Abstract
A prospective multicenter surveillance program on yeast bloodstream infections was implemented in the Paris, France, area without restrictions on ward of hospitalization (intensive care unit, hematology, and surgery) or age (adults and children). The present analysis concerns 2,618 isolates collected over 7 years from 2,441 patients. Centralized species identification and antifungal susceptibility testing using the EUCAST methodology were performed. Almost 10% (232/2,441) of the patients had recently (≤30 days) been treated with antifungal drugs. We analyzed the effect of recent exposure to fluconazole (n = 159) or caspofungin (n = 61) on the proportions of the five major Candida species. For both drugs, preexposure was associated with a decreased prevalence of Candida albicans in favor of less drug-susceptible species (C. glabrata and C. krusei for the former and C. parapsilosis and, to a lesser extent, C. glabrata and C. krusei for the latter; P = 0.001). In the multivariate analysis, the risk of being infected with an isolate with decreased susceptibility to fluconazole was independently associated with an age of ≥15 years (odds ratio [OR] = 2.45; 95% confidence interval [CI] = 1.39 to 4.31; P = 0.002) and with recent exposure to fluconazole (OR = 2.17; 95% CI = 1.51 to 3.13; P < 0.001), while the risk of being infected with an isolate with decreased susceptibility to caspofungin was independently associated with an age <15 years (OR = 2.53; 95% CI = 1.43 to 4.48; P = 0.001) and with recent exposure to caspofungin (OR = 4.79; 95% CI = 2.47 to 9.28; P < 0.001). These findings could influence future recommendations for the management of candidemia.
Yeast fungemia, mostly due to Candida spp., is a public health concern in medical practice (25, 30, 44). With the aim of improving the prognosis of invasive candidiasis, changes in clinical practices have recently occurred, with more antifungal prophylaxis and earlier initiation of therapy to treat possible or probable invasive fungal infections occurring both in hematological wards (8, 43) and in intensive care units (ICUs) (34). Both types of wards have become the predominant hospital setting for candidemia (6, 20, 33), with a proportion of up to 17.1% of Candida isolates being less susceptible or resistant to fluconazole in ICUs (20). The 2009 updated guidelines of the Infectious Diseases Society of America (IDSA) recommend that an echinocandin or fluconazole be used as first-line therapy for candidemia before identification of the causative species in nonneutropenic patients (27) and that an echinocandin be used in the case of exposure to fluconazole (preexposure to caspofungin was not mentioned) (27).
Fluconazole use is associated with a decreased incidence of candidemia in neutropenic patients with hematological malignancies (30, 41). Emergence of species with known decreased susceptibility (Candida glabrata) or even resistance (C. krusei) to fluconazole is observed in hematology populations receiving prolonged fluconazole prophylactically (24, 46) as well as in ICU populations (6, 33). However, lack of a shift is also reported (21, 23, 35, 36). For caspofungin, acquired resistance is reported anecdotally in susceptible Candida species recovered from patients treated with caspofungin (9, 42). Caspofungin usage was once associated with an increased incidence of infections due to Candida parapsilosis, a species with known decreased susceptibility to echinocandins (13).
Overall, global surveillance programs on yeast isolates responsible for fungemia worldwide do not report alterations in the patterns of susceptibility to caspofungin or azoles over time (29, 31) but do not record prior drug exposure. We thus evaluated the impact of recent exposure to caspofungin or fluconazole on candidemia epidemiology through a prospective surveillance program implemented in 2002 (date of caspofungin launching) in the Paris, France, area.
MATERIALS AND METHODS
Population studied.
An active surveillance program (YEASTS) was implemented in the Paris area by the National Reference Center for Mycoses and Antifungals (NRCMA; Institut Pasteur) with participation of all (n = 27) short-stay university hospitals (that included pediatric and adult departments, medicine, surgery, solid organ and allogeneic hematopoietic stem cell transplant programs, and two cancer centers). All episodes of yeast fungemia were recorded, whatever the patient's age and underlying disease. A standardized form recording information on demographics, underlying conditions, treatment, and outcome at day 30 was used through a secured website. The current analysis concerns the episodes of candidemia (incident episode and first recurrence) recorded during the first 7 years of the survey (October 2002 to September 2009).
Species identification and antifungal susceptibility determination.
Upon receipt at the NRCMA, all isolates were checked for purity (BBL Chromagar; bioMérieux, Marcy l'Etoile, France) and identified to the species level using carbon assimilation profiles (ID32C system; bioMérieux). For uncommon (<1.5%) species, the internal transcribed spacer 1 (ITS1)-5.8S-ITS2 region was sequenced. In vitro susceptibility testing was performed according to the EUCAST procedure (11). Fluconazole (Pfizer Central Research, Sandwich, United Kingdom) was tested in RPMI 1640, while caspofungin (Merck & Co., Rahway, NJ) (10) was tested in AM3 medium (Difco, Becton-Dickinson, NJ). Quality control strains (ATCC 22019, ATCC 6258) were included. Resistance was investigated in isolates with caspofungin MICs of ≥0.5 mg/liter by sequencing the hot spot 1 (HS1) and HS2 regions of the fks1 gene for Candida albicans (10) and the HS1 region of the fks1, fks2, and fks3 genes for C. glabrata (18).
Definitions.
The date of candidemia was the day on which blood was sampled. An incident case corresponded to the first blood culture positive for a Candida sp. Recurrences included isolation of the same species at least 10 days later or of a new species with no time limit. Surgery or antifungal drug prescription (whatever their justification) occurring within 30 days prior to candidemia was recorded. Episodes involving Candida spp. and another genus were considered mixed infections, but only the Candida spp. were kept for the analysis (three cases involved C. albicans and Saccharomyces cerevisiae, C. parapsilosis and S. cerevisiae, and Candida kefyr and a Geotrichum sp.). Hospitalization was categorized into medical or surgical ICU and non-ICU wards. We used the EUCAST threshold for fluconazole (MIC ≥ 8 mg/liter) (12). In the absence of consensus for caspofungin, we arbitrarily chose the previously selected threshold of 0.5 mg/liter for caspofungin since Candida isolates exhibiting MICs of ≥0.5 mg/liter in AM3 medium had fks gene mutations (10).
Statistical analysis.
Comparisons between groups were done using chi-square or Fisher exact tests for categorical variables and the Student t test or one-way analysis of variance for continuous variables. For paired comparisons between incident candidemia and first recurrence, the McNemar and signed-rank tests were used for proportions and means, respectively. Tests for trends were performed to assess changes over the study period. Characteristics associated with infection by an isolate with reduced susceptibility to drugs were identified using logistic regression models. First, all covariates with a P value under 0.25 in univariate analysis were simultaneously entered into the regression model. Next, covariates with the largest P values were iteratively removed from the model until all of the covariates remaining in the reduced model had a P value under 0.05. Data were analyzed using Stata statistical software (version 10.0; College Station, TX).
RESULTS
Characteristics of the population.
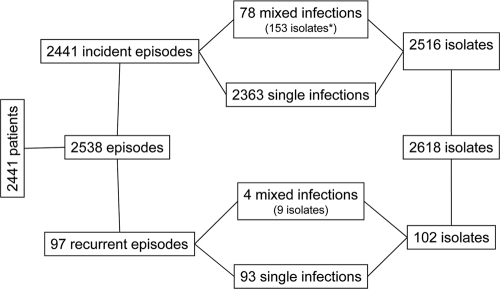
A total of 2,618 isolates were recovered from 2,441 different patients during 2,441 episodes of incident candidemia and 97 recurrent episodes. Mixed infections due to two or three species were recorded in 82 cases, including 4 during recurrences (Fig. 1). To avoid autocorrelation, only the first episodes were considered when the patients were analyzed.
FIG. 1.
Scheme showing the actual number of patients experiencing incident episodes of yeast fungemia or recurrences involving single or multiple isolates (mixed infections) and the corresponding number of isolates studied at NRCMA.
The population studied included 2,315 adults (60% males; mean age ± standard deviation [SD] = 59 ± 17 years; range, 15.1 to 99 years) and 126 children (59.5% males; mean age ± SD, 4.5 ± 4.8 years; range, 0 to 15 years). Hospitalization in an ICU (n = 1,153, 47.2%), recent surgery (n = 916, 38.0%), solid tumor (n = 779, 31.9%), hematological malignancy (n = 442, 18.1%), HIV infection (n = 95, 4.0%), and solid organ transplantation (n = 151, 6.2%) were the major underlying conditions, together with the presence of a central venous catheter (CVC) (n = 1,838, 75.3%).
Recent exposure to antifungal drugs was recorded for 232/2,441 (9.5%) patients during incident candidemia and 55/97 (56.7%) patients during recurrences. Of note, the proportion of children with recent exposure to antifungal drugs was higher than that of adults during incident candidemia (21/126 [16.7%] versus 211/2,315 [9.1%]; P = 0.008) and during recurrences (5/7 [71.4%] versus 50/90 [55.6%]; P = 0.695). The major drugs prescribed before incident or recurrent candidemia were fluconazole (136 [5.6%] and 32 [33%] patients, respectively), caspofungin (50 [2.0%] and 16 [6.5%] patients, respectively), or a combination of both (4 and 2 patients, respectively).
Influence of prior antifungal therapy on yeasts causing fungemia.
When the five major Candida species are considered (incident episodes and recurrences included), their proportions changed significantly when recent exposure (≤30 days) to fluconazole (159 isolates) or caspofungin (61 isolates) was recorded (P < 0.001) (Fig. 2). The proportions of species known for their low MICs (C. albicans, Candida tropicalis) decreased or remained stable, while the proportions of C. glabrata (18 to 29%) and C. krusei (3 to 8%) increased after prescription of fluconazole and those of C. parapsilosis (13 to 31%), C. glabrata (18 to 34%), and C. krusei (3 to 13%) increased after prescription of caspofungin. Overall, after exposure to fluconazole, fluconazole MICs increased significantly for all isolates and more specifically for intrinsically susceptible species (C. parapsilosis, C. tropicalis) but not for species with known decreased susceptibility to fluconazole (C. glabrata). In contrast, the global caspofungin MICs increased significantly after exposure to caspofungin but did not change specifically for any of the five major species (Table 1).
FIG. 2.
Proportion of the five major Candida species responsible for fungemia in patients with (n = 159) or without (n = 2,289) prior exposure to fluconazole (P = 0.001) or with (n = 61) or without (n = 2,387) prior exposure to caspofungin (P < 0.001) (incident episodes and recurrences are included).
TABLE 1.
Antifungal susceptibility of the five most frequent Candida species responsible for bloodstream infections according to recent exposure to fluconazole or caspofungin prior to diagnosis of fungemiaa
| Exposure drug and antifungal | No preexposure recorded |
Preexposure recorded |
Pb | ||
|---|---|---|---|---|---|
| No. of isolates | GMIC (mg/liter) | No. of isolates | GMIC (mg/liter) | ||
| Fluconazole | |||||
| Total | 2,289 | 0.77 (0.71-0.83)c | 159 | 2.31 (1.65-3.23) | <0.001 |
| C. albicans | 1291 | 0.24 (0.23-0.24) | 58 | 0.36 (0.25-0.51) | 0.053 |
| C. glabrata | 413 | 13.97 (12.59-15.50) | 46 | 18.05 (13.19-24.70) | 0.129 |
| C. parapsilosis | 295 | 0.97 (0.86-1.08) | 23 | 1.62 (0.99-2.64) | 0.015 |
| C. tropicalis | 226 | 0.84 (0.70-1.00) | 20 | 1.52 (0.76-3.01) | 0.040 |
| C. krusei | 64 | 34.15 (29.88-39.02) | 12 | 28.51 (10.78-75.41) | 0.402 |
| Caspofungin | |||||
| Total | 1920 | 0.07 (0.07-0.08) | 61 | 0.16 (0.12-0.22) | <0.001 |
| C. albicans | 993 | 0.05 (0.05-0.05) | 13 | 0.09 (0.04-0.22) | 0.252 |
| C. glabrata | 365 | 0.07 (0.07-0.08) | 21 | 0.12 (0.08-0.19) | 0.418 |
| C. parapsilosis | 299 | 0.28 (0.26-0.31) | 19 | 0.32 (0.23-0.45) | 0.893 |
| C. tropicalis | 199 | 0.06 (0.05-0.06) | 0 | ||
| C. krusei | 64 | 0.15 (0.13-0.17) | 8 | 0.19 (0.11-0.33) | 0.571 |
Recent exposure was <30 days prior to diagnosis of fungemia, and data for incident episodes and first recurrences are included. GMIC, geometric mean MIC.
Mann-Whitney test.
Values in parentheses are 95% CIs.
When only incident candidemia is considered, several characteristics distinguished patients reported to be recently exposed to fluconazole or caspofungin from those who were not (Table 2), including the age and risk factors for candidemia but not the outcome. When the isolates recovered from patients reported to be naive for antifungal drug exposure were comparing to those from patients with recent exposure to a drug, the fluconazole MIC and both the fluconazole and caspofungin MICs were significantly higher in the cases of preexposure to fluconazole and caspofungin, respectively (Table 2). In the multivariate analysis, the risk of being infected with an isolate with reduced susceptibility to fluconazole was independently associated with being over 15 years of age (adult) (odds ratio [OR] = 2.45; 95% confidence interval [CI] = 1.39 to 4.31; P = 0.002) and with recent exposure to fluconazole (OR = 2.17; 95% CI = 1.51 to 3.13; P < 0.001). The risk of being infected with an isolate with reduced susceptibility to caspofungin was independently associated with being a child (OR = 2.53; 95% CI = 1.43 to 4.48; P = 0.001) and with recent exposure to caspofungin (OR = 4.79; 95% CI = 2.47 to 9.28; P < 0.001). Of note, two C. albicans and two C. glabrata isolates recovered from patients previously exposed to caspofungin during incident candidemia had high caspofungin MICs and harbored the homozygous C1934A or C1933T mutation in the HS1 region of the fks1 gene, leading to S645Y or S645P substitutions in the deduced protein sequences (10). For one of the patients, C. parapsilosis was also recovered from the same blood culture. One C. glabrata isolate recovered together with C. krusei (caspofungin MICs = 1 and 0.5 mg/liter, respectively) from one patient previously exposed to caspofungin harbored the S629P substitution in the FKS1 protein sequence.
TABLE 2.
Characteristics of patients and Candida sp. isolates responsible for incident fungemia according to recorded or not recorded recent exposured to fluconazole or caspofungin
| Group and characteristic | Recent exposure to fluconazole |
Recent exposure to caspofungin |
||||
|---|---|---|---|---|---|---|
| None recorded (n = 2,305) | Recorded (n = 136) | P | None recorded (n = 2,391) | Recorded (n = 50) | P | |
| Patients | ||||||
| Mean age (yr) | 56.9 (56.1-57.8)c | 50.7 (47.0-54.5) | <0.001 | 56.9 (56.0-57.7) | 42.7 (36.7-48.6) | <0.001 |
| Male gendera | 1,392/2,305 (60.4) | 78/136 (57.4) | 0.528 | 1,435/2,391 (60.0) | 35/50 (70.0) | 0.189 |
| Hematological malignancya | 411/2,305 (17.8) | 31/136 (22.8) | 0.168 | 414/2,391 (17.3) | 28/50 (56.0) | <0.001 |
| Intensive care unita | 1,073/2,305 (46.6) | 80/136 (58.8) | 0.006 | 1,133/2,391 (47.4) | 20/50 (40.0) | 0.320 |
| Central venous cathetera | 1,723/2,305 (74.8) | 115/136 (84.6) | 0.010 | 1,793/2,391 (75.0) | 45/50 (90.0) | 0.012 |
| After diagnosis of fungemia, prescription ofa: | ||||||
| Fluconazole | 1,233/1,938 (63.6) | 43/129 (33.3) | <0.001 | 1,269/2,022 (62.8) | 7/45 (15.6) | <0.001 |
| Caspofungin | 393/1,938 (20.3) | 52/129 (40.3) | <0.001 | 424/2,022 (21.0) | 21/45 (46.7) | <0.001 |
| Death before day 30a | 859/2,185 (39.3) | 54/134 (40.3) | 0.856 | 892/2,271 (39.3) | 21/48 (43.8) | 0.552 |
| Early death (<day 8)a | 481/846 (56.9) | 26/53 (49.1) | 0.318 | 497/878 (56.6) | 10/21 (47.6) | 0.506 |
| Isolatesb | ||||||
| Mixed infectiona | 75/2,305 (3.3) | 3/136 (2.2) | 0.800 | 77/2,391 (3.2) | 1/50 (2.0) | 1.000 |
| Non-C. albicans speciesa | 1,107/2,377 (46.6) | 89/139 (64.0) | <0.001 | 1,157/2,465 (46.9) | 39/51 (76.5) | <0.001 |
| C. glabrata isolatesa | 393/2,377 (16.5) | 35/139 (25.2) | 0.011 | 413/2,465 (16.8) | 15/51 (29.4) | 0.023 |
| Geometric mean MIC (mg/liter) for the isolate causing fungemia | ||||||
| Fluconazole | 0.77 (0.71-0.83)c | 2.14 (1.51-3.04) | <0.001 | 0.80 (0.74-0.86) | 2.17 (1.23-3.82) | <0.001 |
| Caspofungin | 0.07 (0.07-0.08)c | 0.08 (0.07-0.09) | 0.199 | 0.07 (0.07-0.07) | 0.17 (0.13-0.23) | <0.001 |
| Episodes due to at least one isolate with decreased susceptibility toa: | ||||||
| Fluconazole | 452/2,305 (19.6) | 47/136 (34.6) | <0.001 | 480/2,391 (20.1) | 19/50 (38.0) | 0.004 |
| Caspofungin | 121/1,854 (6.5) | 10/106 (9.4) | 0.231 | 119/1,910 (6.2) | 12/51 (24.0) | <0.001 |
The data indicate the number of individuals or isolates for whom the corresponding information was available/total number in the group (%).
The total number of isolates studied during incident candidemia was 2,516.
Values in parentheses in this row are 95% CIs.
Recent exposure was <30 days prior to diagnosis of fungemia.
We then analyzed the subset of patients for whom two episodes of candidemia were available within 3 months (median = 14 days; interquartile range [IQR] = 9 to 62 days) and who were recorded to be naive for antifungal exposure for the first episode and recently exposed to fluconazole or caspofungin for the second one. For those exposed to fluconazole, the proportion of isolates with decreased susceptibility to fluconazole increased between the first and the second episodes (5/30 [16.7%] versus 16/30 [53.3%]; P = 0.003), with modifications in the proportion of C. albicans (17/30 [56.7%] versus 8/30 [26.7%], respectively) and C. glabrata (2/30 [6.7%] versus 10/30 [33.3%]; P = 0.002) isolates occurring and with significant increases in fluconazole MICs for the 30 pairs of isolates considered (geometric mean MIC = 0.66 mg/liter [IQC = 0.33 to 1.31 mg/liter] versus geometric mean MIC = 3.48 mg/liter [IQC = 1.48 to 8.17 mg/liter] for the first and the second isolates, respectively; P = 0.045) being detected. The small sample size (n = 14) prevented a similar analysis for caspofungin-treated patients. Of note, though, for one patient initially infected with a caspofungin-susceptible (MIC = 0.06 mg/liter) C. glabrata isolate, the isolate recovered 19 days later had a MIC of 1 mg/liter and harbored the fks1 gene mutation mentioned above.
DISCUSSION
Despite the apparent stability in the incidence of candidemia and the constant predominance of C. albicans, there is still a need to monitor the disease and track the emergence of resistant species or isolates, especially in high-risk populations, in order to better drive empirical antifungal therapy (25). Thanks to a systematic recording of events taking place within 30 days prior to candidemia, we showed here that recent exposure to caspofungin or fluconazole, the two major drugs recommended for the first-line treatment of candidemia and for preemptive/prophylactic treatment in high-risk patients, was associated with changes in the epidemiology of the infection (22, 28).
This conclusion can be drawn because, despite the restricted geographical area covered, the survey was perennial and multicentric and collected both clinical and mycological data without bias of selection on the basis of age or underlying risk factors. Of importance, the underlying conditions of the population studied here are representative of all major underlying conditions reported so far for patients with candidemia in various countries (2, 5, 7, 16, 40). Species identification and antifungal susceptibility testing using the standardized EUCAST methodology were centralized. The distribution of Candida species, especially of the five major species in the population naive of antifungal drug exposure, is in agreement with previous reports, keeping in mind the wide variations attributed to geographical differences and populations studied (30). The slight modification in the EUCAST technique (AM3 rather than RPMI 1640 was used to test caspofungin) was based on studies from our laboratory and others showing that AM3 was superior to RPMI 1640 for testing of the susceptibilities of both yeasts and filamentous fungi to caspofungin (3) and that it better uncovered mutant strains with decreased susceptibility to caspofungin (10).
We uncovered major changes in the epidemiology of candidemia. Indeed, not only did the species distribution change in patients with recent exposure to fluconazole and also caspofungin, but the overall susceptibility of the isolates to these drugs decreased. Of note, though, this finding was not associated with an alteration in the recorded 30-day outcome.
Specifically, less susceptible or resistant species (i.e., C. glabrata and C. krusei) (32) were significantly more frequent in case of recent fluconazole administration. Candida species with decreased susceptibility/resistance to fluconazole represent 17.1% and 27.4% of isolates in recent studies in ICU patients and hematology/cancer patients, respectively (20, 38). In addition, they have been associated with reduced survival in cancer patients with recent azole administration (17, 38, 45). The relationship between the increased incidence of these species and fluconazole prescription has been debated ever since the introduction of fluconazole as prophylaxis in hematology wards (1, 15, 33, 46) and in ICUs (14). Interestingly, recent studies pointed out that a single dose of fluconazole was associated with an increased risk of candidemia due to non-C. albicans isolates (6) and noted a correlation between reduced fluconazole use and decreasing incidence of non-C. albicans candidemia (4). Previous fluconazole exposure also increased the risk of fluconazole-resistant candidemia (26) in ICU patients (14), in adults with cancer (38), and during C. glabrata candidemia (19). Here, we add evidence on the influence of recent fluconazole prescription on the overall epidemiology of candidemia and during recurrences. Since recent studies failed to uncover robust clinical parameters differentiating ICU patients infected by C. albicans versus non-C. albicans species (9, 21, 39), a careful recording of prior antifungal prescription is necessary before species identification to adapt antifungal drug prescription. Indeed, the 2009 IDSA guidelines recommend that fluconazole not be used in case of previous azole exposure (27).
A drastic shift in species causing candidemia was also found after recent exposure to caspofungin. Candida parapsilosis, known for its reduced susceptibility to echinocandins (reviewed in reference 42), was more frequent in patients recently exposed to caspofungin, but this was also true for C. glabrata and C. krusei. Protracted C. parapsilosis infections were seen during caspofungin treatment in the pivotal clinical trial (25), and the proportion of successful outcomes for C. parapsilosis candidemia tended to be higher with 150 mg/day versus 50 mg/day of caspofungin (7). The outcome of C. parapsilosis candidemia was also less favorable with anidulafungin than with fluconazole (36). Several anecdotal reports showed acquired resistance in normally susceptible species after exposure to echinocandins (5, 12, 42), but only one study suggested an increased incidence of C. parapsilosis infection associated with caspofungin usage (13). None of the studies mentioned a modification in the proportion of C. glabrata and C. krusei isolates that we observed. A possible explanation is the slightly higher caspofungin MICs for C. krusei and, to a lesser extent, for C. glabrata than for C. albicans (30; this study).
Our data demonstrate the importance of large perennial surveillance programs collecting isolates and clinical data, since individual changes that are not reflected by global analysis of susceptibility profiles can occur (30). Even though antifungal susceptibility testing methods are not yet fully accurate to predict treatment efficacy during candidemia (32, 37), decreased susceptibility to fluconazole and caspofungin detected by a standardized method was associated here with recent exposure to the drugs. These data underline the possibility that a new episode of sepsis after recent prescription of antifungals may be due to isolates with decreased susceptibility to the prescribed drug, including caspofungin. Only prescriptions taking place in the 30 days prior to candidemia were recorded here (without details on dose or duration), with earlier prescriptions which could explain why a few isolates with decreased susceptibility to azoles or caspofungin from among normally susceptible species were discovered in patients not reported to have been exposed to the drugs being excluded. In a recent survey in the United States, 43% of the patients (compared to 9.5% in our study) received antifungal agents prior to candidemia, but no detail on the nature of the drugs or on the impact of these prescriptions was provided (18). In a retrospective study involving one hematological center, the authors noted a shift in species distribution (increase in C. parapsilosis and decrease in C. glabrata) associated with decreased and increased use of azoles and caspofungin, respectively (37). Knowing that a recent 1-day prevalence study in French ICUs demonstrated that 7.5% of patients received an antifungal drug (fluconazole for 60%, caspofungin for 24%) but that invasive fungal infections were not documented in two-thirds of these patients (39), there is an urgent need to extend advice for improved usage of antifungals in high-risk patients.
In conclusion, these data emphasize how new therapeutic practices influence the epidemiology of invasive fungal infections. The current international guidelines do not evoke the potential exposure to echinocandins as a risk for the emergence of less susceptible isolates/species, whereas they mention the risks related to azoles. Our results could thus influence future recommendations for the management of patients at risk of or suspected of having invasive candidiasis.
Acknowledgments
We thank all the technicians in the 27 hospitals who made this study possible.
The YEASTS program was supported in part by the Institut de Veille Sanitaire and the Institut Pasteur. The funders had no role in the study design, data collection, analysis, or interpretation.
O.L. and F.D. were involved with designing the study, collecting and reviewing clinical information, analyzing the, data and writing the manuscript. M.D.-O. was involved with performing experiments on yeast isolates, analysis and interpretation of the data, and discussing the manuscript. K.S. was involved with data collection, monitoring and analysis, and reviewing the manuscript. A.F. was involved with analyzing the data and reviewing the manuscript. S.B. was involved with designing the study, interpretation of the data, and discussing the manuscript.
O.L. is member of the speaker's bureaus of Merck, Pfizer, Astellas, Schering, and Gilead and is a consultant for Astellas and Gilead Sciences. S.B. is a consultant for Gilead Sciences and has received speaking honoraria from Gilead Sciences and Pfizer. F.D. has received speaking honoraria from Pfizer. M.D.-O., K.S., and A.F. have no conflicts of interest to declare.
The research described herein was carried out in compliance with the French law and the Declaration of Helsinki (as adopted in 2000) and was approved by the IRB00006966 Institut Pasteur Institutional Review Board. Approval of the Commission Nationale de l'Informatique et des Libertés was obtained, ensuring that the patients' data were kept anonymous, according to French regulation.
The following investigators from the French Mycoses Study Group participated in the YEASTS program by collecting data in each participating center: C. Bouges-Michel (Hôpital Avicenne, Bobigny), I. Poilane (Hôpital Jean Verdier, Bondy), J. Dunan (Hôpital Ambroise Paré, Boulogne), G. Galeazzi (Hôpital Louis Mourier, Colombes), F. Botterel (Hôpital Henri Mondor, Créteil), N. Fauchet (Centre Intercommunal, Créteil), E. Forget (Hôpital Beaujon, Clichy), C. Lawrence (Hôpital Raymond Poincaré, Garches), C. Bonnal, F. Botterel, and P. Bouree (Hôpital du Kremlin Bicêtre, Kremlin-Bicêtre), O. Eloy (Centre Hospitalier, Le Chesnay), M.-F. David, N. Khassis, and L. Milhaila (Hôpital Paul Brousse, Villejuif), and E. Chachaty (Institut Gustave Roussy, Villejuif). The following investigators from the French Mycoses Study Group participated in the YEASTS program by collecting data in Paris: C. Chochillon (Hôpital Bichat), A. Paugam, M.-T. Baixench (Hôpital Cochin), M. Cornet (Hôtel Dieu), M.-C. Escande (Institut Curie), M.-E. Bougnoux, Y. Sterckers, and S. Challier (Hôpital Necker), E. Dannaoui and V. Lavarde (Hôpital Européen Georges Pompidou), A. Datry, B. Lmimouni, S. Brun, and A. Fekkar (Hôpital de la Pitié-Salpétrière), J.-L. Poirot (Hôpital Saint Antoine), C. Lacroix (Hôpital Saint Louis), D. Moissenet (Hôpital Trousseau), M. Develoux (Hôpital Tenon), and S. Bonacorsi (Hôpital Robert Debré).
Technical analysis of the isolates was done by Dorothée Raoux and Damien Hoinard at the National Reference Center for Mycoses and Antifungals.
Footnotes
Published ahead of print on 15 November 2010.
REFERENCES
- 1.Abi-Said, D., E. Anaissie, O. Uzun, I. Raad, H. Pinzcowski, and S. Vartivarian. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24:1122-1128. [DOI] [PubMed] [Google Scholar]
- 2.Almirante, B., D. Rodriguez, B. J. Park, M. Cuenca-Estrella, A. M. Planes, M. Almela, J. Mensa, F. Sanchez, J. Ayats, M. Gimenez, P. Saballs, S. K. Fridkin, J. Morgan, J. L. Rodriguez-Tudela, D. W. Warnock, and A. Pahissa. 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43:1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartizal, C., and F. C. Odds. 2003. Influences of methodological variables on susceptibility testing of caspofungin against Candida species and Aspergillus fumigatus. Antimicrob. Agents Chemother. 47:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassetti, M., F. Ansaldi, L. Nicolini, E. Malfatto, M. P. Molinari, M. Mussap, B. Rebesco, F. Bobbio Pallavicini, G. Icardi, and C. Viscoli. 2009. Incidence of candidaemia and relationship with fluconazole use in an intensive care unit. J. Antimicrob. Chemother. 64:625-629. [DOI] [PubMed] [Google Scholar]
- 5.Chen, S., M. Slavin, Q. Nguyen, D. Marriott, E. G. Playford, D. Ellis, and T. Sorrell. 2006. Active surveillance for candidemia, Australia. Emerg. Infect. Dis. 12:1508-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow, J. K., Y. Golan, R. Ruthazer, A. W. Karchmer, Y. Carmeli, D. Lichtenberg, V. Chawla, J. Young, and S. Hadley. 2008. Factors associated with candidemia caused by non-albicans Candida species versus Candida albicans in the intensive care unit. Clin. Infect. Dis. 46:1206-1213. [DOI] [PubMed] [Google Scholar]
- 7.Colombo, A. L., M. Nucci, B. J. Park, S. A. Nouer, B. Arthington-Skaggs, D. A. da Matta, D. Warnock, and J. Morgan. 2006. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 44:2816-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornely, O. A., J. Maertens, D. J. Winston, J. Perfect, A. J. Ullmann, T. J. Walsh, D. Helfgott, J. Holowiecki, D. Stockelberg, Y. T. Goh, M. Petrini, C. Hardalo, R. Suresh, and D. Angulo-Gonzalez. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348-359. [DOI] [PubMed] [Google Scholar]
- 9.Dannaoui, E., M. Desnos-Olllivier, D. Garcia-Hermoso, D. Raoux, D. Hoinard, F. Dromer, and O. Lortholary for the French Mycoses Study Group. 2010. Infections due to Candida spp. with reduced susceptibility to caspofungin in France, abstr. O346. Abstr. 20th Eur. Congr. Clin. Microbiol. Infect. Dis.
- 10.Desnos-Ollivier, M., S. Bretagne, D. Raoux, D. Hoinard, F. Dromer, and E. Dannaoui. 2008. Mutations in the fks1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the EUCAST method. Antimicrob. Agents Chemother. 52:3092-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Committee on Antimicrobial Susceptibility Testing. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398-405. [DOI] [PubMed] [Google Scholar]
- 12.European Committee on Antimicrobial Susceptibility Testing. 2008. EUCAST technical note on fluconazole. Clin. Microbiol. Infect. 14:193-195. [DOI] [PubMed] [Google Scholar]
- 13.Forrest, G. N., E. Weekes, and J. K. Johnson. 2008. Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J. Infect. 56:126-129. [DOI] [PubMed] [Google Scholar]
- 14.Garnacho-Montero, J., A. Diaz-Martin, E. Garcia-Cabrera, M. Ruiz Perez de Pipaon, C. Hernandez-Caballero, J. Aznar-Martin, J. M. Cisneros, and C. Ortiz-Leyba. 2010. Risk factors for fluconazole-resistant candidemia. Antimicrob. Agents Chemother. 54:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hachem, R., H. Hanna, D. Kontoyiannis, Y. Jiang, and I. Raad. 2008. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 112:2493-2499. [DOI] [PubMed] [Google Scholar]
- 16.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn, D. L., D. Neofytos, E. J. Anaissie, J. A. Fishman, W. J. Steinbach, A. J. Olyaei, K. A. Marr, M. A. Pfaller, C. H. Chang, and K. M. Webster. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the Prospective Antifungal Therapy Alliance Registry. Clin. Infect. Dis. 48:1695-1703. [DOI] [PubMed] [Google Scholar]
- 18.Katiyar, S., M. Pfaller, and T. Edlind. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, I., N. O. Fishman, T. E. Zaoutis, K. H. Morales, M. G. Weiner, M. Synnestvedt, I. Nachamkin, and E. Lautenbach. 2009. Risk factors for fluconazole-resistant Candida glabrata bloodstream infections. Arch. Intern. Med. 169:379-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leroy, O., J. P. Gangneux, P. Montravers, J. P. Mira, F. Gouin, J. P. Sollet, J. Carlet, J. Reynes, M. Rosenheim, B. Regnier, and O. Lortholary. 2009. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005-2006). Crit. Care Med. 37:1612-1618. [DOI] [PubMed] [Google Scholar]
- 21.Lin, M. Y., Y. Carmeli, J. Zumsteg, E. L. Flores, J. Tolentino, P. Sreeramoju, and S. G. Weber. 2005. Prior antimicrobial therapy and risk for hospital-acquired Candida glabrata and Candida krusei fungemia: a case-case-control study. Antimicrob. Agents Chemother. 49:4555-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maertens, J., O. Marchetti, R. Herbrecht, O. A. Cornely, U. Fluckiger, P. Frere, B. Gachot, W. J. Heinz, C. Lass-Florl, P. Ribaud, A. Thiebaut, and C. Cordonnier. 26 July 2010, posting date. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3—2009 update. Bone Marrow Transplant. [Epub ahead of print.] [DOI] [PubMed]
- 23.Marchetti, O., J. Bille, U. Fluckiger, P. Eggimann, C. Ruef, J. Garbino, T. Calandra, M. P. Glauser, M. G. Tauber, and D. Pittet. 2004. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991-2000. Clin. Infect. Dis. 38:311-320. [DOI] [PubMed] [Google Scholar]
- 24.Marr, K. A., K. Seidel, T. C. White, and R. A. Bowden. 2000. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J. Infect. Dis. 181:309-316. [DOI] [PubMed] [Google Scholar]
- 25.Morgan, J. 2005. Global trends in candidemia: review of reports from 1995-2005. Curr. Infect. Dis. Rep. 7:429-439. [DOI] [PubMed] [Google Scholar]
- 26.Oxman, D. A., J. K. Chow, G. Frendl, S. Hadley, S. Hershkovitz, P. Ireland, L. A. McDermott, K. Tsai, F. M. Marty, D. P. Kontoyiannis, and Y. Golan. 2010. Candidaemia associated with decreased in vitro fluconazole susceptibility: is Candida speciation predictive of the susceptibility pattern? J. Antimicrob. Chemother. 65:1460-1465. [DOI] [PubMed] [Google Scholar]
- 27.Pappas, P. G., C. A. Kauffman, D. Andes, D. K. Benjamin, Jr., T. F. Calandra, J. E. Edwards, Jr., S. G. Filler, J. F. Fisher, B. J. Kullberg, L. Ostrosky-Zeichner, A. C. Reboli, J. H. Rex, T. J. Walsh, and J. D. Sobel. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., L. Boyken, R. J. Hollis, J. Kroeger, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2008. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 46:150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., D. J. Diekema, M. G. Rinaldi, R. Barnes, B. Hu, A. V. Veselov, N. Tiraboschi, E. Nagy, and D. L. Gibbs. 2005. Results from the ARTEMIS DISK Global Antifungal Surveillance Study: a 6.5-year analysis of susceptibilities of Candida and other yeast species to fluconazole and voriconazole by standardized disk diffusion testing. J. Clin. Microbiol. 43:5848-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 33.Playford, E. G., D. Marriott, Q. Nguyen, S. Chen, D. Ellis, M. Slavin, and T. C. Sorrell. 2008. Candidemia in nonneutropenic critically ill patients: risk factors for non-albicans Candida spp. Crit. Care Med. 36:2034-2039. [DOI] [PubMed] [Google Scholar]
- 34.Senn, L., P. Eggimann, R. Ksontini, A. Pascual, N. Demartines, J. Bille, T. Calandra, and O. Marchetti. 2009. Caspofungin for prevention of intra-abdominal candidiasis in high-risk surgical patients. Intensive Care Med. 35:903-908. [DOI] [PubMed] [Google Scholar]
- 35.Shorr, A. F., K. Chung, W. L. Jackson, P. E. Waterman, and M. H. Kollef. 2005. Fluconazole prophylaxis in critically ill surgical patients: a meta-analysis. Crit. Care Med. 33:1928-1935. [DOI] [PubMed] [Google Scholar]
- 36.Shorr, A. F., D. R. Lazarus, J. H. Sherner, W. L. Jackson, M. Morrel, V. J. Fraser, and M. H. Kollef. 2007. Do clinical features allow for accurate prediction of fungal pathogenesis in bloodstream infections? Potential implications of the increasing prevalence of non-albicans candidemia. Crit. Care Med. 35:1077-1083. [DOI] [PubMed] [Google Scholar]
- 37.Sipsas, N. V., R. E. Lewis, J. Tarrand, R. Hachem, K. V. Rolston, I. I. Raad, and D. P. Kontoyiannis. 2009. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001-2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 115:4745-4752. [DOI] [PubMed] [Google Scholar]
- 38.Slavin, M. A., T. C. Sorrell, D. Marriott, K. A. Thursky, Q. Nguyen, D. H. Ellis, C. O. Morrissey, and S. C. Chen. 2010. Candidaemia in adult cancer patients: risks for fluconazole-resistant isolates and death. J. Antimicrob. Chemother. 65:1042-1051. [DOI] [PubMed] [Google Scholar]
- 39.Timsit, J., H. Dupont, J. Stahl, A. Tabah, O. Lortholary, C. Martin, A. Francais, B. Guidet, and E. Azoulay. 2009. Use of anitfungal therapy in ICU: a prevalent cohort study in 169 ICUs, abstr. M-1033/523. Abstr. 49th Intersci. Conf. Antimicrob. Chemother. American Society for Microbiology, Washington, DC.
- 40.Tortorano, A. M., J. Peman, H. Bernhardt, L. Klingspor, C. C. Kibbler, O. Faure, E. Biraghi, E. Canton, K. Zimmermann, S. Seaton, and R. Grillot. 2004. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur. J. Clin. Microbiol. Infect. Dis. 23:317-322. [DOI] [PubMed] [Google Scholar]
- 41.Trick, W. E., S. K. Fridkin, J. R. Edwards, R. A. Hajjeh, and R. P. Gaynes. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627-630. [DOI] [PubMed] [Google Scholar]
- 42.Trofa, D., A. Gacser, and J. D. Nosanchuk. 2008. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 21:606-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ullmann, A. J., J. H. Lipton, D. H. Vesole, P. Chandrasekar, A. Langston, S. R. Tarantolo, H. Greinix, W. Morais de Azevedo, V. Reddy, N. Boparai, L. Pedicone, H. Patino, and S. Durrant. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335-347. [DOI] [PubMed] [Google Scholar]
- 44.Vincent, J. L., J. Rello, J. Marshall, E. Silva, A. Anzueto, C. D. Martin, R. Moreno, J. Lipman, C. Gomersall, Y. Sakr, and K. Reinhart. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323-2329. [DOI] [PubMed] [Google Scholar]
- 45.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]
- 46.Wingard, J. R., W. G. Merz, M. G. Rinaldi, T. R. Johnson, J. E. Karp, and R. Saral. 1991. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 18:1274. [DOI] [PubMed] [Google Scholar]