only observed until day 6 of incubation, the reason being that the GS release is occurring mostly at the polymer-water interface. Since the control film degradation is slow and restricted to the surface (15), water percolation into the interior of the film is inadequate, thereby limiting the release of GS in the later phase of incubation.
Our data also show that LGIP coating on catheter material is possible and that the desired leaching characteristics could be achieved. We coated LGIP onto a standard (Foley) urinary catheter and studied the GS release rate from the coating and also the remaining GS concentration present in the coating as a function of time. Although GS release behavior from the coating (Fig. 8) was similar (initial burst release followed by steady release) to that of the LGIP film (Fig. 2A), the release rates and the lifetime were significantly different. In the former case, the entire LGIP preparation was cast into film, whereas in the latter the catheter was coated by dipping it in the preparation. The degradation of the polymer depends on various parameters, including water penetration: the thicker the coating, the longer the time taken for water penetration therefore, leading to a longer lifetime. The thickness of the LGIP was 100 ± 5 μm, whereas the LGIP coating on the catheter was 20 ± 1 μm thick (data not shown). We presume that the lifetime of the catheter coating was shorter (2.5 days, Fig. 8) than that of the LGIP film (16 days, Fig. 2A) due to the difference in their respective thicknesses. This provides us with an option to modulate the lifetime of the coating by adjusting its thickness (for example, by multiple dipping).
Catheters are required for both short- and long-term use; therefore, the anti-infection control strategy should work for both short and long durations. AOT-modified lipase, when used at two different concentrations, resulted in different lifetimes of the LGIP: (i) 7.55 U corresponded to 16 days (Fig. 2B), and (ii) 28 U corresponded to 36 h (Fig. 7A). Similarly, antibiotic dose requirements depend on the type of infection and the organisms involved (30). Higher concentrations of antimicrobials are required for administrating biofilms, because of poor penetration into biofilm and the higher tolerance of biofilm-resident bacteria (11). By increasing the enzyme loading 3.7-fold (from 7.55 to 28 U), the GS release rate in- creases ∼9-fold, the GS-AOT complex loading being the same (Fig. 2A and 7A). Moreover, by manipulating the amount of GS-AOT complex in the film (11 and 3%), keeping the concentration of AOT-modified lipase constant, we were able to obtain two different release rates (Fig. 7A and b).
With regard to application of the coatings on catheters placed within the body, we acknowledge that enzyme biocompatibility could be an issue. However, studies are already in progress on enzymes that are homologous to the enzymes found in animal systems (10). AOT, the surfactant used for coating lipase and ion-pairing with GS, is already accepted for encapsulation and delivery of lipophilic drugs in water-based delivery systems (3). However, further studies are required to substantiate this.
To summarize, the present study demonstrates a facile approach to developing a tunable, self-removing polymer based on the dual incorporation of (i) ion-paired antibiotic and (ii) a surfactant-coated polymer-degrading enzyme. The importance of an impregnation procedure for the development of effective antibacterial material is demonstrated. Surfactant-based HIP and coating methodology ensured complete dissolution of the water-soluble antibiotic (GS) and enzyme (lipase) in the organic solvent, without their activity being compromised. The degradation of the polymer was monitored, based on which a mechanism for the antibiotic release has been proposed. In laboratory experiments, the biomaterial was able to prevent suspended microbial growth and resist surface microbial colonization. In conclusion, we demonstrate here the feasibility of an enzyme-triggered, controlled release of antimicrobial substance from a polymeric base for the control of surface-bound microbial growth on catheters.
Acknowledgments
This research was supported by DST-FAST TRACK grant (SR/FT/LS-031/2007). R.N.D. is the recipient the Young Scientist Fellowship from Department of Science and Technology, Government of India.
We thank Iñigo Lasa, Instituto De Agrobiotechnologia, Universidad Publica de Navarra/Consejo Superior de Investigaciones Científicas, Campus de Arrosadia, Spain, for providing the Staphylococcus aureus strain and Stephen P. Diggle, Institute of Infection, Immunity and Inflammation, Centre for Biomolecular Sciences, University Park, University of Nottingham, Nottingham, United Kingdom, for providing the Pseudomonas aeruginosa strain. We also thank our coworkers S. V. Narasimhan and B. Anupkumar for helpful discussions and M. Radhika, Physical Metallurgy Division, IGCAR, Kalpakkam, India, for SEM analysis.
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Akiyama, H., and S. Okamoto. 1979. Prophylaxis of indwelling urethral catheter infection: clinical experience with a modified Foley catheter and drainage system. J. Urol. 121:40-42. [DOI] [PubMed] [Google Scholar]
- 2.Campbell, A. A., et al. 2000. Development, characterization, and antimicrobial efficacy of hydroxyapatite-chlorhexidine coatings produced by surface-induced mineralization. J. Biomed. Mater. Res. 53:400-407. [DOI] [PubMed] [Google Scholar]
- 3.Chavanpatil, M. D., et al. 2007. Surfactant-polymer nanoparticles overcome P-glycoprotein-mediated drug efflux. Mol. Pharmaceutics 4:730-738. [DOI] [PubMed] [Google Scholar]
- 4.Cho, Y.-H., et al. 2001. Prophylactic efficacy of a new gentamicin-releasing urethral catheter in short-term catheterized rabbits. BJU Int. 87:104-109. [DOI] [PubMed] [Google Scholar]
- 5.Cho, Y.-W., et al. 2003. Gentamicin-releasing urethral catheter for short-term catheterization. J. Biomater. Sci. Polymers 14:963-972. [DOI] [PubMed] [Google Scholar]
- 6.De Jong, E. S., et al. 2001. Antimicrobial efficacy of external fixator pins coated with a lipid stabilized hydroxyapatite/chlorhexidine complex to prevent pin tract infection in a goat model. J. Trauma 50:1008-1014. [DOI] [PubMed] [Google Scholar]
- 7.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldaster, C., B. Erlansson, R. Renstad, A.-C. Albertsson, and S. Karlsson. 2000. The biodegradation of amorphous and crystalline regions in film-blown poly (ɛ-caprolactone). Polymers 41:1297-1304. [Google Scholar]
- 9.Falk, R., T. W. Meyer, J. D. Kelly, and R. M. Manning. 1997. Controlled release of ionic compounds from poly(l-lactide) microspheres produced by precipitation with a compressed antisolvent. J. Control Rel. 44:77-85. [Google Scholar]
- 10.Ganesh, M., R. N. Dave, W. L. L'Amoreaux, and R. A. Gross. 2009. Embedded enzyme biomaterial degradation. Macromolecules 42:6836-6839. [Google Scholar]
- 11.Guggenbichler, J. P., and U. Samuel. 2004. Prevention of catheter-related infections: the potential of a new nano-silver impregnated catheter. Int. J. Antimicrob. Agents 23(Suppl. 1):S75-S78. [DOI] [PubMed] [Google Scholar]
- 12.Ha, U. S., and Y. H. Cho. 2006. Catheter-associated urinary tract infections: new aspects of novel urinary catheters. Int. J. Antimicrob. Agents 28:485-490. [DOI] [PubMed] [Google Scholar]
- 13.Jones, D. S., J. Djokic, J. McCoy, and S. P. Gorman. 2002. Poly(ɛ-caprolactone) and poly(ɛ-caprolactone)-polyvinylpyrrolidone-iodine blends as ureteral biomaterials: characterisation of mechanical and surface properties, degradation and resistance to encrustation in vitro. Biomaterials 23:4449-4458. [DOI] [PubMed] [Google Scholar]
- 14.Joshi, H., R. N. Dave, and V. P. Venugopalan. 2009. Competition triggers plasmid-mediated enhancement of substrate utilisation in Pseudomonas putida. PLoS One 4:e6065. doi: 10.1371/journal.pone.0006065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kister, G., G. Cassanas, M. Bergounhon, D. Hoarau, and M. Vert. 2000. Structural characterization and hydrolytic degradation of solid copolymers of d,l-lactide-co-ɛ-caprolactone by Raman spectroscopy. Polymers 41:925-932. [Google Scholar]
- 16.Lindsay, D., and A. V. Holy. 2006. Bacterial biofilms within the clinical setting: what healthcare professionals should know? J. Hosp. Infect. 64:313-325. [DOI] [PubMed] [Google Scholar]
- 17.Maki, D. G., and P. A. Tambyah. 2001. Engineering out the risk for infection with urinary catheters. Emerg. Infect. Dis. 7:342-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mader, J. T., J. Calhoun, and J. Cobos. 1997. In vitro evaluation of antibiotic diffusion from antibiotic-impregnated biodegradable beads and polymethylmethacrylate beads. Antimicrob. Agents Chemother. 41:415-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFee, R. B. 2009. Nosocomial or hospital-acquired infections. Dis. Monthly 55:422-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer, J. D., et al. 1995. Solution behavior of α-chymotrypsin dissolved in nonpolar organic solvents via hydrophobic ion pairing. Biopolymers 35:451-456. [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 1990. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; M7-A2. NCCLS, Villanova, PA.
- 22.Nickel, J. C., J. W. Costerton, and J. Downey. 1992. Movement of Pseudomonas aeruginosa along catheter surfaces: a mechanism in pathogenesis of catheter-associated infection. Urology 39:93-98. [DOI] [PubMed] [Google Scholar]
- 23.Parker, D., et al. 2009. Nursing interventions to reduce the risk of catheter-associated urinary tract infection. I. Catheter selection. J. Wound Ostomy Continence Nursing 36:23-34. [DOI] [PubMed] [Google Scholar]
- 24.Rauschmann, M. A., et al. 2005. Nanocrystalline hydroxyapatite and calcium sulfate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials 26:2677-2684. [DOI] [PubMed] [Google Scholar]
- 25.Reid, G., et al. 1994. Effects of ciprofloxacin, norfloxacin, and ofloxacin on in vitro adhesion and survival of Pseudomonas aeruginosa AK1 on urinary catheters. Antimicrob. Agents Chemother. 8:1490-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regev-Shoshani, G., M. Ko, C. Miller, and Y. Av-Gay. 2010. Slow release of nitric oxide from charged catheters and its effect on biofilm formation by Escherichia coli. Antimicrob. Agents Chemother. 54:273-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun, H., L. Mei, C. Song, X. Cui, and P. Wang. 2006. The in vivo degradation, absorption, and excretion of a PCL-based implant. Biomaterials 27:1735-1740. [DOI] [PubMed] [Google Scholar]
- 28.Teller, M., U. Gopp, H.-G. Neumann, and K.-D. Kühn. 2007. Release of gentamicin from bone regenerative materials: an in vitro study. J. Biomed. Mater. Res. B Appl. Biomater. 81:23-29. [DOI] [PubMed] [Google Scholar]
- 29.Trotonda, M. P., A. Manna, C. Cheung, A. L. Lasa, I. Penadés, and Y. R. SarA. 2005. Positively controls Bap-dependent biofilm formation in Staphylococcus aureus. J. Bacteriol. 187:5790-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zilberman, M., and J. J. Elsner. 2008. Antibiotic-eluting medical devices for various applications. J. Control Rel. 130:202-215. [DOI] [PubMed] [Google Scholar]