Abstract
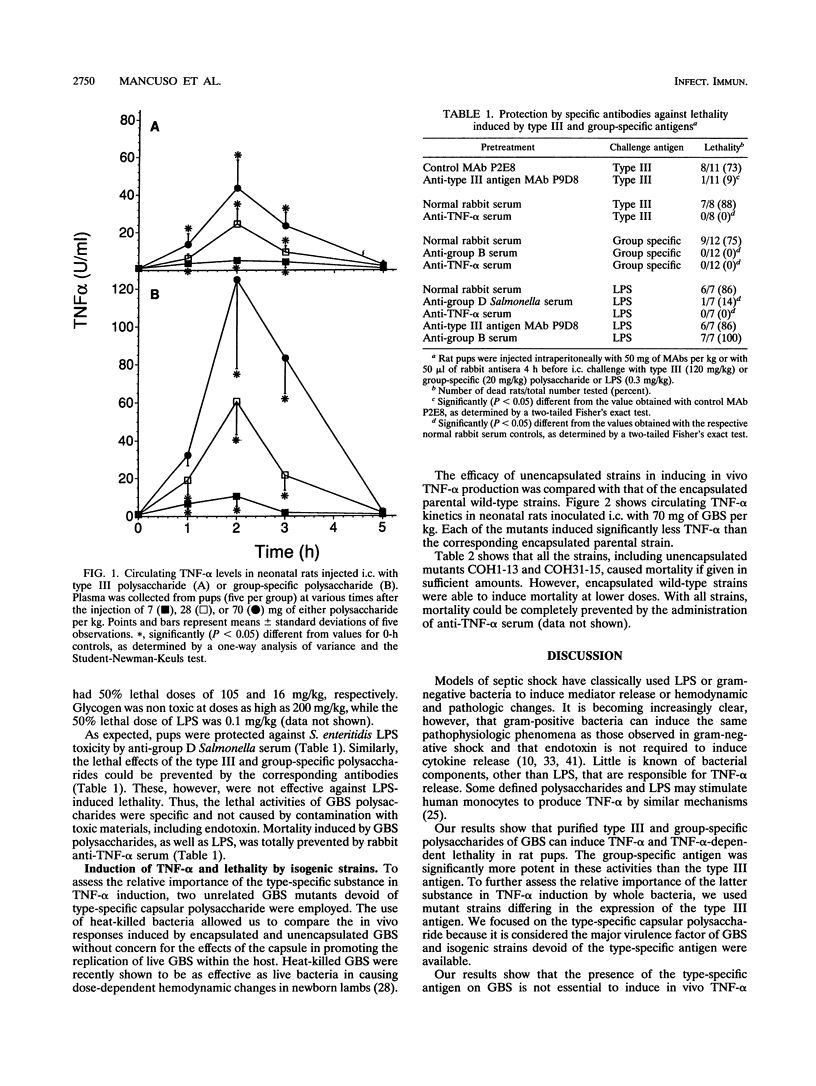
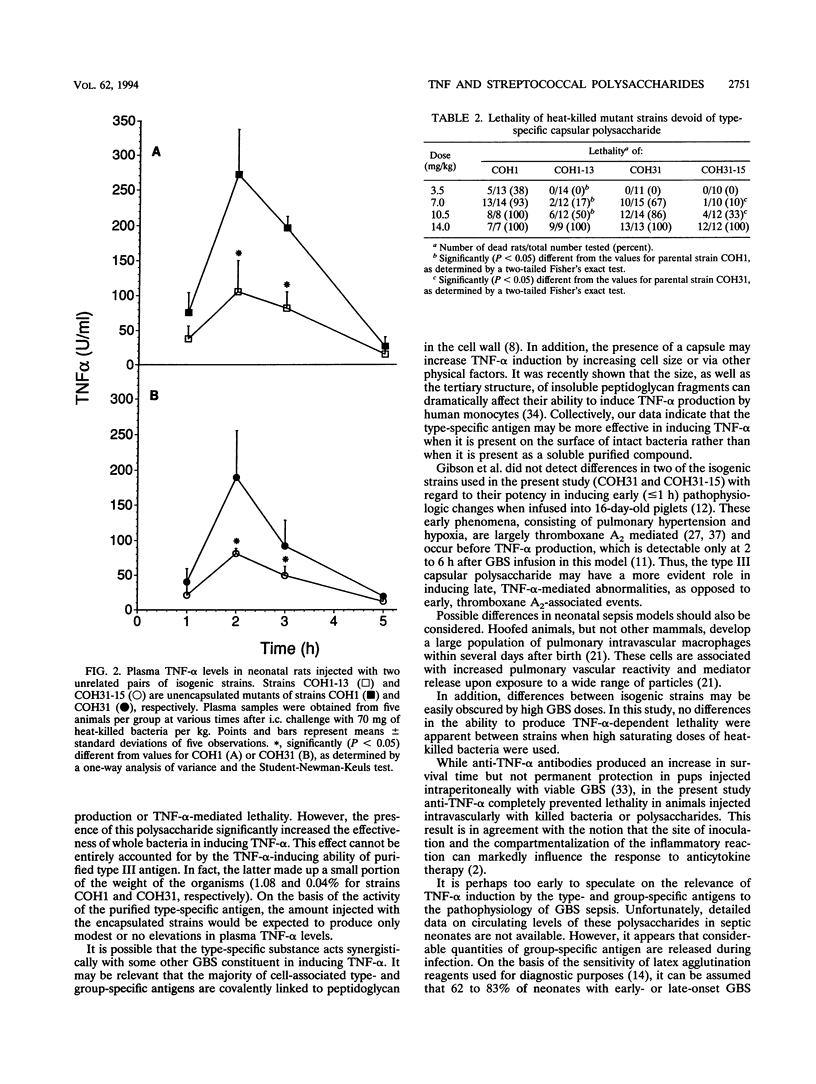
Previous studies suggested that circulating tumor necrosis factor alpha (TNF-alpha) may have a pathophysiologic role in experimental neonatal sepsis induced by group B streptococci (GBS). This study was undertaken to investigate the ability of the type III and group-specific polysaccharides of GBS to induce TNF-alpha production and TNF-alpha-dependent lethality in neonatal rats. The cytokine was detected in plasma samples by the L929 cytotoxicity assay. Intracardiac injections of either polysaccharide induced dose-dependent, transient elevations in plasma TNF-alpha levels that returned to baseline values after 5 h. The group-specific antigen induced significantly higher mean peak TNF-alpha levels than the type III antigen (125 +/- 47 versus 44 +/- 15 U/ml with 70 mg/kg of body weight). Glycogen (70 mg/kg), used as a negative control, did not induce TNF-alpha. The lipopolysaccharide-neutralizing agent polymyxin B did not decrease TNF-alpha levels induced by either polysaccharide, ruling out contamination with endotoxin as a possible cause of TNF-alpha induction. Fifty percent lethal doses of the type III and group-specific antigens given as intracardiac injections were 105 and 16 mg/kg, respectively. Salmonella endotoxin, used as a positive control, had a 50% lethal dose of 0.1 mg/kg. The lethal activities of GBS polysaccharides, as well as endotoxin, were completely prevented by pretreatment of neonatal rats with the respective specific antibodies or anti-murine TNF-alpha serum. To assess the relative importance of the type-specific substance in TNF-alpha induction by whole bacteria, two unrelated GBS transposon mutants devoid of only the type-specific capsular polysaccharide (COH1-13 and COH31-15) were employed. Each of the heat-killed unencapsulated mutants was able to produce plasma TNF-alpha level elevations or TNF-alpha-dependent lethality but was significantly less efficient in these activities than the corresponding encapsulated wild-type strain. These data suggest that the presence of type-specific material on GBS is not necessary for the stimulation of TNF-alpha production. Type III capsular polysaccharide, however, can significantly increase the ability of GBS to induce TNF-alpha. Further studies will be needed to assess the importance of TNF-alpha induction by the group- and type-specific antigens in the pathophysiology of GBS disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony B. F., Concepcion N. F., McGeary S. A., Ward J. I., Heiner D. C., Shapshak P., Insel R. A. Immunospecificity and quantitation of an enzyme-linked immunosorbent assay for group B streptococcal antibody. J Clin Microbiol. 1982 Aug;16(2):350–354. doi: 10.1128/jcm.16.2.350-354.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. J., Plessala K. J., Wilson L. A., Thompson J. J., Nelson S. Divergent efficacy of antibody to tumor necrosis factor-alpha in intravascular and peritonitis models of sepsis. J Infect Dis. 1991 Jan;163(1):83–88. doi: 10.1093/infdis/163.1.83. [DOI] [PubMed] [Google Scholar]
- Baker C. J. Group B streptococcal infection in newborns: prevention at last? N Engl J Med. 1986 Jun 26;314(26):1702–1704. doi: 10.1056/NEJM198606263142609. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976 Apr 1;294(14):753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Klonisch T., Nuber P., Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991 Dec;59(12):4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R. B., Eisenstein T. K., Shockman G. D., Greber T. F., Swenson R. M. Soluble group- and type-specific antigens from type III group B Streptococcus. Infect Immun. 1980 Apr;28(1):195–203. doi: 10.1128/iai.28.1.195-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran T. I., Mattingly S. J. Association of type- and group-specific antigens with the cell wall of serotype III group B streptococcus. Infect Immun. 1982 Jun;36(3):1115–1122. doi: 10.1128/iai.36.3.1115-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtenacher B., Falk W., Männel D. N., Krammer P. H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990 Dec 1;145(11):3762–3766. [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R. L., Redding G. J., Henderson W. R., Truog W. E. Group B streptococcus induces tumor necrosis factor in neonatal piglets. Effect of the tumor necrosis factor inhibitor pentoxifylline on hemodynamics and gas exchange. Am Rev Respir Dis. 1991 Mar;143(3):598–604. doi: 10.1164/ajrccm/143.3.598. [DOI] [PubMed] [Google Scholar]
- Gibson R. L., Redding G. J., Truog W. E., Henderson W. R., Rubens C. E. Isogenic group B streptococci devoid of capsular polysaccharide or beta-hemolysin: pulmonary hemodynamic and gas exchange effects during bacteremia in piglets. Pediatr Res. 1989 Sep;26(3):241–245. doi: 10.1203/00006450-198909000-00017. [DOI] [PubMed] [Google Scholar]
- Hackett S. P., Stevens D. L. Streptococcal toxic shock syndrome: synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J Infect Dis. 1992 May;165(5):879–885. doi: 10.1093/infdis/165.5.879. [DOI] [PubMed] [Google Scholar]
- Hemming V. G., London W. T., Smith L. P., Curfman B. L., Fischer G. W., Sever J. L. Detection of group B streptococcal antigens in amniotic fluid of rhesus monkeys. J Clin Microbiol. 1983 Jun;17(6):1127–1131. doi: 10.1128/jcm.17.6.1127-1131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming V. G., O'Brien W. F., Fischer G. W., Golden S. M., Noble S. F. Studies of short-term pulmonary and peripheral vascular responses induced in oophorectomized sheep by the infusion of a group B streptococcal extract. Pediatr Res. 1984 Mar;18(3):266–269. doi: 10.1203/00006450-198403000-00010. [DOI] [PubMed] [Google Scholar]
- Ikejima T., Okusawa S., van der Meer J. W., Dinarello C. A. Induction by toxic-shock-syndrome toxin-1 of a circulating tumor necrosis factor-like substance in rabbits and of immunoreactive tumor necrosis factor and interleukin-1 from human mononuclear cells. J Infect Dis. 1988 Nov;158(5):1017–1025. doi: 10.1093/infdis/158.5.1017. [DOI] [PubMed] [Google Scholar]
- Ingram D. L., Suggs D. M., Pearson A. W. Detection of group B streptococcal antigen in early-onset and late-onset group B streptococcal disease with the Wellcogen Strep B latex agglutination test. J Clin Microbiol. 1982 Oct;16(4):656–658. doi: 10.1128/jcm.16.4.656-658.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Meyrick B., Jesmok G., Brigham K. L. Human recombinant tumor necrosis factor alpha infusion mimics endotoxemia in awake sheep. J Appl Physiol (1985) 1989 Mar;66(3):1448–1454. doi: 10.1152/jappl.1989.66.3.1448. [DOI] [PubMed] [Google Scholar]
- Keller R., Fischer W., Keist R., Bassetti S. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun. 1992 Sep;60(9):3664–3672. doi: 10.1128/iai.60.9.3664-3672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancefield R. C., McCarty M., Everly W. N. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975 Jul 1;142(1):165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth K. E., Westgate A. M., Grady M. K., Westcott J. Y., Staub N. C. Development of pulmonary intravascular macrophage function in newborn lambs. J Appl Physiol (1985) 1992 Dec;73(6):2608–2615. doi: 10.1152/jappl.1992.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Marques M. B., Kasper D. L., Pangburn M. K., Wessels M. R. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun. 1992 Oct;60(10):3986–3993. doi: 10.1128/iai.60.10.3986-3993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall T. L., Zimmerman G. A., Augustine N. H., Hill H. R. Effect of group B streptococcal type-specific antigen on polymorphonuclear leukocyte function and polymorphonuclear leukocyte-endothelial cell interaction. Pediatr Res. 1987 Jun;21(6):517–523. doi: 10.1203/00006450-198706000-00001. [DOI] [PubMed] [Google Scholar]
- Olson T. A., Fischer G. W., Hemming V. G., O'Brien W. F., Golden S. M., Maybee D. A. A group B streptococcal extract reduces neutrophil counts and induces neutrophil aggregation. Pediatr Res. 1987 Apr;21(4):326–330. doi: 10.1203/00006450-198704000-00002. [DOI] [PubMed] [Google Scholar]
- Otterlei M., Sundan A., Skjåk-Braek G., Ryan L., Smidsrød O., Espevik T. Similar mechanisms of action of defined polysaccharides and lipopolysaccharides: characterization of binding and tumor necrosis factor alpha induction. Infect Immun. 1993 May;61(5):1917–1925. doi: 10.1128/iai.61.5.1917-1925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., Wessels M. R., Heggen L. M., Kasper D. L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkle B., Goldberg R. N., Streitfeld M. M., Clark M. R., Buron E., Setzer E. S., Bancalari E. Cardiovascular changes in group B streptococcal sepsis in the piglet: response to indomethacin and relationship to prostacyclin and thromboxane A2. Pediatr Res. 1984 Sep;18(9):874–878. doi: 10.1203/00006450-198409000-00014. [DOI] [PubMed] [Google Scholar]
- Schreiber M. D., Covert R. F., Torgerson L. J. Hemodynamic effects of heat-killed group B beta-hemolytic streptococcus in newborn lambs: role of leukotriene D4. Pediatr Res. 1992 Feb;31(2):121–126. doi: 10.1203/00006450-199202000-00006. [DOI] [PubMed] [Google Scholar]
- Silva A. T., Bayston K. F., Cohen J. Prophylactic and therapeutic effects of a monoclonal antibody to tumor necrosis factor-alpha in experimental gram-negative shock. J Infect Dis. 1990 Aug;162(2):421–427. doi: 10.1093/infdis/162.2.421. [DOI] [PubMed] [Google Scholar]
- Smith J. G., Magee D. M., Williams D. M., Graybill J. R. Tumor necrosis factor-alpha plays a role in host defense against Histoplasma capsulatum. J Infect Dis. 1990 Dec;162(6):1349–1353. doi: 10.1093/infdis/162.6.1349. [DOI] [PubMed] [Google Scholar]
- Stephens K. E., Ishizaka A., Larrick J. W., Raffin T. A. Tumor necrosis factor causes increased pulmonary permeability and edema. Comparison to septic acute lung injury. Am Rev Respir Dis. 1988 Jun;137(6):1364–1370. doi: 10.1164/ajrccm/137.6.1364. [DOI] [PubMed] [Google Scholar]
- Teti G., Calapai M., Calogero G., Tomasello F., Mancuso G., Galli A., Riggio G. Specificity and protective activity of murine monoclonal antibodies directed against the capsular polysaccharide of type III group B streptococci. Hybridoma. 1992 Feb;11(1):13–22. doi: 10.1089/hyb.1992.11.13. [DOI] [PubMed] [Google Scholar]
- Teti G., Mancuso G., Tomasello F. Cytokine appearance and effects of anti-tumor necrosis factor alpha antibodies in a neonatal rat model of group B streptococcal infection. Infect Immun. 1993 Jan;61(1):227–235. doi: 10.1128/iai.61.1.227-235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman C. P., Mattsson E., Martinez-Martinez L., De Graaf L., Van Strijp J. A., Verbrugh H. A., Verhoef J., Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993 Oct;61(10):4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Truog W. E., Sorensen G. K., Standaert T. A., Redding G. J. Effects of the thromboxane synthetase inhibitor, dazmegrel (UK 38,485), on pulmonary gas exchange and hemodynamics in neonatal sepsis. Pediatr Res. 1986 May;20(5):481–486. doi: 10.1203/00006450-198605000-00020. [DOI] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Vaudaux P., Grau G. E., Huggler E., Schumacher-Perdreau F., Fiedler F., Waldvogel F. A., Lew D. P. Contribution of tumor necrosis factor to host defense against staphylococci in a guinea pig model of foreign body infections. J Infect Dis. 1992 Jul;166(1):58–64. doi: 10.1093/infdis/166.1.58. [DOI] [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Jung W. K., Connolly R. J., Burke J. F., Dinarello C. A. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. J Clin Invest. 1991 Jun;87(6):1925–1935. doi: 10.1172/JCI115218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Magee D. M., Bonewald L. F., Smith J. G., Bleicker C. A., Byrne G. I., Schachter J. A role in vivo for tumor necrosis factor alpha in host defense against Chlamydia trachomatis. Infect Immun. 1990 Jun;58(6):1572–1576. doi: 10.1128/iai.58.6.1572-1576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hunolstein C., D'Ascenzi S., Wagner B., Jelínková J., Alfarone G., Recchia S., Wagner M., Orefici G. Immunochemistry of capsular type polysaccharide and virulence properties of type VI Streptococcus agalactiae (group B streptococci). Infect Immun. 1993 Apr;61(4):1272–1280. doi: 10.1128/iai.61.4.1272-1280.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



