Abstract
The effect of multiple doses of rifabutin (150 mg) on the pharmacokinetics of saquinavir-ritonavir (1,000 mg of saquinavir and 100 mg of ritonavir [1,000/100 mg]) twice daily (BID) was assessed in 25 healthy subjects. Rifabutin reduced the area under the plasma drug concentration-time curve from 0 to 12 h postdose (AUC0-12), maximum observed concentration of drug in plasma (Cmax), and minimum observed concentration of drug in plasma at the end of the dosing interval (Cmin) for saquinavir by 13%, 15%, and 9%, respectively, for subjects receiving rifabutin (150 mg) every 3 days with saquinavir-ritonavir BID. No effects of rifabutin on ritonavir AUC0-12, Cmax, and Cmin were observed. No adjustment of the saquinavir-ritonavir dose (1,000/100 mg) BID is required when the drugs are administered in combination with rifabutin. The effect of multiple doses of saquinavir-ritonavir on rifabutin pharmacokinetics was evaluated in two groups of healthy subjects. In group 1 (n = 14), rifabutin (150 mg) was coadministered every 3 days with saquinavir-ritonavir BID. The AUC0-72 and Cmax of the active moiety (rifabutin plus 25-O-desacetyl-rifabutin) increased by 134% and 130%, respectively, compared with administration of rifabutin (150 mg) once daily alone. Rifabutin exposure increased by 53% for AUC0-72 and by 86% for Cmax. In group 3 (n = 13), rifabutin was coadministered every 4 days with saquinavir-ritonavir BID. The AUC0-96 and Cmax of the active moiety increased by 60% and 111%, respectively, compared to administration of 150 mg of rifabutin once daily alone. The AUC0-96 of rifabutin was not affected, and Cmax increased by 68%. Monitoring of neutropenia and liver enzyme levels is recommended for patients receiving rifabutin with saquinavir-ritonavir BID.
Coinfection with tuberculosis occurs commonly in HIV-infected patients (18) and is a major contributing factor to morbidity and mortality in this population. Not only is there an increased risk of reactivation of latent tuberculosis infections in HIV-coinfected patients, but the progression of new tuberculosis infections is also accelerated (7, 10). The antimycobacterial agent rifabutin is being used for the prevention and treatment of active Mycobacterium tuberculosis infection in patients with advanced HIV infection in combination with other agents (7). Combinatorial regimens involving concurrent treatment of both diseases are complicated by the interactions with the cytochrome P450 3A4 (CYP3A4) isoenzyme that metabolizes protease inhibitors, such as saquinavir (11, 14). Rifabutin and other members of the rifamycin class of agents are inducers of enzymes in the cytochrome P450 3A (CYP3A) subfamily, resulting in reduced systemic exposure of the protease inhibitors (6). When saquinavir (600 mg 3 times daily) and rifabutin (300 mg once daily [QD]) were coadministered to 12 HIV-infected patients, saquinavir exposure was reduced by approximately 40% (14); very similar results were reported in another study of 14 HIV patients using the same rifabutin dosage but twice the saquinavir dosage (13).
In order to boost its exposure, saquinavir is coadministered with ritonavir (2), another HIV protease inhibitor that is also a potent inhibitor of CYP3A (16). In principle, ritonavir thus would be expected to minimize the impact of the induction of CYP3A by the rifamycin antimycobacterials. This question was examined in a recent study involving triple therapy with saquinavir, ritonavir, and rifampin that was terminated owing to an unexpected elevation of liver transaminases (15). The possibility remains, however, that triple therapy using another member of the rifamycin group, such as rifabutin, could provide a successful treatment for both HIV and tuberculosis. The issue is further complicated in the case of rifabutin, since it too is metabolized by CYP3A; when rifabutin and ritonavir were administered together, plasma exposure to rifabutin was increased 4-fold and that of 25-O-desacetyl-rifabutin, the major active metabolite, was increased 38-fold (2). The 25-O-desacetyl metabolite has an in vitro activity equal to that of the parent drug (14). Therefore, the total rifabutin activity should include both rifabutin and the 25-O-desacetyl metabolite. Finally, ritonavir itself is metabolized by CYP3A (2). The multiple interactions among saquinavir, rifabutin, and ritonavir thus make it difficult to predict the respective exposures when the three agents are coadministered to treat patients with both tuberculosis and HIV infections.
Following a request from the Committee for Medicinal Products for Human Use of the European Medicines Agency, the present study was undertaken with the primary objectives of estimating the pharmacokinetics of both saquinavir and rifabutin at steady state when ritonavir-boosted saquinavir was administered together with rifabutin. In addition, as secondary objectives, the study aimed to evaluate the pharmacokinetics of ritonavir in this protocol and the safety and tolerability of the regimen in healthy volunteers.
MATERIALS AND METHODS
This study was conducted at the Roche Clinical Pharmacology Unit in Welwyn, United Kingdom. The study protocol and patient materials were approved by the Welwyn Clinical Pharmacology Ethics Committee, University of Hertfordshire, United Kingdom, and the study was conducted in full compliance with the principles of the Declaration of Helsinki or local country laws, depending on which afforded more patient protection, with strict adherence to the stated provisions in the good clinical practice guidelines. All subjects provided written informed consent prior to screening.
Inclusion and exclusion criteria.
Healthy males and nonpregnant, nonlactating female subjects who were aged 18 to 65 years (inclusive), had a body mass index (BMI) between 18 and 32 kg/m2, and were free of clinically significant disease (as determined by screening assessments) were eligible for enrollment in the study. A screening assessment performed within 28 days prior to study enrollment included a medical history, physical examination, vital signs, BMI, electrocardiogram (ECG), fasting blood and urine samples for safety laboratory tests, an alcohol breath test, a urine pregnancy test, a hormone assay in postmenopausal women, and tests for HIV and hepatitis viruses B and C. Subjects were excluded for the following factors: known personal or family history of congenital QT interval prolongation (QT is a measure of the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle) or sudden death; heart rate at screening greater than 100 beats per minute (bpm) or less than 40 bpm; required the use of contact lenses which may become permanently discolored by rifabutin (14) between day −2 and the follow-up visit; were receiving hormonal contraception or hormone replacement therapy; had a history of alcohol or drug addiction or a positive urine test for drugs of abuse or a positive alcohol breath test; were smokers; or were using CYP3A4 inhibitors or inducers, including, but not limited to, ketoconazole, miconazole, fluconazole, itraconazole, erythromycin, clarithromycin, ranitidine, cimetidine, rifampin, glucocorticoids, carbamazepine, phenytoin, phenobarbital, or St. John's wort within 4 weeks of the first study day; taking any medications or herbal products within 1 week of the first study day; or taking drugs known to prolong the QT/QTc interval (QTc is the QT interval corrected for heart rate). In addition, the consumption of grapefruit or grapefruit juice was not allowed from 2 weeks prior to the first dose and during the study, and the consumption of alcohol, caffeine, and xanthine-containing beverages was not allowed from 48 h prior to the first day of the study and throughout the study. Restriction of caffeine and xanthine-containing beverages was required to isolate the adverse events caused by the potentially increased metabolism of caffeine, since ritonavir is not only an inducer of CYP3A but also an inducer of CYP1A2 (9).
Study design.
Three groups were studied; each group was studied using an open-label, single-sequence, and crossover design, with the third group being adaptive. In group 1, subjects received saquinavir-ritonavir (1,000 mg of saquinavir and 100 mg of ritonavir [1,000/100 mg]) twice daily (BID) for 14 days (period 1) and saquinavir-ritonavir (1,000/100 mg BID) in combination with rifabutin (150 mg) every 3 days (Q3D) for 22 days (period 2). In group 2, subjects received 150 mg rifabutin daily (QD) for 21 days (period 1) and 150 mg rifabutin Q3D in combination with saquinavir-ritonavir (1,000/100 mg BID) for 22 days (period 2). In group 3, subjects received 150 mg rifabutin QD for 21 days (period 1) and 150 mg rifabutin every 4 days (Q4D) in combination with saquinavir-ritonavir (1,000/100 mg BID) for 21 days (period 2). Groups 1 and 2 were studied in parallel. Group 3 was studied after reviewing the pharmacokinetic and safety data obtained for groups 1 and 2. The dosing schedules allowed steady state to be achieved for both rifabutin and saquinavir-ritonavir when administered alone or in triple combination treatment. Owing to the anticipated increase in rifabutin exposure with concomitant saquinavir-ritonavir treatment, rifabutin was administered once every 3 days rather than daily in groups 1 and 2. Recruitment into group 3 was dependent on whether the group 2 regimen led to exposure of rifabutin or its active moiety (sum of rifabutin and its active metabolite, 25-O-desacetyl-rifabutin) that was within the established rifabutin area under the concentration-time curve from 0 to 24 h (AUC0-24) range between 1.6 ± 0.4 μg·h/ml and 3.4 ± 0.7 μg·h/ml following daily administration of rifabutin at doses of 150 mg (9) or 300 mg (12), respectively. Thus, group 3, if required, was to be dosed either every second or every fourth day based on whether the observed deviations were higher or less than these established limits.
In all three groups, adverse events, ECG and vital sign measurements, and laboratory tests were monitored throughout the study period. A physical examination and safety assessment were performed at a follow-up visit 14 to 21 days after the last dose of study drug.
Study drug administration.
Saquinavir was administered as a 500-mg coated tablet (Invirase; Genentech), ritonavir as a 100-mg capsule (Norvir; Abbott Laboratories), and rifabutin as a 150-mg capsule (Mycobutin; Pfizer). Standard meals were provided within 30 min of saquinavir-ritonavir dosing, and rifabutin was taken 2 h after breakfast in the Clinical Pharmacology Unit (CPU). The CPU erroneously provided a standard low-fat breakfast to subjects in groups 1 and 2 (approximately 2 g of fat), consisting of a fruit smoothie, cereal bar, apple or banana, and decaffeinated tea or coffee. The standardized breakfast for group 3 (approximately 26 g fat) was as planned and consisted of a 108-g blueberry muffin, 30 g of cornflakes, 200 g of 2% milk, 2 sugar sachets, and decaffeinated tea or coffee. When subjects were outside the unit, they used the same administration schedule and were reminded by telephone by the unit staff to take and record their doses on diary cards. Triple combination treatment was always administered by the unit staff.
Bioanalysis for pharmacokinetic samples.
For the determination of pharmacokinetics of all four principal analytes, blood samples were collected by venipuncture into tubes containing lithium heparin as the anticoagulant. All analyses were carried out by PRA International-Early Development Services, Assen, Netherlands. Plasma samples were separated by centrifugation at 1,500 × g at 4°C for 10 min. Analysis of total plasma concentrations for saquinavir and ritonavir were performed using a validated specific high-performance liquid chromatography-tandem mass spectroscopy (HPLC-MS-MS) method covering two concentration ranges. For both analytes, the low range varied from 1.00 to 100 ng/ml using 200 μl of plasma, while the high range varied from 10 to 10,000 ng/ml using 100 μl of plasma. The precision of the low-concentration assay ranged from 0.1% to 2.0% for saquinavir and from 0.2% to 4.3% for ritonavir, and the accuracy ranged from 98.6% to 105.8% and from 103.8% to 105.3% for saquinavir and ritonavir, respectively. The precision of the high-concentration assay ranged from 4.7% to 7.2% for saquinavir and from 5.2% to 7.5% for ritonavir. The accuracy ranged from 95.8% to 97.1% and from 96.5% to 98.5% for saquinavir and ritonavir, respectively. For the determination of total plasma rifabutin and 25-O-desacetyl-rifabutin, 500-μl plasma aliquots were analyzed by a validated HPLC assay using UV light (HPLC-UV) having lower limits of quantitation of 5.0 ng/ml and 2.50 ng/ml, respectively. The precision of the assays ranged from 5.8% to 6.7% for rifabutin and from 3.1% to 7.0% for 25-O-desacetyl-rifabutin, while the accuracy ranged from 100.4% to 102.0% and from 97.2% to 99.6%, respectively.
Bioanalysis for plasma protein binding samples.
Plasma protein binding of rifabutin and 25-O-desacetyl-rifabutin were determined based on a dialysis method described in the literature (4). Plasma samples at 2 or 3 h predose (around the time to maximum concentration of drug in plasma [Tmax] of rifabutin) were used to determine plasma protein binding for each subject. The concentrations of rifabutin and 25-O-desacetyl-rifabutin in the samples of the protein binding were determined by a validated liquid chromatography-tandem mass spectrometry method (validation report is on file).
Pharmacokinetic evaluation.
Blood samples for assessment of saquinavir and ritonavir pharmacokinetic parameters were collected predose (immediately prior to saquinavir-ritonavir dosing) and at 0.5, 1, 2, 3, 4, 5, 6, 8, 10, and 12 h postdose. Blood samples for assessment of pharmacokinetic parameters of rifabutin and its metabolite were collected predose (immediately prior to rifabutin dosing) and at 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 16, and 24 h postdose. Additional 48- and 72-h postdose blood samples were collected after the final dose of rifabutin. Subjects were included in the analysis of pharmacokinetic data if they adhered to the study protocol and had evaluable concentration data and pharmacokinetic parameters. Pharmacokinetic parameters were estimated using noncompartmental methods with the WinNonlin Enterprise version 5.2.1 (Pharsight Corporation, Mountain View, CA).
The following pharmacokinetic parameters were estimated for saquinavir and ritonavir on days 14 and 36 (group 1), day 43 (group 2), and day 42 (group 3): the maximum observed concentration of drug in plasma (Cmax), the minimum observed concentration of drug in plasma at the end of the dosing interval (Cmin), the time to Cmax (Tmax), and the area under the plasma drug concentration-time curve from 0 to 12 h postdose (AUC0-12). In addition, trough plasma drug concentrations (Ctrough) of saquinavir and ritonavir were determined from plasma samples obtained predose on days 11, 12, 13, 14, 27, 30, 33, and 36 for group 1, on days 34, 37, 40, and 43 for group 2, and on days 30, 34, 38, and 42 for group 3.
For rifabutin and 25-O-desacetyl-rifabutin, the following pharmacokinetic parameters were estimated on days 36 to 39 for group 1, on days 20 and 21 and days 43 to 46 for group 2, and on days 20 and 21 and days 42 to 45 for group 3: Cmax, Cmin, and AUC0-tau (where tau was the dosing interval [24 h for QD dosing, 72 h for dosing every 3 days, and 96 h for dosing every 4 days]). These parameters were estimated for rifabutin and 25-O-desacetyl-rifabutin separately and as the sum of the two analytes. In addition, Tmax was estimated for rifabutin and 25-O-desacetyl-rifabutin separately. The metabolite-to-parent (M/P) ratio based on AUC0-tau of 25-O-desacetyl-rifabutin to rifabutin was also determined. For group 3, Cmin could not be determined for rifabutin and 25-O-desacetyl-rifabutin separately or for the sum of the two analytes on day 42 as the plasma concentrations of these analytes were not measured up to 96 h postdose (rather only up to 72 h postdose). For group 3, AUC0-96 on day 42 was determined by extrapolation from 72 to 96 h based on the apparent terminal-phase rate constant (Kel). Trough plasma concentrations of rifabutin and 25-O-desacetyl-rifabutin were determined from plasma samples obtained predose on days 17, 18, 20, 34, 37, 40, and 43 for group 2 and on days 17, 18, 20, 30, 34, 38, and 42 for group 3.
For plasma protein binding, the fraction unbound (fu) was calculated by Cb/Cp, where Cb and Cp were the concentrations in buffer and plasma at the end of the dialysis, respectively. Values of fu are presented by summary statistics.
Statistical analysis.
The primary parameters for assessing interaction between the three treatment drugs were the AUC0-12 and Cmax for saquinavir and the AUC0-tau and Cmax for rifabutin, its metabolite 25-O-desacetyl-rifabutin, and active moiety at steady state.
The data were analyzed using PROC MIXED (version 8.2; SAS Institute, Inc., Cary, NC) on Roche's BCE/UNIX platform. An analysis of variance (ANOVA) model that included terms for patient and treatment regimen was applied to logarithmically transformed values of Cmax and AUC0-tau for saquinavir, ritonavir, and the active moiety of rifabutin. Ninety percent confidence intervals (90% CIs) for the difference in computed parameter least-squares means (geometric mean ratio [GMR]) were calculated and expressed as a percentage of the reference (alone). The reference (150 mg rifabutin QD alone) was standardized to an equivalent treatment duration for rifabutin every 3 or 4 days by multiplying by 3 or 4, respectively. For plasma protein binding data, the same ANOVA model was applied to values of fu to derive GMR and its 95% CI. The test and reference regimens were deemed equivalent when the 90% CI for the ratio fell within the equivalence interval of 80% to 125%.
RESULTS
Demographics.
A total of 56 healthy subjects (37 males and 19 females) were enrolled, with 26 assigned to group 1, 14 to group 2, and 16 to group 3. Each of the 56 subjects received at least one dose of study medication, and 39 subjects (19 in group 1, 11 in group 2, and 9 in group 3) completed the study as planned. Seventeen subjects withdrew from the study prematurely, with the highest proportion, 44% (7/16) in group 3 compared to 27% (7/26) in group 1 and 21% (3/14) in group 2; the majority were for safety reasons. The median (range) age, body weight, and BMI were 38.5 (18 to 65) years, 76 (47 to 102) kg, and 25 (19 to 31) kg/m2, respectively. Nineteen of the 56 subjects were female. All but 9 subjects were Caucasian. The safety analysis population included all subjects (n = 56) who received at least one dose of study medication. The pharmacokinetic analysis population included subjects who completed pharmacokinetic assessments in period 1 (n = 52) and period 2 (n = 39). Since the interim analysis of group 2 indicated that dosing every 3 days resulted in exposure (AUC0-72) to activity moiety (13.1 μg·h/ml) greater than 3-fold (normalization to 3 days) of the predefined limit of AUC0-24 (10.2 = 3 × 3.4 μg·h/ml) (13) achieved from 300-mg rifabutin QD dosing alone, group 3 was given rifabutin (150 mg) every 4 days during triple therapy in period 2.
Effect of rifabutin on saquinavir-ritonavir pharmacokinetics.
The effect of rifabutin on saquinavir-ritonavir pharmacokinetics was assessed in group 1.
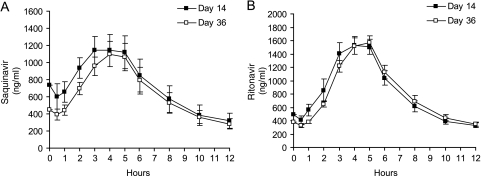
Figure 1 shows the mean saquinavir and ritonavir concentrations versus time on day 14 (the end of period 1) in group 1 following 14 days of treatment with saquinavir-ritonavir and on day 36 (the end of period 2) after 3 weeks of the triple regimen of saquinavir-ritonavir and rifabutin. Key pharmacokinetic parameters and the GMRs for saquinavir and ritonavir before and after triple therapy with rifabutin are summarized in Tables 1 and 2, respectively. As shown in Fig. 1 and Table 2, there was a reduction in exposure for saquinavir following 22 days of combination treatment with rifabutin administered every 3 days, with AUC0-12, Cmax, and Cmin declining by 13%, 15%, and 9%, respectively. The 90% CIs associated with the GMR estimates were 69% to 109%, 68% to 107%, and 72% to 115%, respectively (Table 2). Ritonavir exposure was not changed to a clinically relevant degree in the presence of rifabutin (a 1% decrease in GMR for AUC0-12 and Cmax on days 36 versus day 14 [Table 2]).
FIG. 1.
Mean plasma drug concentration-time profiles of saquinavir (A) and ritonavir (B) on day 14 (saquinavir-ritonavir [1,000 mg of saquinavir and 100 mg of ritonavir] twice daily) and on day 36 (saquinavir-ritonavir [1,000/100 mg] BID and rifabutin [150 mg] every 3 days) for group 1 (pharmacokinetic data for 19 subjects for both day 14 and day 36). The error bars represent the standard errors of the means.
TABLE 1.
Pharmacokinetic parameters for saquinavir and ritonavira
| Study drug | Group | Sampling day | nb | Pharmacokinetic parameterc (mean ± SD) |
|||
|---|---|---|---|---|---|---|---|
| Tmax (h) | Cmax (ng/ml) | Cmin (ng/ml) | AUC0-12 (ng·h/ml) | ||||
| Saquinavir | 1 | 14 | 19 | 3.6 ± 1.1 | 1,264 ± 837 | 322 ± 316 | 8,819 ± 6,899 |
| 1 | 36 | 19 | 4.2 ± 0.5 | 1,144 ± 726 | 274 ± 189 | 7,725 ± 4,878 | |
| 2 | 43 | 11 | 4.2 ± 0.8 | 2,057 ± 970 | 534 ± 425 | 13,321 ± 7,180 | |
| 3 | 42 | 9 | 4.3 ± 0.5 | 4,872 ± 1,910 | 841 ± 417 | 31,169 ± 13,762 | |
| Ritonavir | 1 | 14 | 19 | 3.7 ± 1.1 | 1,683 ± 595 | 292 ± 131 | 9,939 ± 3,844 |
| 1 | 36 | 19 | 4.3 ± 0.7 | 1,688 ± 455 | 293 ± 94 | 9,830 ± 2,505 | |
| 2 | 43 | 11 | 4.2 ± 0.8 | 2,479 ± 538 | 500 ± 102 | 15,239 ± 3,088 | |
| 3 | 42 | 9 | 3.9 ± 1.1 | 1,724 ± 388 | 265 ± 132 | 10,119 ± 3,003 | |
The sanpling days and drug treatments of groups were as follows: day 14, saquinavir-ritonavir (1,000 mg of saquinavir and 100 mg of ritonavir [1,000/100 mg]) twice daily (BID); day 36, saquinavir-ritonavir (1,000/100 mg) BID plus rifabutin (150 mg) once daily (QD); day 43, rifabutin (150 mg) once every 3 days (Q3D) plus saquinavir-ritonavir (1,000/100 mg) BID; and day 42, rifabutin (150 mg) once every 4 days (Q4D) plus saquinavir-ritonavir (1,000/100 mg) BID.
n is the number of subjects with pharmacokinetic data.
AUC0-12, area under the concentration-time curve from 0 to 12 h; Cmax, maximum observed drug concentration in plasma; Cmin, minimum observed drug concentration in plasma at the end of the dosing interval; Tmax, time to Cmax.
TABLE 2.
Geometric least-squares means and geometric mean exposure ratios of saquinavir and ritonavir for group 1
| Study drug | Pharmacokinetic parametera | Sampling day (n)b | Geometric least- squares mean | GMR (%) (90% CI)c | % change in exposured (90% CI) |
|---|---|---|---|---|---|
| Saquinavir | AUC0-12 (ng·h/ml) | 36 (19) | 6,611 | 87 (69-109) | 13↓ (−31 to 9) |
| 14 (25) | 7,599 | ||||
| Cmax (ng/ml) | 36 (19) | 983 | 85 (68-107) | 15↓ (−32 to 7) | |
| 14 (25) | 1,152 | ||||
| Cmin (ng/ml) | 36 (19) | 233 | 91 (72-115) | 9↓ (−28 to 15) | |
| 14 (25) | 255 | ||||
| Ritonavir | AUC0-12 (ng·h/ml) | 36 (19) | 10,022 | 99 (90-109) | 1 ⇆ (−10 to 9) |
| 14 (25) | 10,132 | ||||
| Cmax (ng/ml) | 36 (19) | 1,731 | 99 (92-107) | 1 ⇆ (−8 to 7) | |
| 14 (25) | 1,750 | ||||
| Cmin (ng/ml) | 36 (19) | 349 | 107 (97-118) | 7 ⇆ (−3 to 18) | |
| 14 (25) | 327 |
AUC0-12, area under the concentration-time curve from 0 to 12 h; Cmax, maximum observed plasma drug concentration; Cmin, minimum observed plasma drug concentration at the end of the dosing interval.
n is the number of subjects with pharmacokinetic data.
GMR, geometric mean exposure ratio; 90% CI, 90% confidence interval.
The percent change in exposure comparing the data for day 36 to the data for day 14. Symbols: ↓, decrease; ⇆, no change (the 90% CI surrounding the GMR was within 80% to 125%).
The effect of pretreatment with rifabutin on saquinavir and ritonavir exposure was evaluated and compared during period 2 in group 1 (day 36), group 2 (day 43), and group 3 (day 42) (Table 1). In period 2, the saquinavir exposure (AUC0-12) in group 2 was higher than in group 1 (13,321 ± 7,180 ng·h/ml and 7,725 ± 4,878 ng·h/ml, respectively). In group 3, the saquinavir exposure (31,169 ± 13,762 ng·h/ml) was higher than in groups 1 and 2. In contrast to subjects in groups 1 and 2, who erroneously received a low-fat breakfast, subjects in group 3 received a breakfast with a normal fat content. For ritonavir, the corresponding AUC0-12 on day 43 in group 2 (15,239 ± 3,088 ng·h/ml) was higher than on day 36 in group 1 (9,830 ± 2,505 ng·h/ml) (Table 1) during triple combination period 2. For group 3, in which rifabutin was given only once every 4 days, the AUC0-12 (10,119 ± 3,003 ng·h/ml) was comparable to that seen in group 1 on day 36.
Mean Ctrough saquinavir concentrations following saquinavir-ritonavir (1,000 mg of saquinavir and 100 mg of ritonavir [1,000/100 mg]) BID dosing from days 1 to 14 declined during days 11 to 14 (period 1; P < 0.01) (Table 3) but remained relatively stable on days 27 through 36 following the addition of rifabutin (150 mg Q3D) from days 15 to 36 (period 2; P > 0.05) (Table 3). Mean Ctrough ritonavir concentrations were generally stable in both periods (P > 0.05) (Table 3).
TABLE 3.
Mean trough concentrations for saquinavir, ritonavir, rifabutin, and 25-O-desacetyl-rifabutin
| Group, parameter, or druga | Value for parameter or trough drug concn (mean ± SEM)b |
|||||||
|---|---|---|---|---|---|---|---|---|
| Period 1 | Period 2 | |||||||
| Group 1 | ||||||||
| Time (day) | 11 | 12 | 13 | 14 | 27 | 30 | 33 | 36 |
| n | 25 | 25 | 25 | 19 | 23 | 22 | 20 | 19 |
| SQV (μg/ml) | 1.20 ± 0.13α | 1.24 ± 0.20α | 0.84 ± 0.16α | 0.74 ± 0.15α | 0.65 ± 0.08β | 0.66 ± 0.08β | 0.74 ± 0.14β | 0.45 ± 0.07β |
| RTV (μg/ml) | 0.55 ± 0.05Y | 0.57 ± 0.06Y | 0.56 ± 0.05Y | 0.49 ± 0.07Y | 0.52 ± 0.06δ | 0.44 ± 0.03δ | 0.50 ± 0.06δ | 0.38 ± 0.03δ |
| Group 2 | ||||||||
| Time (day) | 17 | 18 | 20 | NA | 34 | 37 | 40 | 43 |
| n | 14 | 14 | 11 | NA | 12 | 11 | 10 | 11 |
| RFB (ng/ml) | 37 ± 2 | 40 ± 3 | 36 ± 3 | NA | 67 ± 5 | 64 ± 4 | 64 ± 4 | 70 ± 5 |
| 25-RFB (ng/ml) | 1.4 ± 0.4 | 1.5 ± 0.5 | 1.4 ± 0.6 | NA | 57 ± 4 | 53 ± 6 | 53 ± 6 | 57 ± 5 |
| SQV (μg/ml) | NA | NA | NA | NA | 0.8 ± 0.1 | 1.3 ± 0.3 | 0.9 ± 0.3 | 0.6 ± 0.1 |
| RTV (μg/ml) | NA | NA | NA | NA | 0.66 ± 0.09 | 0.8 ± 0.1 | 0.5 ± 0.1 | 0.58 ± 0.03 |
| Group 3 | ||||||||
| Time (day) | 17 | 18 | 20 | NA | 30 | 34 | 38 | 42 |
| n | 15 | 15 | 9 | NA | 11 | 10 | 9 | 9 |
| RFB (ng/ml) | 40 ± 3 | 39 ± 3 | 39 ± 4 | NA | 47 ± 3 | 45 ± 3 | 45 ± 4 | 45 ± 5 |
| 25-RFB (ng/ml) | 1.3 ± 0.1 | 0.8 ± 0.4 | 1.3 ± 0.6 | NA | 42 ± 2 | 46 ± 3 | 47 ± 3 | 48 ± 5 |
| SQV (μg/ml) | NA | NA | NA | NA | 1.2 ± 0.1 | 1.2 ± 0.2 | 0.7 ± 0.1 | 1.2 ± 0.3 |
| RTV (μg/ml) | NA | NA | NA | NA | 0.44 ± 0.05 | 0.47 ± 0.06 | 0.39 ± 0.08 | 0.31 ± 0.04 |
RFB, rifabutin; 25-RFB, 25-O-desaceyl-rifabutin; SQV, saquinavir; RTV, ritonavir.
Statistical significance is indicated by symbols as follows: α, P < 0.01; β, P > 0.05; Y, P > 0.05; δ, P > 0.05. NA, not applicable.
Effect of saquinavir-ritonavir on rifabutin pharmacokinetics.
The effect of saquinavir-ritonavir on rifabutin pharmacokinetics was assessed in groups 2 and 3 in which 21 days of treatment with rifabutin QD was followed by saquinavir-ritonavir BID plus rifabutin every 3 days for 22 days (group 2) or every 4 days for 21 days (group 3). Exposure to rifabutin and its principal metabolite, 25-O-desacetyl-rifabutin was assessed, allowing evaluation of the total active moiety.
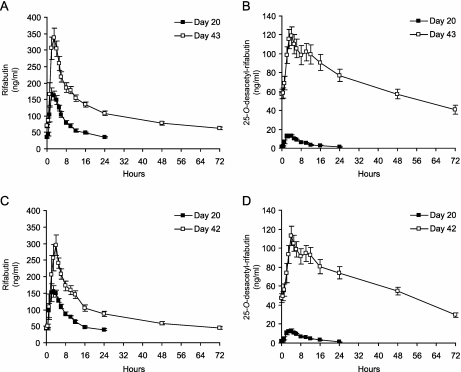
Figure 2 shows the mean rifabutin and 25-O-desacetyl- rifabutin concentrations versus time on day 20 following rifabutin monotherapy and after triple therapy (the end of period 2) in group 2 (on day 43) and group 3 (on day 42). Table 4 summarizes the pharmacokinetic parameters and statistical test results. For group 2, the AUC0-72 of rifabutin increased by 1.53-fold, the AUC0-72 of 25-O-desacetyl-rifabutin increased by 15.4-fold, and the AUC0-72 of the active moiety increased by 2.34-fold comparing day 43 in period 2 with day 20 in period 1. The M/P ratio for AUC0-72 increased from 6.9% on day 20 to 63.8% on day 43 (Table 4). For group 3, the AUC0-96 of rifabutin did not change, but the AUC0-96 of 25-O-desacetyl-rifabutin increased by 10.5-fold and the AUC0-96 of the active moiety increased by 1.6-fold comparing day 42 with day 20. The M/P ratio for AUC0-96 increased from 7.1% on day 20 to 71.0% on day 42 (Table 4).
FIG. 2.
Mean plasma concentration-time profiles of rifabutin and 25-O-desacetyl-rifabutin for group 2 (n = 11) (A and B, respectively) and group 3 (n = 9) (C and D, respectively). The groups were treated as follows: rifabutin (150 mg) QD for both group 2 and group 3 (samples taken on day 20) (▪); rifabutin (150 mg) every 3 days plus saquinavir-ritonavir (1,000/100 mg) BID for group 2 (samples taken on day 43) (□); rifabutin (150 mg) every 4 days plus saquinavir-ritonavir (1,000/100 mg) twice daily for group 3 (samples taken on day 42) (□). The error bars represent the standard errors of the means.
TABLE 4.
Pharmacokinetic parameters and 90% confidence intervals for the geometric mean ratio of exposure to rifabutin, its active metabolite 25-O-desacetyl-rifabutin, and the active moiety
| Study drug and pharmacokinetic parametera | Group 2 |
Group 3 |
||||
|---|---|---|---|---|---|---|
| Value for pharmacokinetic parameter (mean ±SD) |
GMR of exposure to drug (90% CI) (n = 11-14)b | Value for pharmacokinetic parameter (mean ±SD) |
GMR of exposure to drug (90% CI) (n = 9-13) | |||
| Day 20 (n = 11) | Day 43 (n = 11) | Day 20 (n = 9) | Day 42 (n = 9) | |||
| Rifabutin | ||||||
| Tmax (h) | 2.5 ± 1.1 | 2.36 ± 0.93 | NC | 3.0 ± 1.1 | 3.1 ± 0.9 | NC |
| Cmax (ng/ml) | 188 ± 50 | 353 ± 107 | 186 (157-219) | 184 ± 57 | 330 ± 114 | 168 (138-205) |
| AUC0-tau (μg·h/ml) | 1.8 ± 0.4 | 8.1 ± 1.7 | 153 (136-173) | 1.8 ± 0.4 | 7.4 ± 1.7 | 101 (90-113) |
| 25-O-Desacetyl-rifabutin | ||||||
| Tmax (h) | 2.9 ± 0.9 | 4.0 ± 1.0 | NC | 3.3 ± 0.7 | 4.0 ± 0.5 | NC |
| Cmax (ng/ml) | 14.8 ± 5.2 | 123 ± 35 | 873 (743-1,026) | 14.9 ± 5.73 | 115 ± 31 | 773 (642-932) |
| AUC0-tau (μg·h/ml) | 0.13 ± 0.06 | 5.1 ± 1.5 | 1,538 (1,216-1,945) | 0.13 ± 0.04 | 5.2 ± 1.1 | 1,053 (869-1.275) |
| M/P AUC0-tau ratio (%) | 6.9 ± 7.7 | 63.8 ± 17.3 | NC | 7.1 ± 2.3 | 71.0 ± 12.5 | NC |
| Active moiety | ||||||
| Cmax (ng/ml) | 202 ± 54 | 464 ± 124 | 230 [198-267] | 198 ± 62 | 439 ± 135 | 211 [175-253] |
| AUC0-tau (μg·h/ml) | 1.9 ± 0.5 | 13.1 ± 2.6 | 234 [209-262] | 1.9 ± 0.4 | 12.6 ± 2.6 | 160 [143-179] |
Active moiety, rifabutin plus 25-O-desacetyl-rifabutin; AUC0-tau, area under the concentration-time curve from 0 h to the dosing interval tau(24, 72, or 96 h); Cmax, maximum observed plasma drug concentration; M/P AUC0-tau ratio, metabolite-to-parent ratio of the AUC0-tau ratio of 25-O-desacetyl-rifabutin to rifabutin; Tmax, time to Cmax.
NC, not calculated.
Changes in Cmax and AUC0-72 (for group 2) and AUC0-96 (for group 3) are summarized in Table 5 (values were calculated by subtracting 100% from the GMR estimates). In group 2, the rifabutin AUC0-72 was 53% higher when rifabutin was administered once every 3 days in combination with saquinavir-ritonavir (period 2) compared to QD administration without concurrent saquinavir-ritonavir administration (period 1). In group 3, rifabutin AUC0-96 was equivalent when rifabutin was administered once every 4 days in combination with saquinavir-ritonavir (period 2) compared to QD administration without concurrent saquinavir-ritonavir administration (period 1). The overall exposure to the active moiety was higher for the every third and fourth day dosing regimens of rifabutin during triple therapy (134% and 60% increases, respectively) compared to QD dosing of rifabutin alone primarily due to large increases in 25-O-desacetyl-rifabutin exposure (1,438% and 953% for groups 2 and 3, respectively).
TABLE 5.
Changes in exposures of rifabutin (RIF), 25-O-desacetyl-rifabutin (25-O-dRIF), and active moiety with coadministration of saquinavir-ritonavir
| Analytea | Pharmacokinetic parameterb | % change in exposure (90% CI)c |
|
|---|---|---|---|
| Group 2d (day 43 to day 20) | Group 3e (day 42 to day 20) | ||
| Rifabutin | AUC0-72 (ng·h/ml) | 53↑ (36↑-73↑) | 1 ⇆ (−10-13) |
| Cmax (ng/ml) | 86↑ (57↑-119↑) | 68↑ (38↑-105↑) | |
| 25-O-dRIF | AUC0-72 (ng·h/ml) | 1,438↑ (1,116↑-1,845↑) | 953↑ (769↑-1,175↑) |
| Cmax (ng/ml) | 773↑ (643↑-926↑) | 673↑ (542↑-832↑) | |
| Active moiety | AUC0-72 (ng·h/ml) | 134↑ (109↑-162↑) | 60↑ (43↑-79↑) |
| Cmax (ng/ml) | 130↑ (98↑-167↑) | 111↑ (75↑-153↑) | |
25-O-dRIF, 25-O-desacetyl-rifabutin; active moiety, rifabutin plus 25-O-desacetyl-rifabutin.
AUC0-72, area under the concentration-time curve from 0 to 72 h; Cmax, maximum observed plasma drug concentration.
The percent change in exposure with coadministration of saquinavir-ritonavir(SQV-RTV) comparing data from day 43 or 42 to data from day 20 for groups 2 and 3 is shown. Symbols: ↑, increase; ⇆, no change(the 90% confidence interval [90% CI] surrounding the geometric mean ratio [GMR] was within 80% to 125%).
Group 2 was treated as follows: 150 mg of rifatubin(RFB) once daily(QD) for 21 days, followed by saquinavir-ritonavir(SQV-RTV)(1,000 mg of saquinavir and 100 mg of ritonavir) twice daily(BID) and RFB(150 mg) every 3 days(Q3D) for 22 days.
Group 3 was treated as follows: RFB(150 mg) once daily(QD) for 21 days, followed by SQV-RTV(1,000/100 mg) BID and RFB(150 mg) every 4 days(Q4D) for 21 days.
Mean Ctrough levels of rifabutin or 25-O-desacetyl-rifabutin reached stable values from days 17 to 20 (period 1 for both group 2 and group 3) and from days 34 to 43 (period 2 for group 2) or days 30 to 42 (period 2 for group 3) (Table 3). However, the mean Ctrough levels of 25-O-desacetyl-rifabutin in period 2 were significantly higher than in period 1 for both group 2 and group 3 (Table 3).
Plasma protein binding.
Plasma protein binding of rifabutin and 25-O-desacetyl-rifabutin was independent of their concentrations in the range of 10 to 100 ng/ml. Thus, the analysis using samples collected at 2 or 3 h was reasonable. The fraction unbound (fu) was determined for 20 subjects in group 2 (n = 11) and group 3 (n = 9) who completed both periods. The mean plasma protein fu for rifabutin ranged from 9.0% to 12.4%, and for 25-O-desacetyl-rifabutin, it ranged from 29.0% to 33.9% across groups 2 and 3, respectively, during both treatment periods. Results of the ANOVA on the fu for rifabutin and 25-O-desacetyl-rifabutin suggest a slight reduction in fu when rifabutin is coadministered with saquinavir-ritonavir relative to administration of rifabutin alone. For the combined groups 2 and 3, the decrease in GMR for the fu of rifabutin during period 2 compared to period 1 was 14% (90% CI, 9% to 18% decrease), and for the 25-O-desacetyl-rifabutin metabolite, the decrease was 11% (90% CI, 6% to 16% decrease). Since the 90% CIs associated with the GMR estimates for the combined data were within 80% to 125%, the changes were not clinically significant.
Safety results.
Coadministration of rifabutin with saquinavir-ritonavir was generally well tolerated, with no deaths or serious adverse events reported. The percentages of subjects reporting adverse events was higher with triple therapy (period 2) than with saquinavir-ritonavir or rifabutin alone (period 1) (54% and 84%, respectively, for group 1; 50% and 54%, respectively, for group 2; and 63% and 77%, respectively, for group 3). The majority of adverse events were mild (37 of 38 in period 1 and 77 of 97 in period 2 for group 1; 16 of 16 in period 1 and 21 of 23 in period 2 for group 2; and 18 of 22 in period 1 and 20 of 35 in period 2 for group 3), and the rest of the adverse events were moderate. No adverse events were reported as severe. In all treatment groups, the percentage of subjects who withdrew from the study was higher in period 2 (12 subjects; 23.5%) when the subjects were receiving triple combination therapy than in period 1 (5 subjects; 8.9%) when the subjects were treated with either saquinavir-ritonavir or rifabutin alone. Of these 17 withdrawals, the majority (n = 13) were for safety reasons, with 11 of these subjects discontinuing during the period of triple combination therapy. Neutropenia was also the most common adverse event leading to withdrawal from the study (6 cases of neutropenia; 4 cases of grade 3 neutropenia, 1 case of grade 2 neutropenia, and 1 case of grade 1 neutropenia based on National Cancer Institute [NCI] common toxicity critera [CTC] grading [8], all of which resolved following discontinuation of study treatment). Neutropenia was also the most common laboratory abnormality, affecting 22 subjects (12 subjects with grade 3 neutropenia and 8 subjects with grade 2 neutropenia), all of whom were receiving rifabutin either alone or in combination with saquinavir-ritonavir. There was no case of grade 4 neutropenia. Marked abnormalities in liver enzymes (aspartate transaminase, alanine aminotransferase, gamma glutamyl transferase) occurred in ≤15% of subjects in the three treatment groups, with no subject having a grade 4 liver enzyme elevation. No other abnormal laboratory parameter or vital sign, ECG change, or abnormality of clinical relevance was recorded.
DISCUSSION
The rifamycins, including rifampin and rifabutin, are mainstays in the treatment of tuberculosis, but because they induce CYP3A4, they lower the exposure of coadministered protease inhibitors such as saquinavir (7, 10). This complication can in principle be obviated by regimens involving the concomitant administration of ritonavir, an inhibitor of CYP3A4. While the most straightforward candidate for triple therapy regimens is rifampin, this combination is contraindicated owing to the elevation of hepatic transaminases (15). The present study was undertaken to assess the utility of rifabutin as a component of combined treatment with saquinavir and ritonavir. Because rifabutin is metabolized by CYP3A4 (14) and its exposure is enhanced in the presence of coadministered ritonavir (2), two triple therapy regimens, involving rifabutin dosing every 3 days and every 4 days, were employed in order to compensate for this increase.
In the first treatment group, which examined the effect of triple therapy on the exposures to saquinavir and ritonavir, reference exposures were established following 14 days of BID saquinavir-ritonavir (1,000 mg of saquinavir and 100 mg of ritonavir). After 22 days of combination therapy with rifabutin (150 mg) given every 3 days, saquinavir exposure was reduced by 13% for AUC0-12, by 15% for Cmax, and 9% for Cmin. However, this reduction is not considered clinically meaningful, because saquinavir exposure remains within the efficacy and safety range of 1,299 to 19,085 ng·h/ml for AUC0-12 and 70 to 433 ng/ml for Cmin (11). With respect to ritonavir, coadministered rifabutin had no significant effect on exposure. In summary, no dose adjustment for BID saquinavir-ritonavir (1,000/100 mg) is necessary when combined with rifabutin given every 3 or 4 days.
In the component of the study determining an acceptable dosing regimen for rifabutin in combination therapy with the protease inhibitors, one dramatic result was the major increase in the fraction of the active moiety that was contributed by the active metabolite 25-O-desacetyl-rifabutin. This fraction increased from 0.07 when rifabutin was dosed alone (QD at 150 mg) to 0.64 or 0.71 when rifabutin, given at 150 mg every 3 or 4 days, respectively, was combined with BID saquinavir-ritonavir (1,000/100 mg). It has been suggested that CYP3A mediates a larger fraction of 25-O-desacetyl-rifabutin metabolism than rifabutin metabolism (14); thus, inhibition of CYP3A by ritonavir had a larger effect on the clearance of 25-O-desacetyl-rifabutin than for rifabutin.
In the exploration of rifabutin protocols during triple therapy, rifabutin dosing every 3 days resulted in rifabutin exposure that was 53% higher than rifabutin (150 mg) QD given alone and exposure for the active moiety that was 134% higher than rifabutin (150 mg)1 QD given alone. When the rifabutin dosing interval was increased to every 4 days during triple therapy, rifabutin exposure was equivalent to that found when rifabutin was dosed QD alone at 150 mg, while exposure for the active moiety was 60% higher than the rifabutin exposure when it was dosed QD alone at 150 mg. Thus, administration of rifabutin every 4 days during triple therapy resulted in exposure to the active moiety that was within the predefined boundary for daily dosing of rifabutin between 150 and 300 mg.
It should be noted that the rifabutin exposure determined from a drug interaction study of healthy subjects may not be sufficient to control acquired rifamycin resistance in the patient population (5). In the label for Kaletra, it was recommended that 300 mg of rifabutin daily can be reduced to 150 mg three times per week in the presence of lopinavir-ritonavir (1). However, when the regimen was tested in patients with HIV infection and active tuberculosis, the rifabutin AUC0-24 value (2.97 μg·h/ml) (5) fell within the 95% confidence interval (1.9 to 4.5 μg·h/ml) for patients who had acquired rifamycin resistance (17). None of the 10 patients (8) reached mean AUC0-24 of ≥5.2 μg·h/ml which was the mean exposure observed for patients without acquired rifamycin resistance (17). Therefore, although the present study results support the administration of rifabutin (150 mg) Q4D when combined with the saquinavir-ritonavir (1,000/100 mg) BID regimen, it is acknowledged that more frequent dosing for rifabutin (e.g., 150 mg three times per week or every other day) may be needed to treat HIV patients with active tuberculosis in order to prevent acquired rifamycin resistance.
Previously, severe neutropenia among healthy volunteers occurred when the standard 300-mg rifabutin QD dose was given alone or in combination with azithromycin or clarithromycin (3). In the present study, a total of 22 subjects experienced grade 2 or grade 3 neutropenia during triple combination treatment period 2, and no subject experienced severe neutropenia (grade 4), indicating that 150-mg rifabutin administered daily alone or every 3 or 4 days in combination with ritonavir-boosted saquinavir was appropriate. Nevertheless, 6 subjects withdrew from the study due to grade 1 to 3 neutropenia, and 5 of the withdrawals occurred during triple combination period 2. Thus, neutropenia should be monitored when patients receive combination treatment therapy.
In conclusion, the present study examining the coadministration of rifabutin and ritonavir-boosted saquinavir established that triple therapy regimens in which rifabutin (150 mg) was administered every 3 or 4 days did not result in clinically significant effects on either AUC0-12, Cmax, or Cmin for either of the two protease inhibitors. As such, no dose adjustment is required for the latter. With respect to the effect of saquinavir-ritonavir on coadministered rifabutin, however, dosing of rifabutin once every 3 days resulted in an increase in exposure of 53% for the native drug and an increase of 134% for its active moiety, including its principal metabolite, compared to the rifabutin exposure observed with 150-mg QD dosing in the absence of the saquinavir-ritonavir (1,000/100 mg) BID dosing. This increased exposure for rifabutin was not observed, though, when the dosing interval for rifabutin 150 mg was increased to once every 4 days. The increase in exposure for the active moiety was 60% when the dosing interval for rifabutin (150 mg) was increased to once every 4 days. Patients receiving these agents should be monitored for neutropenia and liver enzyme elevations.
Acknowledgments
This study was supported by Hoffmann-La Roche, Ltd., Welwyn, England. Xiaoping Zhang, Scott Fettner, Elke Zwanziger, Lucy Rowell, and Miklos Salgo are all employees of Hoffmann-La Roche.
We acknowledge Adam Foley-Comer, the principal investigator at Roche Clinical Pharmacology Unit in Welwyn, United Kingdom, for his contributions to this study. We thank the study team members at Hoffman-La Roche: Anne-Helene Clugery and Laura Cristea for the study management, Martin Wermelinger for EU regulatory guidance, Patricia Matone for writing the study report, Hagen Zandt and Myriam Riek for the statistical analysis plan and test, and Elke Zwanziger for the management of analytical work. In addition, editorial assistance was provided by Diann Glickman of Zola Associates and was funded by Hoffmann-La Roche.
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Abbott Laboratories. 9 August 2010. PRKaletra® product monograph. PRKaletra® lopinavir/ritonavir film-coated tablets (100 mg/25 mg, 200 mg/50 mg) PRKaletra® lopinavir/ritonavir oral solution (80/20 mg/mL) human immunodeficiency virus (HIV) protease inhibitor. Abbott Laboratories, Ltd., St-Laurent, Quebec, Canada. http://www.abbott.ca/static/content/document/Kaletra-PM-09AUG10.pdf.
- 2.Abbott Laboratories. 2010. Norvir® (ritonavir capsules) soft gelatin (ritonavir oral solution) prescribing information. Abbott Laboratories, North Chicago, IL. http://rxabbott.com/pdf/norpi2a.pdf.
- 3.Apseloff, G. 2003. Severe neutropenia among healthy volunteers given rifabutin in clinical trials. Clin. Pharmacol. Ther. 74:591-592. [DOI] [PubMed] [Google Scholar]
- 4.Banker, M. J., T. H. Clark, and J. A. William. 2003. Development and validation of a 96-well equilibrium dialysis apparatus for measuring plasma protein binding. J. Pharm. Sci. 92:967-974. [DOI] [PubMed] [Google Scholar]
- 5.Boulanger, C., et al. 2009. Pharmacokinetic evaluation of rifabutin in combination with lopinavir-ritonavir in patients with HIV infection and active tuberculosis. Clin. Infect. Dis. 49:1305-1311. [DOI] [PubMed] [Google Scholar]
- 6.Burman, W. J., K. Gallicano, and C. Peloquin. 1999. Therapeutic implications of drug interactions in the treatment of human immunodeficiency virus-related tuberculosis. Clin. Infect. Dis. 28:419-430. [DOI] [PubMed] [Google Scholar]
- 7.Cahn, P., H. Perez, G. Ben, and C. Ochoa. 2003. Tuberculosis and HIV: a partnership against the most vulnerable. J. Int. Assoc. Physicians AIDS Care 2:106-123. [DOI] [PubMed] [Google Scholar]
- 8.Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health. 9 August 2006. Common terminology criteria for adverse events v3.0 (CTCAE). http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, Bethesda, MD.
- 9.Cato, A., et al. 1998. The effect of multiple doses of ritonavir on the pharmacokinetics of rifabutin. Clin. Pharmacol. Ther. 63:414-421. [DOI] [PubMed] [Google Scholar]
- 10.Di Perri, G., et al. 2005. Drug-drug interactions and tolerance in combining antituberculosis and antiretroviral therapy. Expert Opin. Drug Saf. 4:821-836. [DOI] [PubMed] [Google Scholar]
- 11.Genentech USA, Inc. 2010. Invirase® (saquinavir mesylate) capsules and tablets prescribing information. Genentech USA, Inc. South San Francisco, CA. http://www.gene.com/gene/products/information/invirase/pdf/pi.pdf.
- 12.LeBel, M., et al. 1998. Effects of rifabutin and rifampicin on the pharmacokinetics of ethinylestradiol and norethindrone. J. Clin. Pharmacol. 38:1042-1050. [DOI] [PubMed] [Google Scholar]
- 13.Moyle, G. J., et al. 2002. Interaction between saquinavir soft-gel and rifabutin in patients infected with HIV. Br. J. Clin. Pharmacol. 54:178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfizer Inc. 2007. Mycobutin® (rifabutin) capsules, USP prescribing information. Pfizer Inc., New York, NY. http://www.pfizer.com/files/products/uspi_mycobutin.pdf.
- 15.Schmitt, C., M. Riek, E. Winters, M. Schultz, and S. Grange. 2009. Unexpected hepatotoxicity of rifampin and saquinavir/ritonavir in healthy male volunteers. Arch. Drug Info. 2:8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veldkamp, A. I., R. M. W. Hoetelmans, J. H. Beijnen, J. W. Mulder, and P. L. Meenhorst. 1999. Ritonavir enables combined therapy with rifampin and saquinavir. Clin. Infect. Dis. 29:1586. [DOI] [PubMed] [Google Scholar]
- 17.Weiner, M., et al. 2005. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin. Infect. Dis. 40:1481-1491. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. 2008. Global tuberculosis control 2008: surveillance, planning, financing. WHO Report 2008. WHO publication WHO/HTM/TB/2008.393. World Health Organization, Geneva, Switzerland http://www.who.int/tb/publications/global_report/2008/pdf/fullreport.pdf.