Abstract
In a single quantitative study, we measured acrA, acrB, tolC, mdfA, and norE expression in Escherichia coli clinical isolates by using real-time PCR. acrA and acrB overexpression strongly correlated with fluoroquinolone and multidrug resistance; tolC, mdfA, and norE expression did not. The order of abundance of efflux pump transcripts in all fluoroquinolone-susceptible isolates was tolC (highest), then acrA and acrB, and then mdfA and norE. Our findings suggest acrAB overexpression is an indicator of multidrug resistance.
Multidrug resistance (MDR) is an increasing public health concern worldwide (7, 11). There is a growing epidemic of multidrug-resistant Gram-negative pathogens and a dwindling arsenal of antibiotic options. MDR is most commonly defined as resistance to ≥3 classes of antibiotics (4). Increased efflux pump expression has been documented in association with resistance to several antibiotic classes, including the fluoroquinolones (reviewed in reference 11). Of more than 40 putative transporters in Escherichia coli, acrAB-tolC, mdfA, and norE affect fluoroquinolone MICs when expressed with their own promoters under laboratory growth conditions (14, 17). Only AcrAB-TolC overproduction, however, has been shown to contribute to clinical fluoroquinolone resistance. Additionally, plasmid-borne efflux pump gene qepA was found in a small percentage of E. coli isolates (10, 16), and it confers resistance to fluoroquinolones and aminoglycosides (9). Despite these findings, the link between efflux pump expression and multidrug resistance in the clinical setting is unclear.
We quantified expression of the efflux pump genes known to affect fluoroquinolone resistance in a single quantitative study. From our earlier study of 214 fluoroquinolone-resistant isolates and 27 fluoroquinolone-susceptible isolates from Ben Taub General Hospital in Houston, TX (2), 24 susceptible isolates and 36 resistant isolates that represented a full range of fluoroquinolone MICs were analyzed. RNA was stabilized in RNAprotect bacterial reagent. RNA was isolated using RNeasy minicolumns (Qiagen, Valencia, CA). RNase-free DNase I was incubated on-column for digestion of genomic DNA. RNA concentrations were assessed by using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE). Reverse transcription was completed using the ABI high-capacity reverse transcription kit (Applied Biosystems, Foster City, CA). Quantitative PCRs (qPCRs) were performed in triplicate on a 7500 Fast PCR system from Applied Biosystems using 2× Power SYBR green chemistry. PCR-grade water served as a negative control. Genomic DNA from the E. coli ATCC 25922 strain was the positive control, and its cDNA was the calibrator. The primer concentrations (Table 1) equaled 300 nM, and melt curve analysis ensured that only a single PCR product was amplified.
TABLE 1.
Primers used in this study
| Primer | Sequence (5′-3′) | Amplicon length (bp)a | % primer efficiency (E)a,b |
|---|---|---|---|
| acrA-F | CTCTCAGGCAGCTTAGCCCTAA | 107 | 95 |
| acrA-R | TGCAGAGGTTCAGTTTTGACTGTT | ||
| acrB-F | GGTCGATTCCGTTCTCCGTTA | 107 | 95 |
| acrB-R | CTACCTGGAAGTAAACGTCATTGGT | ||
| rpsL-F | GCAAAAACGTGGCGTATGTACTC | 104 | 97 |
| rpsL-R | TTCGAAACCGTTAGTCAGACGAA | ||
| mdfA-F | CATTGGCAGCGATCTCCTTT | 103 | 97 |
| mdfA-R | TTATAGTCACGACCGACTTCTTTCA | ||
| norE-F | CTGGCGGCAGCGGTAA | 108 | 94 |
| norE-R | TGCCATACAGACACCCACCATA | ||
| tolC-F | AAGCCGAAAAACGCAACCT | 100 | 95 |
| tolC-R | CAGAGTCGGTAAGTGACCATC |
Amplicon lengths and primer efficiencies in the rows for forward (F) primers correspond to the respective primer pairs.
As measured by the efficiency equation, E = 10(−1/m) − 1, where m is ΔCT/Δ[cDNA].
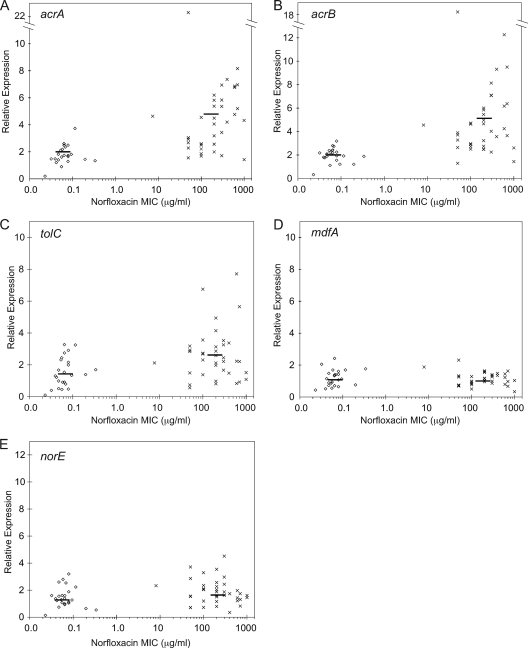
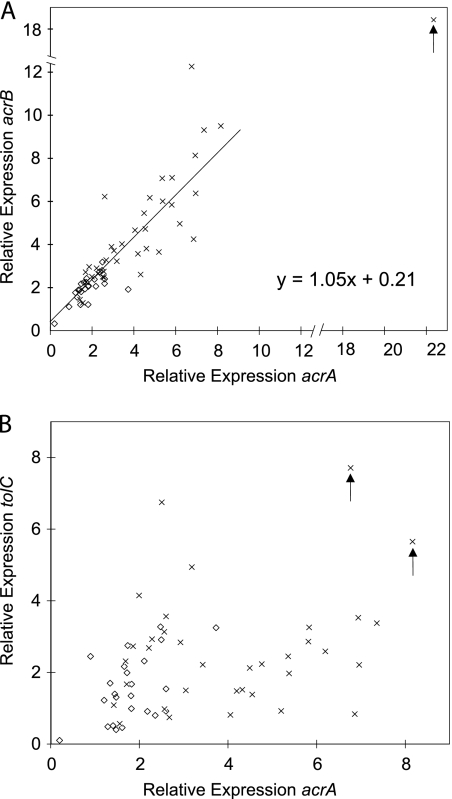
In our previous study, ∼30% of fluoroquinolone-resistant isolates overproduced AcrA, but fluoroquinolone-susceptible isolates had normal AcrA levels (6). Relative to the housekeeping gene rpsL, the average levels of expression of acrA and acrB in the fluoroquinolone-susceptible strains were 1.8- ± 0.7-fold and 2.0- ± 0.6-fold, respectively, compared to those of the ATCC 25922 standard E. coli strain. In fluoroquinolone-resistant isolates, the expression level of acrA averaged 4.5- ± 2.0-fold (Fig. 1A) and the expression level of acrB averaged 4.6- ± 2.5-fold (Fig. 1B). For both genes, the difference between the two groups was significant by Wilcoxon rank sum (P < 0.001). Overall, of 37 fluoroquinolone-resistant isolates, 22 overexpressed acrA and 25 overexpressed acrB more than two standard deviations above the respective means for the fluoroquinolone-susceptible isolates. acrA and acrB coexpression was plotted (r2 = 0.75; Fig. 2A). The best-fit line had a slope of 1.05, fitting the 1:1 ratio expected.
FIG. 1.
Multidrug efflux pump expression in E. coli clinical isolates. Transcript levels of acrA (A), acrB (B), tolC (C), mdfA (D) and norE (E) were determined by qPCR and are shown normalized to their expression in the standard E. coli strain ATCC 25922, which had a norfloxacin MIC of 0.032 μg/ml. The housekeeping gene rpsL was used to calculate relative expression. Data are displayed relative to the MIC (μg/ml) of the historically relevant fluoroquinolone norfloxacin as measured in our laboratory. Isolates were classified as either susceptible (⋄) or resistant (×) to fluoroquinolones as determined by the hospital. Each point is the average of three experiments. Lines represent the average relative expression values for all of the isolates in the norfloxacin-susceptible (⋄) and -resistant (×) groups. Overexpression was defined as greater than two standard deviations above the mean for the 24 fluoroquinolone-susceptible isolates.
FIG. 2.
Correlation of efflux pump expression levels. For each isolate, the transcript levels of acrB and acrA (A) and tolC and acrA (B) were plotted. Clinical isolates were either susceptible (⋄) or resistant (×) to fluoroquinolones as determined by the hospital. In panel A, isolate ELZ4033 (arrow) was determined to be an outlier by the extreme studentized deviate test statistic and was removed from the best-fit regression. The arrows in panel B denote isolates ELZ4000 and ELZ4001, which significantly overexpressed acrA and tolC relative to the fluoroquinolone-susceptible isolates (P < 0.05).
Two fluoroquinolone-resistant Shigella clinical isolates overexpressed tolC in response to ciprofloxacin (5). Otherwise, tolC expression has not been previously assessed. In three fluoroquinolone-resistant isolates, tolC expression was increased ∼7-fold. Two of the isolates that overexpressed tolC also overexpressed acrA and acrB (Fig. 2B, arrows). Overall, the average tolC expression levels (Fig. 1C) of the fluoroquinolone-susceptible and fluoroquinolone-resistant clinical isolates did not differ statistically. tolC did not correlate with either acrA (Fig. 2B) or acrB (data not shown). It does not appear that tolC is overexpressed with acrAB for acquisition of fluoroquinolone resistance despite the ability of all three genes to respond to MarA regulation (1), which indicates complex regulation differences between these two promoters.
We previously found that known genotypic alterations could not explain the fluoroquinolone MICs in ∼30% of the fluoroquinolone-resistant clinical isolates, suggesting that additional unknown mechanisms exist (2, 6). In laboratory strains, the overexpression of mdfA or norE causes 2- to 4-fold increased ciprofloxacin and norfloxacin MICs but has no effect on levofloxacin MICs (17). Overexpression of acrAB and either mdfA or norE synergistically increases fluoroquinolone MICs (17). In Shigella, transcript levels of ydhE (norE) and mdfA were increased in two fluoroquinolone-resistant isolates exposed to ciprofloxacin (5). Thus, increased expression of mdfA and norE could contribute to fluoroquinolone resistance, especially if combined with overproduction of AcrAB-TolC. Expression levels in fluoroquinolone-susceptible and fluoroquinolone-resistant clinical isolates, respectively, were 1.2- ± 0.5-fold and 1.1- ± 0.4-fold for mdfA and 1.5- ± 0.8-fold and 1.8- ± 0.9-fold for norE (Fig. 1D and E). Thus, E. coli isolates do not stably overexpress mdfA or norE in fluoroquinolone-resistant clinical isolates.
Although the genes encoding each of the three pumps, AcrAB-TolC, MdfA, and NorE, when overexpressed, increase fluoroquinolone MICs similarly, only the deletion of acrAB decreases MICs (8, 17). There are several possible explanations for these findings, but one simple explanation is that acrAB is normally expressed at higher levels than mdfA and norE and thus masks their contributions. Earlier exponential amplification of a gene, as shown by a lower threshold cycle (CT) value, may be inferred as greater transcript abundance in the template (3); every 3.32 CT values indicates a 10-fold difference in abundance. In the ATCC 25922 strain, the CT value was 18.5 for tolC, 20.3 for acrA and acrB (each), and 22.6 and 22.7 for mdfA and norE, respectively. This order was observed for all fluoroquinolone-susceptible isolates (see Table S1 in the supplemental material).
In spite of the very low prevalence of the qepA gene (0.3% in Japan [15] and, to our knowledge, none yet in the United States), we screened 78 of our fluoroquinolone-resistant isolates for qepA by colony PCR using the primers 5′-CGAACCGATGACGAAGCACAG and 5′-CTCGCTTCCTGCCCGAGTAT. We found no isolate that harbored this gene.
AcrAB-TolC overproduction affects the MICs of several antimicrobial agents. To determine whether increased acrAB expression correlated with MDR, we analyzed drug resistance data generated at the hospital (described in references 2 and 6) for each E. coli isolate. We classified the antibiotics that were tested at the hospital into the following classes: aminoglycosides, carbapenems, cephalosporins, fluoroquinolones, monobactams, nitrofurans, penicillins, combination penicillins, and sulfamethoxazole-trimethoprim. We then grouped the isolates into MDR classifications as follows: “non-MDR” if the isolate was resistant to fewer than three drug classes, “MDR(≥3)” if the isolate was resistant to three or more drug classes, and “MDR(≥5)” if the isolate was resistant to five or more drug classes. The non-MDR and MDR(≥3) classifications were chosen to model the most common definition of MDR (4). MDR(≥5) isolates are highly multidrug resistant, akin to extremely drug-resistant (XDR) Mycobacterium tuberculosis.
In general, the more severe the MDR phenotype, the higher the probability that the isolate also overexpressed acrAB (Table 2). Interestingly, no isolate categorized as MDR(≥5) was fluoroquinolone susceptible. While direct drug efflux has been demonstrated for some fluoroquinolones and a few additional agents (12), most of the drugs to which these clinical isolates were resistant have been shown to be unaffected by acrAB overexpression or deletion in laboratory experiments (12). This introduces the possibility of an underlying correlation between fluoroquinolone resistance and MDR. Because fluoroquinolones are heavily prescribed (13), there is strong selective pressure for bacteria to become resistant to them. If exposure to other antibiotics occurs prior to exposure to fluoroquinolones, an isolate that subsequently overexpresses acrAB following fluoroquinolone treatment might simultaneously become both fluoroquinolone resistant and MDR. Thus, acrAB may not cause MDR but rather is indicative of an MDR phenotype in isolates that overexpress it. Regardless of the specific mechanisms through which bacteria become MDR, these data indicate that acrAB overexpression is a biomarker for MDR.
TABLE 2.
acrAB overexpression in MDR isolates
| Resistance | No. (%) of isolates with acrAB expression |
|
|---|---|---|
| Normal | Increased | |
| Non-MDR | 19 | 3 (13.6) |
| MDR(≥3) | 17 | 17a (50.0) |
| MDR(≥5) | 5 | 8b (61.5) |
Significantly increased relative to the value for the non-MDR classification (P < 0.05).
Significantly increased relative to the value for the non-MDR classification (P < 0.01).
Supplementary Material
Acknowledgments
We thank Lauren Becnel Boyd, Rainer B. Lanz, and Richard Sucgang of Baylor College of Medicine (Houston, TX) for helpful discussions and critically reading the manuscript, Joseph F. Petrosino of Baylor College of Medicine (Houston, TX) for equipment, and Samuel Eguae of Paul Quinn College (Dallas, TX) for technical assistance during his visiting professorship funded by the American Society of Cell Biology.
M.C.S. is a fellow of the Pharmacoinformatics Training Program of the W. M. Keck Center for Computational and Structural Biology of the Gulf Coast Consortia (NIH grant T90 DK070109). S.K.M.-L. was supported by the Houston Area Molecular Biophysics training programs of the W. M. Keck Center of the Gulf Coast Consortia (NIH grant T32 GM008280). This work was supported by NIH grant R01 AI054830 (to L.Z.).
Footnotes
Published ahead of print on 22 November 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Barbosa, T. M., and S. B. Levy. 2000. Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J. Bacteriol. 182:3467-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becnel Boyd, L., et al. 2009. Relationships among ciprofloxacin, gatifloxacin, levofloxacin, and norfloxacin MICs for fluoroquinolone-resistant Escherichia coli clinical isolates. Antimicrob. Agents Chemother. 53:229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'haene, B., J. Vandesompele, and J. Hellemans. 2010. Accurate and objective copy number profiling using real-time quantitative PCR. Methods 50:262-270. [DOI] [PubMed] [Google Scholar]
- 4.Falagas, M. E., P. K. Koletsi, and I. A. Bliziotis. 2006. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J. Med. Microbiol. 55:1619-1629. [DOI] [PubMed] [Google Scholar]
- 5.Kim, J. Y., et al. 2008. Resistance to fluoroquinolones by the combination of target site mutations and enhanced expression of genes for efflux pumps in Shigella flexneri and Shigella sonnei strains isolated in Korea. Clin. Microbiol. Infect. 14:760-765. [DOI] [PubMed] [Google Scholar]
- 6.Morgan-Linnell, S. K., L. Becnel Boyd, D. Steffen, and L. Zechiedrich. 2009. Mechanisms accounting for fluoroquinolone resistance in Escherichia coli clinical isolates. Antimicrob. Agents Chemother. 53:235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikaido, H. 2009. Multidrug resistance in bacteria. Annu. Rev. Biochem. 78:119-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Périchon, B., et al. 2008. Sequence of conjugative plasmid pIP1206 mediating resistance to aminoglycosides by 16S rRNA methylation and to hydrophilic fluoroquinolones by efflux. Antimicrob. Agents Chemother. 52:2581-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Périchon, B., P. Courvalin, and M. Galimand. 2007. Transferable resistance to aminoglycosides by methylation of G1405 in 16S rRNA and to hydrophilic fluoroquinolones by QepA-mediated efflux in Escherichia coli. Antimicrob. Agents Chemother. 51:2464-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poole, K. 2005. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 56:20-51. [DOI] [PubMed] [Google Scholar]
- 12.Poole, K. 2004. Efflux-mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 10:12-26. [DOI] [PubMed] [Google Scholar]
- 13.SDI/Verispan. 14 May 2009, posting date. 2008 top 100 branded drugs by retail dollars. http://formularyjournal.modernmedicine.com/formulary/ArticleStandard/Article/detail/598276.
- 14.Sulavik, M. C., et al. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamane, K., J. Wachino, S. Suzuki, and Y. Arakawa. 2008. Plasmid-mediated qepA gene among Escherichia coli clinical isolates from Japan. Antimicrob. Agents Chemother. 52:1564-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamane, K., et al. 2007. New plasmid-mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 51:3354-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang, S., S. R. Clayton, and E. L. Zechiedrich. 2003. Relative contributions of the AcrAB, MdfA and NorE efflux pumps to quinolone resistance in Escherichia coli. J. Antimicrob. Chemother. 51:545-556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.