Abstract
Alveolar echinococcosis (AE) is caused by the metacestode stage of the fox tapeworm Echinococcus multilocularis and causes severe disease in the human liver, and occasionally in other organs, that is fatal when treatment is unsuccessful. The present chemotherapy against AE is based on mebendazole and albendazole. Albendazole treatment has been found to be ineffective in some instances, is parasitostatic rather than parasiticidal, and usually involves the lifelong uptake of large doses of drugs. Thus, new treatment options are urgently needed. In this study we investigated the in vitro and in vivo efficacy of mefloquine against E. multilocularis metacestodes. Treatment using mefloquine (20 μM) against in vitro cultures of metacestodes resulted in rapid and complete detachment of large parts of the germinal layer from the inner surface of the laminated layer within a few hours. The in vitro activity of mefloquine was dependent on the dosage. In vitro culture of metacestodes in the presence of 24 μM mefloquine for a period of 10 days was parasiticidal, as determined by murine bioassays, while treatment with 12 μM was not. Oral application of mefloquine (25 mg/kg of body weight administered twice a week for a period of 8 weeks) in E. multilocularis-infected mice was ineffective in achieving any reduction of parasite weight, whereas treatment with albendazole (200 mg/kg/day) was highly effective. However, when the same mefloquine dosage was applied intraperitoneally, the reduction in parasite weight was similar to the reduction seen with oral albendazole application. Combined application of both drugs did not increase the treatment efficacy. In conclusion, mefloquine represents an interesting drug candidate for the treatment of AE, and these results should be followed up in appropriate in vivo studies.
In humans, infection with the larval stage of Echinococcus multilocularis causes alveolar echinococcosis (AE), a mainly hepatic disease, which is fatal if not treated appropriately. E. multilocularis metacestodes proliferate asexually by forming small daughter vesicles, leading to a parasite mass that exhibits tumor-like properties and progressively infiltrates the neighboring tissue (8). The treatment options for AE are surgery and/or chemotherapy. Surgery is, of course, an invasive procedure, and feasibility depends on the location of the metacestode tissue. In addition, care must be taken to remove the entire parasite mass (otherwise, recurrences are certain), and there is the risk of metastasis formation caused by accidental dissemination of small daughter vesicles or even parasitic cells. Surgery is always accompanied by pre- and postoperative chemotherapy. In cases where surgery is not possible, chemotherapy remains the only option. For chemotherapeutical treatment of AE, the only two drugs licensed to date are the benzimidazole carbamate derivatives albendazole and mebendazole (42). However, therapy using these drugs does not result in parasiticidal activity in vivo or in vitro, and treatment failures and/or the occurrence of adverse effects have been reported, leading to discontinuation of treatment or to progressive disease. Therefore, the search for novel drug candidates for the treatment of AE has to be pursued (7).
In order to identify potentially better chemotherapeutical treatment options, a number of compounds has been investigated in the past, using cultured parasites in vitro and/or applying in vivo rodent models. Tested compounds include other benzimidazole derivatives such as flubendazole, itraconazole, and methiazole, as well as other anti-infective agents such as arthemether, caspofungin, ivermectin, miltefosine, rifampin, trimethoprim-sulfamethoxazole, amphotericin B (29), isoprinosine and derivatives (20), nitazoxanide, nitazoxanide and albendazole in combination (36, 37), alpha-difluoromethyl-ornithine (24), genistein and derivatives (26), p38 mitogen-activated protein kinase inhibitors (5), thioureides (25), and anticancer agents such as 2-methoxyestradiol (32) and doxorubicin and cyclosporine (21, 22). Although some of these compounds showed promising activities in vitro and, to some extent, also in the rodent models (for recent reviews, see references 7 and 8), these findings have not translated into clinical applications. Two exceptions are (i) amphotericin B desoxycholate, which has been applied as a salvage treatment but cannot be used for extended time periods (29), and (ii) nitazoxanide, which was not as effective as had been indicated by the results of in vivo studies in mice (28, 37) and did not lead to improved disease outcome in human patients, whether applied as monotherapy or in combination with albendazole (39). Other members of the thiazolides have been recently investigated in vitro by employing a novel screening assay that measures the activity of phosphoglucose isomerase (PGI) released from metacestodes as a marker for impaired viability due to drug treatment (35).
A plethora of potential novel antiparasitic compounds have been generated during the last years through consortia sponsored by public-private partnerships, which are mostly designated to develop novel drugs to combat neglected tropical diseases such as malaria, trypanosomiasis, leishmaniasis, or schistosomiasis (27). Nevertheless, regardless of how effective the activity of a novel compound might be against E. multilocularis metacestodes in experimental models, it is unlikely that this drug candidate will ever reach clinical application. AE is a disease that affects only a small number of people, and the pharmaceutical industry is reluctant to invest financial resources in a disease for which no significant market exists. As a consequence, it is more sensible to search for effective compounds or compound classes that are at an advanced stage of development or, even more realistically, are present in already marketed drugs. In the case of echinococcosis, these potentially interesting compounds are either broad-spectrum anti-infective drugs (8) or compounds that have been developed for cancer treatment (18, 22, 32).
Mefloquine, developed in 1971, is a synthetic analogue of quinine. Due to its long half-life, mefloquine is commonly used in malaria prophylaxis and treatment of chloroquine-resistant Plasmodium falciparium malaria (19). In vitro and in vivo studies demonstrated promising activities of mefloquine and mefloquine enantiomers in mice infected with young or adult stages of Schistosoma mansoni or S. japonicum (12, 23). Mefloquine was also reported to exhibit considerable efficacy against Opisthorchis viverrini in vitro and in infected hamsters (13) and against larval and adult stages of Brugia patei and B. malayi in vitro (43).
The present report presents data showing the in vitro efficacy of mefloquine against E. multilocularis metacestodes and demonstrates the effects of the drug, either alone or in combination with albendazole, when applied in an murine model employing experimental infection to investigate efficacy against secondary AE.
MATERIALS AND METHODS
Media and biochemicals.
If not stated otherwise, all culture media and reagents were purchased from Gibco-BRL (Zürich, Switzerland) and biochemical reagents were from Sigma (St. Louis, Mo). Mefloquine was kindly supplied by Mepha Pharma AG (Aesch BL, Switzerland).
In vitro culture of E. multilocularis metacestodes.
Culture of E. multilocularis (isolate H95; kindly provided by Klaus Brehm, Institute for Hygiene and Microbiology, University of Würzburg) was carried out as previously described (34). In short, metacestodes dissected from experimentally infected BALB/c mice were pressed through a metal tea strainer. The metacestodes were incubated in an antibiotic solution (20 μg/ml levoflaxin [Aventis, Meyrin, Switzerland]-20 μg/ml ciprofloxacin [Bayer, Zürich, Switzerland]-phosphate-buffered saline [PBS]) overnight. A 1-ml volume of the sedimented material, washed in PBS, was added to a culture flask containing 5 × 106 rat hepatocytes/ml (kindly provided by Klaus Brehm, Institute for Hygiene and Microbiology, University of Würzburg) in medium (Dulbecco's modified Eagle medium [DMEM], 10% fetal calf serum [FCS], 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate). These cocultures were incubated at 37°C in a 5% CO2 atmosphere, with changes of the medium once a week. Splitting of cultures was carried out when the total metacestode volume exceeded 15 ml. Metacestodes were used for experimental procedures when they reached diameters of 2 to 4 mm.
In vitro drug treatment of Echinococcus multilocularis metacestodes.
E. multilocularis metacestodes were collected after 1 to 2 months of culture and were washed three times in PBS. Treatments were carried out as described by Stadelmann et al. (35). In short, medium without phenol red (RPMI medium, 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate) was added to the same volume of vesicles and distributed to 24-well plates (Greiner Bio-One; Frickenhausen, Germany) (2 ml per well, ∼25 to 35 vesicles). Mefloquine was kept as a 40 mM stock in dimethyl sulfoxide (DMSO). Dilutions were prepared in medium and added to the metacestodes at concentrations from 1 to 100 μM. As a negative control, the corresponding dilution of DMSO was used. As positive controls, nontreated metacestodes were kept in (i) 20 μM nitazoxanide (35) or (ii) 1% Triton X-100 in PBS. Treatments were carried out at 37°C in a 5% CO2 atmosphere for 5 days. Aliquots of medium supernatant (200 μl) were collected and stored at −20°C until further measurements of phosphoglucose isomerase (PGI) activity. In parallel, specimens were viewed by light microscopy to morphologically assess potential drug-induced damage.
Determination of PGI activity in medium supernatants.
Drug-induced damage of metacestodes was measured indirectly by detecting the release of PGI, as previously described by Stadelmann et al. (35). The evaluation is presented as a percentage relative to that obtained by treatment of vesicles with 1% Triton X-100. Linear regression analysis was performed using Microsoft Excel (2007).
Morphological and ultrastructural investigations of mefloquine-treated metacestodes.
To visualize the structural alterations in metacestodes imposed by mefloquine treatment, parasites were processed for scanning and transmission electron microscopy (SEM and TEM) at different time points after the initiation of treatment with 24 μM mefloquine. Metacestode material collected from the in vivo treatment study was also processed for TEM. Briefly, metacestodes were fixed in 2.5% glutaraldehyde in 100 mM sodium cacodylate buffer (pH 7.2) for 2 h at room temperature, followed by postfixation in 2% OsO4 in 100 mM sodium cacodylate buffer (pH 7.2) for 2 h at room temperature. For SEM analysis, the fixed specimens were extensively washed in distilled water and dehydrated by sequential incubations in increasing concentrations of ethanol. They were finally immersed in hexamethyldisilazane, air dried under a fume hood, sputter-coated with gold, and inspected on a JEOL 840 scanning electron microscope operating at 25 kV. For TEM analysis, the fixed specimens were washed in distilled water and were treated with 1% uranyl acetate for 30 min. Parasites obtained from in vitro experiments were subsequently embedded in epoxy resin (Epon 812), and those obtained from in vivo studies were embedded in LR White acrylic resin. Polymerization of the resin was carried out at 65°C overnight. Sections were cut on a Reichert and Jung ultramicrotome (1 to 2 μm in thickness for light microscopy or 80 to 90 nm for TEM). Sections for light microscopy were loaded onto poly-l-lysine-coated glass coverslips and were stained with methylene-blue/basic fuchsin (36). TEM sections were loaded onto 300-mesh copper grids (Plano GmbH, Marburg, Germany) and stained with uranyl acetate and lead citrate as previously described (6).
Mouse bioassays.
In order to investigate the viability of drug-treated metacestodes, female BALB/c mice (five animals per group [age, 8 weeks; body weight, 20 to 25 g]) were housed in a temperature-controlled light-cycle room with food and water available ad libitum. Experiments were carried out according to the Swiss Animal Welfare regulations. Mice were infected by intraperitoneal injection of 50 to 100 drug-treated vesicles. Prior to inoculation, the vesicles had been treated in vitro with either 12 μM or 24 μM mefloquine for a period of 10 days (see above). A control group was infected with untreated vesicles. At 5 months postinoculation, the mice were euthanized and necroscopy was performed in order to determine whether growth of parasite tissue had occurred.
Studies of the efficacy of mefloquine treatment in experimentally infected BALB/c mice.
Forty-eight female BALB/c mice (8 animals per group [age, 9 weeks; body weight, 20 to 25 g]) were housed under the conditions described above. They were injected intraperitoneally with 200 μl of homogenized metacestode material (H95 strain). At 6 weeks postinfection, treatments were carried out as follows: albendazole was applied on a daily basis at a dose of 200 mg/kg of body weight, and treatments with mefloquine were performed twice a week at a dose of 25 mg/kg. Oral applications (of albendazole and mefloquine) were performed by suspending the drugs in a 1:1 mixture of honey (M-Budget honey; Migros, Zürich, Switzerland) and 0.5% carboxymethyl cellulose sodium salt (CMC) and feeding the appropriate amount to the animals. Intraperitoneal treatment (mefloquine only) was administered twice a week, with the drug suspended in 0.5% CMC.
The animals were divided in 6 groups of 8 animals each, with the animals receiving the following treatment schemes for a period of 8 weeks: group 1 was not administered any drug (infection control group); group 2 received mefloquine orally (25 mg/kg twice a week); group 3 was treated with mefloquine intraperitoneally (25 mg/kg twice a week), group 4 was treated with albendazole orally (200 mg/kg/day); group 5 received albendazole orally (200 mg/kg/day) combined with mefloquine orally (25 mg/kg twice a week); and group 6 received a combination of albendazole orally (200 mg/kg/day) and mefloquine intraperitoneally (25 mg/kg twice a week). At the end of the study, the animals were euthanized, necroscopy was performed, and the total parasite material was collected in order to determine parasite weight. Several samples of the recovered material were processed for transmission electron microscopy (TEM), and the residual tissue was processed for in vitro cultivation.
The experimental data were analyzed with a box plot, and outliers were identified by the extreme studentized deviate (ESD) method. Only one outlier was identified and discarded. The remaining data were submitted to a two-tailed distributed Student t test, with two-sample equal variance determinations performed using the untreated group and each of the treatment groups. For the analysis of variance (ANOVA) between the groups, a one-way ANOVA computation was performed with an online calculator (Statistics Calculator version 2.0).
RESULTS
In vitro activity of mefloquine against E. multilocularis metacestodes.
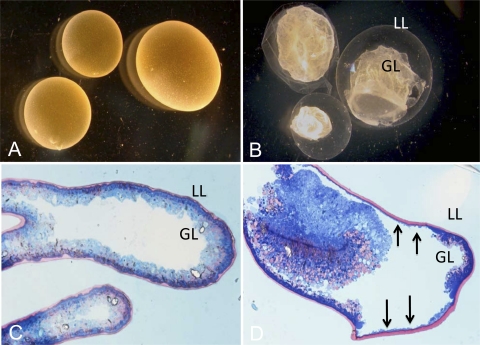
As can be seen in Fig. 1, incubation of E. multilocularis metacestode vesicles at 37°C in the presence of 20 μM mefloquine resulted in dramatic alterations within 4 to 6 h after initiation of treatment (Fig. 1A and B). At lower concentrations, similar morphological changes occurred, although at a lower rate (data not shown). Already visible macroscopically, the parasite tissue detached from the interior lining of the laminated layer and formed a densely packed aggregate inside the vesicles. The laminated layer became translucent, mostly because large parts of the parasite tissue had been redistributed to another site. On methylene-blue/basic fuchsin-stained sections of Epon 812-embedded parasites, the inner germinal layer (GL [blue]) and the outer laminated layer (LL [pink]) could be clearly distinguished (Fig. 1C and D). Mefloquine-treated parasites formed large clumps of parasite tissue within the vesicular lumen, and the germinal layer was strongly reduced in many regions (vertical arrows in Fig. 1D).
FIG. 1.
Morphological effects of mefloquine treatment on metacestodes. In vitro-cultivated E. multilocularis metacestodes were kept either in normal cultivation medium (A and C) or in medium containing 24 μM mefloquine (B and D) for 4 to 6 h. Macroscopical pictures (A and B) show the dramatic morphological changes of metacestodes upon treatment: The germinal layer (GL) detaches rapidly from the laminated layer (LL). Upon methylene-blue/basic fuchsin staining of Epon-embedded parasites (C and D), the separation of the GL and LL was further confirmed. The residual GL, still attached to the LL but strongly reduced in thickness, is indicated by arrows.
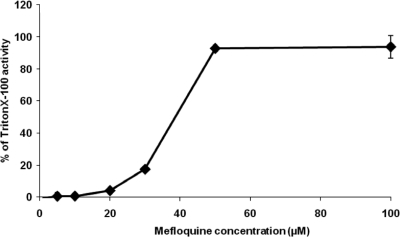
These dramatic alterations induced by mefloquine were accompanied by release of PGI activity into the medium supernatant in a dose-dependent manner (Fig. 2).
FIG. 2.
Concentration-dependent in vitro release of E. multilocularis PGI activities after metacestode damage induced by mefloquine. Metacestodes were incubated for 5 days with different concentrations (1 to 100 μM) of mefloquine. Levels of concentration-dependent parasite damage were assessed by the PGI assay and are depicted as percentages of total metacestode damage (obtained by addition of Triton X-100 to achieve a 1% concentration).
SEM and TEM of in vitro mefloquine treatment of E. multilocularis metacestodes.
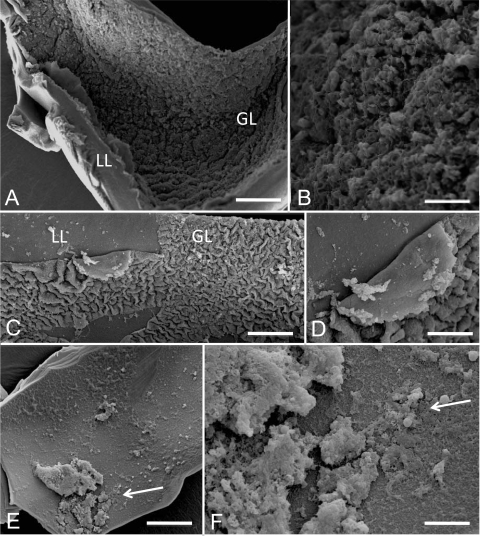
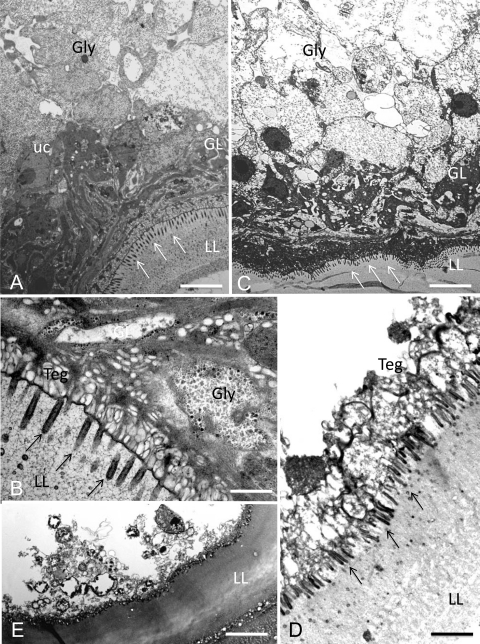
Electron microscopy was employed to visualize the effects of mefloquine treatment and to analyze the changes in a time-dependent manner (Fig. 3 and 4). SEM (Fig. 3) demonstrated that when untreated parasites were used, the inner surface of the vesicle wall was densely covered by parasite tissue (Fig. 3A and B). Metacestode vesicle specimens, which had been fixed and processed after 2 h of mefloquin incubation (Fig. 3C and D), already showed some degree of detachment in various parts of the germinal layer tissue, and after 6 h (Fig. 3E and F), only residual cellular material could be observed. The damage induced by mefloquine in vitro treatment was also assessed by TEM. Untreated E. multilocularis metacestode vesicles exhibited typical features, with an acellular laminated layer surrounding the entire parasite (Fig. 4A and B). The proximal surface of the laminated layer contains the parasite tissue, with microtriches that protrude from the tegument into the laminated layer. The germinal layer is constituted by relatively densely packed tissue containing muscle cells, connective tissue, undifferentiated cells, and mostly fully loaded glycogen storage cells. TEM micrographs taken after 2 h of 24 μM mefloquine exposure already revealed the depletion of glycogen storage cells, and the germinal layer tissue exhibited a less dense appearance, but microtriches were still present (Fig. 4C). After 12 h, most tegumental residues remained attached to the laminated layer and often associated with electron-dense microtriches, but structurally intact parasite tissue could no longer be seen (Fig. 4D). Mefloquine treatment for 48 h resulted in complete breakdown of the structural integrity of the germinal layer (Fig. 4E). In addition, the ultrastructure of the laminated layer changed from a meshwork-like appearance (seen in Fig. 4B and D) to an amorphous appearance (Fig. 4E).
FIG. 3.
Ultrastructural effects of mefloquine on E. multilocularis metacestodes in vitro as visualized by SEM. Scanning electron microscopy (SEM) pictures were prepared for untreated metacestodes from in vitro-cultured parasites (A and B) and metacestodes treated for 2 h (C and D) and 6 h (E and F) with 24 μM mefloquine. Overviews of the vesicle tissue consisting of a laminated layer (LL) and a germinal layer (GL) are given in panels A, C, and E, whereas in panels B, D, and F, the focus is directed to GL cells detaching from the LL with increasing incubation times. Bars: panel A, 1,100 μm; panel B, 350 μm; panel C, 600 μm; panel D, 220 μm; panel E, 950 μm; panel F, 220 μm.
FIG. 4.
Ultrastructural effects of mefloquine on E. multilocularis metacestodes in vitro visualized by TEM. Transmission electron microscopy (TEM) pictures were prepared from in vitro-cultured parasites for untreated metacestodes (A and B) and metacestodes treated for 2 h (C), 12 h (D), and 48 h (E) with 24 μM mefloquine. Control vesicles showed a meshwork-like laminated layer (LL) with microtriches (indicated by arrows) protruding from the tegument (Teg) into the LL. The adjacent germinal layer (GL) consisted of densely packed cells (glycogen storage cells [Gly], undifferentiated cells [uc], and others). With increasing treatment time, glycogen storage cells were depleted (C) and the parasite tissue was no longer structurally intact (D); finally, the structural integrity of the GL broke down, microtriches were lost, and the LL acquired an amorphous appearance (E). Bars: panel A, 3.8 μm; panel B, 1.3 μm; panel C, 4.2 μm; panel D, 2.2 μm; panel E, 3.6 μm.
Murine bioassay.
To investigate whether the effects of mefloquine were truly parasiticidal, drug-treated parasites were tested by a murine bioassay. At 5 months after inoculation of parasite tissue, mice that had received either untreated parasites or metacestodes previously treated with 12 μM mefloquine for 10 days harbored new metacestode tissue. However, mice inoculated with parasite tissue treated with 24 μM mefloquine for 10 days remained uninfected (data not shown).
Mefloquine treatment of mice experimentally infected with E. multilocularis metacestodes.
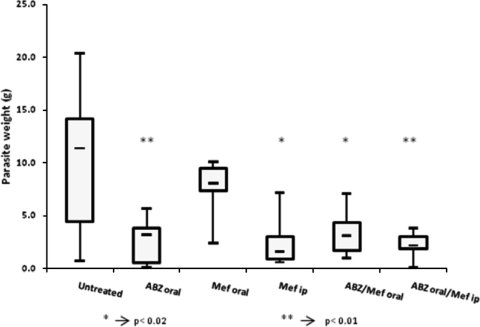
Intraperitoneally infected BALB/c mice were exposed to different treatment regimens involving albendazole and mefloquine as indicated in Materials and Methods and in Fig. 5. Treatments were performed for 8 weeks, at the end of which animals were euthanized and total parasite weight was measured. Figure 5 shows box plots of the recovered parasite weights in the different treatment groups and their respective average parasite weights. Daily oral application of albendazole yielded the expected result, namely, significantly decreased parasite weights (37). Oral application of mefloquine twice a week did not have any noticeable impact on the growth of the metacestodes in vivo (P = 0.414). However, a significant decrease in parasite weight was noted for the mice treated with mefloquine intraperitoneally (P = 0.013), with average weights in a range similar to those seen with the albendazole-treated group (P = 0.009). Combining albendazole with mefloquine did not further improve treatment efficacy compared to albendazole or mefloquine monotherapy (P = 0.016 and P = 0.009 for oral albendazole combined with oral and intraperitoneal mefloquine, respectively). The analysis of variance between the treatment groups (one-way, two-tailed ANOVA) resulted in F = 6.168 and P = 0.000.
FIG. 5.
In vivo mefloquine treatment. Treatment was initiated 6 weeks postinfection. Each treatment group comprised 8 animals. Oral treatments were administered with honey combined with CMC, intraperitoneal treatments (ip) with the drug resuspended in 0.5% CMC. Albendazole (ABZ) was given daily (200 mg/kg), mefloquine (Mef) twice a week (25 mg/kg). Asterisks indicate scores obtained using Student's t test in comparison with the untreated control group results.
For each treatment group, metacestode material was collected and processed for in vitro culture in order to assess the viability of the recovered parasite material. After 3 weeks in culture, metacestode formation was observed in the untreated group as well as in the groups treated orally with mefloquine and orally with a combination of mefloquine and albendazole. For both groups treated intraperitoneally with mefloquine (i.e., with or without albendazole) and for the group treated orally with albendazole alone, no viable parasites could be recovered (data not shown).
TEM of metacestodes recovered from mice following experimental treatments with mefloquine and albendazole.
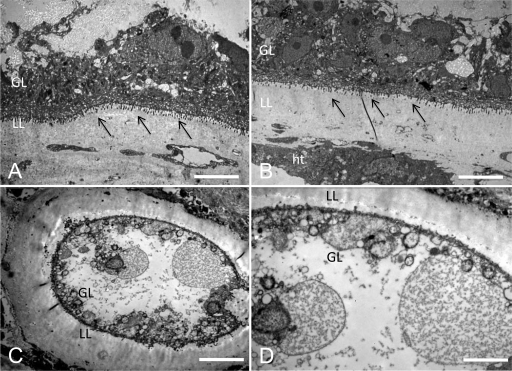
Extensive parasite tissue damage could also be observed in the material collected from the in vivo study (Fig. 6). Untreated metacestodes, and those subjected to oral mefloquine treatment, presented intact structural features such as a well-defined laminated layer, an intact and densely packed germinal layer, clearly delineated microtriches, and abundant glycogen storage cells, as can be seen in the Fig. 6B. Mice subjected to intraperitoneal mefloquine treatments presented metacestodes with a germinal layer that was largely composed of rounded cells or cellular debris, depleted glycogen storage cells, and largely reduced or even invisible microtriches (Fig. 6C and D). Parasites from mice exposed to albendazole treatment exhibited similar damage (results not shown).
FIG. 6.
Ultrastructural effects of mefloquine on E. multilocularis metacestodes in vivo. Images from transmission electron microscopy (TEM) of metacestodes recovered from mice by the end of the in vivo treatment study are shown. (A) Untreated metacestodes show a well-defined laminated layer (LL) against host tissue (ht) and densely packed adjacent germinal layers (GL). (B) Metacestodes from animals treated orally with mefloquine present the same well defined structured and, at a higher magnification, undifferentiated cells and glycogen storage cells are visible, as well as well-defined microtriches (indicated by arrows). (C and D) The metacestodes recovered from mice treated intraperitoneally with mefloquine show a less-defined laminated layer as well as a thinner germinal layer. The undifferentiated cells are rounder in shape, and the glycogen storage cells are no longer visible. Bars: panel A, 4.2 μm; panel B, 4.2 μm; panel C, 5.4 μm; panel D, 2.6 μm.
DISCUSSION
In this report we demonstrate that the antimalarial compound mefloquine exhibits promising activity against E. multilocularis metacestodes in vitro and in mice. Antimalarial drugs were shown to be active against a broad spectrum of other parasitic diseases such as trypanosomiasis, leishmaniasis, amoebiasis, and/or fungal infections (for reviews, see references 10, 31, and 44). In addition, trioxolanes and mefloquine also exhibited profound activities against several trematode species (11-15, 23). In particular, a mefloquine-artesunate combination has shown high efficacy against S. haematobium in a randomized, exploratory open-label trial using school children (16).
The effects of mefloquine treatment against E. multilocularis metacestodes in vitro were striking (see Fig. 1). Within 4 to 6 h, a large part of the germinal layer collapsed and aggregated. The germinal layer tissue detached from the tegument, leaving only a thin layer of tegumental tissue still attached to the laminated layer, fixed in place by microtriches protruding into the matrix of the laminated layer. Following detachment of the germinal layer, the laminated layer adopted the appearance of a translucent shell. TEM micrographs taken at 48 h after initiation of drug treatment (Fig. 4E) indicated that the matrix of the laminated layer had become amorphous and that most of the parasite tissue, including the tegument, had been completely destroyed by that time. In schistosomes, the tegument is considered to be a likely drug target that exhibits numerous alterations upon in vitro and in vivo mefloquin treatment (23). This also appears to be the case for E. multilocularis, and the rapid destruction of this layer at the parasite-host interface must play an important role in mefloquine toxicity. While mefloquine exerts its toxic effects within a short time, other previously characterized drugs such as albendazole (9), nitazoxanide (36), artesunate, and dihydroartemisinin (33) presented distinct effects only after 5 to 12 days of treatment. The rapid response to mefloquine suggests that this compound could be a useful additive to the current, very limited, antimetacestodal arsenal based on benzimidazoles.
As assessed by PGI assays and by murine bioassays performed with mefloquine-treated parasites, the parasiticidal activity of mefloquine in vitro was dependent on the dosage. This had to be taken into consideration when designing experiments to assess the efficacy of mefloquine in E. multilocularis-infected mice. In humans, the oral absorption of mefloquine is relatively rapid, reaching peak concentrations within 24 h. Metabolism takes place in the liver, with carboxy-mefloquine being a major metabolite. A total of 98% of the drug is bound to plasma, and elimination of mefloquine takes approximately 2 weeks. Serum concentrations of mefloquine reach levels of approximately 1 μg/ml (2.41 μM) and metabolite concentrations of 3 μg/ml (9.7 μM) (40, 41). Since we did not know whether it would have been possible to achieve such high serum levels in mice by oral application of the drug, we applied the compound through the intraperitoneal route as well. Moreover, the neurotoxicity of mefloquine was recognized as a major problem and was taken into account during the in vivo trial (41). Treatment with albendazole was carried out daily at a dose of 200 mg/kg, as previously described (37). Mefloquine was administered either orally or intraperitoneally only twice a week, due to its longer half-life compared to albendazole (30). The propylactic dosage for humans, as indicated by one manufacturer of mefloquine, is approximately 5 mg/kg of body weight applied once a week, while the treatment dosage consists of 20 to 25 mg/kg of body weight applied only once. In this study in mice, animals were treated by administration of 25 mg/kg twice a week. Daily doses above 12.5 mg/kg have been reported to cause severe side effects, and increased lethality was observed in mice at doses above 30 mg/kg (41). Thus, adverse events such as epilepsy or impairment in motor performance were not observed in any of the animals over the course of treatment. However, and probably as a consequence of the low dosage of mefloquine, oral treatment of infected mice did not result in any noticeable effects with regard to parasite weight and number. Metacestodes collected from animals submitted to oral mefloquine treatment exhibited intact parasite structures comparable to those of the untreated group. For comparison, high single oral doses of 200 to 400 mg/kg were necessary to achieve significant worm burden reductions in S. mansoni- and S. japonicum-infected mice (12). The lack of efficacy of mefloquine treatment against murine AE indicates a problem with the bioavailability of the drug when given orally, and dose-response studies should be undertaken in order to explore whether a more frequent oral application of mefloquin, or application of mefloquine at a higher dosage, could result in a more beneficial treatment outcome.
On the other hand, the in vivo study showed that the reduction of parasite weight caused by intraperitoneal mefloquine treatment was comparable to that caused by oral albendazole therapy, and TEM confirmed that the parasites were indeed substantially damaged (Fig. 6). Whether the treatment was parasiticidal is not entirely proven. However, in vitro culture of the corresponding metacestode tissues did not result in regrowth of metacestodes within a culture period of 3 weeks.
How mefloquine acts against E. multilocularis metacestodes is not known. In Plasmodium species, the exact mechanism of action has not yet been elucidated, but it has been suggested that, in similarity to what has been described for chloroquine, there occurs a disturbance of hemoglobin metabolism and formation of an insoluble polymer, hemozoin, which is toxic for the parasite's host cell. For mefloquine, however, alternative sites of action have previously been postulated (38). A hemozoin-related effect is unlikely to occur against the E. multilocularis metacestode in vitro, which implies that other targets must be involved. Combined application of mefloquine with albendazole did not augment activity against the parasite compared to albendazole monotherapy, which indicates that the two drugs are metabolized through different pathways. This is in contrast to previous studies employing nitazoxanide, artemisinin derivatives, and 2-methoxy-estradiol, which, upon combined application with albendazole, were all more effective than albendazole alone (32, 33, 37).
One of the possible advantages of mefloquine over novel drugs for the treatment of AE is that this drug is commercially available and has been extensively characterized in terms of bioavailability, pharmacokinetics, and toxicity (1-4, 41). Mefloquine treatment has a variety of documented adverse side effects, including neurotoxicity and elevation of liver enzyme levels. In therapy in conjunction with albendazole, the latter side effect could turn out to become a serious problem clinically. Nevertheless, inclusion of mefloquine in an albendazole-based treatment regimen at a prophylactic dosage in addition to continuous albendazole treatment might be an intriguing new concept.
Despite the promising results obtained with mefloquine intraperitoneal treatment, this administration route is not a viable option for human AE patients, and other means of drug delivery should be taken into account. In conclusion, mefloquine represents an interesting and promising drug candidate that should get further attention for treatment of AE. More in vivo and in vitro studies should be performed to optimize treatment with mefloquine. Investigations to elucidate this drug's mode of action in its effect on E. multilocularis metacestodes are under way.
Acknowledgments
We gratefully acknowledge the technological help of Markus Spiliotis during the culture of E. multilocularis metacestodes and of Joachim Müller for continuing advice and help during the study. The rat hepatocyte cell line and H95 E. multilocularis metacestode isolate were kindly provided by Klaus Brehm, University of Würzburg. Mepha Pharma AG contributed mefloquine for animal studies free of charge.
B.S. has been supported by a fellowship provided through the Karl Enigk Stiftung. We also gratefully acknowledge the financial support of the Swiss Life Jubiläumsstiftung, Fondation Sana, and the Opo-Foundation. This study was carried out within the framework of the Swiss National Science Foundation (grant 31-111780).
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.AlKadi, H. 2007. Antimalarial drug toxicity: a review. Chemotherapy 53:385-391. [DOI] [PubMed] [Google Scholar]
- 2.Barraud de Lagerie, S., et al. 2004. Cerebral uptake of mefloquine enantiomers with and without the P-gp inhibitor elacridar (GF1210918) in mice. Br. J. Pharmacol. 141:1214-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basco, L., C. Gillotin, F. Gimenez, R. Farinotti, and J. Le Bras. 1992. In vitro activity of the enantiomers of mefloquine, halofantrine and enpiroline against Plasmodium falciparum. Br. J. Clin. Pharmacol. 33:517-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baudry, S., et al. 1997. Stereoselective passage of mefloquine through the blood-brain barrier in the rat. J. Pharm. Pharmacol. 49:1086-1090. [DOI] [PubMed] [Google Scholar]
- 5.Gelmedin, V., R. Caballero-Gamiz, and K. Brehm. 2008. Characterization and inhibition of a p38-like mitogen-activated protein kinase (MAPK) from Echinococcus multilocularis: antiparasitic activities of p38 MAPK inhibitors. Biochem. Pharmacol. 76:1068-1081. [DOI] [PubMed] [Google Scholar]
- 6.Hemphill, A., and S. L. Croft. 1997. Electron microscopy in parasitology. Springer Verlag, Heidelberg, Germany.
- 7.Hemphill, A., and J. Müller. 2009. Alveolar and cystic echinococcosis: towards novel chemotherapeutical treatment options. J. Helminthol. 83:99-111. [DOI] [PubMed] [Google Scholar]
- 8.Hemphill, A., et al. 2010. Echinococcus metacestodes as laboratory models for the screening of drugs against cestodes and trematodes. Parasitology 137:569-587. [DOI] [PubMed] [Google Scholar]
- 9.Ingold, K., et al. 1999. Efficacies of albendazole sulfoxide and albendazole sulfone against in vitro-cultivated Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 43:1052-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jefford, C. W. 2001. Why artemisinin and certain synthetic peroxides are potent antimalarials. Implications for the mode of action. Curr. Med. Chem. 8:1803-1826. [DOI] [PubMed] [Google Scholar]
- 11.Keiser, J. 2010. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology 137:589-603. [DOI] [PubMed] [Google Scholar]
- 12.Keiser, J., et al. 2009. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis. 3:e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keiser, J., P. Odermatt, and S. Tesana. 2009. Dose-response relationships and tegumental surface alterations in Opisthorchis viverrini following treatment with mefloquine in vivo and in vitro. Parasitol. Res. 105:261-266. [DOI] [PubMed] [Google Scholar]
- 14.Keiser, J., and J. Utzinger. 2007. Artemisinins and synthetic trioxolanes in the treatment of helminth infections. Curr. Opin. Infect. Dis. 20:605-612. [DOI] [PubMed] [Google Scholar]
- 15.Keiser, J., and J. Utzinger. 2007. Food-borne trematodiasis: current chemotherapy and advances with artemisinins and synthetic trioxolanes. Trends Parasitol. 23:555-562. [DOI] [PubMed] [Google Scholar]
- 16.Keiser, J., et al. 2010. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin. Infect. Dis. 50:1205-1213. [DOI] [PubMed] [Google Scholar]
- 17.Reference deleted.
- 18.Klinkert, M., and V. Heussler. 2006. The use of anticancer drugs in antiparasitic chemotherapy. Mini Rev. Med. Chem. 6:131-143. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, N., R. Singh, and D. Rawat. 21 December 2009, posting date. Tetraoxanes: synthetic and medicinal chemistry perspective. Med. Res. Rev. doi: 10.1002/med.20189. [DOI] [PubMed]
- 20.Lawton, P., N. Walchshofer, and M. Sarciron. 2001. In vitro effects of isoprinosine and a dipeptide methyl ester on Echinococcus multilocularis protoscoleces. J. Helminthol. 75:251-257. [PubMed] [Google Scholar]
- 21.Liance, M., et al. 1992. Effects of cyclosporin A on the course of murine alveolar echinococcosis and on specific cellular and humoral immune responses against Echinococcus multilocularis. Int. J. Parasitol. 22:23-28. [DOI] [PubMed] [Google Scholar]
- 22.Liance, M., F. Nemati, C. Bories, and P. Couvreur. 1993. Experience with doxorubicin-bound polyisohexylcyanoacrylate nanoparticles on murine alveolar echinococcosis of the liver. Int. J. Parasitol. 23:427-429. [DOI] [PubMed] [Google Scholar]
- 23.Manneck, T., Y. Haggenmüller, and J. Keiser. 2010. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology 137:85-98. [DOI] [PubMed] [Google Scholar]
- 24.Miyaji, S., et al. 1993. Failure of treatment with alpha-difluoromethylornithine against secondary multilocular echinococcosis in mice. Parasitol. Res. 79:75-76. [DOI] [PubMed] [Google Scholar]
- 25.Müller, J., et al. 2009. Thioureides of 2-(phenoxymethyl)benzoic acid 4-R substituted: a novel class of anti-parasitic compounds. Parasitol. Int. 58:128-135. [DOI] [PubMed] [Google Scholar]
- 26.Naguleswaran, A., et al. 2006. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob. Agents Chemother. 50:3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nwaka, S., et al. 2009. Advancing drug innovation for neglected diseases—criteria for lead progression. PLoS Negl. Trop. Dis. 3:e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuter, S., B. Manfras, M. Merkle, G. Härter, and P. Kern. 2006. In vitro activities of itraconazole, methiazole, and nitazoxanide versus Echinococcus multilocularis larvae. Antimicrob. Agents Chemother. 50:2966-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuter, S., M. Merkle, K. Brehm, P. Kern, and B. Manfras. 2003. Effect of amphotericin B on larval growth of Echinococcus multilocularis. Antimicrob. Agents Chemother. 47:620-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozman, R. S., N. A. Molek, and R. Koby. 1978. The absorption, distribution, and excretion in mice of the antimalarial mefloquine, erythro-2,8-bis(trifluoromethyl)-alpha-(2-piperidyl)-4-quinolinemethanol hydrochloride. Drug Metab. Dispos. 6:654-658. [PubMed] [Google Scholar]
- 31.Soeiro, M. N., et al. 2009. Experimental chemotherapy for Chagas disease: 15 years of research contributions from in vivo and in vitro studies. Mem. Inst. Oswaldo Cruz 104(Suppl. 1):301-310. [DOI] [PubMed] [Google Scholar]
- 32.Spicher, M., et al. 2008. In vitro and in vivo effects of 2-methoxyestradiol, either alone or combined with albendazole, against Echinococcus metacestodes. Exp. Parasitol. 119:475-482. [DOI] [PubMed] [Google Scholar]
- 33.Spicher, M., et al. 2008. In vitro and in vivo treatments of echinococcus protoscoleces and metacestodes with artemisinin and artemisinin derivatives. Antimicrob. Agents Chemother. 52:3447-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiliotis, M., et al. 2008. Transient transfection of Echinococcus multilocularis primary cells and complete in vitro regeneration of metacestode vesicles. Int. J. Parasitol. 38:1025-1039. [DOI] [PubMed] [Google Scholar]
- 35.Stadelmann, B., S. Scholl, J. Müller, and A. Hemphill. 2010. Application of an in vitro drug screening assay based on the release of phosphoglucose isomerase to determine the structure-activity relationship of thiazolides against Echinococcus multilocularis metacestodes. J. Antimicrob. Chemother. 65:512-519. [DOI] [PubMed] [Google Scholar]
- 36.Stettler, M., et al. 2003. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 47:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stettler, M., et al. 2004. Secondary and primary murine alveolar echinococcosis: combined albendazole/nitazoxanide chemotherapy exhibits profound anti-parasitic activity. Int. J. Parasitol. 34:615-624. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan, D. J., I. Gluzman, D. Russell, and D. Goldberg. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. U. S. A. 93:11865-11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tappe, D., A. Müller, M. Frosch, and A. Stich. 2009. Limitations of amphotericin B and nitazoxanide in the treatment of alveolar echinococcosis. Ann. Trop. Med. Parasitol. 103:177-181. [DOI] [PubMed] [Google Scholar]
- 40.Todd, G., A. Hopperus Buma, M. Green, C. Jaspers, and H. Lobel. 1997. Comparison of whole blood and serum levels of mefloquine and its carboxylic acid metabolite. Am. J. Trop. Med. Hyg. 57:399-402. [DOI] [PubMed] [Google Scholar]
- 41.Toovey, S. 2009. Mefloquine neurotoxicity: a literature review. Travel Med. Infect. Dis. 7:2-6. [DOI] [PubMed] [Google Scholar]
- 42.Vuitton, D. 2009. Benzimidazoles for the treatment of cystic and alveolar echinococcosis: what is the consensus? Expert Rev. Anti Infect. Ther. 7:145-149. [DOI] [PubMed] [Google Scholar]
- 43.Walter, R., R. Wittich, and F. Kuhlow. 1987. Filaricidal effect of mefloquine on adults and microfilariae of Brugia patei and Brugia malayi. Trop. Med. Parasitol. 38:55-56. [PubMed] [Google Scholar]
- 44.Woodrow, C., R. Haynes, and S. Krishna. 2005. Artemisinins. Postgrad. Med. J. 81:71-78. [DOI] [PMC free article] [PubMed] [Google Scholar]