Abstract
The human pathogen Giardia lamblia is an anaerobic protozoan parasite that causes giardiasis, one of the most common diarrheal diseases worldwide. Although several drugs are available for the treatment of giardiasis, drug resistance has been reported and is likely to increase, and recurrent infections are common. The search for new drugs that can overcome the drug-resistant strains of Giardia is an unmet medical need. New drug screen methods can facilitate the drug discovery process and aid with the identification of new drug targets. Using a bioluminescent ATP content assay, we have developed a phenotypic drug screen method to identify compounds that act against the actively growing trophozoite stage of the parasite. This assay is homogeneous, robust, and suitable for high-throughput screening of large compound collections. A screen of 4,096 pharmacologically active small molecules and approved drugs revealed 43 compounds with selective anti-Giardia properties, including 32 previously reported and 11 novel anti-Giardia agents. The most potent novel compound was fumagillin, which showed 50% inhibitory concentrations of 10 nM against the WB isolate and 2 nM against the GS isolate.
The flagellated protozoan Giardia lamblia is the most common human gastrointestinal parasite in the United States and in most developed countries (21a, 48). The parasite causes the waterborne diarrheal disease giardiasis, which has an estimated worldwide prevalence of 280 million cases annually. Furthermore, giardial infections contribute substantially to the 2.5 million annual deaths from diarrheal disease (2, 51). Disease prevalence is the highest in developing countries due to poor sanitation. In Asia, Africa, and Latin America, approximately 200 million people have symptomatic giardiasis, with some 500,000 new cases being reported each year (2, 48). Clinical manifestations range from asymptomatic carriage to diarrhea, vomiting, abdominal pain, weakness, and weight loss. The common length of infection is 2 to 4 weeks, with 30 to 50% of cases evolving into chronic infections with intermittent diarrhea and substantial weight loss (18, 51).
Of the seven genetically distinct assemblages of G. lamblia, only two assemblages, A and B, are known to infect humans (28, 43). Of these, the WB isolate of assemblage A and the GS isolate of assemblage B are the only two Giardia isolates that have been successfully cultured and studied at the molecular level in vitro (2). The WB and GS isolates are biologically distinct (36), and the GS isolate is currently the only Giardia isolate that has been used successfully in experimental infections in humans (38) and adult mice (9). Therefore, the drug screening study described here is focused on the G. lamblia WB and GS isolates.
Currently, treatments of choice for giardiasis are metronidazole (Mnz) or tinidazole, with single-course cure rates being 60 to 90%, while other drugs, such as nitazoxanide, furazolidone, albendazole, and paromomycin, are used to a lesser extent with similar and/or lower success rates (32). Although these drugs are generally effective (albeit with undesirable side effects), reports of treatment failures and drug-resistant strains raise concern that these drugs will become increasingly ineffective, underscoring the need for new chemotherapeutic agents (19, 47, 51).
The standard assays for Giardia drug sensitivity rely on visual counting of trophozoites in liquid culture (46) and evaluation of Giardia attachment to inorganic surface or Caco-2 monolayer cells (14, 35). The reliance on visual evaluation induces human bias and limits throughput. Other nonbiased assays that monitor [3H]thymidine incorporation (6), oxygen usage (42), nuclear dye incorporation (5), and ATP content (15, 49) have been developed. Of these, only the recently developed ATP content assay is in a homogeneous format that is amenable to high-throughput screens (HTSs), while the other assays require multiple wash steps and/or specialized equipment. ATP is a main energy storage and carrier molecule in all cells. Hence, the cellular content of ATP is an important marker for the functional integrity of live cells. ATP content decreases quickly during apoptosis and necrosis and is completely lost within a few hours of cell lysis. Thus, measurements of ATP content have been extensively used to determine compound cytotoxicity in mammalian cells and have recently been applied toward determination of Giardia trophozoite growth (15, 49). To facilitate screening of new anti-Giardia agents, we report here the optimization and miniaturization of the ATP content assay to a 1,536-well format suitable for HTS and the results of a pilot screen against a collection of 4,096 pharmacologically active compounds.
MATERIALS AND METHODS
Materials.
Mnz, 5-aza-2′-deoxycytidine (decitabine), nitarsone, carbadox, GW9662, and hydroxocobalamin acetate were purchased from Sigma-Aldrich (St. Louis, MO). Fumagillin was purchased from Enzo Life Sciences (Plymouth Meeting, PA), bortezomib from Santa Cruz Biotechnology (Santa Cruz, CA), and BTO-1 from EMD Chemicals (Gibbstown, NJ). All compounds were dissolved in dimethyl sulfoxide (DMSO) to either 50 mM or 10 mM, depending on solubility. The ATPLite one-step luminescence assay kit was purchased from PerkinElmer (Waltham, MA).
Small-molecule libraries and compound management.
The library of 1,280 pharmacologically active compounds (LOPAC1,280) consists of a collection of small molecules with characterized biological activities. The LOPAC1,280 library has been extensively used for HTS assay validations (29, 44) and was purchased from Sigma-Aldrich. The NIH Chemical Genomics Center Pharmaceutical (NPC) collection was constructed in-house through a combined effort of compound purchasing and custom synthesis (33). Briefly, the NPC library consists of 2,816 small-molecule compounds, 52% of which are drugs approved for human or animal use by the United States Food and Drug Administration (FDA), 22% are drugs approved in Europe, Canada, or Japan, and the remaining 25% are compounds that have entered clinical trials or are research compounds commonly used in biomedical research. Compounds from both libraries were obtained as powder samples and dissolved in DMSO as 10 mM stock solutions, except several hundred from the NPC library that were prepared as 4.47 mM stock solutions due to solubility limitations. All library compounds were serially diluted in DMSO via 1:5 interplate titrations for primary screens and then formatted to 1,536-well compound plates using an Evolution P3 system (PerkinElmer, Inc., Wellesley, MA) (52). After formatting of the compounds, compound plates were stored desiccated at room temperature for as long as 6 months when in use or were heat sealed and stored at −80°C for long-term storage. Compound cherry-picking for hit confirmation was done as follows: 10 mM compound solutions were thawed from storage, diluted in DMSO as 12-point 1:3 intraplate titrations, and then formatted to 1,536-well compound plates.
Giardia culture.
Trophozoites of G. lamblia isolates WB and GS (9, 37) were grown at pH 7.0 in modified TYI-S-33 medium as described previously (27). The medium was supplemented with 10% heat-inactivated bovine serum (Sigma) and 0.05% bovine bile (Sigma) in borosilicate glass screw-cap culture tubes (Fisherbrand). To attain low-oxygen-tension conditions, the tubes were filled to 85 to 90% of their total volume capacity and incubated without shaking at 37°C. Subcultures (2 × 105 trophozoites per tube) were made three times a week. Detachment of trophozoites for preparation of inocula was achieved by chilling the cultures on ice for 20 min.
Giardia viability assay in 96-well format.
Giardia trophozoites were plated at a density of 2,500 cells/well in 120 μl medium in sterile 96-well black clear-bottom assay plates. Mnz was serially diluted from a 100 mM stock solution 1:4 in DMSO, and then 0.5 μl/well of Mnz titrations or DMSO control was transferred in duplicate to the assay wells using a multichannel pipette. The assay plates were covered with plastic low-evaporation lids and individually sealed with anaerobic generators (type A Bio-Bag; BD Diagnostics) to create an anaerobic growth environment. The sealed Bio-Bags were incubated at 37°C for the indicated periods. Following incubation, 80 μl/well of the ATPLite reagent (PerkinElmer) was added to the assay plates for one-step lysis and ATP level detection. The plates were centrifuged briefly (1,000 rpm, 30 s) and incubated at room temperature for 20 min. The luminescent signals of the assay plates were measured on a ViewLux plate reader (PerkinElmer). Total-to-background (S/B) ratios and Z′ factors were calculated from eight wells containing 0.42% DMSO (total signal) and eight wells containing 41.7 μM Mnz (basal signal).
Giardia viability assay in 1,536-well format.
For the viability assay in the 1,536-well format, 2 μl medium was dispensed per well into 1,536-well white solid-bottom plates using a Multidrop Combi dispenser (Thermo Scientific). Subsequently, 23 nl/well compound solutions or DMSO controls was dispensed into the assay plates containing medium via a pintool workstation (Kalypsys, San Diego, CA). Trophozoites were chilled on ice to detach them from the glass tubes and, unless otherwise noted, were diluted to 250,000 trophozoites/ml with ice-cold medium. The trophozoite suspension was kept on ice and dispensed at 4 μl/well with the Multidrop Combi dispenser. Attachment of trophozoites to the dispensing apparatus was avoided with continuous dispensing and icing of the trophozoite suspension. The plates were covered with plastic low-evaporation lids, individually sealed in type A Bio-Bags, and incubated at 37°C for 48 h. Following incubation, 4 μl/well of ATPLite reagent was dispensed with the Multidrop Combi dispenser. The assay plates were briefly centrifuged at 1,000 rpm, and the luminescence signal was detected on a ViewLux plate reader (PerkinElmer) after 20 min incubation at room temperature. The S/B ratio, Z′ factor, and coefficient of variation (CV) were calculated from 32 wells with 0.38% DMSO (total signal) and 32 wells with 38.3 μM Mnz (basal signal).
CHO cell cytotoxicity assay in 1,536-well format.
For the viability assay in the 1,536-well format, 2 μl medium was dispensed into each well of 1,536-well white solid-bottom plates using the Multidrop Combi dispenser. Subsequently, 23 nl/well compound solutions or DMSO controls was dispensed into the assay plates via a pintool workstation. Chinese hamster ovary (CHO) cells were grown in T225 flasks to 70% confluence under a standard cell culture condition (ATCC), detached with 0.25% trypsin-EDTA, and seeded at 250 cells/well in 4 μl medium (Dulbecco's modified Eagle medium, 10% fetal bovine serum, 1× penicillin-streptomycin). The plates were incubated at 37°C with 5% CO2 and 95% humidity for 48 h. Subsequently, ATP content was measured following the addition of 4 μl/well of ATPLite reagent and a 20-min incubation on a ViewLux plate reader.
Trophozoite enumeration assay.
The Giardia lamblia GS isolate was plated into sterile 96-well black clear-bottom assay plates at a 10,000-cell/well density and 120-μl/well volume in culture medium. Compounds tested were prepared as 50 mM DMSO solutions in a 12-point 1:3 titration series in DMSO, with exceptions being carbadox, bortezomib, and BTO-1, which were used at a 10 mM top concentration due to solubility limitations. Compound titration series were added to duplicate assay wells at 0.5 μl/well, and the assay plates were incubated anaerobically in a type A Bio-Bag (BD Diagnostics) for 48 h at 37°C. The cell density in each well was visually scored, and the six wells surrounding the visually determined 50% inhibitory concentrations (IC50s) were quantitated by cell counting. For enumeration, trophozoites were detached on ice for 30 min and resuspended via pipetting. Seventy-five microliters of culture was removed from each well and mixed with 65 μl of 0.4% trypan blue and 10 μl of 30% bleach (final bleach concentration, 2%) to immobilize trophozoites. Trophozoites were then counted in a hemocytometer.
Data analysis.
Statistical values for assay robustness were calculated as follows: Z′ factor = 1 − 3(SDtotal + SDbasal)/(meantotal − meanbasal), where SDtotal is the standard deviation of DMSO-treated wells, SDbasal is the standard deviation of Mnz-treated wells, meantotal is the mean of DMSO-treated wells, and meanbasal is the mean of Mnz-treated wells (53), and CV = SDtotal/meanbasal, expressed as a percentage.
Data normalization and curve fitting were performed as previously described (24). Briefly, raw plate reads for each titration point were first normalized relative to those for the DMSO-only wells (0% activity) and 38.3 μM Mnz-treated wells (100% activity) and then corrected by applying a pattern correction algorithm using compound-free control plates (DMSO plates). Concentration-response titration points for each compound were fitted to the Hill equation, yielding concentrations of half-maximal inhibition (IC50) and maximal response (efficacy) values. Compounds from the quantitative HTS (qHTS) were classified into four major classes using the set of criteria listed in previous studies (24). In brief, the highest confidence curve classes (classes 1.1 and 2.1) were well fit (R2 ≥ 0.9) and showed a full response (efficacy, >80%), except that class 1.1 curves exhibited upper and lower asymptotes, while class 2.1 curves only showed one asymptote. From the LOPAC and NPC library screens, compounds from curve classes 1.1 and 2.1 with IC50s of <15 μM were selected as hits.
Data from the CHO cell counterscreen underwent the same initial analysis described above, with the exception that raw luminescence counts were normalized relative to those for DMSO-only wells (0% activity) and 38.3 μM camptothecin-treated wells (100% activity). For evaluation of cytotoxicity, compounds that were at least 10-fold less potent (IC50s, over 10-fold higher) or 50% less efficacious (maximal response, less than 50%) in the CHO cell assay compared with the respective values in the Giardia assay were considered selective anti-Giardia compounds.
RESULTS
ATP content assay development.
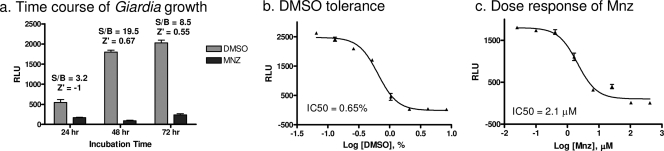
We used a commercially available ATP detection kit to measure the viability of G. lamblia after compound treatment. This bioluminescence assay utilizes the luciferase enzyme reaction with two substrates, luciferin and the ATP derived from the live cell lysate, to produce light. The assay was developed in a 96-well plate format, and the known giardiacidal agent Mnz was used as a positive control (8). The time course of G. lamblia WB growth showed an increase in the ATP signal up to 48 h, and this reached a plateau at between 48 and 72 h (Fig. 1 a). On the basis of these growth characteristics, the 48-h time point was selected for compound treatments. At all time points tested, 41.7 μM Mnz treatment reduced the ATP content to <5% of the DMSO control values (Fig. 1a). The DMSO tolerance of trophozoites was assessed in this assay, as DMSO is used to dissolve the compounds in the library. DMSO suppressed the assay signal in a concentration-dependent manner. At the 0.38% DMSO concentration, which was used for the compound screening, there was a 20 to 30% reduction in the ATP signal (Fig. 1b). Although this is a significant decrease, the S/B of 19.5 and the Z′ factor of 0.67 indicate that this DMSO concentration is acceptable for HTS, as long as the same concentration is used in all wells (Fig. 1a). Mnz, the giardiacidal control compound, showed a concentration-dependent inhibition of the ATP content signal with an IC50 of 2.1 μM (Fig. 1c), which agrees with previously reported values obtained via trophozoite counting (14, 35) as well as ATP content assays (15, 49). These results indicated that the ATP content Giardia assay is valid for compound screening.
FIG. 1.
Assay development in 96-well format. (a) G. lamblia WB trophozoite samples were treated with 0.42% DMSO control or 41.7 μM Mnz and were incubated anaerobically at 37°C for the indicated periods of time; (b) DMSO tolerance of the growth assay was tested between 0.07% and 8.3% DMSO; (c) dose-response of Mnz on Giardia growth. RLU, relative luminescence units.
Miniaturization of ATP content assay.
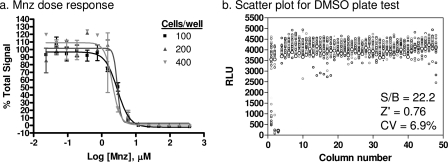
Assay miniaturization in a 1,536-well plate format was undertaken to increase throughput and facilitate screening of large compound libraries. The volume of the trophozoite suspension was reduced to 6 μl/well and that of the ATP detection reagent was reduced to 4 μl/well in the 1,536-well assay plates. The assay was tested at three different trophozoite densities of 100, 200, and 400 trophozoites/well to determine the activity of Mnz. All three cell densities showed similar sensitivities toward Mnz, and IC50s were also comparable to the results from the 96-well format assay (Fig. 2 a), indicating that the miniaturization retained assay sensitivity and robustness. The 100-trophozoite/well density was selected for further experiments to reduce the work for preparation of Giardia cultures. By following these conditions, a DMSO test plate was used to assess the statistical parameters of the assay in the 1,536-well plate format. The S/B, Z′ factor, and CV values were 22.2, 0.76, and 6.9%, respectively (Fig. 2b), indicating that the miniaturized assay is robust and suitable for HTS.
FIG. 2.
Assay miniaturization to 1,536-well format. (a) Dose-response curves for Mnz treatment on three plating densities of Giardia. Calculated 50% effective concentrations are 2.9 μM for 100 cells/well, 2.9 μM for 200 cells/well, and 2.1 μM for 300 cells/well Giardia density. Percent total signal is calculated on the basis of 32 wells each for DMSO (100%) and Mnz (0%) treatment for each of the cell densities. (b) Scatter plot for a DMSO test plate. Columns 1 and 2 were treated with Mnz titration, column 3 was treated with 38.3 μM Mnz, and columns 4 to 48 were treated with 0.38% DMSO. RLU, relative luminescence units.
Compound library screen and hit confirmation.
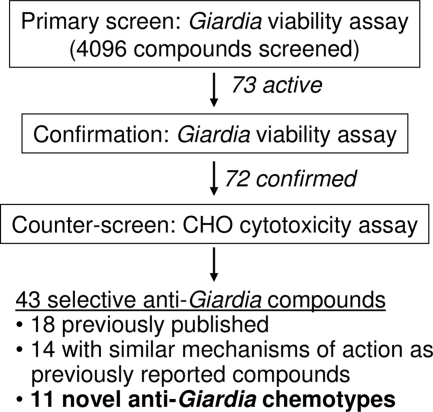
Using the above established conditions in the 1,536-well format, the assay was used in a pilot screen against two small-molecule libraries: the 1,280 compounds in LOPAC (Sigma-Aldrich) and the 2,816 compounds in the NPC library. The screen was conducted in qHTS format, in which each compound was tested at five concentration points, ranging from 61 nM to 38.3 μM, in a 1:5 dilution ratio (24). The qHTS mode provided concentration-response curves, potencies, and efficacies from the outset. Compounds with high-confidence curve classes (classes 1.1 and 2.1; see Materials and Methods for details) and potencies of <15 μM were selected for use in follow-up studies. Although the 15 μM cutoff is much higher than the standard 1 to 10 μM employed by the HTS field, the fact that Giardia infections are restricted to the intestinal lumen means that anti-Giardia drugs do not need to be absorbed into the body. Therefore, low-potency compounds could still have therapeutic potential if they have poor gastrointestinal permeability and/or low toxicity. A total of 73 hits were found in the screen, resulting in a total hit rate of 1.8%. These compounds were cherry-picked and retested with the ATP content assay at 12 concentration points with 1:3 titrations. All IC50 and efficacy values reported here are from the confirmation results, since the 12-point 1:3 titration produces more accurate concentration-response values than the 5-point 1:5 titration used in the primary screen. The results for all but one of the compounds were confirmed. The unconfirmed compound had a relatively high IC50 in the primary screen and was therefore more susceptible to day-to-day experimental variations (see Fig. 3 for schematic of the screen and confirmation).
FIG. 3.
Flowchart of Giardia viability screen and follow-up.
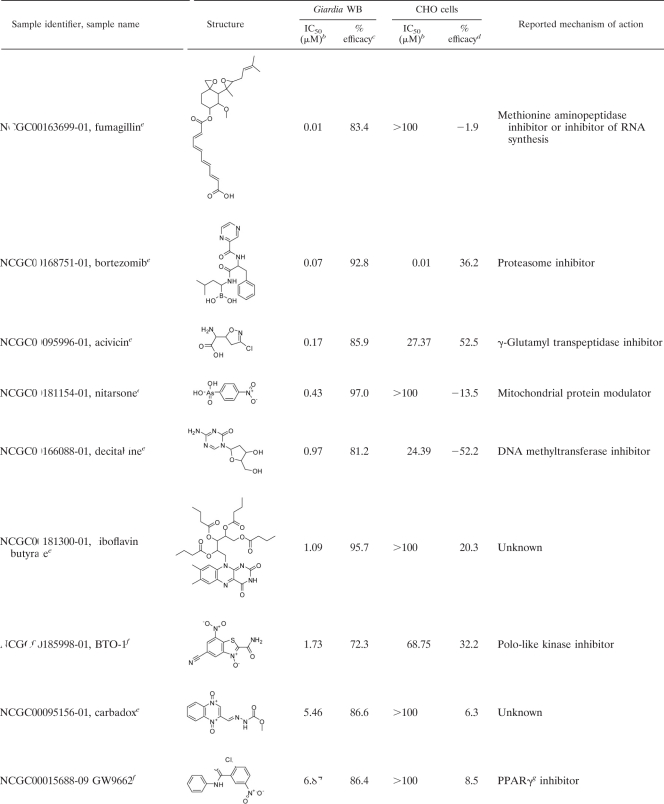
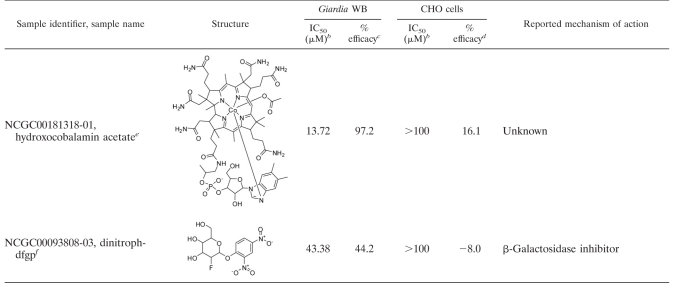
In order to assess the specificity of the confirmed hits toward Giardia and to eliminate false positives such as luciferase inhibitors, a counterscreen was developed using CHO cells and the same ATP content assay. CHO cells, commonly used in high-throughput compound screening, were selected to determine the cytotoxicities of the compounds toward a mammalian cell line. When the confirmed compounds were incubated with CHO cells for 48 h and the cytotoxicity was detected by the ATP content assay, 43 compounds were found to be selective toward Giardia. Compounds were considered selective if they showed either greater than 10-fold selectivity in IC50 or greater than 2-fold selectivity in efficacy. The other 29 compounds were either general cytotoxic agents or interferers with the detection system. The selective agents include 32 compounds that either were previously reported to have anti-Giardia properties or share mechanisms of action with known compounds (Table 1) and 11 compounds with unknown or novel targets (Table 2). In fact, all novel and selective anti-Giardia compounds found in the screen are listed in Table 2.
TABLE 1.
Hit compounds reported previously to exhibit anti-Giardia activitya
| Sample identifier, sample name | Library |
Giardia WB |
CHO cells |
Previous report(s) | Compound class | Mechanism of action | ||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM)b | % efficacyc | IC50 (μM)b | % efficacyd | |||||
| NCGC00160481-01, tioxidazole | NPC | 0.97 | 90.9 | >100 | 34.9 | None | Antimicrotubule | |
| NCGC00094226-07, piceatannol | LOPAC1,280 | 2.17 | 81.3 | >100 | −16.6 | None | Antimicrotubule | |
| NCGC00181306-01, docetaxel | NPC | 2.74 | 66.1 | >100 | 23.0 | None | Antimicrotubule | |
| NCGC00091810-01, dinitrofluorobenzene | NPC | 2.74 | 90.8 | >100 | −0.7 | None | Antimicrotubule | |
| NCGC00024995-07, taxol | NPC | 2.74 | 80.2 | >100 | 3.6 | 5 | Antimicrotubule | |
| NCGC00015894-02, resveratrol | NPC | 3.29 | 80.7 | 26.16 | 22.2 | None | Antimicrotubule | |
| NCGC00181131-01, epothilone B | NPC | 8.65 | 71.0 | >100 | −7.4 | None | Antimicrotubule | |
| NCGC00016806-01, mebendazolee | NPC | 0.12 | 91.5 | 13.11 | 53.0 | 11, 26 | Benzimidazole | Antimicrotubule |
| NCGC00016855-01, fenbendazole | NPC | 0.17 | 90.8 | 52.19 | 39.2 | 26 | Benzimidazole | Antimicrotubule |
| NCGC00016876-01, albendazolee | NPC | 0.23 | 93.5 | 13.11 | 55.1 | 11, 26 | Benzimidazole | Antimicrotubule |
| NCGC00181110-01, cambendazole | NPC | 0.31 | 95.8 | 4.34 | 92.2 | 26 | Benzimidazole | Antimicrotubule |
| NCGC00018238-04 oxibendazole | NPC | 0.87 | 89.2 | >100 | 34.1 | 26 | Benzimidazole | Antimicrotubule |
| NCGC00015220-04, CB1954 | LOPAC1,280 | 0.08 | 96.4 | >100 | −16.9 | None | DNA cross-linker | |
| NCGC00017057-01, quinacrine HCle | NPC | 0.22 | 93.8 | 2.17 | 95.8 | 6, 11 | DNA cross-linker | |
| NCGC00095258-01, mitomycin C | NPC | 2.17 | 82.0 | 21.74 | 67.4 | 5 | DNA cross-linker | |
| NCGC00095304-02, furazolidonee | NPC | 0.19 | 96.6 | >100 | −10.2 | 6, 11 | Nitrofuran | Free radical generation |
| NCGC00164507-01, nifuroxime | NPC | 0.31 | 97.1 | 27.37 | −50.3 | None | Nitrofuran | Free radical generation |
| NCGC00016774-01, nifurtimox | NPC | 0.37 | 94.8 | >100 | −21.1 | None | Nitrofuran | Free radical generation |
| NCGC00016638-01, furaltadone | NPC | 0.47 | 94.7 | >100 | 0.6 | None | Nitrofuran | Free radical generation |
| NCGC00016554-01, nifuroxazide | NPC | 0.93 | 95.2 | >100 | 20.2 | 11 | Nitrofuran | Free radical generation |
| NCGC00164501-01, nihydrazone | NPC | 1.73 | 92.5 | >100 | −13.7 | None | Nitrofuran | Free radical generation |
| NCGC00166238-01, benznidazole | NPC | 0.39 | 92.9 | >100 | 8.5 | 23 | Nitroimidazole | Free radical generation |
| NCGC00016685-05, ronidazole | NPC | 0.43 | 96.2 | >100 | 28.3 | 6 | Nitroimidazole | Free radical generation |
| NCGC00016741-01, tinidazolee | NPC | 0.47 | 95.4 | >100 | −14.0 | 6, 11 | Nitroimidazole | Free radical generation |
| NCGC00016723-01, ornidazolee | NPC | 0.52 | 95.0 | >100 | 1.0 | 6, 11 | Nitroimidazole | Free radical generation |
| NCGC00181095-01, ipronidazole | NPC | 0.97 | 94.6 | >100 | 1.6 | 1 | Nitroimidazole | Free radical generation |
| NCGC00095158-01, secnidazolee | NPC | 4.34 | 92.6 | >100 | 9.3 | 6, 11 | Nitroimidazole | Free radical generation |
| NCGC00016446-06 metronidazolee | NPC | 8.65 | 90.6 | 17.27 | −44.8 | 6, 11, 15, 49 | Nitroimidazole | Free radical generation |
| NCGC00016277-01, niridazole | NPC | 0.08 | 95.4 | >100 | 11.5 | None | Nitrothiazole | PFORf inhibition |
| NCGC00160655-01, tenonitrozol | NPC | 0.19 | 93.1 | >100 | 21.5 | None | Nitrothiazole | PFOR inhibition |
| NCGC00164494-01, nithiamide | NPC | 0.39 | 94.6 | >100 | 25.9 | None | Nitrothiazole | PFOR inhibition |
| NCGC00090774-01, nitazoxanidee | NPC | 0.61 | 95.5 | 21.74 | 68.4 | 11 | Nitrothiazole | PFOR inhibition |
Giardia WB isolate and CHO viability assay data for confirmed and selective compounds either that have been previously published or that function through mechanisms similar to those of previously known agents.
IC50 was calculated as the concentration of compound at which 50% of DMSO control ATP levels are detected.
Efficacy was normalized to percent reduction in ATP levels compared with those achieved with 38.3 μM Mnz at the maximal compound concentration.
Efficacy was normalized to percent reduction in ATP levels compared those achieved with 38.3 μM camptothecin at the maximal compound concentration.
Drugs used for treatment of giardiasis.
PFOR, pyruvate:ferredoxin oxidoreductase.
TABLE 2.
Novel anti-Giardia chemotypesa
Giardia WB isolate and CHO viability assay data for selective compounds that might have mechanisms of action different from those of previously known anti-Giardia agents.
IC50 was calculated as the concentration of compound at which 50% of the DMSO control ATP levels are detected.
Efficacy was normalized to percent reduction in ATP levels compared with that achieved with 38.3 μM Mnz at the maximal compound concentration.
Efficacy was normalized to percent reduction in ATP levels compared with that achieved with 38.3 μM camptothecin at the maximal compound concentration.
Compound isolated from NPC library.
Compound isolated from LOPAC1,280 library.
PPARγ, peroxisome proliferator-activated receptor gamma.
As a further confirmatory step, 8 out of the 11 novel compounds that were commercially available were purchased as powder samples and tested against the G. lamblia GS isolate using both the ATP content and enumeration assays (Table 3). The choice of using the GS isolate was made to facilitate forward progress toward susceptibility testing in adult mouse models (9). The ATP content and enumeration assays provided good correlation of IC50s, with an R2 value of 0.95 (Table 3). For two of the compounds, BTO-1 and GW9662, >2-fold lower IC50s were obtained from the ATP content assay than from the enumeration assay. This discrepancy might be due to differences in assay principles, since metabolically inactive trophozoites would not give a signal in the ATP content assay but might still maintain an intact cell shape that would be counted in the enumeration assay. Overall, these results validated the use of ATP measurement as a surrogate for visual counting of trophozoites. We have also demonstrated that the ATP content assay can be used for HTS to isolate novel anti-Giardia compounds.
TABLE 3.
Confirmation of powder compoundsa
| Sample name | ATP content |
IC50 (μM) by enumeration method | |
|---|---|---|---|
| IC50 (μM) | % efficacy | ||
| Metronidazole | 1.6 | 99.7 | 2.2 |
| Fumagillin | 0.002 | 98.9 | 0.003 |
| Bortezomib | 0.06 | 98.5 | 0.13 |
| Decitabine | 0.15 | 95.1 | 0.07 |
| Carbadox | 0.24 | 99.4 | 0.29 |
| Nitarsone | 0.9 | 99.6 | 1.2 |
| BTO-1 | 2.0 | 99.1 | 9.3 |
| GW9662 | 12.7 | 99.2 | 32.9 |
| Hydroxocobalamin acetate | 78.3 | 98.4 | 54.2 |
The novel anti-Giardia compounds were purchased as powder samples and tested against the GS isolate using both the ATP content and trophozoite enumeration methods.
DISCUSSION
Of the existing methods for Giardia drug susceptibility testing, assay for the utilization of ATP content as an indicator of trophozoite viability is the only assay that is both unbiased and homogeneous (15, 49). We have successfully optimized and miniaturized the ATP content assay to a 1,536-well format to allow high-throughput screening for anti-Giardia compounds. Being a phenotypic screen, the mechanisms of action of the HTS hits might be unknown, thus providing opportunities to identify potential new drug targets through additional follow-up studies. The ATP content assay was validated in a screen against 4,096 small molecules from two libraries. One was the commercially available LOPAC1,280 library (Sigma-Aldrich), consisting of 1,280 pharmacologically active compounds, which has been extensively used for HTS assay validations (29, 44). The other was an investigational and approved drugs library comprising 2,816 compounds (33).
The Giardia viability assay showed high reproducibility, as 72 out of 73 hits were confirmed when they were cherry-picked for follow-up studies. Of the 72 confirmed hits, the CHO cell cytotoxicity counterscreen further narrowed down the compounds of interest to 43 selective compounds (Fig. 3). The great majority (88%) of the 43 selective compounds was present in the NPC library, which is expected, as this is a known drug library and is therefore enriched in drug-like compounds. A validation of our methodology is shown by the capacity of the assay to identify several known anti-Giardia compounds. In fact, all drugs currently used to treat giardiasis (summarized in reference 18) were identified in the primary screen and were selective toward Giardia in the counterscreen (Table 1). One exception was paramomycin, which was present in the NPC library but which was inactive due to its low in vitro potency (IC50 exceeding 100 μM) (6, 16).
In addition to giardiasis drugs, the compound screen also isolated many diverse chemotypes that share common underlying mechanisms with those of known anti-Giardia agents (Table 1). We found previously reported drugs with antitubulin effects, such as mebendazole, fenbendazole, albendazole, cambendazole, oxibendazole, and taxol, as well as new molecules potentially having the same mechanism of action, such as tioxidazole, piceatannol, docetaxel, dinitrofluorobenzene, resveratrol, and epothilone B (22, 30, 54). We also found reported DNA binders/cross-linkers, quinacrine and mitomycin C, and a new bioactivated alkylating agent, CB1954 (7). Another traditionally in-use class of anti-Giardia compounds includes several types of bioactivated radical inducers, such as the nitroimidazoles (benznidazole, ronidazole, tinidazole, ornidazole, ipronidazole, secnidazole, metronidazole) and the nitrofurans (furazolidone, nifuroxazide) (51). Table 1 shows additional nitrofurans (nifuroxime, nifurtimox, furaltadone, nihydrazone) having anti-Giardia activity. Moreover, we also found a number of old (nitazoxanide) and new (niridazole, tenonitrozol, nithiamide) nitrothiazoles possibly functioning via inhibition of pyruvate:ferredoxin oxidoreductase (51).
Most significantly, the assay revealed a number of molecules which previously have not been reported to exhibit anti-Giardia activity. These compounds probably possess mechanisms of action different from those of the current drugs for the treatment of giardiasis. The most potent inhibitor found was fumagillin, a biomolecule drug used to treat microsporidiosis. Although fumagillin has been described to be a potential inhibitor of RNA synthesis and of methionine aminopeptidase (MetAP2) in Octosporea muscaedomesticae and Plasmodium falciparum (12, 25), its antimicrobial mode of action is still unclear. Bortezomib is a known proteasome inhibitor with low gastrointestinal permeability (17). This compound is particularly interesting because proteasomes have been shown to play significant roles in the replication and transformation of several parasitic protozoa and have been suggested to be an attractive anti-Giardia target (39). Other compounds with well-defined targets are decitabine, a DNA methyltranferase (DNMT) inhibitor (13); BTO-1, a polo-like kinase (Plk) inhibitor (31); and nitarsone, a modulator of mitochondrial proteins involved in regulating the production of reactive oxygen species (40). While homologs of MetAP2, DNMT, and Plk are present in the Giardia genome, more studies are needed to validate these targets. Likewise, while Giardia does not have mitochondria (40), it contains mitochondrial remnant organelles (mitosomes), which may explain the action of nitarsone (45). Lastly, we identified several compounds with unknown mechanisms of action. Although the mammalian targets of GW9662, 2,4-dinitrophenyl 2-fluoro-2-deoxy-beta-d-glucopyranoside (dinitroph-dfgp), and acivicin are known, these enzymes are absent in Giardia (3, 50). Carbadox is an antimicrobial compound, but its mechanism of action remains unknown. There is also no clear mechanism for riboflavin butyrate or hydroxocobalamin acetate. Hydroxocobalamin acetate is a stable synthetic analogue of the naturally occurring hydroxocobalamin, one of the isoforms of vitamin B12. Vitamin B12 is considered very safe and is used in acute cyanide poisoning up to a dosage of 5 g/day (41). When administered orally, only 1% is absorbed by passive diffusion (4), and the absorption is nonlinear: 50% at a 1-μg dose, 20% at a 5-μg dose, and 5% at a 25-μg dose (20). These properties may be ideal for the treatment of giardiasis. Needless to say, repurposing novel drugs to fight giardiasis may be useful for combating resistant G. lamblia strains. While animal testing of these novel compounds was beyond the scope of this study, we report here the sensitivities of eight commercially available novel compounds not only against the WB isolate but also against the GS isolate, which is used in adult mouse models of Giardia infection (Table 3) (9). Animal testing and mechanism-of-action studies conducted with these compounds would further evaluate their usefulness as well as enhance our understanding of Giardia biology.
The commercially available novel compounds were also tested, along with Mnz, against the WB and GS isolates using the ATP content assay. A comparison of compound potencies found that five out of nine compounds (Mnz, fumagillin, decitabine, carbadox. and hydroxocobalamin acetate) exhibited >2-fold variations in potency (Table 2 and 3). Of these five compounds, all but one (hydroxocobalamin acetate) showed greater potency against the GS strain than the WB strain, suggesting that this isolate might be generally more susceptible to antimicrobial agents. These differences in compound sensitivity might reflect the genomic differences between the two isolates. The genome sequence of assemblage A isolate WB (34) and a draft genome sequence of the assemblage B GS isolate (21) have been published. Comparison of these revealed approximately 75% amino acid sequence identity between proteins from the two isolates. In addition, 28 unique GS proteins and 3 unique WB proteins have been identified, the variable surface protein (VSP) repertoires of the two isolates are completely different, and differences in encystation-specific promoters have been found. The successful use of the ATP content assay against two genetically distinct assemblages indicates that this assay might have general applications for generating Giardia drug susceptibility profiles.
In summary, the capability to test large numbers of compounds in parallel using a phenotypic cell-killing screen establishes a powerful tool for multiple applications, including screening for novel anti-Giardia agents, generation of drug sensitivity profiles for different Giardia isolates, and interrogation of Giardia biological pathways through chemical intervention. This can ultimately lead to the discovery of new drug targets, development of novel drugs, refinement of combinatorial drug therapy, and increased understanding of Giardia biology. Using a pilot screen, we have demonstrated that the ATP content assay can be implemented in a high-throughput format to identify known and novel anti-Giardia compounds with wide-ranging chemotypes and mechanisms of action.
Acknowledgments
This study was supported by National Institutes of Health grant R01 AI059733 (to O.H.) and the Molecular Libraries Initiative of the NIH Roadmap for Medical Research.
Footnotes
Published ahead of print on 15 November 2010.
REFERENCES
- 1.Abbitt, B., R. L. Huey, A. K. Eugster, and J. Syler. 1986. Treatment of giardiasis in adult greyhounds, using ipronidazole-medicated water. J. Am. Vet. Med. Assoc. 188:67-69. [PubMed] [Google Scholar]
- 2.Adam, R. D. 2001. Biology of Giardia lamblia. Clin. Microbiol. Rev. 14:447-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahluwalia, G. S., J. L. Grem, Z. Hao, and D. A. Cooney. 1990. Metabolism and action of amino acid analog anti-cancer agents. Pharmacol. Ther. 46:243-271. [DOI] [PubMed] [Google Scholar]
- 4.Baik, H. W., and R. M. Russell. 1999. Vitamin B12 deficiency in the elderly. Annu. Rev. Nutr. 19:357-377. [DOI] [PubMed] [Google Scholar]
- 5.Bonilla-Santiago, R., Z. Wu, L. Zhang, and G. Widmer. 2008. Identification of growth inhibiting compounds in a Giardia lamblia high-throughput screen. Mol. Biochem. Parasitol. 162:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boreham, P. F., R. E. Phillips, and R. W. Shepherd. 1985. A comparison of the in-vitro activity of some 5-nitroimidazoles and other compounds against Giardia intestinalis. J. Antimicrob. Chemother. 16:589-595. [DOI] [PubMed] [Google Scholar]
- 7.Boyle, R. G., and S. Travers. 2006. Hypoxia: targeting the tumour. Anticancer Agents Med. Chem. 6:281-286. [DOI] [PubMed] [Google Scholar]
- 8.Busatti, H. G., J. F. Santos, and M. A. Gomes. 2009. The old and new therapeutic approaches to the treatment of giardiasis: where are we? Biologics 3:273-287. [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd, L. G., J. T. Conrad, and T. E. Nash. 1994. Giardia lamblia infections in adult mice. Infect. Immun. 62:3583-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Cedillo-Rivera, R., and O. Munoz. 1992. In-vitro susceptibility of Giardia lamblia to albendazole, mebendazole and other chemotherapeutic agents. J. Med. Microbiol. 37:221-224. [DOI] [PubMed] [Google Scholar]
- 12.Chen, X., S. Xie, S. Bhat, N. Kumar, T. A. Shapiro, and J. O. Liu. 2009. Fumagillin and fumarranol interact with P. falciparum methionine aminopeptidase 2 and inhibit malaria parasite growth in vitro and in vivo. Chem. Biol. 16:193-202. [DOI] [PubMed] [Google Scholar]
- 13.Creusot, F., G. Acs, and J. K. Christman. 1982. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2′-deoxycytidine. J. Biol. Chem. 257:2041-2048. [PubMed] [Google Scholar]
- 14.Cruz, A., M. Isaura Sousa, Z. Azeredo, M. Carolina Silva, J. C. Figueiredo de Sousa, O. Manso, and M. Cabral. 2003. Comparison between two common methods for measuring Giardia lamblia susceptibility to antiparasitic drugs in vitro. Acta Trop. 88:131-135. [DOI] [PubMed] [Google Scholar]
- 15.Dunn, L. A., A. G. Burgess, K. G. Krauer, L. Eckmann, P. Vanelle, M. D. Crozet, F. D. Gillin, P. Upcroft, and J. A. Upcroft. 2010. A new-generation 5-nitroimidazole can induce highly metronidazole-resistant Giardia lamblia in vitro. Int. J. Antimicrob. Agents 36:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edlind, T. D. 1989. Susceptibility of Giardia lamblia to aminoglycoside protein synthesis inhibitors: correlation with rRNA structure. Antimicrob. Agents Chemother. 33:484-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Einsele, H. 2010. Bortezomib. Recent Results Cancer Res. 184:173-187. [DOI] [PubMed] [Google Scholar]
- 18.Escobedo, A. A., and S. Cimerman. 2007. Giardiasis: a pharmacotherapy review. Expert Opin. Pharmacother. 8:1885-1902. [DOI] [PubMed] [Google Scholar]
- 19.Farbey, M. D., J. A. Reynoldson, and R. C. Thompson. 1995. In vitro drug susceptibility of 29 isolates of Giardia duodenalis from humans as assessed by an adhesion assay. Int. J. Parasitol. 25:593-599. [DOI] [PubMed] [Google Scholar]
- 20.Food and Nutrition Board, Institute of Medicine. 1998. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. National Academy Press, Washington, DC. [PubMed]
- 21.Franzen, O., J. Jerlstrom-Hultqvist, E. Castro, E. Sherwood, J. Ankarklev, D. S. Reiner, D. Palm, J. O. Andersson, B. Andersson, and S. G. Svard. 2009. Draft genome sequencing of Giardia intestinalis assemblage B isolate GS: is human giardiasis caused by two different species? PLoS Pathog. 5:e1000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Furness, B. W., M. J. Beach, and J. M. Roberts. 2000. Giardiasis surveillance: United States, 1992-1997. MMWR CDC Surveill. Summ. 49:1-13. [PubMed] [Google Scholar]
- 22.Harikumar, K. B., and B. B. Aggarwal. 2008. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle 7:1020-1035. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Nunez, E., H. Tlahuext, R. Moo-Puc, H. Torres-Gomez, R. Reyes-Martinez, R. Cedillo-Rivera, C. Nava-Zuazo, and G. Navarrete-Vazquez. 2009. Synthesis and in vitro trichomonicidal, giardicidal and amebicidal activity of N-acetamide(sulfonamide)-2-methyl-4-nitro-1H-imidazoles. Eur. J. Med. Chem. 44:2975-2984. [DOI] [PubMed] [Google Scholar]
- 24.Inglese, J., D. S. Auld, A. Jadhav, R. L. Johnson, A. Simeonov, A. Yasgar, W. Zheng, and C. P. Austin. 2006. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc. Natl. Acad. Sci. U. S. A. 103:11473-11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaronski, S. T. 1972. Cytochemical evidence for RNA synthesis inhibition by fumagillin. J. Antibiot. (Tokyo) 25:327-331. [DOI] [PubMed] [Google Scholar]
- 26.Katiyar, S. K., V. R. Gordon, G. L. McLaughlin, and T. D. Edlind. 1994. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob. Agents Chemother. 38:2086-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keister, D. B. 1983. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 77:487-488. [DOI] [PubMed] [Google Scholar]
- 28.Lalle, M., E. Pozio, G. Capelli, F. Bruschi, D. Crotti, and S. M. Caccio. 2005. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 35:207-213. [DOI] [PubMed] [Google Scholar]
- 29.Lea, W. A., J. Xi, A. Jadhav, L. Lu, C. P. Austin, A. Simeonov, and R. G. Eckenhoff. 2009. A high-throughput approach for identification of novel general anesthetics. PLoS One 4:e7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, Y. C., R. A. Yaple, R. Baldridge, M. Kirsch, and R. H. Himes. 1981. Inhibition of tubulin self-assembly in vitro by fluorodinitrobenzene. Biochim. Biophys. Acta 671:71-77. [DOI] [PubMed] [Google Scholar]
- 31.McInnes, C., A. Mazumdar, M. Mezna, C. Meades, C. Midgley, F. Scaerou, L. Carpenter, M. Mackenzie, P. Taylor, M. Walkinshaw, P. M. Fischer, and D. Glover. 2006. Inhibitors of Polo-like kinase reveal roles in spindle-pole maintenance. Nat. Chem. Biol. 2:608-617. [DOI] [PubMed] [Google Scholar]
- 32.McPhee, S. J., and M. A. E. Papadakis. 2010. Current medical diagnosis & treatment, 2010 ed. The McGraw-Hill Companies, Inc., New York, NY.
- 33.Miller, S. C., R. Huang, S. Sakamuru, S. J. Shukla, M. S. Attene-Ramos, P. Shinn, D. Van Leer, W. Leister, C. P. Austin, and M. Xia. 2010. Identification of known drugs that act as inhibitors of NF-kappaB signaling and their mechanism of action. Biochem. Pharmacol. 79:1272-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison, H. G., A. G. McArthur, F. D. Gillin, S. B. Aley, R. D. Adam, G. J. Olsen, A. A. Best, W. Z. Cande, F. Chen, M. J. Cipriano, B. J. Davids, S. C. Dawson, H. G. Elmendorf, A. B. Hehl, M. E. Holder, S. M. Huse, U. U. Kim, E. Lasek-Nesselquist, G. Manning, A. Nigam, J. E. Nixon, D. Palm, N. E. Passamaneck, A. Prabhu, C. I. Reich, D. S. Reiner, J. Samuelson, S. G. Svard, and M. L. Sogin. 2007. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science 317:1921-1926. [DOI] [PubMed] [Google Scholar]
- 35.Muller, J., G. Ruhle, N. Muller, J. F. Rossignol, and A. Hemphill. 2006. In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob. Agents Chemother. 50:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nash, T. E. 2002. Surface antigenic variation in Giardia lamblia. Mol. Microbiol. 45:585-590. [DOI] [PubMed] [Google Scholar]
- 37.Nash, T. E., A. Aggarwal, R. D. Adam, J. T. Conrad, and J. W. Merritt, Jr. 1988. Antigenic variation in Giardia lamblia. J. Immunol. 141:636-641. [PubMed] [Google Scholar]
- 38.Nash, T. E., D. A. Herrington, G. A. Losonsky, and M. M. Levine. 1987. Experimental human infections with Giardia lamblia. J. Infect. Dis. 156:974-984. [DOI] [PubMed] [Google Scholar]
- 39.Paugam, A., A. L. Bulteau, J. Dupouy-Camet, C. Creuzet, and B. Friguet. 2003. Characterization and role of protozoan parasite proteasomes. Trends Parasitol. 19:55-59. [DOI] [PubMed] [Google Scholar]
- 40.Ralph, S. J. 2008. Arsenic-based antineoplastic drugs and their mechanisms of action. Met. Based Drugs 2008:260146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shepherd, G., and L. I. Velez. 2008. Role of hydroxocobalamin in acute cyanide poisoning. Ann. Pharmacother. 42:661-669. [DOI] [PubMed] [Google Scholar]
- 42.Sousa, M. C., and J. Poiares-Da-Silva. 1999. A new method for assessing metronidazole susceptibility of Giardia lamblia trophozoites. Antimicrob. Agents Chemother. 43:2939-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson, R. C., and P. T. Monis. 2004. Variation in Giardia: implications for taxonomy and epidemiology. Adv. Parasitol. 58:69-137. [DOI] [PubMed] [Google Scholar]
- 44.Titus, S. A., X. Li, N. Southall, J. Lu, J. Inglese, M. Brasch, C. P. Austin, and W. Zheng. 2008. A cell-based PDE4 assay in 1536-well plate format for high-throughput screening. J. Biomol. Screen. 13:609-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tovar, J., G. Leon-Avila, L. B. Sanchez, R. Sutak, J. Tachezy, M. van der Giezen, M. Hernandez, M. Muller, and J. M. Lucocq. 2003. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 426:172-176. [DOI] [PubMed] [Google Scholar]
- 46.Upcroft, J. A., and P. Upcroft. 2001. Drug susceptibility testing of anaerobic protozoa. Antimicrob. Agents Chemother. 45:1810-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Upcroft, J. A., P. Upcroft, and P. F. Boreham. 1990. Drug resistance in Giardia intestinalis. Int. J. Parasitol. 20:489-496. [DOI] [PubMed] [Google Scholar]
- 48.Upcroft, P., and J. A. Upcroft. 2001. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 14:150-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valdez, C. A., J. C. Tripp, Y. Miyamoto, J. Kalisiak, P. Hruz, Y. S. Andersen, S. E. Brown, K. Kangas, L. V. Arzu, B. J. Davids, F. D. Gillin, J. A. Upcroft, P. Upcroft, V. V. Fokin, D. K. Smith, K. B. Sharpless, and L. Eckmann. 2009. Synthesis and electrochemistry of 2-ethenyl and 2-ethanyl derivatives of 5-nitroimidazole and antimicrobial activity against Giardia lamblia. J. Med. Chem. 52:4038-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willson, T. M., J. E. Cobb, D. J. Cowan, R. W. Wiethe, I. D. Correa, S. R. Prakash, K. D. Beck, L. B. Moore, S. A. Kliewer, and J. M. Lehmann. 1996. The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J. Med. Chem. 39:665-668. [DOI] [PubMed] [Google Scholar]
- 51.Wright, J. M., L. A. Dunn, P. Upcroft, and J. A. Upcroft. 2003. Efficacy of antigiardial drugs. Expert Opin. Drug Saf. 2:529-541. [DOI] [PubMed] [Google Scholar]
- 52.Yasgar, A., P. Shinn, A. Jadhav, D. Auld, S. Michael, W. Zheng, C. P. Austin, J. Inglese, and A. Simeonov. 2008. Compound management for quantitative high-throughput screening. JALA Charlottesv. Va. 13:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, J. H., T. D. Chung, and K. R. Oldenburg. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4:67-73. [DOI] [PubMed] [Google Scholar]
- 54.Zhao, J., J. E. Kim, E. Reed, and Q. Q. Li. 2005. Molecular mechanism of antitumor activity of taxanes in lung cancer (review). Int. J. Oncol. 27:247-256. [PubMed] [Google Scholar]