Abstract
Blastocystis is an emerging protistan parasite of controversial pathogenesis. Although metronidazole (Mz) is standard therapy for Blastocystis infections, there have been accumulating reports of treatment failure, suggesting the existence of drug-resistant isolates. Furthermore, very little is known about Blastocystis susceptibility to standard antimicrobials. In the present study, we established resazurin and XTT viability microassays for Blastocystis spp. belonging to subtypes 4 and 7, both of which have been suggested to represent pathogenic zoonotic subtypes. The optimized resazurin assay was used to screen a total of 19 compounds against both subtypes. Interestingly, subtype 7 parasites were resistant to Mz, a 1-position-substituted 5-nitroimidazole (5-NI), while subtype 4 parasites were sensitive. Some cross-resistance was observed to tinidazole, another 1-position 5-NI. Conversely, subtype 4 parasites were resistant to emetine, while subtype 7 parasites were sensitive. Position 2 5-NIs were effective against both subtypes, as were ornidazole, nitazoxanide, furazolidone, mefloquine, quinicrine, quinine, cotrimoxazole (trimethoprim-sulfamethoxazole), and iodoacetamide. Both subtypes were resistant to chloroquine, doxycycline, paromomycin, ampicillin, and pyrimethamine. This is the first study to report extensive variations in drug sensitivities among two clinically important subtypes. Our study highlights the need to reevaluate established treatment regimens for Blastocystis infections and offers clear new treatment options for Mz treatment failures.
Blastocystis is an emerging enteric protistan parasite with zoonotic potential (39, 57, 58). It is one of the most common parasites colonizing the human gut, with prevalences ranging between 10% of the population in developed countries and 50% in developing countries (58). It frequently infects immunocompromised individuals (27, 40, 59) and has a high prevalence in impoverished children (35) and HIV/AIDS (27) and cancer (59) patients. Individuals infected with Blastocystis present with common intestinal symptoms, such as abdominal pain, vomiting, and bloating, as well as mucous and watery diarrhea (58). Blastocystis infections are commonly associated with dermatological disorders (25, 67) and irritable bowel syndrome (54).
Although metronidazole (Mz) treatment is considered first-line therapy for Blastocystis infections, therapeutic intervention is equivocal because of the large number of asymptomatic carriers and frequent reports of treatment failure (3, 23, 37, 53, 55). The confusion concerning the status of Blastocystis as a pathogen is primarily due to limitations of diagnostic techniques, purported subtype-dependent variations in parasite virulence, and variable host responses (55). The variation in treatment response suggests the presence of metronidazole-resistant (Mzr) subtypes of the parasite, but there are currently no in vitro or in vivo data to support this hypothesis. Despite these controversies, interest in the parasite has increased in recent years, as signified by the establishment of organizations like the Blastocystis Research Foundation, which actively support studies on subtype-dependent variations in Blastocystis pathobiology and treatment (6). The clinical significance of the intestinal parasite Giardia intestinalis was recognized only after it became possible to effectively eliminate it from the gut (33). To understand the role of Blastocystis as a human pathogen, there is an urgent need to identify standardized and effective treatment options for various Blastocystis subtypes.
At least 9 out of the 11 subtypes of Blastocystis are known to colonize the human gut (57). The identification of antibiotic-resistant subtypes of the parasite and development of new therapeutic options to counter antimicrobial resistance require a high-throughput screening tool. Conventional drug susceptibility assays for Blastocystis (16, 68, 72, 75) are not suitable for high-throughput drug screening (HTS) because they are expensive, laborious, time-consuming, potentially hazardous, and prone to bias. Since the incidence of Blastocystis is higher in developing countries (58), the cost and availability of sophisticated equipment are also limitations for such screenings.
In this study, we evaluated two high-throughput viability assays and applied them to drug susceptibility microassays for Blastocystis. Resazurin (7-hydroxy-3H-phenoxazin-3-one 10-oxide) is the active compound of a propriety solution, Alamar blue (41). The resazurin assay measures intrinsic cellular metabolic activity, which reduces resazurin and changes its color as a measurable indicator of the number of viable cells that are present in a test sample (34, 47). Resazurin-based assays are commonly used for drug susceptibility analysis of prokaryotic (29) and eukaryotic (20, 34, 41, 46) cells. Much like resazurin, the tetrazolium salt 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) is reduced by mitochondrial and cytoplasmic redox enzymes to a colored formazan compound with a direct correlation with cell proliferation. Tetrazolium compounds have also been widely utilized for cytotoxic evaluation of both prokaryotic (14) and eukaryotic (5) organisms.
In the current study, we report that optimized resazurin and XTT redox-based assays are suitable for viability studies of the parasite. Blastocystis subtype 4 and subtype 7 isolates are most commonly found in rats and birds, respectively (57). Both subtypes are known to colonize the human gut, and studies suggest that both subtypes have pathogenic potential (54). We utilized the optimized assays to determine the susceptibility of Blastocystis isolates to a range of antimicrobial agents. We observed extensive subtype-dependent variations in Blastocystis susceptibility to a panel of conventional and experimental antiprotozoal agents and identified Mz- and emetine (EM)-resistant subtypes of the parasite. Importantly, we identified several new and potentially effective treatment options for Mzr Blastocystis infections.
MATERIALS AND METHODS
Cell culture.
Four axenized isolates of Blastocystis were used (Table 1). All four isolates were subtyped previously by small-subunit rRNA gene analyses (39). Isolates WR-1 and S-1 belong to subtype 4, while isolates B and E belong to subtype 7, according to a recent Blastocystis sp. classification system (52). Cultures of all four isolates were maintained as described previously (36). In brief, the parasites were maintained in 10 ml of prereduced Iscove's modified Dulbecco's medium (IMDM) containing 10% horse serum in an anaerobic jar (Oxoid) with an AnaeroGen gas pack (Oxoid) at 37°C. The parasites were subcultured alternately at 72 and 96 h. Under these culture conditions, all four parasites exhibited noncystic vacuolar morphology. This morphological state is advantageous for assessment of MZ resistance because Blastocystis cysts are known to be resistant to the drug (73), complicating our study. Cultures were harvested from log-phase in vitro cultures for viability studies in 96-well plates.
TABLE 1.
Sources of Blastocystis isolates
Microculture technique.
In order to establish and validate the analytical methods for Blastocystis viability determination, the microculture conditions were optimized for standard 96-well plates. Subtype 7 parasites (isolate B) were employed for the optimization experiments. Several parasite numbers between 103 and 106 cells were incubated in Blastocystis culture medium in a final volume of 200 μl/well in standard 96-well plates, unless otherwise stated. The 96-well plates were then incubated at 37°C under anaerobic conditions for 24 h unless otherwise stated. After 24 h, the cultures were incubated with redox dyes for an additional 3 h and 5 h for quantitative and semiquantitative evaluation, respectively. Unless otherwise stated, a 5% final dilution of the resazurin dye solution (Sigma) was used for resazurin assays, whereas XTT (Sigma) was used at a final concentration of 50 μg/ml. At the end of incubation, readings of resazurin fluorescence were taken at 550-nm excitation and 570-nm emission wavelengths, while XTT assay measurements were made at an absorbance wavelength of 450 nm. A Tecan Infinite M200 reader was used for both fluorimetric and colorimetric measurements. For semiquantitative evaluation, the color change in each well was visually observed and recorded after 5 h.
Drug preparation.
Compounds purchased from Sigma included Mz, ornidazole (Oz), ronidazole (Rz), furazolidone (FUR), mefloquine (MQ), quinacrine (QC), quinine (QN), chloroquine (CQ), emetine (EM), doxycycline (DOX), trimethoprim sulfate-sulfamethoxazole (TMP-SMZ), paromomycin (PAR), ampicillin (AMP), pyrimethamine (PYR), and iodoacetamide (IA). Tinidazole (Tz) was purchased from AK Scientific, whereas nitazoxanide (NTZ) was purchased from Romark Laboratory. C-17 is an experimental, chemically synthesized, 2-position 5-nitroimidazole (NI) compound (66). Stock solutions of each compound to be tested were prepared fresh in dimethyl sulfoxide (DMSO). For drug sensitivity determination, stock solutions were diluted in prereduced Blastocystis medium and transferred to 96-well plates. A total of 0.5 × 106 cells/well were incubated for 24 h with different dilutions of the drugs ranging between 0 and 100 μg/ml. The final DMSO concentration was kept constant at 0.5%.
Confocal microscopy.
Confocal micrographs of the parasites were taken in order to determine whether the alteration in Blastocystis redox activity under drug tension observed in previous assays was also associated with morphological changes. Metronidazole-susceptible (Mzs) ST-4 (isolate WR-1) and Mzr ST-7 (isolate E) were treated for 24 h with a 12.5-μg/ml concentration of FUR and Mz. After drug exposure, the parasites were washed and resuspended in annexin V binding buffer (BioVision). Annexin V and propidium iodide (PI) (BioVision) were then added to the cell suspension. Confocal imaging of cell suspensions was done using an Olympus Fluoview FV1000 (Japan) equipped with a dual filter set for fluorescein isothiocyanate (FITC) and rhodamine. Images were captured using Olympus Fluoview version 1.6b.
Statistical analysis and validation of reproducibility.
Before a particular assay was used for a full-scale HTS, smaller pilot screenings were used to predict its usefulness for large-scale applications. The Z′ factor predicts the robustness of an assay for HTS by taking into account the mean and standard deviation of both positive and negative controls of the pilot screening (74). We calculated the Z′ factors of both assays for Blastocystis drug screening using the following equation: Z′ factor = 1 − [(3σc+ + 3σc−)/|μc+ − μc−|], where, c+ is the positive control (0.5% DMSO), c− is the negative control (6.25 μg/ml FUR), σ is the standard deviation, and μ is the mean.
Assays having a Z′ factor score between 0.5 and 1 are considered excellent for HTS (74).
Comparison of data sets with wide differences between their means should be made using the coefficient of variation (Cv) instead of the standard deviation (σ). It represents the σ in the context of the mean (μ) and is another test used to evaluate the robustness of an assay for HTS. We calculated the Cvs of both assays using the following formula (30): Cv = σ/μ, where, Cv is the coefficient of variation, σ is the standard deviation of the positive control (5 × 105 parasites in 200 μl culture medium plus 0.5% DMSO), and μ is the mean of the positive control (5 × 105 parasites in 200 μl culture medium plus 0.5% DMSO).
Assays with a Cv of <1 are considered low variance and fit for HTS (30).
The final validation step was the screening of the dose-dependent antiprotozoal activity of Mz against 4 different isolates of Blastocystis repeated twice in triplicate. The results were statistically compared for reproducibility.
The statistical significance of variations between the drug susceptibility values of 4 isolates was determined using one-way analysis of variance (ANOVA). A one-way ANOVA test is ideal to test the statistical significance of the variations observed between means of three or more groups of data.
RESULTS
Resazurin and XTT result in fluorimetric and colorimetric reactions with Blastocystis in a cell density-dependent manner.
For semiquantitative analysis, visible color changes were observed after 5 h of incubation of resazurin and XTT with Blastocystis sp. subtype 7 in 200 μl parasite culture medium. Several shades of resazurin dye, ranging from blue to pink, developed with increasing cell density. Similarly, XTT developed shades ranging from yellow to deep orange with increasing cell density. Minimums of 105 parasites/well were needed to obtain visual evidence of color change for both dyes, although the color change was more obvious in the resazurin dye than with XTT.
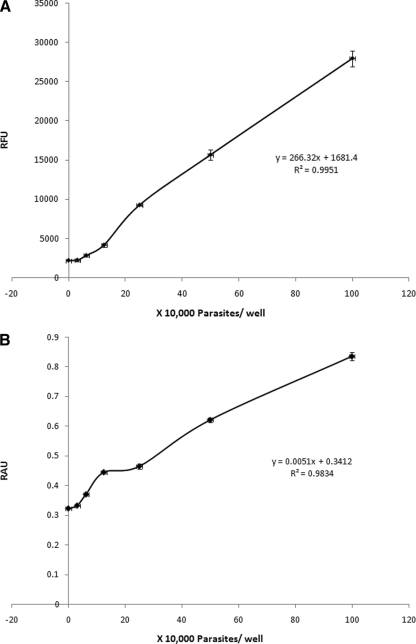
For quantitative analysis, fluorescence and absorbance measurements were taken for resazurin and XTT dyes, respectively, after 3 h of incubation. Negligible changes in absorbance and fluorescence measurements were observed between the blank medium control and up to 104 parasites/well (Fig. 1), but a linear increase in fluorimetric, as well as colorimetric, measurements was noted from 104 parasites to 106 parasites/well (Fig. 1). The R2 values for resazurin and XTT dyes were calculated to be 0.995 and 0.983, respectively (Fig. 1; see Table 3). A density of 5 × 105 parasites/well was chosen as the optimal cell density for further experiments because it lies within the linear range of cell density versus dye reduction for both assays (Fig. 1) and provides visible color changes in a short time.
FIG. 1.
Correlation between the number of subtype 7 parasites and relative fluorescence units (RFU) (A) and relative absorbance units (RAU) (B) after 24 h of incubation and 3 h of development with resazurin and XTT, respectively. Each point represents an average of 6 values derived from two independent sets of experiments. The error bars represent standard errors.
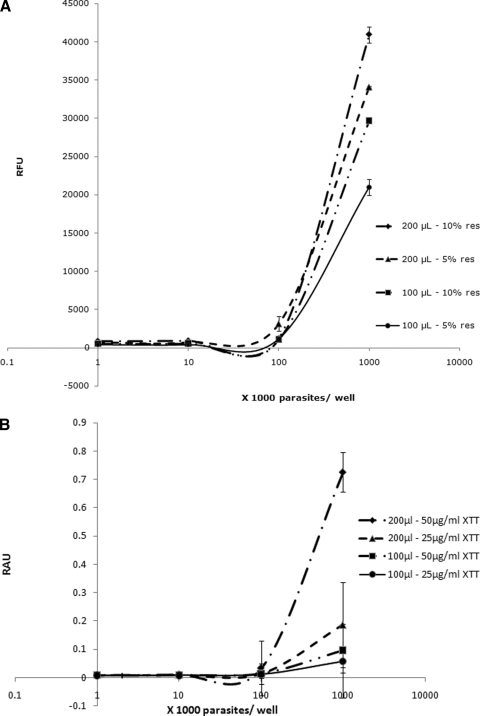
Blastocystis requires 200-μl/well volumes for optimal metabolic activity.
For viability assays, cells should be at their optimal metabolic activity. A recent study reported an increase in metabolic activity of Acanthamoeba with a reduction of the culture volume from 200 to 100 μl/well (34). In this study, a decrease in volume per well resulted in a drop in Blastocystis metabolic activity (Fig. 2). Blastocystis, an anaerobic organism (57), should have higher metabolic activity in high well volumes as opposed to Acanthamoeba, which is an aerobic protozoan (34). Therefore, a 200-μl/well volume was used in all subsequent experiments (Table 2 and Fig. 2).
FIG. 2.
Correlation of total volume per well and dye concentration with relative fluorescence units (RFU) (A) and relative absorbance units (RAU) (B) for resazurin (res) and XTT dyes, respectively. Higher volumes per well and dye concentrations resulted in higher sensitivity of resazurin and XTT, denoted by higher RFU and RAU readings, respectively. Each point represents a mean of 6 values derived from two independent sets of experiments. The error bars represent standard errors.
TABLE 2.
Optimized parameters for resazurin and XTT assays
| Parameter | Value |
|---|---|
| Dye concn | |
| Resazurin | 5% |
| XTT | 50 μg/ml |
| Growth medium | IMDM + 10% HSa + 0.5% DMSO |
| Volume/well | 200 μl |
| Temperature | 37°C |
| Culture conditions | Anaerobic |
| Contact time with dye (h) | |
| Semiquantitative/visual | 5 |
| Quantitative | 3 |
| Excitation/emission λ (nm) | |
| Resazurin | 550/580 |
| XTT | 450 |
| Optimal cell density (parasites/well) | 0.5 × 106 |
HS, heat-inactivated horse serum.
Blastocystis exhibits exponential growth in microcultures.
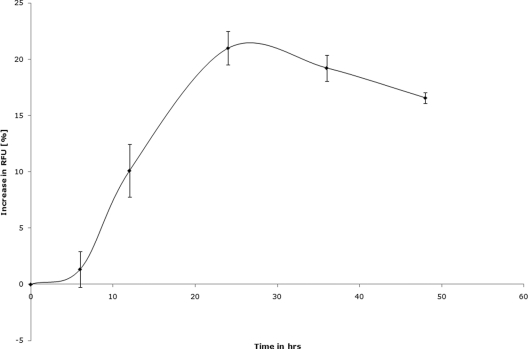
Blastocystis sp. subtype 7, when incubated under optimal microplate growth conditions, exhibited an increasing degradation of resazurin over time, suggesting a rise in the redox activity of the culture (Fig. 3). This increase in redox activity could be due to an increase in either parasite numbers or metabolic activity. The redox activity of the parasite cultures peaked at 24 h, followed by a drop, suggesting a slowing down of the culture growth or metabolism due to overcrowding. The 24-h time point was chosen for drug susceptibility assays (Table 2). The complete optimized parameters for both resazurin and XTT assays are summarized in Table 2.
FIG. 3.
Blastocystis subtype 7 exhibits a time-dependent increase in redox activity when cultured in a 96-well plate under the resazurin assay conditions described in this study. The starting parasite density was 0.5 × 106 cells in 200 μl of IMDM supplemented with 10% horse serum and 0.5% DMSO. The redox activity of the culture peaked at 24 h, followed by a steady decline. A drug contact duration of 24 h was chosen based on these results. Each point represents a mean of 6 values derived from two independent experiments, with each experiment conducted in triplicate. The error bars represent standard errors.
Resazurin and XTT are suitable for HTS of antimicrobials against Blastocystis.
HTS quality control parameters, i.e., a Z′ factor of >0.5 (74) and a Cv of <10% (30), were met by both resazurin and XTT assays (Table 3). Both assays exhibited statistical reproducibility for dose-dependent activity assays of antimicrobial agents against Blastocystis (Fig. 4).
TABLE 3.
Statistical evaluation of the quality of resazurin and XTT assaysa
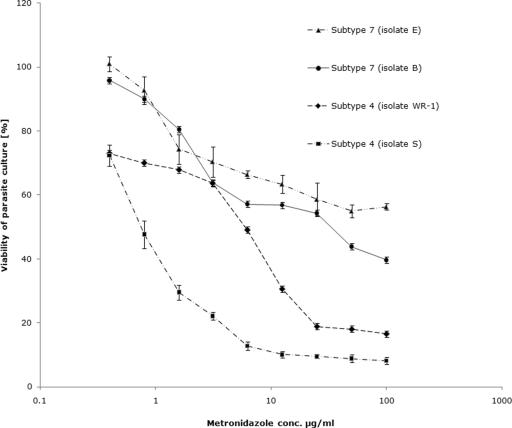
FIG. 4.
Graph representing percent inhibition of Blastocystis subtype 4 and 7 cultures by Mz using the resazurin assay. The IC50s of Mz against subtype 4 isolates were found to be significantly lower than those of subtype 7 isolates (P < 0.01). Mz induced 50% inhibition of subtype 7 isolate B cultures at a concentration (conc.) of 32.5 ± 3.4 μg/ml, whereas isolate E cultures exhibited only minimal inhibition even at concentrations as high as 100 μg/ml. Each point represents a mean of six readings derived from two independent experiments. The error bars represent standard errors.
Blastocystis exhibits subtype-dependent variation in susceptibility and resistance to Mz.
Using the optimized resazurin assay, the 50% inhibitory concentrations (IC50s) of Mz against subtype 4 and subtype 7 isolates of Blastocystis were calculated. Mz inhibited 50% of growth of subtype 4 isolates WR-1 and S-1 at concentrations of 5.5 ± 2.89 μg/ml and 1.9 ± 1.32 μg/ml, respectively (Table 4; Fig. 4 and 5). These values were within the range of previously reported values of Mz susceptibility for Blastocystis (16, 75). The IC50 of Mz against isolate B (subtype 7) was 32.5 ± 3.4 μg/ml. This value is significantly higher than the IC50 of subtype 4 isolates (P < 0.01) and exceeds the average fecal Mz concentration of 9.5 μg/ml (26). Isolate E of subtype 7 exhibited minimal susceptibility to Mz (Table 4 and Fig. 4), even at concentrations as high as 100 μg/ml. These results suggest that isolates B and E of subtype 7 are Mzr strains of Blastocystis. The XTT assay further confirmed these strains to be Mzr (Table 4).
TABLE 4.
IC50 values of Blastocystis susceptibility to Mz
| Viability assay | IC50 [μg/ml (μM)] |
|||
|---|---|---|---|---|
| Subtype 7 isolates |
Subtype 4 isolates |
|||
| B | E | WR-1 | S | |
| Resazurin | 32.5 ± 3.4 (189.8) | NSa | 5.5 ± 2.89 (32.16) | 0.75 ± 0.04 (4.38) |
| XTT | 29 ± 3.4 (169.36) | NS | 1.76 ± 0.39 (10.27) | 1.1 ± 0.08 (6.4) |
NS, not susceptible to drug concentrations of ≤100 μg/ml.
FIG. 5.
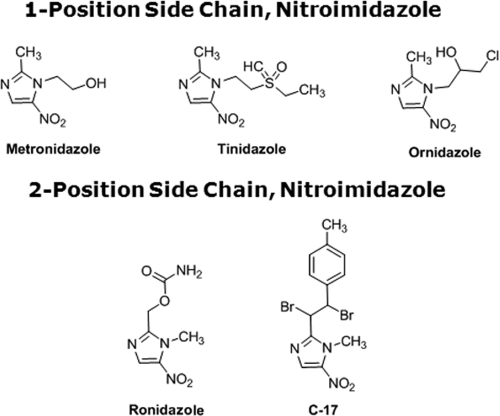
Chemical structures of position 1 and position 2 5-nitroimidazoles.
An Mzs isolate of Blastocystis exhibits typical morphological features of cell death after exposure to Mz, as opposed to an Mzr isolate.
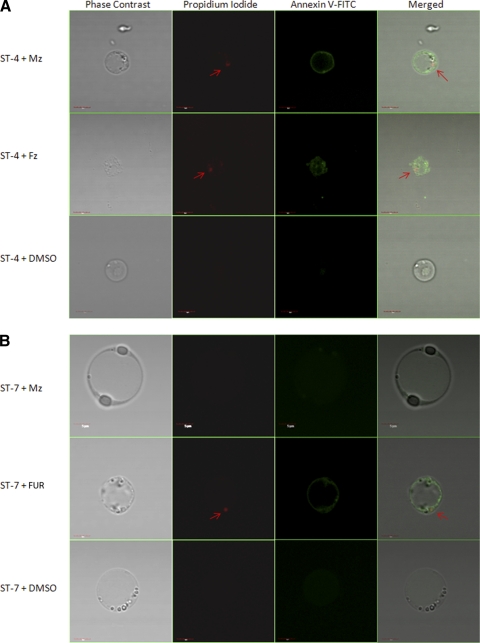
Our findings, based on resazurin and XTT assays, indicate suppression of parasite redox activity under drug tension. Concomitantly, to determine whether Blastocystis undergoes morphological changes after drug exposure, parasites were stained with propidium iodide and annexin V-FITC. Both PI and annexin V stain only dying parasites (71). PI binds to the parasite nuclear material (71). Annexin V binds with high affinity to phosphatidylserine (PS). PS is located at the cytosolic face of the cell membrane and has access to annexin V only when it becomes exposed at cell death (71). Healthy parasites are impermeable to both PI and annexin V (71). Mzs ST-4 (isolate WR-1) exhibited nuclear incorporation of PI and annexin V binding after 24 h of exposure to a 12.5-μg/ml concentration of Mz, suggesting a breach in the parasite cell membrane (Fig. 6 A). No changes were observed in Mzr ST-7 (isolate E) after Mz treatment (Fig. 6B). MZs and Mzr isolates exhibited cell death morphology after treatment with a 12.5-μg/ml concentration of FUR (Fig. 6A and B), whereas neither of the isolates incorporated PI or annexin V after treatment with the DMSO control (Fig. 6A and B). These findings suggest that after treatment with Mz, morphological alterations typical of dying cells were observed in the Mzs isolate, while the Mzr isolate remained unaffected.
FIG. 6.
Confocal micrographs of Blastocystis stained with propidium iodide (arrow) and annexin V-FITC. (A) Mzs ST-4 (WR-1) exhibited nuclear incorporation of PI and annexin V-FITC binding after 24 h of exposure to 12.5 μg/ml Mz. (B) Mzr ST-7 (isolate E) did not exhibit these classical signs of cell death after Mz treatment. Both Mzs and Mzr isolates exhibited PI incorporation and annexin V-FITC binding after 24-h treatment with 12.5 μg/ml FUR, while no changes were observed in healthy parasites incubated with DMSO. Bars, 5 μm.
Mzr isolates of Blastocystis exhibit cross-resistance with a 1-position-substituted 5-NI.
Tz, a compound closely related to Mz due to the presence of its side chain at position 1 of the imidazole ring (Fig. 5), was effective in killing both Mzr and Mzs isolates. Interestingly, Mzs subtype 4 isolates WR-1 and S exhibited IC50s (0.51 ± 0.02 and 0.3 ± 0.1 μg/ml, respectively) of Tz lower than those of Mzr subtype 7 isolates B and E (5.13 ± 0.16 and 9.33 ± 0.45 μg/ml, respectively) (P < 0.01) (Table 5). Even within subtype 7, the IC50 of Tz for Mzr isolate E was significantly higher than that for isolate B (Table 5). These findings in Blastocystis suggest a cross-resistance pattern similar to those exhibited by other parasites (7, 12). Oz, another closely related 5-NI (Fig. 5), despite having a position 1 side chain, was found to be equally effective against both Mzr and Mzs isolates. Interestingly, Mzr subtype 7 isolates exhibited significantly higher susceptibility to the position 2 side chain 5-NIs Rz and C-17 (Fig. 5) than to position 1 5-NI. No significant subtype-dependent variation in Blastocystis susceptibility to position 2 5-NIs was observed.
TABLE 5.
IC50 values of Blastocystis for 5-NIs by resazurin assay
| Drug | IC50 [μg/ml (μM)] |
|||
|---|---|---|---|---|
| Subtype 7 isolates |
Subtype 4 isolates |
|||
| B | E | WR-1 | S | |
| 1-Position 5-NIsa | ||||
| Mzd | 32.5 ± 3.4 (189.8) | NSc | 5.5 ± 2.89 (32.16) | 0.75 ± 0.04 (4.38) |
| Tzd | 5.13 ± 0.16 (20.52) | 9.33 ± 0.45 (37.32) | 0.51 ± 0.02 (2.04) | 0.3 ± 0.1 (1.2) |
| Oze | 1.42 ± 0.02 (6.44) | 1.23 ± 0.15 (5.58) | 1.1 ± 0.3 (4.9) | 1.15 ± 0.05 (5.22) |
| 2-Position 5-NIsb | ||||
| Rze | 0.52 ± 0.02 (2.6) | 0.31 ± 0.08 (1.55) | 0.32 ± 0.1 (1.6) | 0.37 ± 0.08 (1.85) |
| C-17f | 0.63 ± 0.1 (1.56) | 0.36 ± 0.13 (0.89) | 0.42 ± 0.08 (1.04) | 0.5 ± 0.05 (1.24) |
Side chain at position 1 of the imidazole ring of 5-NI.
Side chain at position 2 of the imidazole ring of 5-NI.
NS, not susceptible to drug concentrations of ≤100 μg/ml.
FDA-approved antimicrobial agent.
Veterinary antiparasitic agent.
Experimental antiparasitic agent effective against Trichomonas and Giardia (66).
Blastocystis subtype 4 exhibits EM resistance.
EM is an antiamoebic agent with limited clinical use, reported to be effective against Blastocystis in vitro (16, 75). Our study found EM to be effective against Mzr subtype 7 isolates (Table 6). Subtype 4 isolates S and WR-1, on the other hand, exhibited no inhibition even at the highest test concentrations of 100 μg/ml, suggesting EM resistance in subtype 4 isolates.
TABLE 6.
IC-50 values of anti-protozoal agents effective against Blastocystis isolates using the resazurin assay
| Drug | IC50 [μg/ml (μM)] |
|||
|---|---|---|---|---|
| Subtype 7 (Mzr) isolates |
Subtype 4 (Mzs) isolates |
|||
| B | E | WR-1 | S | |
| NTZb | 0.62 ± 0.07 (2.01) | 1.14 ± 0.49 (3.7) | 4.15 ± 0.41 (13.48) | 8 ± 4.7 (26) |
| FURb | 0.65 ± 0.05 (2.88) | 1.06 ± 0.4 (4.7) | 0.49 ± 0.01 (2.17) | 0.475 ± 0.05 (2.1) |
| MQb | 1.49 ± 0.83 (3.93) | 1.85 ± 0.88 (4.88) | 4.7 ± 0.35 (12.4) | 5.1 ± 0.58 (13.46) |
| QCb | 2.8 ± 0.56 (7) | 1.9 ± 0.2 (4.75) | 5.1 ± 0.47 (12.75) | 4.9 ± 0.53 (12.25) |
| QNb | 5.1 ± 1.1 (15.7) | 4.3 ± 2.4 (13.24) | 3.2 ± 0.52 (9.8) | 5.4 ± 1.4 (16.63) |
| EMc | 1.03 ± 0.4 (2.13) | 1.32 ± 0.9 (2.73) | NSa | NS |
| TMP:SMZ 1:2b | 4.7 ± 0.5 | 5.3 ± 0.62 | 3.2 ± 0.8 | 4.3 ± 0.48 |
| TMP:SMZ 1:5b | 22 ± 3.2 | 18.5 ± 1.3 | 24.5 ± 2.4 | 19 ± 0.46 |
| IAd | 0.34 ± 0.05 (1.83) | 0.2 ± 0.03 (1.08) | 0.33 ± 0.06 (1.78) | 0.26 ± 0.02 (1.4) |
N/S, not susceptible to ≤100-μg/ml concentration of the drug.
FDA-approved antimicrobial agent.
Antiparasitic agent with adverse side effects; not currently used in clinical practice.
Carcinogenic cysteine protease inhibitor; not clinically useful.
Blastocystis exhibits subtype-dependent variations in susceptibility to NTZ, MQ, and QC.
NTZ, a well-documented pyruvate-ferredoxin oxidoreductase (PFOR) inhibitor (43), was found to be more effective against Mzr strains of the parasite in this study (Table 6). Subtype 7 (avian) isolates were significantly more sensitive to NTZ than subtype 4 (rodent) isolates (P < 0.01). Similarly, the anti-malarial MQ and a closely related drug, QC, were also found to be significantly more effective against subtype 7 isolates than subtype 4 (Table 6).
No subtype-dependent variations in FUR and QN susceptibility.
Both Mzr and Mzs isolates exhibited sensitivity to FUR and QN (Table 6), two well-known antiprotozoal agents.
Higher susceptibility of Blastocystis spp. to a TMP/SMZ ratio of 1:2 than to one of 1:5.
SMZ and TMP are administered in two different ratios for protozoan infections. TMP/SMZ ratios of 1:5 and 1:2 were tested for Blastocystis inhibition. All isolates exhibited susceptibility to both combinations, but all four isolates were significantly more sensitive (P < 0.01) to a TMP/SMZ ratio of 1:2 than to one of 1:5 (Table 6).
Nonsusceptibility of Blastocystis to broad-spectrum antibiotics.
PAR, PYR, CQ, DOX, and AMP were found to be ineffective against all four isolates of the parasite (data not shown).
Cysteine protease inhibition causes parasite death.
The significance of cysteine proteases in Blastocystis pathobiology is well reported (36, 48, 58, 71). In this study, Inhibition of cysteine protease activity of the parasite by IA resulted in complete inhibition of all four isolates with similar IC50s, suggesting the importance of cysteine proteases in parasite survival.
DISCUSSION
We found both resazurin and XTT assays to be suitable for high-throughput analysis of drug susceptibility in Blastocystis isolates. The HTS parameters (a Z′ factor of >0.5 and a Cv of <10%) provide a highly conservative estimate of the sensitivity of an assay (30, 74). The high Z′ factor value, low Cv, and reproducibility of both resazurin and XTT assays suggest that they are robust and suitable for HTS. The option of semiquantitative visual evaluation of color gives these assays the flexibility to be applied in the field without the need for sophisticated equipment. The suppression of metabolic activity observed in these redox assays was also found to be associated with morphological signs of cell death (71), i.e., nuclear incorporation of PI and annexin V binding to the cell membrane, further validating these assays in determining drug susceptibilities. Considering the large number of variant Blastocystis isolates and the predominance of the parasite in developing countries (58) with limited research funding, these assays will be particularly useful due to their low cost and high yield.
Subtype 7 isolates were shown to be resistant to Mz and cross-resistant to Tz, the 1-position-substituted 5-NI of choice to treat a wide variety of anaerobic organisms (4, 22). This is consistent with previous reports of cross-resistance between the two drugs in Trichomonas (12, 31) and Giardia (7, 61). In these organisms, resistance is proposed to be due to downregulation of the enzymes PFOR (65) and thioredoxin oxidoreductase (28), which in conjunction with the electron acceptor ferredoxin are believed to activate the 5-NI prodrugs to the toxic radical states inside the parasite (28, 65). However, this mechanism of activation has not been shown for Blastocystis, although PFOR and other oxidoreductase enzymes are present in the organism (70). The subtype 4 isolates showed no convincing uniformity in susceptibility to Mz and Tz, indicating that new, unknown mechanisms of activation and/or resistance may be involved.
All isolates were similarly susceptible to another 1-position 5-NI, Oz. Compared to Mz, the drug has significantly higher efficacy against Mzr isolates of Blastocystis (P < 0.01), as observed in other parasites (10, 64) and also reported for Blastocystis previously (16). However, its superior efficacy against Mzs isolates is not as obvious, again suggesting new, unknown mechanisms of activation and/or resistance to 1-position 5-NIs in the parasite. Oz is frequently used to treat amoebiasis in India (21). Although the IC50s of Oz against all four isolates tested here (4.9 to 6.44 μM) were higher than the MIC of the drug against Entamoeba (0.25 μM) (10), its effectiveness against both Mzr and Mzs isolates suggests the drug would be a useful alternative to Mz to treat Blastocystis infections.
Similarly to Oz, 2-position 5-NIs, the commercially available poultry drug Rz and the experimental drug C-17, were uniformly effective against the isolates of both subtypes tested. These 2-position 5-NIs exhibited significantly higher efficacy against Mzr isolates than 1-position 5-NIs (P < 0.01), as observed in Giardia and Trichomonas (66). Again, the improved efficacy of 2-position 5-NIs against Mzs subtype 4 isolates is not as obvious, suggesting a different mechanism of action in Blastocystis than in other organisms (66). The IC90 of C-17 against Giardia was recently reported to be 0.5 μM (17), whereas against Trichomonas it exhibited a MIC of 6.3 μM (66). In this study, the IC50 of C-17 against Blastocystis ranged from 0.89 to 1.54 μM, suggesting the potential of the drug as a broad-spectrum antiprotozoal agent against Mzr parasites. Two-position 5-NIs may prove to be effective alternatives to treat Blastocystis infections in cases of Mz treatment failure.
The susceptibility of the Mzr subtype 7 isolates to NTZ and the reduced susceptibility to the Mzs subtype 4 isolates are also evidence for different mechanisms of action of NTZ in Blastocystis than in Giardia and Trichomonas, where cross-resistance between Mz and NTZ is apparent (2). These data suggest that Mz treatment failures in blastocystosis may well respond to NTZ, as in the case of Cryptosporidium parvum infections. C. parvum infections do not respond well to Mz (19), and NTZ is the treatment of choice, with in vitro IC50s of <10 μg/ml (60), similar to the IC50s of the drug against both Mzr and Mzs isolates of Blastocystis in this study. Recent in vitro (68) and clinical data (55) also suggest the usefulness of the drug in Blastocystis infections.
Another alternative to treat Mzr Blastocystis isolates is FUR, which was equally effective against all isolates in this study. FUR is a nitrofuran commonly used to treat giardiasis (49). It is activated inside the cell by NADH oxidase and generates toxic products that interfere with DNA processes in the parasite (9). The IC50s of FUR against both Mzr and Mzs isolates of Blastocystis were found to be similar to that against Giardia (2 μM) (5).
The prophylactic antimalarial MQ and a closely related drug, QC, were also found to be more effective against Mzr subtype 7 isolates than Mzs subtype 4 isolates. These findings are surprising because in Giardia, cross-resistance against QC has been observed between Mzr (8) and Tzr (63) strains, suggesting a different mode of action of the drug in Blastocystis. The exact mechanisms of action of these drugs against luminal parasites are not known, although they have been suggested to act on protozoan cell membranes (62). The activity of QC against Blastocystis has been reported previously (16, 68), but the current study is the first to report the potential usefulness of MQ as an anti-Blastocystis drug.
EM is an effective antiamoebic agent with unpleasant side effects. It targets ribosomes and limits protein synthesis (43). The in vitro activity of EM against Blastocystis has been evaluated in two previous studies. While both studies suggested its effectiveness against Blastocystis, Zierdt et al. reported strain-to-strain variation in the susceptibility of the parasite to the drug (75). The multidrug resistance (MDR) phenotype of Entamoeba histolytica exhibits resistance to a wide range of drugs, including EM, while responding to Mz (43), but no such MDR phenotypes have been reported in Blastocystis spp. Our study describes the existence of EM resistance in Mzs isolates of Blastocystis, suggesting that MDR phenotypes might be present in the parasite. Clinically, however, EM has limited use because of its severe side effects (32, 56).
TMP and SMZ are often prescribed in combination at a 1:5 ratio as an alternative to Mz in Blastocystis infections. Clinical studies suggest that this drug combination successfully eradicates Blastocystis infections in 95% to 100% of cases (53, 54). There are no reports of the effectiveness of a 1:2 combination against Blastocystis. Our findings suggest the superiority of a 1:2 combination over a 1:5 combination with no subtype-dependent variation in susceptibility. We suggest that the 1:2 combination is likely to be more effective than the 1:5 combination in treatment of clinical infections of Blastocystis.
Cysteine proteases play an important role in the cell cycle and pathophysiology of protozoan parasites. Blastocystis cysteine proteases have been reported to cleave human secretory IgAs (58) and to induce upregulation of proinflammatory cytokines (48). A prosurvival role of legumain, a cysteine protease, has also been reported recently for Blastocystis (71). Accumulating data in recent years suggest the therapeutic potential of protease inhibitors in parasitic infections (1, 42). Several cysteine protease inhibitors are being investigated as potential chemotherapeutic agents against parasites as diverse as Plasmodium (42, 44, 50), trypanosomes (18), and schistosomes (69). In this study, we found all four isolates to be highly susceptible to IA, a cysteine protease inhibitor, irrespective of their susceptibility to Mz. These findings suggest a potential role of cysteine protease inhibitors as a therapeutic option for Blastocystis isolates resistant to conventional antiprotozoal agents.
PAR is a broad-spectrum aminoglycoside (13). Although clinical studies suggest its effectiveness in the treatment of Blastocystis infections (3, 45, 67), in vitro data are equivocal (68, 72). In this study, PAR was found to be ineffective against the isolates of both subtypes tested. The high clinical efficacy of the drug against Blastocystis could be due to its broad-spectrum antibiotic activity (13). Although predominantly used for parasitic infections, PAR is also bactericidal (15). It might act by destruction of the gut bacterial flora essential for Blastocystis survival (57).
All four isolates tested were found to be nonsensitive to several other broad-spectrum antibiotics, PAR, PYR, CQ, DOX, and AMP. This feature could be exploited for the isolation and axenization of Blastocystis from clinical samples.
Clinical (54) and animal infection (24) studies, as well as in vitro data (36), suggest a subtype-dependent variation in the pathobiology of Blastocystis. Although strain-to-strain variation in parasite susceptibilities to drugs has been reported previously, subtype-dependent variation in parasite responses to chemotherapeutic agents has not been described before. To the best of our knowledge, this is the first study of its kind suggesting a variation in parasite susceptibilities to six common antiparasitic agents between isolates of two subtypes known to infect humans (54). It will be interesting to conduct a more extensive evaluation analyzing variability in the drug responses of different isolates across all 11 subtypes of the parasite.
Although the vacuolar form is the most commonly reported form of the parasite, Blastocystis is also known to exist in amoeboid, granular, and cyst forms. Blastocystis cysts have been reported to be Mzr, suggesting that different forms might respond differently to drug pressure (73). Since there are no standardized methods available for maintaining axenic cultures of other Blastocystis forms, only vacuolar forms were evaluated in this study, limiting the application of our findings across different life cycle stages of the parasite. Despite this limitation, this is the first study suggesting subtype-dependent variation in the parasite response to chemotherapeutic pressure.
In conclusion, this study describes two cost-effective assays for high-throughput antimicrobial susceptibility analysis of Blastocystis. Using one of these assays, we demonstrated for the first time subtype-dependent variations in the susceptibility of Blastocystis to six different antiprotozoal agents. We identified 4 new potential therapeutic options against Blastocystis, namely, MQ, TMP-SMZ (1:2), Oz, and FUR. Furthermore, we confirmed the antiprotozoal activities of 10 compounds already reported to be effective against Blastocystis. We also demonstrated in vitro Mz and EM resistance in Blastocystis. By assessing the susceptibility of the parasite to different 5-NIs, we also demonstrated that 5-NI resistance could be overcome in Blastocystis with more effective 5-NI compounds. Based on our findings, there is clearly a need to reevaluate currently established treatment regimens for Blastocystis infections.
Acknowledgments
This work was supported by a generous grant from the National Medical Research Council (NMRC/1071/2006). H.M. and J.D.W.T. are graduate students supported by National University of Singapore (NUS) research scholarships. This work was also supported in part by U01 Cooperative Research Agreement AI75527 from the National Institutes of Health. The study was facilitated by the commissioning of synthesis of C-17 by the NIH from the Southern Research Institute.
We are grateful to Martin Lear and Oliver Simon for providing the chemical structures of 5-nitroimidazoles.
Footnotes
Published ahead of print on 22 November 2010.
REFERENCES
- 1.Abdulla, M. H., K. C. Lim, M. Sajid, J. H. McKerrow, and C. R. Caffrey. 2007. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Med. 4:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adagu, I. S., D. Nolder, D. C. Warhurst, and J. F. Rossignol. 2002. In vitro activity of nitazoxanide and related compounds against isolates of Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. J. Antimicrob. Chemother. 49:103-111. [DOI] [PubMed] [Google Scholar]
- 3.Armentia, A., et al. 1993. Urticaria by Blastocystis hominis. Successful treatment with paromomycin. Allergol. Immunopathol. 21:149-151. [PubMed] [Google Scholar]
- 4.Bassily, S., Z. Farid, N. A. el-Masry, and E. M. Mikhail. 1987. Treatment of intestinal E. histolytica and G. lamblia with metronidazole, tinidazole and ornidazole: a comparative study. J. Trop. Med. Hyg. 90:9-12. [PubMed] [Google Scholar]
- 5.Bénéré, E., R. A. da Luz, M. Vermeersch, P. Cos, and L. Maes. 2007. A new quantitative in vitro microculture method for Giardia duodenalis trophozoites. J. Microbiol. Methods 71:101-106. [DOI] [PubMed] [Google Scholar]
- 6.Boorom, K. F., et al. 2008. Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection. Parasit. Vectors 1:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boreham, P. F. L., N. C. Smith, and R. W. Shepherd. 1988. Drug resistance and treatment of giardiasis, p. 3-7. In P. M. Wallis and B. R. Hammond (ed.), Advances in Giardia research. University of Calgary Press, Calgary, Alberta, Canada.
- 8.Brasseur, P., and L. Favennec. 1995. Two cases of giardiasis unsuccessfully treated by albendazole. Parasite 2:422. [PubMed] [Google Scholar]
- 9.Brown, D. M., J. A. Upcroft, and P. Upcroft. 1996. A H2O-producing NADH oxidase from the protozoan parasite Giardia duodenalis. Eur. J. Biochem. 241:155-161. [DOI] [PubMed] [Google Scholar]
- 10.Chintana, T., P. Sucharit, V. Mahakittikun, C. Siripanth, and W. Suphadtanaphongs. 1986. In vitro studies on the sensitivity of local Entamoeba histolytica to anti-amoebic drugs. Southeast Asian J. Trop. Med. Public Health 17:591-594. [PubMed] [Google Scholar]
- 11.Chen, X. Q., et al. 1997. A survey of Blastocystis sp. in rodents. Lab. Anim. Sci. 47:91-94. [PubMed] [Google Scholar]
- 12.Crowell, A. L., K. A. Sanders-Lewis, and W. E. Secor. 2003. In vitro metronidazole and tinidazole activities against metronidazole-resistant strains of Trichomonas vaginalis. Antimicrob. Agents Chemother. 47:1407-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson, R. N., M. den Boer, and K. Ritmeijer. 2009. Paromomycin. Trans. R. Soc. Trop. Med. Hyg. 103:653-660. [DOI] [PubMed] [Google Scholar]
- 14.De Logu, A., et al. 2003. Comparison of the susceptibility testing of clinical isolates of Mycobacterium tuberculosis by the XTT colorimetric method and the NCCLS standards method. Int. J. Antimicrob. Agents 21:244-250. [DOI] [PubMed] [Google Scholar]
- 15.Donald, P. R., et al. 2000. Early bactericidal activity of paromomycin (aminosidine) in patients with smear-positive pulmonary tuberculosis. Antimicrob. Agents Chemother. 44:3285-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn, L. A., and P. F. Boreham. 1991. The in-vitro activity of drugs against Blastocystis hominis. J. Antimicrob. Chemother. 27:507-516. [DOI] [PubMed] [Google Scholar]
- 17.Dunn, L. A., et al. 2010. A new-generation 5-nitroimidazole can induce highly metronidazole-resistant Giardia lamblia in vitro. Int. J. Antimicrob. Agents 36:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engel, J. C., P. S. Doyle, and J. H. McKerrow. 1999. Trypanocidal effect of cysteine protease inhibitors in vitro and in vivo in experimental Chagas disease. Medicina (Buenos Aires) 59:171-175. [PubMed] [Google Scholar]
- 19.Gargala, G. 2008. Drug treatment and novel drug target against Cryptosporidium. Parasite 15:275-281. [DOI] [PubMed] [Google Scholar]
- 20.Glass, R. H., et al. 1991. The resazurin reduction test provides an assessment of sperm activity. Fertil. Steril. 56:743-746. [DOI] [PubMed] [Google Scholar]
- 21.Güven, A. 2003. Amebiasis in the newborn. Indian J. Pediatr. 70:437-438. [DOI] [PubMed] [Google Scholar]
- 22.Harder, A., G. Greif, and A. Haberkorn. 2001. Chemotherapeutic approaches to protozoa: Giardia, Trichomonas and Entamoeba—current level of knowledge and outlook. Parasitol. Res. 87:785-786. [DOI] [PubMed] [Google Scholar]
- 23.Haresh, K., K. Suresh, A. Khairul Anus, and S. Saminathan. 1999. Isolate resistance of Blastocystis hominis to metronidazole. Trop. Med. Int. Health 4:274-277. [DOI] [PubMed] [Google Scholar]
- 24.Hussein, E. M., A. M. Hussein, M. M. Eida, and M. M. Atwa. 2008. Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol. Res. 102:853-860. [DOI] [PubMed] [Google Scholar]
- 25.Katsarou-Katsari, A., et al. 2008. Acute urticaria associated with amoeboid forms of Blastocystis sp. subtype 3. Acta Derm. Venereol. 88:80-81. [DOI] [PubMed] [Google Scholar]
- 26.Krook, A., B. Lindström, J. Kjellander, G. Järnerot, and L. Bodin. 1981. Relation between concentrations of metronidazole and Bacteroides spp in faeces of patients with Crohn's disease and healthy individuals. J. Clin. Pathol. 34:645-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurniawan, A., et al. 2009. Intestinal parasitic infections in HIV/AIDS patients presenting with diarrhoea in Jakarta, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 103:892-898. [DOI] [PubMed] [Google Scholar]
- 28.Leitsch, D., D. Kolarich, and M. Duchêne. 2010. The flavin inhibitor diphenyleneiodonium renders Trichomonas vaginalis resistant to metronidazole, inhibits thioredoxin reductase and flavin reductase, and shuts off hydrogenosomal enzymatic pathways. Mol. Biochem. Parasitol. 171:17-24. [DOI] [PubMed] [Google Scholar]
- 29.Leonard, B., et al. 2008. Inter- and intra-assay reproducibility of microplate Alamar Blue assay results for isoniazid, rifampicin, ethambutol, streptomycin, ciprofloxacin, and capreomycin drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 46:3526-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, Q., C. Maddox, L. Rasmussen, J. V. Hobarth, and L. E. White. 2009. Assay development and high-throughput antiviral drug screening against Bluetongue virus. Antiviral Res. 83:267-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Löfmark, S., C. Edlund, and C. E. Nord. 2010. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin. Infect. Dis. 50(Suppl. 1):S16-S23. [DOI] [PubMed] [Google Scholar]
- 32.Marino, A., R. Costa, and G. De Natale. 1990. Cardiotoxicity of emetine. Clin. Ter. 133:131-143. [PubMed] [Google Scholar]
- 33.Markell, E. K. 1995. Is there any reason to continue treating Blastocystis infections? Clin. Infect. Dis. 21:104-105. [DOI] [PubMed] [Google Scholar]
- 34.McBride, J., P. R. Ingram, F. L. Henriquez, and C. W. Roberts. 2005. Development of colorimetric microtiter plate assay for assessment of antimicrobials against Acanthamoeba. J. Clin. Microbiol. 43:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehraj, V., J. Hatcher, S. Akhtar, G. Rafique, and M. A. Beg. 2008. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One 3:e3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirza, H., and K. S. Tan. 2009. Blastocystis exhibits inter- and intra-subtype variation in cysteine protease activity. Parasitol. Res. 104:355-361. [DOI] [PubMed] [Google Scholar]
- 37.Moghaddam, D. D., E. Ghadirian, and M. Azmi. 2005. Blastocystis hominis and the evaluation of efficacy of metronidazole and trimethoprim/sulfamethoxazole. Parasitol. Res. 96:273-275. [DOI] [PubMed] [Google Scholar]
- 38.Muzaffar, J., K. Madan, M. P. Sharma, and P. Kar. 2006. Randomized, single-blind, placebo-controlled multicenter trial to compare the efficacy and safety of metronidazole and satranidazole in patients with amebic liver abscess. Dig. Dis. Sci. 51:2270-2273. [DOI] [PubMed] [Google Scholar]
- 39.Noël, C., et al. 2005. Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J. Clin. Microbiol. 43:348-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noureldin M. S., A. A. Shaltout, E. M. El Hamshary, and M. E. Ali. 1999. Opportunistic intestinal protozoal infections in immunocompromised children. J. Egypt. Soc. Parasitol. 29:951-961. [PubMed] [Google Scholar]
- 41.O'Brien, J., I. Wilson, T. Orton, and F. Pognan. 2000. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267:5421-5426. [DOI] [PubMed] [Google Scholar]
- 42.Olson, J. E., G. K. Lee, A. Semenov, and P. J. Rosenthal. 1999. Antimalarial effects in mice of orally administered peptidyl cysteine protease inhibitors. Bioorg. Med. Chem. 7:633-668. [DOI] [PubMed] [Google Scholar]
- 43.Orozco, E., L. A. Marchat, C. Gómez, C. López-Camarillo, and D. G. Pérez. 2009. Drug resistance mechanisms in Entamoeba histolytica, Giardia lamblia, Trichomonas vaginalis, and opportunistic anaerobic protozoa, p. 549-559. In G. A. Jacoby, R. Elston, P. R. Bonneau, and I. M. Douglas (ed.), Antimicrobial drug resistance; mechanisms of drug resistance, vol. 1. Humana Press, Totowa, NJ. [Google Scholar]
- 44.Parikh, S., et al. 2005. Antimalarial activity of human immunodeficiency virus type 1 protease inhibitors. Antimicrob. Agents Chemother. 49:2983-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasqui, A. L., et al. 2004. Chronic urticaria and Blastocystis hominis infection: a case report. Eur. Rev. Med. Pharmacol. Sci. 8:117-120. [PubMed] [Google Scholar]
- 46.Perrot, S., H. Dutertre-Catella, C. Martin, J. M. Warnet, and P. Rat. 2003. A new nondestructive cytometric assay based on resazurin metabolism and an organ culture model for the assessment of corneal viability. Cytometry A 55:7-14. [DOI] [PubMed] [Google Scholar]
- 47.Petrenko, Y. A., N. A. Gorokhova, E. N. Tkachova, and A. Y. Petrenko. 2005. The reduction of Alamar Blue by peripheral blood lymphocytes and isolated mitochondria. Ukr. Biokhim. Zh. 77:100-105. [PubMed] [Google Scholar]
- 48.Puthia, M. K., J. Lu, and K. S. Tan. 2008. Blastocystis ratti contains cysteine proteases that mediate interleukin-8 response from human epithelial cells in an NF-kappaB-dependent manner. Eukaryot. Cell 7:435-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quiros-Buelna, E. 1989. Furazolidone and metronidazole for treatment of giardiasis in children. Scand. J. Gastroenterol. Suppl. 169:65-69. [DOI] [PubMed] [Google Scholar]
- 50.Rosenthal, P. J., G. K. Lee, and R. E. Smith. 1993. Inhibition of a Plasmodium vinckei cysteine proteinase cures murine malaria. J. Clin. Invest. 91:1052-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossignol, J. F., S. M. Kabil, M. Said, H. Samir, and A. M. Younis. 2005. Effects of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis. Clin. Gastroenterol. Hepatol. 3:987-991. [DOI] [PubMed] [Google Scholar]
- 52.Stensvold, C. R., et al. 2007. Terminology for Blastocystis subtypes—a consensus. Trends Parasitol. 23:93-96. [DOI] [PubMed] [Google Scholar]
- 53.Stensvold, C. R., M. C. Arendrup, H. V. Nielsen, A. Bada, and S. Thorsen. 2008. Symptomatic infection with Blastocystis sp. subtype 8 successfully treated with trimethoprim-sulfamethoxazole. Ann. Trop. Med. Parasitol. 102:271-274. [DOI] [PubMed] [Google Scholar]
- 54.Stensvold, C. R., et al. 2009. Blastocystis: unravelling potential risk factors and clinical significance of a common but neglected parasite. Epidemiol. Infect. 137:1655-1663. [DOI] [PubMed] [Google Scholar]
- 55.Stensvold, C. R., H. V. Smith, R. Nagel, K. E. Olsen, and R. J. Traub. 2010. Eradication of Blastocystis carriage with antimicrobials: reality or delusion? J. Clin. Gastroenterol. 44:85-90. [DOI] [PubMed] [Google Scholar]
- 56.Sugie, H., R. Russin, and M. A. Verity. 1984. Emetine myopathy: two case reports with pathobiochemical analysis. 7:54-59. [DOI] [PubMed]
- 57.Tan, K. S. 2008. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 21:639-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan, K. S., H. Mirza, D. W. T. Joshua, B. Wu, and P. A. MacAry. 2010. Current views on the clinical relevance of Blastocystis spp. Curr. Infect. Dis. Rep. 12:28-35. [DOI] [PubMed] [Google Scholar]
- 59.Taçsova, Y., B. Sahin, S. Koltaçs, and S. Paydaçs. 2000. Clinical significance and frequency of Blastocystis hominis in Turkish patients with hematological malignancy. Acta Med. Okayama 54:133-136. [DOI] [PubMed] [Google Scholar]
- 60.Theodos, C. M., J. K. Griffiths, J. D'Onfro, A. Fairfield, and S. Tzipori. 1998. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 42:1959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Upcroft, J. A., and P. Upcroft. 1993. Drug resistance and Giardia. Parasitol. Today 9:187-190. [DOI] [PubMed] [Google Scholar]
- 62.Upcroft, J. A., R. Mitchell, N. Chen, and P. Upcroft. 1996. Albendazole resistance in Giardia is correlated with cytoskeletal changes but not with a mutation at amino acid 200 in beta-tubulin. Microb. Drug Resist. 2:303-308. [DOI] [PubMed] [Google Scholar]
- 63.Upcroft, J. A., R. W. Campbell, and P. Upcroft. 1996. Quinacrine resistant Giardia duodenalis. Parasitology 112:309-313. [DOI] [PubMed] [Google Scholar]
- 64.Upcroft, J. A., R. W. Campbell, K. Benakli, P. Upcroft, and P. Vanelle. 1999. Efficacy of 5-nitroimidazoles against metronidazole-susceptible and resistant Giardia, Trichomonas and Entamoeba spp. Antimicrob. Agents Chemother. 43:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Upcroft, P., and J. A. Upcroft. 2001. Drug targets and mechanisms of resistance in anaerobic protozoa. Clin. Microbiol. Rev. 14:150-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Upcroft, J. A., et al. 2006. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob. Agents Chemother. 50:344-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valsecchi, R., P. Leghissa, and V. Greco. 2004. Cutaneous lesions in Blastocystis hominis infection. Acta Derm. Venereol. 84:322-323. [DOI] [PubMed] [Google Scholar]
- 68.Vdovenko, A. A., and J. E. Williams. 2000. Blastocystis hominis: neutral red supravital staining and its application to in vitro drug sensitivity testing. Parasitol. Res. 86:573-581. [DOI] [PubMed] [Google Scholar]
- 69.Wasilewski, M. M., K. C. Lim, J. Phillips, and J. H. McKerrow. 1996. Cysteine protease inhibitors block schistosome hemoglobin degradation in vitro and decrease worm burden and egg production in vivo. Mol. Biochem. Parasitol. 81:179-189. [DOI] [PubMed] [Google Scholar]
- 70.Wawrzyniak, I., et al. 2008. Complete circular DNA in the mitochondria-like organelles of Blastocystis hominis. Int. J. Parasitol. 38:1377-1382. [DOI] [PubMed] [Google Scholar]
- 71.Wu, B., J. Yin, C. Texier, M. Roussel, and K. S. Tan. 2010. Blastocystis legumain is localized on the cell surface, and specific inhibition of its activity implicates a pro-survival role for the enzyme. J. Biol. Chem. 285:1790-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yakoob, J., W. Jafri, N. Jafri, M. Islam, and M. A. Beg. 2004. In vitro susceptibility of Blastocystis hominis isolated from patients with irritable bowel syndrome. Br. J. Biomed. Sci. 61:75-77. [DOI] [PubMed] [Google Scholar]
- 73.Zaman, V., and M. Zaki. 1996. Resistance of Blastocystis hominis cysts to metronidazole. Trop. Med. Int. Health 1:677-678. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, J. H., T. D. Chung, and K. R. Oldenburg. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4:67-73. [DOI] [PubMed] [Google Scholar]
- 75.Zierdt, C. H., J. C. Swan, and J. Hosseini. 1983. In vitro response of Blastocystis hominis to antiprotozoal drugs. J. Protozool. 30:332-334. [DOI] [PubMed] [Google Scholar]