Abstract
Staphylococcus aureus is a major human pathogen responsible for a number of serious and sometimes fatal infections. One of its reservoirs on the human body is the skin, which is known to be a source of invasive infection. The potential for an engineered staphylococcus-specific phage lysin (ClyS) to be used for topical decolonization is presented. We formulated ClyS into an ointment and applied it to a mouse model of skin colonization/infection with S. aureus. Unlike the standard topical antibacterial agent mupirocin, ClyS eradicated a significantly greater number of methicillin-susceptible S. aureus (MSSA) and -resistant S. aureus (MRSA) bacteria: a 3-log reduction with ClyS as opposed to a 2-log reduction with mupirocin in our model. The use of ClyS also demonstrated a decreased potential for the development of resistance by MRSA and MSSA organisms compared to that from the use of mupirocin in vitro. Because antibodies may affect enzyme function, we tested antibodies developed after repeated ClyS exposure for their effect on ClyS killing ability. Our results showed no inhibition of ClyS activity at various antibody titers. These data demonstrate the potential of developing ClyS as a novel class of topical antimicrobial agents specific to staphylococcus.
Methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA) are Gram-positive bacteria known to cause both superficial and invasive disease in a variety of human hosts. Their ability to colonize skin, mucous membranes, and catheters puts certain populations, such as dialysis and atopic dermatitis/eczema patients, at risk for recurrent staphylococcal infections (10, 11, 13, 14, 17). Efforts at decolonizing patients who carry staphylococci in their nares and oropharynx and on their skin are not always successful and are often cumbersome and costly (26, 29). Use of oral or intravenous antibiotics for decolonization puts patients at risk for side effects, results in killing of beneficial flora along with the staphylococci, and increases the potential for antibiotic resistance. The key topical agent employed for decolonization is mupirocin, administered nasally and topically. However, mupirocin resistance rates have been increasing (19% to 24%) in recent years (7, 20). Mupirocin is preferentially active against Gram-positive bacteria, and as such, the ointment formulation is typically prescribed for presurgical patients and patients with indwelling catheters. It is recommended that patients be treated twice daily for 5 days prior to surgery, which poses a challenge for patient compliance, breeding further resistance. Due to the current deficiencies of available topical antistaphylococcal agents and with the advent of rapid microbial diagnostics, there is a need for effective topical agents for which the potential for the development of resistance is low, that work quickly, and that specifically target staphylococci.
Bacteriophage endolysins (lysins) are one such class of novel antimicrobials that are emerging as alternative agents for the prophylaxis and treatment of bacterial infections. Bacteriophages (phages) are viruses that infect bacteria. Phages are specific to the bacteria that they infect and have evolved to bind to unique and essential bacterial cell wall targets (12). The early widespread use of phages for therapeutic purposes was eclipsed by the discovery of antibiotics, such as penicillin. As the rate of resistance to antibiotics continues to rise, phage therapy may come into vogue again, now through the lysins that they produce. Lysins are bacterial cell wall hydrolases generated during the infection cycle of double-stranded DNA phages, enabling release of progeny virions by cleaving essential bonds in the cell wall peptidoglycan, resulting in hypotonic lysis. Lysins consist of a catalytic domain, which cleaves specific bonds in bacterial peptidoglycan and which tends to be conserved among the same class of hydrolases. The binding domain allows each lysin to target a specific substrate in the bacterial cell wall (usually carbohydrate), offering some species specificity to lysin molecules. When they are applied exogenously to Gram-positive bacteria, purified native or recombinant lysins are able to degrade the cell wall of susceptible bacteria and cause log fold cell lysis within seconds to minutes (12). ClyS (chimeric lysin for staphylococci) is a unique lysin specific to staphylococcus made recombinantly in our laboratory. ClyS is a chimera because it contains a staphylococcus-specific catalytic domain fused to a unique cell wall-targeting binding domain (9). The purified protein is highly active against all staphylococcal species, including lysostaphin- and mupirocin-resistant strains of S. aureus. ClyS kills methicillin-resistant and vancomycin-intermediate strains of S. aureus by 3 to 4 log units within a few minutes in vitro (9). Lysins pose a viable alternative to conventional antibiotics by being specific and highly effective, and the potential for the development of resistance to lysins is low.
We have developed a topical formulation of ClyS and assessed its therapeutic efficacy using a mouse skin infection model with MSSA and MRSA strains. We demonstrate the in vivo efficacy of ClyS compared to the efficacies of placebo and mupirocin after a single application, nonneutralization of the antibodies produced, and potential for the development resistance to ClyS in vitro compared to that for mupirocin.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains were stored at −80°C and grown at 37°C. The staphylococcal strains used were grown in Trypticase soy broth (TSB) medium. Staphylococcus MW2 is a MRSA strain that was purchased from the ATCC collection, and S. aureus strain 8325-4 is from The Rockefeller University collection.
ClyS production.
The chimeric lysin ClyS was constructed by fusing the N-terminal catalytic domain of the S. aureus Twort phage lysin with the C-terminal cell wall binding domain from another S. aureus phage lysin (phiNM3) as previously described (9). The chimeric gene was cloned into expression vector pJML6 and transformed into Escherichia coli DH5α cells. The ClyS molecule was purified to >90% homogeneity by two-step ion-exchange chromatography. The specificity of ClyS against various Gram-positive and Gram-negative bacterial species, including staphylococcus, streptococcus, enterococcus, bacillus, and pseudomonas species, was determined (9).
The in vitro activity of ClyS was quantified by lytic and killing assays. MICs were determined according to the Clinical and Laboratory Standards Institute (CLSI) guidelines for broth microdilution susceptibility testing methods in Mueller-Hinton broth (MHB) (6). ClyS was stored in phosphate-buffered saline (PBS; pH 7.4) at −80°C, with activity being retained for at least 6 months postfreezing. The frozen solution was aliquoted into milligram/ml dosage units and lyophilized. When this lyophilized powder was stored at −80°C, total activity was maintained for at least 6 months.
Topical ClyS formulation.
Lyophilized ClyS is incorporated into commercially prepared Aquaphor, which is an emollient ointment whose active ingredient is petrolatum.
In vitro activity of topical ClyS.
The in vitro activity of topical ClyS was assessed by mixing 1 ml of PBS with a dose of 10% ClyS in Aquaphor and centrifuging the mixture at 4,000 rpm for 10 min. This separated out Aquaphor from soluble ClyS. We then performed a lytic assay of the ClyS supernatant using S. aureus 8325-4 or MRSA MW2 cells to assess activity, as described in reference 9.
To illustrate the efficacy of ClyS on the staphylococcal cell wall, a 2-liter culture of S. aureus 8325-4 in MHB was centrifuged and the pellet was resuspended in 100 ml PBS. Agar was added to this solution to achieve a 1.5% concentration. The mixture was then autoclaved, to produce nonviable cells. The autoclaved staphylococcus-agar suspension was placed in empty 150-mm petri plates and allowed to solidify. A 10% dose of topical ClyS was streaked onto the surface of the agar, with Aquaphor alone being streaked onto the other side of the plate. The plates (activity of cell wall lysis) were observed and imaged after 12 h.
Histology.
Mouse skin biopsy specimens (diameter, 6 mm) were obtained from noninfected, control, placebo, and ClyS-treated mice (4 mice per group). Skin biopsy specimens were taken after one and two doses administered 18 h apart, as well as after an additional dose 2 months later. Biopsy specimens were frozen in OCT (Sakura, Tokyo, Japan) embedding material and stored at −80°C until required. Hematoxylin (Thermo Fisher) and eosin (Shandon) (H&E) staining was performed to inspect the dermal components before and after treatments; modified Brown and Brenn (American MasterTech) stain was used for visualization of bacteria. Staphylococci in mouse tissue were visualized by immunofluorescence using the purified binding domain of ClyS labeled with Alexa Fluor 647 (Invitrogen, Eugenia, OR). Briefly, the ∼10-kDa ClyS binding domain protein was expressed in E. coli DH5α and then purified in one step by cation-exchange chromatography. The ClyS binding domain (1 mg/ml) was complexed to Alexa Fluor 647, according to the manufacturer's instructions. Alexa Fluor-labeled ClyS was incubated with 0.6-mm-diameter mouse skin sections, fixed in acetone overnight, and washed with PBS twice on the following day. Slides were observed using appropriate filters of a Zeiss Axioplan 2 wide-field fluorescence microscope with a Plan Neofluar 20-by-0.7-numerical-aperture lens and a Hamamatsu Orca ER-cooled charge-coupled-device camera, controlled by METAVUE software (MDS Analytical Technologies, Downington, PA). This technique was used on control, placebo, and ClyS-treated mouse skin sections. Staphylococcal burden was determined by measuring the depth from the epidermis to the dermis, where staphylococci could be found (treatment depth), using National Institutes of Health software (NIH IMAGE software, version 6.1), which captures an image of a 1-mm length of epidermis and an approximately 1-mm depth of dermis below this length. A semiquantitative scale from 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe) was used for the evaluation of mean dermal cell infiltration per square millimeter in H&E-stained tissue.
Mouse skin model and topical treatment.
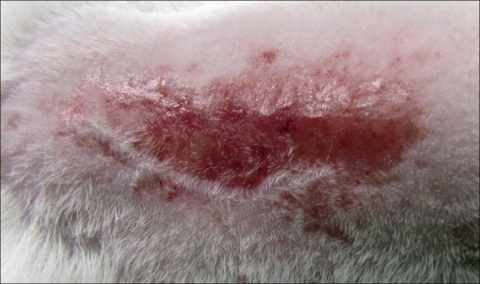
Animal experiments were performed at The Rockefeller University and were approved by the Institutional Animal Care and Use Committee. A modified approach from Kugelberg et al. was used to induce topical skin colonization with S. aureus on 6- to 8-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) (16). Mice were anesthetized by intraperitoneal injection of ketamine (1.2 mg/animal; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (0.25 mg/animal; Miles Inc., Shawnee Mission, KS). A 2-cm2 area of the dorsum of each mouse was shaved with an electric razor; the shaved area was then tape stripped, using autoclave tape, approximately 15 to 20 times in succession, using a fresh piece of tape each time. This allowed a significant portion of the epidermis to be removed and reddening of the skin (Fig. 1). In order to standardize the degree of irritation among the mice, the transepidermal water loss (TEWL) was measured using a VapoMeter instrument (Delfin Technologies, Kuopio, Finland). Measurements were made according to the guidelines of the Standardization Group of the European Dermatitis Society (23). Our shaving and then tape-stripping method resulted in a TEWL of 75 g/m2 h, on average. H&E-stained skin sections were prepared for mice with the same TEWL reading to grade the degree of inflammation. We replicated our model for each mouse by attaining the same TEWL reading and grade of inflammation, thus minimizing the variability of epidermal excoriation between animals. A group of mice was killed, and the skin was cultured after abrasion to ensure that there was no colonization with any bacteria on the skin (data not shown). The shaved/tape-stripped area of the mice was topically colonized with overnight cultures of S. aureus 8325-4 or MRSA MW2 using 5 μl containing 1 ×107 cells in logarithmic or stationary growth phases in TSB. Colonization by each strain was verified by killing groups of mice at 24, 48, and 72 h postcolonization to assess bacterial counts on the skin. This was done by excising the infected skin area, homogenizing the tissue in 1 ml of PBS, and plating dilutions on mannitol salt agar (an S. aureus-selective medium). Colonies were counted at 24 and 48 h postincubation at 37°C.
FIG. 1.
Mouse skin infection model. Shaved and tape-stripped mouse.
At 24 h postcolonization, mice were randomly separated into five groups with five mice in each group: untreated, control, placebo, ClyS treated, and mupirocin treated. Untreated mice were colonized with S. aureus and left untreated; control mice were treated with a weighed quantity of Aquaphor ointment; mice in the placebo group were treated with 10% of the purified ClyS binding domain alone in Aquaphor; mice in the treatment group were treated with one dose of either 1%, 6%, or 10% (wt/wt) topical ClyS; and mice in a comparator arm were treated with one dose of commercially available topical mupirocin (2%). Mice were observed daily for signs of stress and were weighed before and after treatments.
For all experiments, topical agents stayed on the skin for 18 h, at which time mice were killed; the wound area was excised and weighed to ensure uniformity among samples. Skin samples were then individually homogenized in 1 ml of PBS using a stomacher 80 Biomaster machine (Seward Ltd., United Kingdom). Dilutions of the homogenates were plated onto mannitol salt agar to calculate the number of viable bacteria by determination of the numbers of CFU. To ensure that no ClyS remained on the surface of skin samples prior to homogenization, which might result in postprocessing lysis of staphylococci, S. aureus 8325-4 was added to the remaining solutions of homogenates and lytic assays were run; no drop in optical density was detected (data not shown). Each experiment was repeated three times for each group of mice, resulting in a total of 15 mice per treatment group per bacterial strain.
In vitro resistance studies.
The potential for the development of resistance to ClyS was compared to that for mupirocin via exposure of MRSA or MSSA to increasing concentrations (1/32× to 4× MIC) of either agent over 8 days. On day 1, 1 × 108 CFU/ml of MRSA MW2 was grown in the presence of 1.25 μg/ml of ClyS (1/32× MIC) in 10 ml MHB at 37°C overnight. On the next day, the culture was divided in half and centrifuged to pellet the bacteria. One half of the cells received 10 ml of MHB-2.5 μg/ml (1/16× MIC) of ClyS and were incubated overnight in the manner described above. The second portion was plated onto Mueller-Hinton agar incorporated with 40 μg/ml of ClyS and incubated for 48 h at 37°C.
The MICs of colonies grown on Mueller-Hinton agar were determined by broth microdilution. This experiment was repeated for eight consecutive rounds with a doubling of the ClyS concentration incubated with MHB on each successive day, until 4× the ClyS MIC was used on day 8. The experiments were repeated in the same manner for mupirocin at concentrations 1/32× to 4× MIC as described by Rouse et al. (27). Experiments for determination of resistance development were repeated using strain 8325-4. In previous experiments, the MICs of ClyS determined by the CLSI broth microdilution method for S. aureus 8325-4 and MRSA MW2 were found to be between 30 and 40 μg/ml (9).
Immunologic assays.
The mice were assessed for the development of antibodies against ClyS after topical administration. BALB/c mice were shaved/tape stripped and exposed to 10% topical ClyS: four mice were treated with two consecutive doses 18 h apart, four mice were treated with weekly doses for 4 weeks, and four mice were treated with weekly doses for 8 weeks. Serum from each group was obtained by retroorbital puncture before ClyS administration and at 14 and 30 days after the last ClyS dose. Titers of antibodies to ClyS were determined by enzyme-linked immunosorbent assay (ELISA). Serum from a rabbit hyperimmunized with ClyS was used as a control. This animal received a primary immunization with 100 μg of ClyS in complete Freund's adjuvant and three monthly boosts in incomplete Freund's adjuvant, and serum was collected 10 days after the last boost.
Sera from mice exposed to topical ClyS were also assayed for neutralization of ClyS activity in vitro. Thirty microliters of serum from mice that were ClyS exposed and ClyS naive was placed in the wells of microtiter plates. To assess the activity of ClyS for low titers of serum antibodies, ClyS was serially diluted 2-fold in PBS beginning at a concentration of 900 μg. The serum and ClyS were allowed to react in each well for 15 min at room temperature before 100 μl of 108 S. aureus 8325-4 bacteria was added. Optical densities (595 nm) were determined every 12 min for 60 min. The highest dilution of ClyS that prevented a 25% or more decrease in optical density was considered the endpoint. Controls included (i) bacteria and ClyS plus serum from an unexposed mouse and (ii) bacteria in PBS plus ClyS without mouse serum.
Statistical analysis.
For testing if there are differences in colony counts among those groups, both analysis of variance (with necessary transformation to better fit the normality assumption) and the Kruskal-Wallis test were used for MSSA and MRSA strains (data were not pooled between the strains). Standard deviations were slightly enlarged to take into account human error for the shave/tape-stripping technique. A P value of <0.01 was considered significant.
RESULTS
Topical ClyS formulation.
ClyS solution was lyophilized, allowing us to formulate different dose concentrations without being limited by solution volume. The ideal ointment base in which to incorporate lyophilized ClyS had to be hydrophilic enough to allow lyophilized ClyS to go into solution and be activated and hydrophobic enough for adequate absorption into the skin. Initially, lyophilized ClyS was incorporated into glycerol with good maintenance of activity, but absorption into the skin was limited. White petrolatum did not permit Clys to go into solution. Ultimately, we found Aquaphor to be an optimal ointment base. Aquaphor is an emollient ointment whose active ingredient is petrolatum (41%); inactive ingredients include mineral oil, ceresin, lanolin alcohol, panthenol, glycerin, and bisabolol. The combination of active and inactive ingredients allows lyophilized ClyS to go into solution and have good penetration into the skin. The in vitro activity of ClyS incorporated in Aquaphor was equivalent to that of ClyS solution; the activity of ClyS ointment was maintained for up to 2 weeks when it was stored at −4°C.
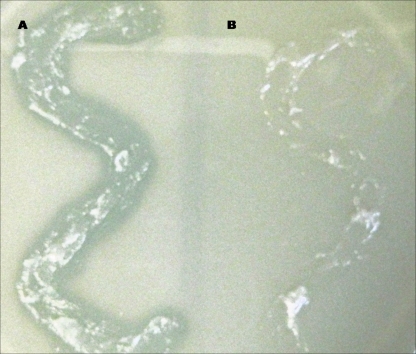
We tested 1%, 6%, and 10% (wt/wt) concentrations of ClyS in Aquaphor, with superior in vivo effects being achieved using a 10% ClyS dose. The lytic activity of ClyS in Aquaphor may be seen visually by applying 10% ClyS in Aquaphor on S. aureus colloid (bacteria suspended in an agar matrix) (Fig. 2). ClyS is released from the Aquaphor and penetrates the agar, lysing S. aureus cell walls. Aquaphor alone shows no lytic activity (Fig. 2).
FIG. 2.
Lysis of agar-embedded staphylococci by ClyS ointment. Topical ClyS in Aquaphor (A) or Aquaphor alone (B) was applied to S. aureus colloid.
Histology studies.
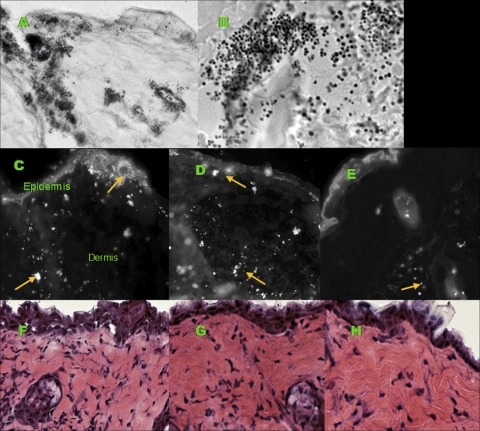
Gram stains were performed on untreated, control, and ClyS-treated mouse skin sections to verify infection with S. aureus (Fig. 3 A and B) and to compare bacterial loads among mouse groups (data not shown). We determined the treatment depth via immunofluorescence staining. When comparing the locations of S. aureus, we found the surface of the skin and dermis to be heavily infected with S. aureus for untreated and Aquaphor-treated mice, while ClyS-treated mice had bacteria visible only deep in the dermis (Fig. 3C to E). The depth of treatment for untreated and Aquaphor-treated mice was approximately 20 μm, and that for ClyS-treated mice was, on average, 300 μm. Mouse skin sections stained with H&E were used to evaluate the degree of inflammation among control, placebo, and ClyS-treated mice. ClyS-treated mice appeared to have fewer inflammatory cells than untreated mice after one dose (grade 2 versus grade 3 dermal cell infiltration) (Fig. 3F to H).
FIG. 3.
Histological appearance of mouse skin infected with Staphylococcus aureus 8325-4 or MRSA MW2. (A) Modified Brown and Brenn stain of mouse skin infected with S. aureus (magnification, ×400); (B) magnified inset of the image in panel A (magnification, ×1,000), revealing Gram-positive cocci in clusters. (C) Immunofluorescence staining of S. aureus with ClyS binding domain complexed with Alexa Fluor 647 in skin sections from an untreated mouse; (D) after treatment with Aquaphor ointment alone (magnification, ×400); (E) after treatment with ClyS in Aquaphor ointment (magnification, ×400). Bright cocci represent S. aureus, as denoted by arrows. (F) Hematoxylin-eosin-stained 6-mm skin sections of an untreated mouse; (G) after one dose of Aquaphor ointment alone (magnification, ×400); (H) after one dose of ClyS in Aquaphor ointment (magnification, ×400). Note the decreased inflammatory infiltrate in treated skin. The images are representative of those from experiments using S. aureus strain 8325-4 or MRSA strain MW2.
ClyS in vivo efficacy.
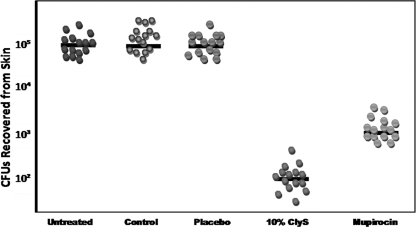
After infection with S. aureus was verified through histology, we proceeded to demonstrate the efficacy of ClyS in vivo. One dose of 6% (wt/wt) ClyS resulted in a 2-log reduction in the amount of bacteria (data not shown); a 10% (wt/wt) dose caused a 3-log reduction compared to the amount detected after treatment with Aquaphor alone (Fig. 4). There was no statistically significant difference between the control and placebo arms, emphasizing that the catalytic domain of ClyS is needed for activity (Fig. 4). The difference in the efficacy of a 10% dose of ClyS (3-log reduction in numbers of CFU) and that of one dose of 2% topical mupirocin (2-log reduction) was statistically significant (P = 0.001) (Fig. 4). No statistically significant difference in results was found when using either strain 8325-4 or strain MW2. The weight and macroscopic appearance between mice groups were not significantly different.
FIG. 4.
In vivo activity of ClyS ointment versus that of placebo or mupirocin on tape-stripped mice infected with S. aureus 8325-4 or MRSA MW2. Topical treatments included Aquaphor (control), 10% ClyS binding domain in Aquaphor (placebo), 10% (wt/wt) ClyS in Aquaphor, and 2% mupirocin. The median value of the data for each group is shown as a horizontal bar; each sphere represents one mouse. No statistically significant difference was seen between untreated, control, and placebo groups. A statistically significant difference (P < 0.0001) was noted between untreated/control/placebo and ClyS groups, with a 3-log drop in bacteria (numbers of CFU) recovered. A statistically significant difference (P = 0.001) was noted between the ClyS- and mupirocin-treated groups, with a 1-log-drop difference between them. The data presented here are representative of those from experiments using S. aureus strain 8325-4 or MRSA strain MW2.
In vitro resistance studies.
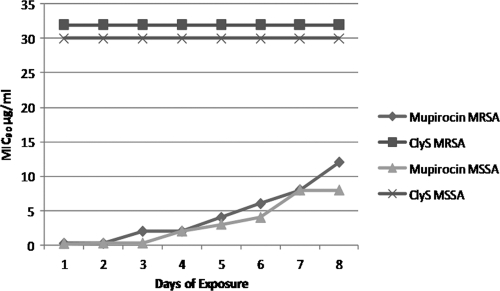
We exposed MRSA MW2 cells to increasing concentrations of either ClyS or mupirocin over an 8-day time period. The MIC for mupirocin began to increase by day 3, with MRSA bacteria developing low-level resistance (MICs, 8 to 512 μg/ml) by day 8, while the MIC remained unchanged for ClyS over the same time period. Similar results were found using strain 8325-4 (Fig. 5).
FIG. 5.
In vitro resistance studies of ClyS and mupirocin. MIC90 values for MRSA strain MW2 and MSSA strain 8325-4 remain the same for ClyS but increase for mupirocin after an 8-day exposure to 1/32× to 4× the MIC of the respective agent.
Immune response to ClyS.
ELISA of mouse serum exposed to topical ClyS revealed the formation of antibodies at a low titer after repeat dosing (Table 1). Given this low titer of antibodies, we serially diluted ClyS with the same concentration of mouse serum and ran a lytic assay. Even at the lowest dilution of ClyS, ClyS antibodies did not exhibit any inhibition of ClyS activity (Table 1). Serum from a hyperimmunized rabbit with a high titer of antibody to ClyS (1:100,000) did not demonstrate inhibition of ClyS activity either (Table 1) (9).
TABLE 1.
ClyS antibody and neutralization titers for the topical model
| Animal serum | ClyS dose | Time (days) of ELISA after last dose | Antibody titera | Neutralization titerb |
|---|---|---|---|---|
| Mouse | Two consecutive | 14 | 128 | 0 |
| Mouse | Weekly for 4 wk | 14 | 64 | 0 |
| Mouse | Weekly for 8 wk | 30 | 16 | 0 |
| Mouse | No ClyS exposure | NAc | 2 | 0 |
| Rabbit | 3-mo boosters in Freund's adjuvant | NA | 512,000 | 0 |
Median ClyS antibody titer from treated and untreated animals (≥4 animals per group) determined by ELISA.
The antibody neutralization titer of ClyS was determined by incubating sera from treated or untreated animals with serial dilutions of ClyS before the addition of S. aureus strain 8325-4. Titers were determined by the lowest dilution of ClyS that inhibited 75% of the lysis of S. aureus.
NA, not applicable.
DISCUSSION
New topical therapies are needed for the decolonization and treatment of superficial skin infections by S. aureus and, in particular, MRSA due to the paucity of available effective agents with low resistance potential. Our animal studies provide the first description of in vivo efficacy of a prospective candidate, ClyS, as a topical ointment. ClyS is able to eliminate staphylococci (MSSA and MRSA) in the epidermis and upper dermis. We have demonstrated ClyS to be more effective and to have less resistance development potential than the leading available topical therapeutic, mupirocin.
Mupirocin remains the standard agent used for MSSA and MRSA nasal and skin decolonization (1, 28, 30). There are several patient populations that benefit from staphylococcal skin decolonization. Patients with chronic skin conditions such as atopic dermatitis and eczema are well-known to be colonized with S. aureus (4, 25), and treatment with topical mupirocin has been found to be beneficial in these patients (18). Patients with chronic indwelling catheters are also at increased risk for infections caused by staphylococci, both coagulase positive and coagulase negative (2, 15). Casey et al. conducted a study that included 291 peritoneal dialysis patients. Of these patients, 143 were administered mupirocin at the exit site daily, while no treatment was applied to the remaining 148 patients. After 1 year, the authors observed a 49% reduction in the rate of exit-site infection and a 31% reduction in the rate of peritonitis (5). A recent Cochrane review of 10 studies (786 patients) with central venous catheters for hemodialysis looked at application of mupirocin ointment at the catheter site. Mupirocin ointment reduced the risk of catheter-related bacteremia (relative risk, 0.17; 95% confidence interval, 0.07 to 0.43) (19).
The implication of mupirocin resistance with repeated use is of major concern. Perez-Fontan et al. found that in peritoneal dialysis patients receiving chronic mupirocin, the accumulated incidence of S. aureus exit-site infection from 1997 to 2000 was 32.3% in patients colonized by mupirocin-resistant S. aureus, whereas it was 14.5% in those colonized by mupirocin-sensitive S. aureus (P = 0.03) (22). In our study, one dose of ClyS was able to significantly decrease the bacterial burden in infected/colonized skin compared to the decrease achieved with mupirocin. Contrary to the finding for staphylococci exposed to mupirocin, bacteria exposed to ClyS did not develop any resistance, nor did ClyS lose potency with repeat administration. This offers an advantage because skin decolonization is often a continual process, as patients at risk are often recolonized with S. aureus.
A recent article in the New England Journal of Medicine by Bode et al. found that decolonization of the nares with mupirocin and extranasal sites with chlorhexidine during hospital admission may decrease the risk of surgical site infections (3). These data may lead to widespread use of mupirocin, increasing the resistance rate beyond its current 19% to 24% (20), significantly decreasing its efficacy. Mupirocin binds specifically to isoleucyl-tRNA synthetase (IRS) to inhibit protein synthesis. Resistance to mupirocin is related to alterations in the host IRS (21). We were able to observe the development of mupirocin-resistant S. aureus strains after repeated exposure to the agent in vitro after just 1 week. ClyS-resistant strains could not be created after repeat exposure, as demonstrated by an unchanging ClyS MIC over the same time period. This may be explained by the way in which phage lysins have evolved. Lysin binding domains bind tightly to a critical component in the bacterial cell wall (usually carbohydrate). Through this evolutionary process, phages have selected binding domains that are difficult for the bacteria to change. To date, resistance has not been seen for any reported lysin (24).
As a protein, ClyS has the potential of being immunogenic with the production of antibodies. We performed studies to determine if sera from mice exposed to topical ClyS for up to 8 weeks had an effect on the killing ability of ClyS. While low titers of antibodies were produced in topically treated animals, they had no effect on ClyS activity, even at low concentrations of ClyS. Hyperimmune rabbit serum with an ELISA titer of over 100,000 showed no effect on the catalytic activity of the lysin (9). This lack of antibody neutralization differs from the neutralization ability of lysins that are not phage encoded, such as lysostaphin (8).
In summary, we present a novel topical antimicrobial agent that works specifically against staphylococci. Previous studies with ClyS have shown it to be effective in vivo for the decolonization of S. aureus and MRSA in the nares (9). Our S. aureus and MRSA mouse skin model found that ClyS formulated in an ointment is effective and statistically significantly superior to mupirocin after one dose. We expect ClyS to be useful as a decolonizing agent, as it is highly efficient at removing staphylococci on the skin surface. It may also be developed as a topical agent for soft tissue infections and as a prophylactic ointment at the skin-device interface in catheter and dialysis patients. Its efficacy, specificity, quick onset of action, and low risk for resistance substantiate it as a plausible therapeutic to be used in humans.
Acknowledgments
The project described here was supported by grant award number UL1RR024143 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research.
The contents of this article are solely our responsibility and do not necessarily represent the official view of the NCRR or NIH.
Footnotes
Published ahead of print on 22 November 2010.
REFERENCES
- 1.Ammerlaan, H. S., J. A. Kluytmans, H. F. Wertheim, J. L. Nouwen, and M. J. Bonten. 2009. Eradication of methicillin-resistant Staphylococcus aureus carriage: a systematic review. Clin. Infect. Dis. 48:922-930. [DOI] [PubMed] [Google Scholar]
- 2.Aykut, S., et al. 2010. Mupirocin application at the exit site in peritoneal dialysis patients: five years of experience. Ren. Fail. 32:356-361. [DOI] [PubMed] [Google Scholar]
- 3.Bode, L. G., et al. 2010. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 362:9-17. [DOI] [PubMed] [Google Scholar]
- 4.Breuer, K., H. A. S. A. Kapp, and T. Werfel. 2002. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br. J. Dermatol. 147:55-61. [DOI] [PubMed] [Google Scholar]
- 5.Casey, M., et al. 2000. Application of mupirocin cream at the catheter exit site reduces exit-site infections and peritonitis in peritoneal dialysis patients. Perit. Dial. Int. 20:566-568. [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing. 16th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Coates, T., R. Bax, and A. Coates. 2009. Nasal decolonization of Staphylococcus aureus with mupirocin: strengths, weaknesses and future prospects. J. Antimicrob. Chemother. 64:9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dajcs, J. J., et al. 2002. Immunity to lysostaphin and its therapeutic value for ocular MRSA infections in the rabbit. Invest. Ophthalmol. Vis. Sci. 43:3712-3716. [PubMed] [Google Scholar]
- 9.Daniel, A., et al. 2010. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 54:1603-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, K. A., J. J. Stewart, H. K. Crouch, C. E. Florez, and D. R. Hospenthal. 2004. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin. Infect. Dis. 39:776-782. [DOI] [PubMed] [Google Scholar]
- 11.de Lourdes Ribeiro de Souza da Cunha, M., et al. 2005. Predictive factors of outcome following staphylococcal peritonitis in continuous ambulatory peritoneal dialysis. Clin. Nephrol. 64:378-382. [DOI] [PubMed] [Google Scholar]
- 12.Fischetti, V. A. 2008. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 11:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong, J. Q., et al. 2006. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br. J. Dermatol. 155:680-687. [DOI] [PubMed] [Google Scholar]
- 14.Huang, S. S., and R. Platt. 2003. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin. Infect. Dis. 36:281-285. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, D. W., et al. 2002. A randomized controlled trial of topical exit site mupirocin application in patients with tunnelled, cuffed haemodialysis catheters. Nephrol. Dial. Transplant. 17:1802-1807. [DOI] [PubMed] [Google Scholar]
- 16.Kugelberg, E., et al. 2005. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 49:3435-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lentino, J. R., L. M. Baddour, M. Wray, E. S. Wong, and V. L. Yu. 2000. Staphylococcus aureus and other bacteremias in hemodialysis patients: antibiotic therapy and surgical removal of access site. Infection 28:355-360. [DOI] [PubMed] [Google Scholar]
- 18.Lever, R., K. Hadley, D. Downey, and R. Mackie. 1988. Staphylococcal colonization in atopic dermatitis and the effect of topical mupirocin therapy. Br. J. Dermatol. 119:189-198. [DOI] [PubMed] [Google Scholar]
- 19.McCann, M., and Z. E. Moore. Interventions for preventing infectious complications in haemodialysis patients with central venous catheters. Cochrane Database Syst. Rev. CD006894. [DOI] [PubMed]
- 20.Mongkolrattanothai, K., P. Mankin, V. Raju, and B. Gray. 2008. Surveillance for mupirocin resistance among methicillin-resistant Staphylococcus aureus clinical isolates. Infect. Control Hosp. Epidemiol. 29:993-994. [DOI] [PubMed] [Google Scholar]
- 21.Patel, J. B., R. J. Gorwitz, and J. A. Jernigan. 2009. Mupirocin resistance. Clin. Infect. Dis. 49:935-941. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Fontan, M., M. Rosales, A. Rodriguez-Carmona, T. G. Falcon, and F. Valdes. 2002. Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am. J. Kidney Dis. 39:337-341. [DOI] [PubMed] [Google Scholar]
- 23.Pinnagoda, J., R. A. Tupker, T. Agner, and J. Serup. 1990. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 22:164-178. [DOI] [PubMed] [Google Scholar]
- 24.Rashel, M., et al. 2007. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J. Infect. Dis. 196:1237-1247. [DOI] [PubMed] [Google Scholar]
- 25.Ravenscroft, J. C., et al. 2003. Short-term effects of topical fusidic acid or mupirocin on the prevalence of fusidic acid resistant (FusR) Staphylococcus aureus in atopic eczema. Br. J. Dermatol. 148:1010-1017. [DOI] [PubMed] [Google Scholar]
- 26.Robicsek, A., J. L. Beaumont, R. B. Thomson, Jr., G. Govindarajan, and L. R. Peterson. 2009. Topical therapy for methicillin-resistant Staphylococcus aureus colonization: impact on infection risk. Infect. Control Hosp. Epidemiol. 30:623-632. [DOI] [PubMed] [Google Scholar]
- 27.Rouse, M. S., et al. 2005. In vitro and in vivo evaluations of the activities of lauric acid monoester formulations against Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3187-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rijen, M., M. Bonten, R. Wenzel, and J. Kluytmans. 2008. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst. Rev. CD006216. [DOI] [PMC free article] [PubMed]
- 29.Wertheim, H. F., et al. 2004. Mupirocin prophylaxis against nosocomial Staphylococcus aureus infections in nonsurgical patients: a randomized study. Ann. Intern. Med. 140:419-425. [DOI] [PubMed] [Google Scholar]
- 30.Xu, G., W. Tu, and C. Xu. 2010. Mupirocin for preventing exit-site infection and peritonitis in patients undergoing peritoneal dialysis. Nephrol. Dial. Transplant. 25:587-592. [DOI] [PubMed] [Google Scholar]