Abstract
Tuberculosis is a serious global health threat for which new treatments are urgently needed. This study examined the safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple ascending doses of the oxazolidinone PNU-100480 in healthy volunteers, using biomarkers for safety and efficacy. Subjects were randomly assigned to PNU-100480 or placebo (4:1) at schedules of 100, 300, or 600 mg twice daily or 1,200 mg daily for 14 days or a schedule of 600 mg twice daily for 28 days to which pyrazinamide was added on days 27 and 28. A sixth cohort was given linezolid at 300 mg daily for 4 days. Signs, symptoms, and routine safety tests were monitored. Bactericidal activity against Mycobacterium tuberculosis was measured in ex vivo whole-blood culture. Plasma drug and metabolite concentrations were compared to the levels required for inhibition of M. tuberculosis growth and 50% inhibition of mitochondrial protein synthesis. All doses were safe and well tolerated. There were no hematologic or other safety signals during 28 days of dosing at 600 mg twice daily. Plasma concentrations of PNU-100480 and metabolites at this dose remained below those required for 50% inhibition of mitochondrial protein synthesis. Cumulative whole-blood bactericidal activity of PNU-100480 at this dose (−0.316 ± 0.04 log) was superior to the activities of all other doses tested (P < 0.001) and was significantly augmented by pyrazinamide (−0.420 ± 0.06 log) (P = 0.002). In conclusion, PNU-100480 was safe and well tolerated at all tested doses. Further studies in patients with tuberculosis are warranted. Biomarkers can accelerate early development of new tuberculosis treatments.
Drug-resistant Mycobacterium tuberculosis is a serious, growing global health concern, causing an estimated 444,000 tuberculosis (TB) cases and 150,000 deaths worldwide in 2008 (30). Although improved treatments for these life-threatening infections are urgently needed, their development has been slow, in part due to the lack of predictive biomarkers suitable for early-phase clinical trials.
PNU-100480 and linezolid (LZD) are oxazolidinone antimicrobials that act by blocking translation and thereby inhibiting protein synthesis. Both are active against M. tuberculosis, including strains with multidrug and extensive drug resistance (MDR-TB and XDR-TB, respectively) (1). Linezolid has been used to treat difficult cases of MDR- and XDR-TB with apparent clinical benefit (2, 5, 7, 13, 16, 18, 19, 21, 22, 31). However, reports of these uncontrolled studies indicate that its dose must often be reduced and/or the duration of its use must be limited due to potentially serious neurologic, ophthalmologic, and hematologic toxicities. These toxicities, which typically occur only after months of treatment, are thought to be due to inhibition of mitochondrial protein synthesis (MPS) (15, 17). Thus, oxazolidinones with greater efficacy and reduced toxicity during prolonged administration are needed.
The activity of PNU-100480 against M. tuberculosis was first described in 1996 (4). Although its MICs against M. tuberculosis are similar to those of linezolid, PNU-100480 shows efficacy superior to that of linezolid against experimental murine tuberculosis, whether it is used alone or in combination with other drugs (6, 28, 29). Two metabolites (PNU-101603, a sulfoxide, and PNU-101244, a sulfone) contribute to its activity but do not account for its superiority, the basis of which is not understood.
In the present study, multiple ascending doses of PNU-100480 up to 1,200 mg per day were administered to healthy volunteers for 14 or 28 days to assess its safety, tolerability, pharmacokinetics (PKs), and pharmacodynamics (PDs). Two biomarkers were used to assess endpoints otherwise not readily measured during a relatively short trial with healthy volunteers: bactericidal activity against intracellular M. tuberculosis was measured ex vivo using whole-blood culture (whole-blood activity [WBA]), and drug concentrations in plasma were compared to those required for half-maximal MPS inhibition in vitro. During the final 2 days of dosing, subjects in the 28-day cohort also received pyrazinamide (PZA). The goals of the study were to identify an optimally safe and efficacious dose of PNU-100480 to advance in clinical trials and, secondarily, to determine if the superior activity of the combination of PNU-100480 plus pyrazinamide observed in the mouse could be confirmed in the human whole-blood infection model.
MATERIALS AND METHODS
Four cohorts of healthy volunteers (n = 10 each) were randomly assigned at a 4:1 ratio to receive PNU-100480 or placebo suspension for 14 days. PNU-100480 doses were of 100, 300, or 600 mg twice a day (BID) and 1,200 mg once a day (QD) (cohorts 1 to 4, respectively). A fifth cohort received PNU-100480 at 600 mg or placebo BID as 200-mg tablets at a 4:1 ratio for 28 days. On days 27 and 28, cohort 5 also received pyrazinamide at 25 mg/kg of body weight/day. In all but the first two cohorts, doses were administered with food. Dosing with PNU-100480 and placebo was double-blind. A sixth unblinded cohort (n = 8) received linezolid as a 300-mg suspension QD for 4 days, on the basis of one report that this might be the maximum adequately tolerated dose in patients with MDR- and XDR-TB (13). Subjects were ages 18 to 55 years; had laboratory parameters indicating normal hematologic, hepatic, and renal function; were not concurrently being treated with immunosuppressive medications; and were known not to have HIV-1 infection. Women of childbearing potential were excluded. The study was conducted at the Pfizer Clinical Research Unit in New Haven, CT. All subjects provided written informed consent according to International Conference on Harmonization guidelines. The study protocol was approved by the Integreview Institutional Review Board and U.S. FDA and was registered as trial NCT00990990. Some data from the linezolid cohort have been reported previously (23).
Symptoms, electrocardiogram results, and routine blood and urine safety test results were monitored. Blood was collected for PK analysis on days 1, 4 (linezolid cohort), 14 (PNU-100480 cohorts), and 28 (PNU-100480 cohort 5). Specimens were obtained at 0, 0.25, 0.5, 1, 2, 3, 6, 12, 24, 48, and 72 h postdosing on days 14 and 28. Total concentrations of PNU-100480, PNU-101603, and PNU-101244 in plasma were assayed using a validated high-pressure liquid chromatography (HPLC)-tandem mass spectrometry (MS/MS) method by Advion BioServices (Ithaca, NY). A 100-μl aliquot of plasma was extracted using protein precipitation with acetonitrile. A Phenomenex Gemini C18 column (50 by 2.0 mm; particle size, 3 μm) was used for HPLC separation; methanol-10 mM ammonium acetate (60:40, vol/vol) was used to elute samples. A 10-μl aliquot of the extracted sample was injected into a Sciex API-4000 liquid chromatograph (LC)-MS/MS (Applied Biosystems) utilizing a heated nebulizer (atmospheric pressure chemical ionization) source. PF-00184033, a structure analogue, was used as the internal standard. The ranges of detection of the assay were 2.5 to 2,500, 0.5 to 500, and 10 to 10,000 ng/ml for PNU-100480, PNU-101603, and PNU-101244, respectively. Total concentrations of linezolid in plasma were assayed using a validated HPLC-MS/MS method by WuXi AppTec, Inc. (Shanghai, China). Plasma samples were extracted using protein precipitation with acetonitrile. A Capcell Pak C18 column (2.0 mm by 50 mm; particle size, 5 μm) was used for HPLC separation, and a gradient mobile phase that consisted of 1.0 mM ammonium acetate in water and methanol was used to elute samples. A 10-μl aliquot of the extracted sample was injected into a Sciex API-4000 LC-MS/MS (Applied Biosystems) utilizing a heated nebulizer (atmospheric pressure chemical ionization) source. Deuterium-labeled linezolid served as the internal standard. The range of detection of the assay was 20 to 10,000 ng/ml for linezolid. The accuracies (percent difference from nominal value) of the quality control samples used during sample analysis ranged from 4.7 to 10.6% with a precision (as measured by the percent relative standard deviation [SD]) of ≤5.2% for linezolid, from −1.0 to 3.0% with a precision of ≤6.3% for PNU-100480, from −0.5 to 9.8% with a precision of ≤12.2% for PNU-101603, and from 6.6 to 1.8% with a precision of ≤7.4% for PNU-101244. PK parameters were calculated using noncompartmental analysis of plasma concentration-time data. Data for samples with values below the lower limit of quantitation were set equal to 0 ng/ml for the analysis.
Blood was obtained for WBA measurements on days 4 (linezolid cohort), on days 14 and 28 (PNU-100480 cohorts), and at 0, 2, 3, 4, 8, and 12 h postdosing. WBA was measured as previously described (11, 23, 25, 27). Briefly, M. tuberculosis H37Rv was grown in mycobacterial growth indicator tubes (MGIT; Becton-Dickinson) and frozen in aliquots. A titration experiment determined the relationship between inoculum size and time to positivity (TTP) and identified the volume positive in 5.5 days. Whole-blood cultures consisted of heparinized blood, an equal volume of RPMI 1640 tissue culture medium, and the specified volume of mycobacterial stock. After 72 h, cells were sedimented, the liquid phase was removed, and blood cells were disrupted by hypotonic lysis. Bacilli were recovered and inoculated into MGIT. Log change in viability was calculated as log(final) − log(initial), where final and initial are the volumes corresponding to TTP of the completed cultures and its inoculum, respectively, on the basis of the titration curve. The laboratory protocol and software developed by one of the authors (R.S.W.) are available on request. WBA was reported as log change per day. Cumulative WBA was calculated as the area under the concentration-time curve (AUC) from 0 h to 24 h (AUC0-24) using the trapezoidal method and is reported as log change.
The MPS 50% inhibitory concentrations (IC50s) of PNU-100480, PNU-101603, PNU-101244, and linezolid were determined by [35S]methionine incorporation by rat heart mitochondria (17). Fold MPS IC50s and MIC levels for PNU-100480 were determined as the sum of the ratios of the parent and metabolites to their respective reference concentrations (e.g., conc1/MIC1 + conc2/MIC2 + conc3/MIC3, where 1, 2, and 3 represent the parent and 2 metabolites). For these calculations, MIC values of 500 ng/ml were used for M. tuberculosis H37Rv for PNU-101603, PNU-101244, and linezolid and an MIC of 250 ng/ml was used for PNU-100480, although MIC values of 250 ng/ml for the PNU metabolites have also been reported (29). Groups were compared by one-way analysis of variance (ANOVA); post hoc testing used the Holm-Sidak method. The threshold for significance was 0.05. Regression analysis using a four-parameter formula describing a sigmoid curve was performed using Sigmaplot (version 11) software (Systat Software).
RESULTS
Safety and tolerability.
All of the doses studied were generally safe and well tolerated. The most commonly reported adverse events (AEs) deemed related or possibly related to study treatment were gastrointestinal and central nervous system symptoms (Table 1). All but four events were mild in intensity. No subjects discontinued the study due to an abnormal safety laboratory finding. No subjects exhibited laboratory values that met the safety parameters of potential clinical concern or that resulted in AEs. No subjects had an abnormal electrocardiography reading that was considered clinically significant or reported as an adverse event.
TABLE 1.
Adverse events regarded as related or possibly related to study treatment by the blinded site investigator during multidose treatment with PNU-100480 or placeboa
| Organ system and symptom | No. of volunteers |
|||||
|---|---|---|---|---|---|---|
| Placebo | 14 days of treatment |
28 days of treatment at 600 mg BID | ||||
| 100 mg BID | 300 mg BID | 600 mg BID | 1200 mg QD | |||
| GI (total) | 3 | 2 | 2 | 4 | 4 | 5 |
| Anorexia | 1 | |||||
| Abdominal pain | 1 | 2b | 2 | 1 | 1 | |
| Colitis | 1c | |||||
| Diarrhea | 1 | 1 | 1 | 2 | 2 | |
| Dysgeusia | 1 | |||||
| Nausea | 1 | 1 | ||||
| Urgency | 1 | |||||
| CNS (total) | 2 | 0 | 4 | 4 | 2 | 5 |
| Dizziness | 1 | 1 | 2d | |||
| Headache | 2 | 3 | 3d,e | |||
| Euphoria | 1 | |||||
| Fatigue | 1 | 1 | ||||
| Restlessness | 1 | |||||
| Somnolence | 1 | |||||
| Other (total) | 1 | 0 | 0 | 1 | 0 | 3 |
| Flank pain | 1 | |||||
| Urinary frequency | 1 | |||||
| Chest pain | 1 | |||||
| Intertrigo | 1d | |||||
| Rhinitis | 1 | |||||
Each cohort except for the placebo cohort consisted of eight subjects. The placebo cohort included eight subjects dosed for 14 days and two dosed for 28 days. Events were of mild intensity and could be attributed solely to study drug or placebo, except as indicated. GI, gastrointestinal; CNS, central nervous system.
One episode of abdominal pain was of moderate intensity.
One episode of colitis was classified as a serious adverse event.
One episode each of intertrigo, dizziness, and headache occurred during cotreatment with pyrazinamide.
Two episodes of headache were of moderate intensity.
One serious adverse event (SAE), colitis, occurred in a 36-year-old male of Asian descent randomized to PNU-100480 at a dose of 600 mg BID for 14 days. The event began on study day 5 as abdominal cramping and diarrhea, accompanied by an elevated leukocyte count and, later, heme-positive stool. Pancolitis was diagnosed by computed tomography scan. The study drug was discontinued after the subject had received nine doses. He was treated with metronidazole and ciprofloxacin and was permanently withdrawn from the study. He quickly improved clinically; colonoscopy was not performed. No bacterial pathogens (including Clostridium difficile) were identified. Adenovirus was identified and confirmed in one stool specimen; however, the significance of its presence without histologic confirmation was uncertain. The assessment by the gastrointestinal consultant was that the study drug could not be excluded as the cause of the colitis.
Hematologic oxazolidinone toxicities are generally dose and duration dependent. A cohort receiving PNU-100480 at 600 mg BID for 28 days was therefore included in this study to better understand the risk of these toxicities. Neither the reticulocyte nor the platelet counts of this cohort were significantly affected by 28 days of dosing (reticulocyte percentages, 1.21 ± 0.27 on day 0 versus 1.05 ± 0.42 on day 28 [P = 0.20]; numbers of platelets per nl, 296 ± 78 on day 0 versus 275 ± 52 on day 28 [P = 0.26]; both sets of P values were determined by paired t test). No safety signals were apparent in any other laboratory parameters. There were no episodes of colitis or other SAEs in this cohort.
PKs.
Absorption of PNU-100480 was rapid, with a median time to the maximum concentration of drug in plasma (Tmax) of 1 to 2 h after dosing under fasting conditions. Absorption was delayed by food, which shifted the median Tmax to 3 h. Steady-state conditions were reached after approximately 3 days. Plasma concentrations of PNU-101603 and PNU-101244 tended to rise and fall in tandem with those of the parent, with mean concentrations being 5.98 ± 3.0 and 0.146 ± 0.08 times those of PNU-100480, respectively. Concentrations of PNU-100480 were highly correlated with those of PNU-101603 (R = 0.904), which in turn were highly correlated with those of PNU-101244 (R = 0.928), consistent with their stepwise oxidation. The sum of exposures (maximum concentration of drug in plasma [Cmax] and AUC from 0 to 12 h for BID dosing and 0 to 24 h for QD dosing [AUCtau]) of PNU-100480 and its active metabolites increased proportionally with an increase in the dose across the range studied. There was little or no accumulation during 14 days of dosing, with accumulation ratios ranging from 1.05 to 1.24 for the PNU-101603 AUC from 0 h to the last measurement (AUC0-last) for all regimens studied. PK parameters for PNU-100480 and PNU-101603 at steady state are summarized in Table 2. Plasma concentrations of PNU-101244 remained below its MIC at all dose levels.
TABLE 2.
Pharmacokinetic parameters of PNU-100480 and PNU-101603 in healthy volunteers at steady state (day 14) after oral dosing of PNU-100480
| Analyte and PK parameter | Result for the following PNU-100480 dose: |
|||
|---|---|---|---|---|
| 100 mg BID (n = 8) | 300 mg BID (n = 8) | 600 mg BID (n = 7) | 1,200 mg QD (n = 8) | |
| PNU-100480 | ||||
| AUCtau (ng·h/ml)a | 845.8 (43) | 2,133 (23) | 4,294 (23) | 10,100 (30) |
| Cmax (ng/ml)a | 252.4 (43) | 458.7 (45) | 942.7 (20) | 2,016 (50) |
| Tmax (h)b | 1.0 (0.5-2.0) | 2.0 (0.5-2.0) | 3.0 (2.0-3.0) | 3.0 (2.0-3.0) |
| t1/2 (h)c,d | 2.725 (5) | 2.550 (27) | 2.925 (41) | 3.380 (10) |
| Raca,e (AUCtau) | 1.565 (12) | 1.080 (19) | 1.194 (14) | 1.051 (14) |
| PNU-101603 | ||||
| AUCtau (ng·h/ml) | 3,289 (16) | 8,882 (19) | 23,460 (9) | 42,950 (15) |
| Cmax (ng/ml) | 941.7 (30) | 1,678 (31) | 3,895 (16) | 6,469 (15) |
| Tmax (h) | 0.5 (0.5-1.0) | 1.0 (0.5-2.0) | 3.0 (3.00-3.03) | 3.0 (2.0-6.0) |
| t1/2 (h) | 2.850 (9) | 3.328 (32) | 3.53 (26) | 3.493 (14) |
| Rac (AUCtau) | 1.237 (14) | 1.049 (17) | 1.188 (10) | 1.129 (14) |
Data represent the geometric mean(percent coefficient of variation).
Data represent the median (range).
Data represent the arithmetic mean (percent coefficient of variation).
t1/2, half-life.
Rac, accumulation ratio.
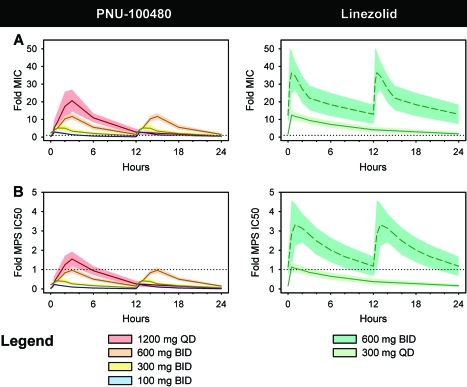
Summed ratios of plasma concentrations of PNU-100480 and metabolites in relation to their respective MICs are shown in Fig. 1 A. Dosing at 1,200 mg QD resulted in maximum PNU concentrations of 20.6-fold the MIC, whereas only dosing at 600 mg BID maintained trough concentrations at or above the MIC. Corresponding values for linezolid at 600 mg BID, included for comparison, are based on published PK data for healthy volunteers (20). At this dose, maximum linezolid concentrations were 36.4-fold the MIC. Trough linezolid levels remained above the MIC whether linezolid was dosed at 300 mg QD or 600 mg BID. PNU-100480 at 600 mg BID and linezolid at 300 mg QD produced similar peak concentrations (11.7 and 12.5-fold the MICs, respectively).
FIG. 1.
Plasma concentrations of PNU-100480 (left) and linezolid (right) in relation to those required for inhibition of growth of M. tuberculosis H37Rv (MIC) (A) and MPS (IC50) (B). Lines indicate means; shaded areas indicate SDs. For linezolid at 600 mg BID, values are calculated on the basis of published plasma drug concentrations in healthy volunteers. For PNU-100480, values represent summed ratios of concentrations of the parent compound and its metabolites and the respective MIC or MPS IC50. Of all regimens tested, only PNU-100480 at 600 mg BID resulted in mean levels above the MIC but below the MPS IC50 throughout the dosing interval.
MPS IC50s for PNU-100480, PNU-101603, and PNU-101244 were 15,478, 4,395, and 6,706 ng/ml, respectively. Summed plasma PNU concentrations in relation to the respective MPS IC50s are shown in the left panel of Fig. 1B. Mean PNU concentrations did not exceed the MPS IC50 at any time point for any BID dosing schedules but reached 1.5-fold the MPS IC50 at 3 h after the 1,200-mg-QD dose. The MPS IC50 of linezolid was 5,500 ng/ml. Plasma linezolid concentrations in relation to this MPS IC50 are shown in the right panel of Fig. 1B. Dosing at 300 mg QD resulted in mean plasma concentrations only briefly above the MPS IC50, reaching a maximum of 1.1-fold the MPS IC50 at 0.5 h postdosing. Dosing at 600 mg BID would be expected to result in levels above the MPS IC50 throughout the dosing interval, ranging from 1.1- to 3.3-fold the MPS IC50.
WBA.
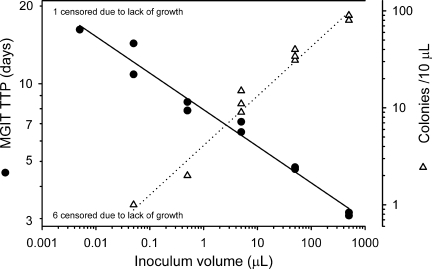
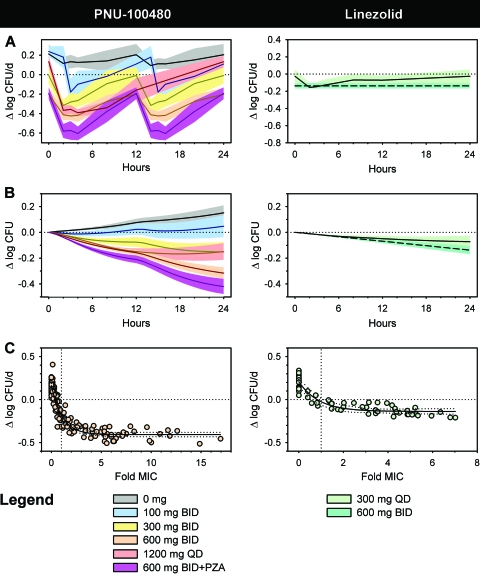
The suitability of the use of TTP in MGIT as a surrogate for CFU counts in the whole-blood model is illustrated by the highly significant inverse relationship between these parameters in Fig. 2 (R = −0.970, P = 0.006). Two approaches to displaying WBA data are illustrated in Fig. 3. Figure 3A shows bactericidal activity at discrete time points. Figure 3B shows cumulative activity as it evolves over 24 h. In both panels, killing is indicated by negative values.
FIG. 2.
Inverse relationship between TTP (black circles; in duplicate) in MGIT and colony count (white triangles; in triplicate) of 10-fold serial dilutions of cultured stock of M. tuberculosis H37Rv. Lines indicate linear regressions. Six colony count data points were censored due to lack of growth, whereas only one corresponding MGIT culture data point was censored. All negative cultures occurred at small inoculum volumes. Log mean TTP and log mean CFU were highly correlated (R = −0.970, P = 0.006).
FIG. 3.
WBA of PNU-100480 (left) and linezolid (right). Zero on the vertical axis indicates bacteriostasis; negative values indicate killing. (A) Values at discrete time points after dosing. (B) Cumulative activity over 24 h. (C) Concentration-activity relationship. Drug concentrations have been reduced by half to account for dilution in whole-blood culture. Values for linezolid at 600 mg BID are based on published PK data and the relationship between concentration and activity that was established from the data in panel C. The cumulative bactericidal activity of PNU-100480 at 600 mg BID exceeded that of any other oxazolidinone dosing schedule tested. This was significantly augmented by the addition of pyrazinamide.
Prior to dosing, whole-blood cultures of placebo recipients showed mean growth of M. tuberculosis of 0.15 log/day, corresponding to a doubling time of 34 h. This value was unaffected by dosing with placebo (Fig. 3A, left panel). As a result, the corresponding curve in Fig. 3B shows steadily progressive cumulative growth of M. tuberculosis throughout the dosing interval. Bactericidal activity was evident at least transiently after each PNU-100480 dose at each dose level. The maximal bactericidal effect of approximately −0.42 log/day was present 3 h after 600-mg-BID dosing, coinciding with maximal plasma PNU-100480 concentrations. However, there was no further increment in maximal effect at 1,200 mg QD. The PNU-100480 dosing schedule with the greatest cumulative activity was 600 mg BID at −0.316 ± 0.04 log. Comparison of the cumulative WBA of all PNU cohorts and the linezolid cohort by ANOVA revealed highly significant differences among the groups (P < 0.001). Post hoc testing revealed significant differences between each possible pair of cohorts with the exception of the 300-mg-BID and 1,200-mg-QD PNU-100480 cohorts, which did not differ.
The corresponding effects of linezolid at 300 mg QD are shown as the solid lines in the right panels of Fig. 3A and B. Its maximal bactericidal activity, −0.16 log/day, was significantly less than that of PNU-100480 at 600 mg BID (P < 0.001), despite similar plasma drug concentrations with respect to the MIC. To better understand the basis of this observation, an analysis of the relationship between drug concentration and bactericidal activity for PNU-100480 and linezolid was performed. As shown in Fig. 3C, both drugs showed concentration-dependent increases in activity between the MIC and twice the MIC, reaching approximately 90% of maximal activity at twice the MIC. However, both drugs showed concentration-independent activity at higher concentrations. The maximal effect of PNU-100480 was more than twice that of linezolid. This indicates the superior bactericidal activity of PNU-100480 to be intrinsic rather than to be due to higher plasma concentrations or greater exposure.
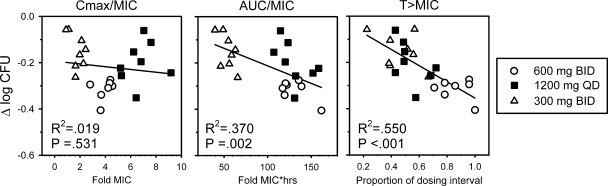
The PK/PD relationship is examined in Fig. 4. Three PNU-100480 dosing schedules were included in this analysis: 600 mg BID, which showed the greatest cumulative WBA; 1,200 mg QD, which was equal to the 600-mg dose with regard to total dose; and 300 mg BID, which was equal to the 1,200-mg dose with respect to cumulative WBA. The values in Fig. 4 reflect the drug concentrations in whole-blood culture, which are half those in vivo. In plasma, PNU-100480 given at 300 mg BID, 1,200 mg QD, and 600 mg BID resulted in fractions of the dosing interval for which drug concentrations were above the MIC of 0.61 ± 0.14, 0.64 ± 0.13, and 0.97 ± 0.06, respectively. Both time above the MIC and AUC/MIC were significantly correlated with cumulative WBA, but with relatively low correlation coefficients (R2 = 0.550 and 0.370, respectively). Additional studies may help clarify the basis of this observation and identify other parameters with greater predictive power.
FIG. 4.
Relationships of three PK parameters to cumulative WBA during dosing of PNU-100480 at 300 or 600 mg BID or 1,200 mg QD to healthy volunteers. Drug concentrations have been reduced to half those measured in plasma to account for dilution in whole-blood culture. T > MIC, time that the concentration remains above the MIC.
The optimal dose of linezolid in MDR- and XDR-TB is not known. To determine the potential activity of standard adult linezolid doses, its WBA was predicted on the basis of published PK data and the concentration-activity relationship described in Fig. 3C. The dashed lines in the right panels of Fig. 3A and B were calculated on the basis of published mean plasma concentrations, from which expected WBA values were calculated using the regression curve in Fig. 3C. The upper and lower bounds were calculated using published ± 1 SD plasma concentrations and the lower and upper values of the 95% confidence interval of the regression curve, respectively. Although this was not a formal modeling exercise, these data indicate that even when both drugs are dosed equally at 600 mg BID, the activity of linezolid does not equal that of PNU-100480. This is consistent with the relationship established in Fig. 3C, as doses resulting in higher plasma concentrations (above twice the MIC) would not be anticipated to result in substantially superior biologic activity.
Lastly, the effects of concomitant pyrazinamide administration on the safety, tolerability, PKs, and bactericidal activity of PNU-100480 were assessed during the final 2 days of dosing of cohort 5 (PNU-100480 at 600 mg BID). No significant safety signals were noted during the period of combined dosing. There was no significant effect of pyrazinamide on PNU-100480 pharmacokinetics. Combined dosing resulted in improved cumulative WBA compared to that achieved with PNU-100480 alone (−0.420 ± 0.06 versus −0.316 ± 0.04; P = 0.002; Fig. 3A and B). Two subjects in the 4-week cohort had been assigned to placebo; they received pyrazinamide plus placebo during the last 2 days of dosing. In these subjects, pyrazinamide alone was mainly bacteriostatic, with a cumulative WBA of −0.002 ± 0.02. Although the difference between this effect and that of pyrazinamide when it was added to PNU-100480 is substantial (−0.104 log), it does not meet the criteria set by this journal for synergy (which require a 2-log effect over 24 h and which appear to be better suited to studies of rapidly growing bacteria). Nonetheless, the markedly enhanced effect of pyrazinamide when it is added to PNU-100480 appears to be consistent with reports of accelerated sterilization with this combination in the mouse TB model (28).
DISCUSSION
This is the second study of PNU-100480 in human subjects. The first trial, of single ascending doses up to 1,500 mg, found that all doses were safe and well tolerated, that doses up to 1,000 mg were adequately and predictably absorbed, and that plasma levels of the main metabolite, PNU-101603, were several times those of the parent compound and apparently contributed substantially to overall activity (23). That study, like the present one, used whole-blood culture as a biomarker to assess the PK/PD relationship and found killing of M. tuberculosis by PNU-100480 to be independent of drug concentration above twice the MIC. That finding, together with our analysis of the pharmacokinetics of PNU-100480 and its metabolites, contributed to our decision to include twice-daily dosing schedules in the present study. To our knowledge, this program is the first in which biomarkers for efficacy and safety were used to guide dose selection in early anti-TB drug development.
Whole-blood culture is an emerging biomarker to assess the efficacies of antimycobacterial drugs and vaccines (24). Mycobacteria added to whole-blood culture are rapidly ingested by phagocytic cells (10). Interactions of infected cells with lymphocytes and other cells result in restriction of intracellular mycobacterial growth: partial restriction in the blood of healthy volunteers and full bacteriostasis in the blood of TB patients (10, 26, 27). Cumulative WBA during anti-TB therapy correlates with 2-month sputum culture status and serial sputum CFU counts, is greater for rifampin than isoniazid, and is greater for the standard four-drug therapy than for current treatment regimens for MDR-TB (11, 25, 27). It is therefore indirectly related to treatment outcome (24). The predictive value of the whole-blood model in TB patients likely rests on its ability to measure killing by administered therapy of an otherwise elusive mycobacterial subpopulation characterized by nonreplicating persistence. Additional studies are warranted to examine the relationship of this marker and other indicators of tissue sterilization in patients with pulmonary tuberculosis.
The main findings of the present study were that all PNU-100480 doses were safe and generally well tolerated, that exposures increased approximately linearly with dose, that maximal cumulative whole-blood bactericidal activity was associated with a dose of 600 mg BID, and that this dose appeared to have a low likelihood of mitochondrial protein synthesis inhibition. These important observations will inform dose selection for future studies of PNU-100480 in patients with tuberculosis. At the same time, it is important to recognize their specific limitations and implications.
This study found that both PNU-100480 and linezolid reached 90% maximum bactericidal activity at approximately twice the MIC, thus confirming the findings of our single-dose trial. The lack of additional activity at higher concentrations may indicate saturation of binding to mycobacterial rRNA. This study also confirmed the significantly greater maximal bactericidal effect of PNU-100480 compared with that of linezolid, a finding not predicted by MICs or other in vitro measures. The basis of this superiority is uncertain but may be due to interactions of PNU-100480 and/or its metabolites with host cell killing mechanisms, as has been reported for killing of Staphylococcus aureus by torezolid (14). The finding that twice-daily PNU-100480 dosing resulted in optimal cumulative WBA is consistent with its relatively short plasma half-life and the lack of incremental killing at higher concentrations. Dose fractionation studies of PNU-100480 in mice had not clearly indicated that its activity was either time or concentration dependent (29). Mycobacterial adaptations to specific growth conditions are recognized to affect drug activity. The combination of meropenem plus clavulanic acid, for example, shows concentration-dependent killing of M. tuberculosis in vitro only under hypoxic conditions (8). Studies with patients will be required to determine the potential efficacy of once-daily PNU-100480 dosing.
Drug concentrations remain constant in each individual whole-blood culture. Cumulative activity is calculated as the integral over time of multiple cultures of blood collected throughout the dosing interval, thereby reflecting total activity as drug concentrations change in vivo. This approach has the recognized shortcoming that postantibiotic effects (PAEs) are not represented. PAEs can delay the resumption of bacterial growth in vivo as drug concentrations fall. Two factors mitigate this potential shortcoming. First, the PAE of linezolid against M. tuberculosis is short compared to the PAEs of other TB drugs (9). Second, PAE at best could be expected to prevent the slight upward inflection of the 1,200-mg-QD curve in the left graph of Fig. 3B, maintaining bacteriostasis but not preventing the loss of cidality at later time points. At most, the effect of the absence of a PAE in this study therefore would be small.
It is uncommon for studies of a new antibiotic to be continued for 28 days in healthy volunteers. The duration of dosing was extended in this study to better appreciate potential oxazolidinone toxicities due to MPS inhibition. Effects on platelet counts have been noted as early as 10 days into dosing with linezolid at 600 mg BID (3, 20). No significant untoward hematologic effects were observed in the present study when PNU-100480 was similarly dosed, despite more than two additional weeks of dosing. Clinical trials of greater size and longer duration will be required to better understand the full safety profile of PNU-100480, including its gastrointestinal safety. However, on the basis of its present MPS profile, it appears likely that the frequency of adverse events related to MPS inhibition by PNU-100480 when it is dosed at 600 mg BID will be similar to that for linezolid given at 300 mg once daily.
Lastly, this study found favorable effects in the whole-blood model when PNU-100480 and pyrazinamide were combined. Although pyrazinamide is an important sterilizing drug in animals and in TB patients, its activity is difficult to detect in short clinical trials (12). Indeed, when it was first discovered, pyrazinamide had no apparent activity in vitro (32). Subsequent experiments showed its dependence on an acidic pH, which arises in tissues in the context of a cellular inflammatory response and in macrophages after phagolysosomal fusion. Previous studies had indicated that pyrazinamide shows activity in the whole-blood model (25, 27). Drug combinations including PNU-100480 and pyrazinamide show an unusual capacity to shorten the duration of TB treatment in mice (28). Clinical trials to test this combination in TB patients are warranted.
In summary, this study showed that at doses of 600 mg BID, PNU-100480 shows an MPS safety profile similar to that of linezolid at 300 mg QD, superior whole-blood bactericidal activity compared to the activities achieved with standard linezolid doses, and additive or synergistic activity when it is added to standard doses of pyrazinamide. Further studies with patients with drug-sensitive and -resistant TB are warranted.
Acknowledgments
This study was supported by Pfizer Inc.
Footnotes
Published ahead of print on 15 November 2010.
REFERENCES
- 1.Alcala, L., M. J. Ruiz-Serrano, T. C. Perez-Fernandez, D. Garcia de Viedma, M. Diaz-Infantes, M. Marin-Arriaza, and E. Bouza. 2003. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob. Agents Chemother. 47:416-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anger, H. A., F. Dworkin, S. Sharma, S. S. Munsiff, D. M. Nilsen, and S. D. Ahuja. 2010. Linezolid use for treatment of multidrug-resistant and extensively drug-resistant tuberculosis, New York City, 2000-06. J. Antimicrob. Chemother. 65:775-783. [DOI] [PubMed] [Google Scholar]
- 3.Attassi, K., E. Hershberger, R. Alam, and M. J. Zervos. 2002. Thrombocytopenia associated with linezolid therapy. Clin. Infect. Dis. 34:695-698. [DOI] [PubMed] [Google Scholar]
- 4.Barbachyn, M. R., D. K. Hutchinson, S. J. Brickner, M. H. Cynamon, J. O. Kilburn, S. P. Klemens, S. E. Glickman, K. C. Grega, S. K. Hendges, D. S. Toops, C. W. Ford, and G. E. Zurenko. 1996. Identification of a novel oxazolidinone (U-100480) with potent antimycobacterial activity. J. Med. Chem. 39:680-685. [DOI] [PubMed] [Google Scholar]
- 5.Condos, R., N. Hadgiangelis, E. Leibert, G. Jacquette, T. Harkin, and W. N. Rom. 2008. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest 134:187-192. [DOI] [PubMed] [Google Scholar]
- 6.Cynamon, M. H., S. P. Klemens, C. A. Sharpe, and S. Chase. 1999. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob. Agents Chemother. 43:1189-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortun, J., P. Martin-Davila, E. Navas, M. J. Perez-Elias, J. Cobo, M. Tato, E. G. de la Pedrosa, E. Gomez-Mampaso, and S. Moreno. 2005. Linezolid for the treatment of multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 56:180-185. [DOI] [PubMed] [Google Scholar]
- 8.Hugonnet, J. E., L. W. Tremblay, H. I. Boshoff, C. E. Barry III, and J. S. Blanchard. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui, M., C. Au-Yeang, K. T. Wong, C. Y. Chan, W. W. Yew, and C. C. Leung. 2008. Post-antibiotic effects of linezolid and other agents against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 31:395-396. [DOI] [PubMed] [Google Scholar]
- 10.Janulionis, E., C. Sofer, S. Schwander, D. Simmons, B. Kreiswirth, E. Shashkina, and R. S. Wallis. 2005. Survival and replication of clinical Mycobacterium tuberculosis isolates in the context of human innate immunity. Infect. Immun. 73:2595-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janulionis, E., C. Sofer, H. Y. Song, and R. S. Wallis. 2004. Lack of activity of oral clofazimine against intracellular Mycobacterium tuberculosis in whole-blood culture. Antimicrob. Agents Chemother. 48:3133-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jindani, A., C. J. Dore, and D. A. Mitchison. 2003. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am. J. Respir. Crit. Care Med. 167:1348-1354. [DOI] [PubMed] [Google Scholar]
- 13.Koh, W. J., O. J. Kwon, H. Gwak, J. W. Chung, S. N. Cho, W. S. Kim, and T. S. Shim. 2009. Daily 300 mg dose of linezolid for the treatment of intractable multidrug-resistant and extensively drug-resistant tuberculosis. J. Antimicrob. Chemother. 64:388-391. [DOI] [PubMed] [Google Scholar]
- 14.Louie, A., C. Fregeau, W. Liu, H. Conde, R. Kulawy, and G. L. Drusano. 2009. Defining the impact of granulocytes on the kill of methicillin-resistant Staphylococcus aureus by the new oxazolidinone prodrug TR-701, abstr. A1-1935. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 15.McKee, E. E., M. Ferguson, A. T. Bentley, and T. A. Marks. 2006. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob. Agents Chemother. 50:2042-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Migliori, G. B., B. Eker, M. D. Richardson, G. Sotgiu, J. P. Zellweger, A. Skrahina, J. Ortmann, E. Girardi, H. Hoffmann, G. Besozzi, N. Bevilacqua, D. Kirsten, R. Centis, and C. Lange. 2009. A retrospective TBNET assessment of linezolid safety, tolerability and efficacy in multidrug-resistant tuberculosis. Eur. Respir. J. 34:387-393. [DOI] [PubMed] [Google Scholar]
- 17.Nagiec, E. E., L. Wu, S. M. Swaney, J. G. Chosay, D. E. Ross, J. K. Brieland, and K. L. Leach. 2005. Oxazolidinones inhibit cellular proliferation via inhibition of mitochondrial protein synthesis. Antimicrob. Agents Chemother. 49:3896-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nam, H. S., W. J. Koh, O. J. Kwon, S. N. Cho, and T. S. Shim. 2009. Daily half-dose linezolid for the treatment of intractable multidrug-resistant tuberculosis. Int. J. Antimicrob. Agents 33:92-93. [DOI] [PubMed] [Google Scholar]
- 19.Park, I. N., S. B. Hong, Y. M. Oh, M. N. Kim, C. M. Lim, S. D. Lee, Y. Koh, W. S. Kim, D. S. Kim, W. D. Kim, and T. S. Shim. 2006. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J. Antimicrob. Chemother. 58:701-704. [DOI] [PubMed] [Google Scholar]
- 20.Pfizer. 2010. Zyvox package insert. Pfizer Inc., New York. http://media.pfizer.com/files/products/uspi_zyvox.pdf. Last accessed 12 July 2010.
- 21.Udwadia, Z. F., T. Sen, and G. Moharil. 2010. Assessment of linezolid efficacy and safety in MDR- and XDR-TB: an Indian perspective. Eur. Respir. J. 35:936-938. [DOI] [PubMed] [Google Scholar]
- 22.von der Lippe, B., P. Sandven, and O. Brubakk. 2006. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB)—a report of ten cases. J. Infect. 52:92-96. [DOI] [PubMed] [Google Scholar]
- 23.Wallis, R. S., W. Jakubiec, V. Kumar, A. M. Silvia, D. Paige, D. Dimitrova, X. Li, L. Ladutko, S. Campbell, G. Friedland, M. Mitton-Fry, and P. F. Miller. 2010. Pharmacokinetics and whole blood bactericidal activity against Mycobacterium tuberculosis of single ascending doses of PNU-100480 in healthy volunteers. J. Infect. Dis. 202:745-751. [DOI] [PubMed] [Google Scholar]
- 24.Wallis, R. S., M. Pai, D. Menzies, T. M. Doherty, G. Walzl, M. D. Perkins, and A. Zumla. 2010. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 375:1920-1937. [DOI] [PubMed] [Google Scholar]
- 25.Wallis, R. S., M. Palaci, S. Vinhas, A. G. Hise, F. C. Ribeiro, K. Landen, S. H. Cheon, H. Y. Song, M. Phillips, R. Dietze, and J. J. Ellner. 2001. A whole blood bactericidal assay for tuberculosis. J. Infect. Dis. 183:1300-1303. [DOI] [PubMed] [Google Scholar]
- 26.Wallis, R. S., S. Vinhas, and E. Janulionis. 2009. Strain specificity of antimycobacterial immunity in whole blood culture after cure of tuberculosis. Tuberculosis (Edinb.) 9:221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallis, R. S., S. A. Vinhas, J. L. Johnson, F. C. Ribeiro, M. Palaci, R. L. Peres, R. T. Sa, R. Dietze, A. Chiunda, K. Eisenach, and J. J. Ellner. 2003. Whole blood bactericidal activity during treatment of pulmonary tuberculosis. J. Infect. Dis. 187:270-278. [DOI] [PubMed] [Google Scholar]
- 28.Williams, K. N., S. J. Brickner, C. K. Stover, T. Zhu, A. Ogden, R. Tasneen, S. Tyagi, J. H. Grosset, and E. L. Nuermberger. 2009. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am. J. Respir. Crit. Care Med. 180:371-376. [DOI] [PubMed] [Google Scholar]
- 29.Williams, K. N., C. K. Stover, T. Zhu, R. Tasneen, S. Tyagi, J. H. Grosset, and E. Nuermberger. 2008. Promising anti-tuberculosis activity of the oxazolidinone PNU-100480 relative to linezolid in the murine model. Antimicrob. Agents Chemother. 53:1314-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. WHO/HTM/TB/2010.3. World Health Organization, Geneva, Switzerland. http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf. Last accessed 12 July 2010.
- 31.Yew, W. W., C. H. Chau, and K. H. Wen. 2008. Linezolid in the treatment of ‘difficult’ multidrug-resistant tuberculosis. Int. J. Tuber. Lung Dis. 12:345-346. [PubMed] [Google Scholar]
- 32.Zhang, Y., and D. Mitchison. 2003. The curious characteristics of pyrazinamide: a review. Int. J. Tuber. Lung Dis. 7:6-21. [PubMed] [Google Scholar]