Abstract
Monensin is a polyether ionophore antibiotic that is widely used in the control of coccidia in animals. Despite its significance in veterinary medicine, little is known about its mode of action and potential mechanisms of resistance in coccidian parasites. Here we show that monensin causes accumulation of the coccidian Toxoplasma gondii at an apparent late-S-phase cell cycle checkpoint. In addition, experiments utilizing a monensin-resistant T. gondii mutant show that this effect of monensin is dependent on the function of a mitochondrial homologue of the MutS DNA damage repair enzyme (TgMSH-1). Furthermore, the same TgMSH-1-dependent cell cycle disruption is observed with the antiparasitic ionophore salinomycin and the DNA alkylating agent methyl nitrosourea. Our results suggest a novel mechanism for the mode of action of monensin and salinomycin on coccidial parasites, in which the drug activates an MSH-1-dependent cell cycle checkpoint by an unknown mechanism, ultimately leading to the death of the parasite. This model would indicate that cell cycle disruption is an important mediator of drug susceptibility and resistance to ionophoric antibiotics in coccidian parasites.
The polyether ionophore monensin has broad-spectrum antibiotic and antiparasitic actions. Monensin catalyzes the exchange of Na+ for H+ across cellular membranes, and it has been shown to be effective against a number of parasites in the phylum Apicomplexa, including members of the genera Plasmodium, Toxoplasma, and Eimeria (17, 23, 25). It is especially important in controlling Eimeria spp., parasites responsible for as much as $800 million in losses to the poultry industry worldwide (31). However, despite the economic importance of this drug and the evolution of natural resistance in Eimeria populations (17), little is understood about its mode of action or mechanisms of resistance. Monensin causes osmotic swelling of apicomplexan parasites (7), presumably due to the inability to regulate ionic homeostasis as a result of Na+ influx (38). In addition, monensin appears to interfere with vesicular traffic (35), by an undescribed mechanism. Either of these effects could contribute to parasite death, but any direct link has not been demonstrated. Likewise, authors have speculated that resistance to monensin may be due to changes in fluidity or other components of membrane structure (5, 37), which would prevent the association of monensin with the cell membrane, but experimental evidence for this is currently lacking.
Contributing to the difficulty of identifying modes of action and mechanisms of resistance to monensin is the difficulty of rearing and experimentally manipulating Eimeria spp. This contrasts with Toxoplasma gondii, which is easily reared in vitro and for which a number of tools have been developed to allow its molecular and genetic manipulation (22). Like Eimeria spp., T. gondii is highly susceptible to monensin, and indeed, monensin has been used to treat toxoplasmosis in several animal species (23). Thus, our laboratory has used T. gondii as a model to study monensin's mode of action and possible mechanisms of resistance in apicomplexans. We recently demonstrated that disruption of a mitochondrial MutS homologue (TgMSH-1) results directly in resistance to monensin as well as to the related antiparasitic drug salinomycin (13). This proved to be a surprising result, given that the disrupted gene was not one involved in membrane dynamics or ionic balance or even located in proximity to the cellular membrane but instead encoded a DNA damage repair enzyme localized to the parasite's mitochondrion. Nonetheless, the relation between disruption of this gene and monensin resistance was confirmed by phenotypic complementation using a wild-type copy of the gene (13).
MutS homologues function in DNA repair, as well as detection of DNA damage and initiation of a signal transduction cascade that results typically in the arrest of cells in the G2 phase of the cell cycle (8, 28). Damaged DNA is then repaired, or if the damage cannot be repaired, the cell undergoes programmed cell death. Because of this function of MutS homologues in DNA damage-induced cell cycle arrest and consequent cell death, MutS-deficient mutants are frequently resistant to a number of DNA-damaging drugs (1). Within this context, our hypothesis is that monensin treatment results in some type of cellular stress, potentially DNA damage, and that TgMSH-1-deficient mutants are resistant to the action of the drug because they do not exhibit a perturbation of their cell cycle that is mediated by TgMSH-1. Here we show that, indeed, monensin appears to cause disruption of the cell cycle in T. gondii in a MSH-1-dependent manner.
MATERIALS AND METHODS
Parasite and host cell maintenance and reagents.
RH strain parasites expressing the green fluorescent protein (GFP) and parasites of the TgMSH-1-deficient strain MRC5 (here referred to as the Tgmsh-1 mutant) (13) were maintained by passage through human foreskin fibroblasts (HFFs) at 37°C and 5% CO2. Normal culture medium was Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 100 units penicillin/100 μg streptomycin per ml. For drug treatment experiments, normal culture medium was supplemented with monensin (Sigma), salinomycin (Sigma), methylnitrourea (MNU) (Sigma), pyrimethamine (Sigma), artemisinin (Sigma), PX-866 (Chemdea), or KU-55933 (EMD Chemicals) at the concentration indicated below.
RNA preparation and microarray analysis.
Total RNA was collected by Trizol (Invitrogen) extraction from either RH strain tachyzoites 24 h after invasion of human foreskin fibroblasts or 24 h after invasion followed by 24-h exposure to 2.5 ng monensin/ml tissue culture medium. Microarray assays were performed in the Molecular Biology and Genomics Core lab, a unit of the School of Molecular Biosciences at Washington State University. A total of 5 μg starting RNA was used to produce cRNA using the Affymetrix one-cycle kit (Affymetrix, Santa Clara, CA). Fragmented cRNA (15 μg) was hybridized to the custom Toxoplasma gondii Affymetrix microarray according to standard hybridization protocols. Array processing was performed on the Fluidics Station 450 (Affymetrix) using GeneChip operating system (GCOS) software (version 1.2; Affymetrix), yielding cell fluorescence intensity (CEL) files. The distribution of fluorescent material on the array was determined with the confocal laser scanner (GeneChip scanner 3000; Affymetrix). CEL files containing raw data were processed and analyzed using R software (R Foundation for Statistical Computing) and Bioconductor (14) packages. The CEL files have been deposited with the NCBI gene expression and hybridization array data repository (GEO no. GSE25177; http://www.ncbi.nlm.nih.gov/geo). Microarray hybridization data were examined for physical anomalies on the chip by pseudo-chromatin immunoprecipitation (chIP) and residual error visualizations. Quality assurance for microarray data was completed using the affyQAReport function from the Bioconductor package affyQCReport.
Quantitative PCR.
RNA was extracted from developing parasites using the RNeasy Plus minikit (Qiagen) and reverse transcribed using Superscript III (Invitrogen). A PCR was then run using T. gondii-specific histone primers on a StepOne real-time PCR machine (Applied Biosystems), and relative quantification determined by the comparative threshold cycle (CT) method. The cDNA quantity was normalized between samples using primers for the T. gondii 60S ribosomal protein L29 (GeneID TGME49 034550), which microarray analysis showed did not vary in transcriptional level between treatments. All assays were performed in triplicate, and results were compared for statistical significance between control and test groups by the use of a two-tailed t test (P < 0.05) using JMP software (SAS Institute).
Flow cytometry.
Intracellular parasites were isolated by the passage of host cells through a 30-gauge needle followed by filtration through a 3.0-μm-pore-size membrane (Whatman). Parasites were then fixed in 70% ethanol, and their DNA was stained using 1 μm Sytox green (Invitrogen) plus 50 units RNase A and 200 units RNase T1 (Ambion) per ml, in 50 mM Tris, pH 7.5. Samples were analyzed on a FACSAria flow cytometer (BD Biosciences). Results were analyzed using FlowJo software (Tree Star), and the percentage of parasites in each phase of the cell cycle was estimated by gating. All assays were performed in triplicate, and percentages of parasites in each phase of the cell cycle were compared for statistical significance between control and test groups by the use of a two-tailed t test (P < 0.05) using JMP software (SAS Institute).
RESULTS
Monensin causes changes in transcriptional regulation of many T. gondii genes, including upregulation of canonical histones.
In order to better understand how monensin affects T. gondii, we analyzed the effect of monensin on gene expression of intracellular parasites through microarrays. We compared RNA collected from RH strain tachyzoites either 24 h after invasion of human foreskin fibroblasts or 24 h after invasion followed by 24 h exposure to 2.5 ng monensin/ml in tissue culture medium. Addition of monensin arrests the division of the parasites and kills them, so both the treated and untreated parasites are at the same stage of development (approximately 16 to 32 parasites per vacuole). Each treatment was done in triplicate and hybridized to three separate Affymetrix array chips, containing probe sets for 8,393 putative T. gondii genes. Hybridization and housekeeping controls, RNA degradation, sample clustering, normalized unscaled standard error (NUSE) plots, local pooled error (LPE) plots, and relative log expression (RLE) plots all showed high-quality data (not shown), and no chips were removed. The arrays were then preprocessed using the robust multiarray average (RMA) procedure using the AFFY package (4, 20, 21). Next, unexpressed and low-variability genes were removed by unbiased filtering. Probe sets with a median log2 signal value of 5.00 were removed from further analysis. This approach ultimately removed 2,752 genes presumed to be unexpressed, leaving 6,241 genes for further analysis. A filter on the interquartile range was also applied to remove low-variability genes. Genes with an interquartile range of less than 0.5 across all chips in the experiment were excluded, reducing the data set to 3,000 genes for further analysis. The Linear Models for Microarray Data (LIMMA) package was used to perform differential expression analysis on the filtered gene list by the use of a linear model on log2 signal values, with an empirical Bayes correction to the variance (34). Comparisons of interest were extracted through contrasts, and P values were corrected for multiple comparisons using the Benjamini and Hochberg method (3). This analysis revealed that exposure to monensin caused specific and unique sets of genes to be up- or downregulated in wild-type parasites. A total of 383 genes were significantly upregulated (P < 0.05), while 1,544 genes were significantly downregulated after exposure to monensin. Table 1 lists the 30 most strongly downregulated genes, while Table 2 lists the 30 most strongly upregulated genes. Of the 30 most strongly downregulated genes for which some function is known or can be inferred based on homology, most appear to relate to cellular metabolism, particularly macromolecular biosynthesis (Table 1). Of the significantly upregulated genes, the major class with a known function were the histones (Table 2). Because a number of stressors can induce T. gondii tachyzoites to switch to the encysted bradyzoite stage, we also checked the microarray data to see how monensin affected transcription of known bradyzoite markers (6, 39). Monensin exposure did not significantly alter transcription of the bradyzoite markers BAG1, SAG4, SAG2C, LDH2, and ENO1, while transcription of BSR4, MAG1, and SRS9 was significantly downregulated by monensin exposure. Thus, it does not appear that monensin induces stage conversion to bradyzoites, at least in this T. gondii strain.
TABLE 1.
Microarray analysis shows that exposure to monensin causes significant downregulation in transcription of Toxoplasma genesa
| GeneID | Function/homology | Fold downregulation |
|---|---|---|
| TGME49 017740 | Oxoacyl-ACP reductase | 33.6 |
| TGME49 090200 | Opine dehydrogenase | 24.4 |
| TGME49 055450 | Hypothetical protein | 20.6 |
| TGME49 055450 | FUN14 family domain-containing protein | 18.8 |
| TGME49 094640 | Ribonucleoside-diphosphate reductase, large subunit | 18.8 |
| TGME49 039530 | Alanine-glyoxylate aminotransferase | 18.1 |
| TGME49 097120 | Hypothetical protein | 16.1 |
| TGME49 120110 | Proliferating cell nuclear antigen | 16.1 |
| TGME49 045670 | Pyruvate dehydrogenase | 16.0 |
| TGME49 043200 | Hypothetical protein | 15.6 |
| TGME49 067140 | SRS38B | 15.1 |
| TGME49 018540 | Hypothetical protein | 14.1 |
| TGME49 063500 | Vacuolar protein sorting 26 | 14.0 |
| TGME49 069870 | Hypothetical protein | 13.7 |
| TGME49 062730 | Rhoptry kinase family protein ROP16 | 12.8 |
| TGME49 116280 | Hypothetical protein | 12.2 |
| TGME49 097860 | Hypothetical protein | 12.1 |
| TGME49 064080 | Acyl carrier protein | 11.9 |
| TGME49 025050 | Adenosylhomocysteinase | 11.9 |
| TGME49 007060 | Ribonucleotide-diphosphate reductase, small subunit | 11.8 |
| TGME49 105510 | Hypothetical protein | 11.6 |
| TGME49 018270 | Hypothetical protein | 11.4 |
| TGME49 040630 | Protein kinase | 11.4 |
| TGME49 116260 | Hypothetical protein | 11.3 |
| TGME49 085860 | SRS20C | 11.3 |
| TGME49 007140 | SRS49B | 11.2 |
| TGME49 039750 | Hypothetical protein | 11.0 |
| TGME49 009160 | Glycylpeptide N-tetradecanoyltransferase | 11.0 |
| TGME49 118430 | Malate dehydrogenase | 11.0 |
| TGME49 024570 | Hypothetical protein | 10.9 |
Shown are the 30 genes with the largest transcriptional downregulation in response to monensin exposure (2.5 ng/ml for 24 h). ACP, acyl carrier protein.
TABLE 2.
Microarray analysis shows that exposure to monensin causes significant upregulation in transcription of Toxoplasma genesa
| GeneID | Function/homology | Fold upregulation |
|---|---|---|
| TGME49 039260 | Histone H4 | 23.1 |
| TGME49 105160 | Histone H2Ba | 11.6 |
| TGME49 045880 | Hypothetical protein | 8.8 |
| TGME49 068970 | Hypothetical protein | 8.3 |
| TGME49 000400 | Protein phosphatase 2A | 8.2 |
| TGME49 065320 | Cell cycle control protein encoded by cwf2 | 7.2 |
| TGME49 061240 | Histone H3 | 7.0 |
| TGME49 018740 | Hypothetical protein | 6.8 |
| TGME49 014410 | Hypothetical protein | 6.5 |
| TGME49 100050 | Hypothetical protein | 6.3 |
| TGME49 077230 | Hypothetical protein | 6.1 |
| TGME49 061580 | Histone H2AX | 5.9 |
| TGME49 061390 | Hypothetical protein | 5.1 |
| TGME49 070260 | Hypothetical protein | 5.0 |
| TGME49 092980 | Hypothetical protein | 4.9 |
| TGME49 045770 | Hypothetical protein | 4.8 |
| TGME49 026540 | Protein kinase | 4.8 |
| TGME49 106510 | Hypothetical protein | 4.8 |
| TGME49 108820 | Hypothetical protein | 4.7 |
| TGME49 089920 | Hypothetical protein | 4.7 |
| TGME49 061250 | Histone H2A | 4.5 |
| TGME49 017610 | Hypothetical protein | 4.3 |
| TGME49 023560 | Hypothetical protein | 4.1 |
| TGME49 008370 | Myosin heavy chain | 4.0 |
| TGME49 106620 | Platelet binding protein GspB (AP2 domain containing) | 4.0 |
| TGME49 115570 | Histone H1 | 3.9 |
| TGME49 038440 | SRS22A | 3.9 |
| TGME49 050120 | Hypothetical protein | 3.8 |
| TGME49 119360 | SRS17 | 3.8 |
| TGME49 105930 | Hypothetical protein | 3.8 |
Shown are the 30 genes with the largest transcriptional upregulation in response to monensin exposure (2.5 ng/ml for 24 h).
Further examination of transcriptional changes for all putative T. gondii histone genes showed significant upregulation of all the canonical histones (Table 3). These include H2A (TGME49 061250), H2B (TGME49 105160, i.e., H2Ba; the T. gondii H2Bb genomic variant does not appear to be transcribed [9]), H3 (TGME49 061240), H4 (TGME49 039260), and a homologue to the H1 linker histone of kinetoplastids (TGME49 115570). Of the variant histone genes, H2AX (TGME49 061580) is also upregulated by monensin exposure, while H2AZ (TGME49 100200) and an H3 variant (TGME49 025410, potentially the CenH3 centrosome-associated histone) are downregulated and H3.3 (TGME49 018260) and an H2B variant (H2Bv1; TGME49 009910) did not show significant change. Thus, upregulation of histones in response to monensin treatment is limited to the canonical histones and to H2AX.
TABLE 3.
Changes in transcript abundance of all known putative Toxoplasma histone subunits in response to monensina
| GeneID | Putative histone subunit | Fold change |
|---|---|---|
| TGME49 115570 | H1 | 3.9 up |
| TGME49 061250 | H2A | 4.5 up |
| TGME49 105160 | H2B | 11.6 up |
| TGME49 061240 | H3 | 7.0 up |
| TGME49 039260 | H4 | 23.1 up |
| TGME49 061580 | H2AX | 5.9 up |
| TGME49 100200 | H2AZ | 2.3 down |
| TGME49 009910 | H2Bv1 | NSC |
| TGME49 018260 | H3.3 | NSC |
| TGME49 025410 | CenH3 | 1.8 down |
All of the transcripts were validated by qPCR. NSC, no significant change in transcript abundance. Canonical histone subunits are shown in bold. See text for discussion of methods of microarray analysis and statistical comparison. up, upregulation; down, downregulation.
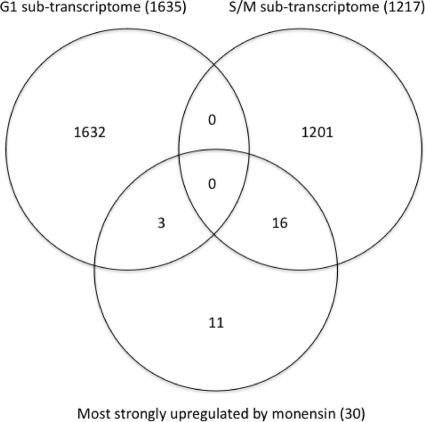
Transcription of canonical histones, but not variant histones, is largely restricted to S phase of the cell cycle (26). Such cell cycle-dependent regulation has been shown specifically in T. gondii for the canonical H2A subunit (30). The specific transcriptional upregulation of T. gondii canonical histones therefore suggests that monensin alters the parasite's cell cycle. We examined published subtranscriptomes for T. gondii in the G1 and S/M phases (2) to see if any of the 30 genes most upregulated in response to monensin also appeared to be cell cycle regulated. Figure 1 shows that of these 30 genes, 16 are also transcriptionally upregulated in S/M phase, 3 are transcriptionally upregulated in G1 phase, and 11 do not show cell cycle-specific transcriptional regulation. Thus, monensin appears to affect the parasite's cell cycle, specifically enriching for cells in S/M.
FIG. 1.
Venn diagram showing overlap of genes that are transcriptionally upregulated in G1 and S/M phases of the T. gondii cell cycle (2) and the 30 most strongly transcriptionally upregulated genes in T. gondii exposed to monensin. Values in parentheses indicate number of genes.
Transcriptional upregulation of canonical histones and H2AX by monensin is TgMSH-1 dependent.
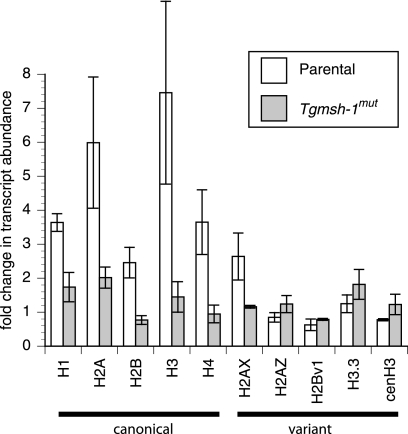
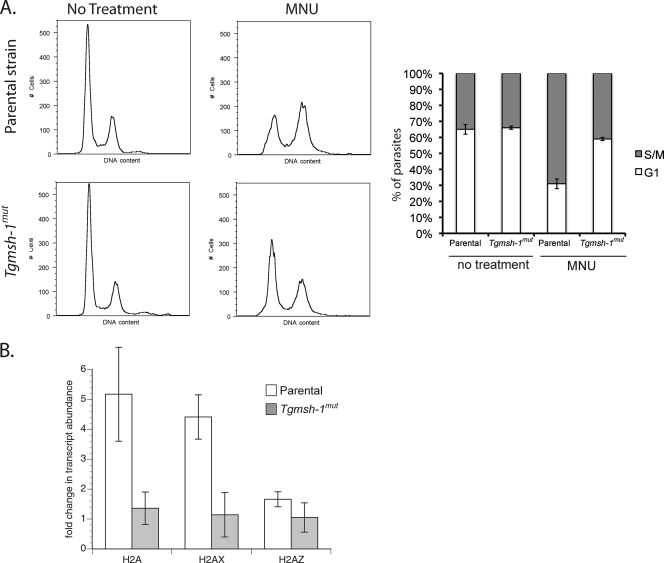
To confirm the upregulation in the levels of mRNAs for specific histones in response to monensin, we performed quantitative PCR (qPCR) comparing mRNA levels for all T. gondii histones in monensin-treated and untreated wild-type parasites (RH). Furthermore, to determine whether the effect on histone levels is related to the TgMSH-1-dependent monensin sensitivity that we recently reported (13), we performed the qPCR analysis using TgMSH-1-deficient parasites, referred to as the Tgmsh-1 mutant strain. For this purpose, RNA was isolated from RH or Tgmsh-1 mutant strains 24 h after invasion of human foreskin fibroblasts (HFFs) or 24 h after invasion of HFFs followed by 24-h exposure to monensin at 0.75 ng/ml. This lower concentration of monensin was used because it is the ideal one to observe the TgMSH-1 dependence of monensin sensitivity in terms of parasite death (13). The relative abundance of transcript for all 10 putative T. gondii histones in monensin-treated parasites was compared to the samples from parasites grown in the absence of monensin. There was no significant difference in transcript abundance for any of the histones between RH and Tgmsh-1 mutant strains grown under normal conditions (t test, P ≤ 0.05). After exposure to monensin, the RH strain showed significant increases in transcript abundance for the canonical histones H1, H2A, H2B, H3, and H4, as well as the H2AX variant (Fig. 2). The Tgmsh-1 mutant strain did not show statistically significant increases for any of these histones except H2A and H2AX. However, the upregulation of H2A and H2AX in Tgmsh-1 mutant parasites was significantly less than that for the RH strain (Fig. 2). Transcriptional abundance of the histone variants H2AZ, H3.3, and putative CenH3 was not significantly altered by exposure to monensin in either the mutant or parental parasite strain, while H2Bv1 was significantly downregulated in both strains. Thus, transcriptional upregulation of canonical histones and H2AX upon monensin treatment shows TgMSH-1 dependence, just as is observed for parasite death.
FIG. 2.
Real-time quantitative PCR (RT-qPCR) of histones in treated and untreated parasites. Relative transcript abundance (in comparison to that of RH under control conditions) for all known T. gondii histone subunits was determined by RT-qPCR for both parental (RH) and TgMSH1-deficient (Tgmsh-1mut) strains after exposure to monensin (0.75 ng/ml, 24 h). Each bar represents the mean value for three independent replicates. Error bar = 1 standard deviation.
Monensin causes TgMSH-1-dependent accumulation of parasites in late S phase.
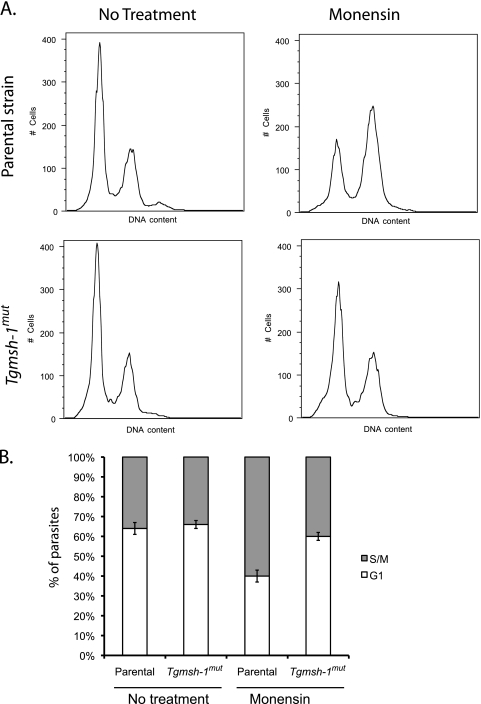
Because our microarray data suggested monensin was increasing the proportion of parasites in S/M phases, we examined monensin's effect on the T. gondii cell cycle by the use of flow cytometry (Fig. 3). Both parental RH and Tgmsh-1 mutant strains of T. gondii were allowed to invade and develop in HFFs for 24 h (control) or allowed to develop for 24 h and exposed for 24 h to 0.75 ng/ml monensin. Parasites were then syringe lysed to remove them from host HFFs and fixed, and their DNA was stained. It has been shown that T. gondii does not exhibit a typical accumulation of post-S-phase cells (i.e., 2N, or G2 phase of the cell cycle) (30). Instead there is a large subpopulation of cells at 1.8N, indicating chromosomal replication pauses or slows at this point. We found that when grown with normal growth mediums, both parental and Tgmsh-1 mutant strains showed a cell cycle distribution typical for asynchronously dividing populations of T. gondii: 64% ± 3% of the RH parasites in G1 (1N) and 36% ± 3% in S/M phases (primarily late S phase, 1.8N), and 66% ± 2% of Tgmsh-1 mutant parasites in G1 and 34% ± 2% in S/M (values represent means ± standard deviations for three independent experiments). In parental strain parasites exposed to monensin, there was a significant (t test, P ≤ 0.05) increase of parasites in late S phase, so that 40% ± 3% of parasites were in G1, while 60% ± 3% were in S/M. However, there was only a small change in cell cycle distribution of Tgmsh-1 mutant parasites exposed to monensin (G1 = 60% ± 2%, S/M = 40% ± 2%) that was shown to be not significantly different from the untreated sample by paired t test, indicating that the observed effect of monensin on the T. gondii cell cycle is TgMSH-1 dependent.
FIG. 3.
Flow cytometry analysis of parental (RH) and TgMSH1-deficient (Tgmsh-1mut) T. gondii strains in response to monensin. Intracellular parasites were exposed to either normal culture medium or culture medium plus 0.75 ng monensin/ml for 24 h. DNA content was measured using Sytox green. (A) Representative histograms are shown. Each histogram represents 10,000 total events. (B) Percentage ± standard deviation of parasites in G1 or S/M phases determined by gating for three separate experiments is indicated in the bar graphs.
Salinomycin and MNU cause TgMSH-1-dependent accumulation of parasites in late S phase.
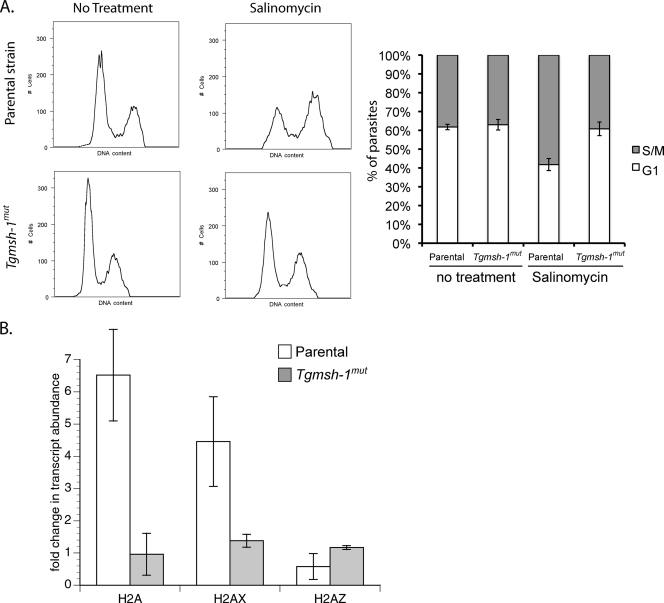
Previously, we showed that TgMSH-1-deficient mutants were also resistant to another coccidiostat ionophore, salinomycin (13). Accordingly, we investigated whether salinomycin had the same effect on histone transcript levels and cell cycle distribution. As can be seen in Fig. 4 A, salinomycin caused an accumulation of parental RH parasites in late S phase, and this effect was TgMSH-1 dependent. Asynchronously dividing populations of the RH strain showed a distribution of 62% ± 1% of parasites in G1 and 38% ± 1% in S/M phases, while asynchronously dividing populations of the Tgmsh-1 mutant showed 63% ± 3% in G1 and 37% ± 3% in S/M. After 24-h exposure to 15 ng/ml salinomycin, the relative percentage of RH strain parasites in G1 phase was 42% ± 3%, with 58% ± 3% of parasites in S/M phases, a significantly different distribution than the one seen for the untreated parental parasites (determined by t test, P ≤ 0.05). In Tgmsh-1 mutant parasites exposed to salinomycin, there was no significant change (determined by t test, P ≤ 0.05) in the cell cycle distribution of the parasites compared to untreated parasites, with 61% ± 4% in G1 and 39% ± 4% in S/M. Thus, the effect of salinomycin on the cell cycle, like that of monensin, is TgMSH-1 dependent. We also performed qPCR on RNA extracted from RH and Tgmsh-1 mutant parasites on the histones H2A, H2AX, and H2AZ to see if salinomycin had an effect on histone transcription similar to that of monensin. Exposure to 15 ng/ml salinomycin for 24 h caused significant increases (t test, P ≤ 0.05) in the transcript abundance of the canonical histone H2A and the variant histone H2AX, but not the variant H2AZ, similar to what was observed for monensin (Fig. 4B). In addition, salinomycin did not cause significant increases in transcription of canonical H2A or variant H2AX in the Tgmsh-1 mutant strain, indicating this response is TgMSH-1 dependent.
FIG. 4.
Effect of salinomycin on T. gondii cell cycle and histone transcription. (A) Flow cytometry analysis of parental (RH) and TgMSH1-deficient (Tgmsh-1mut) T. gondii in response to salinomycin. Intracellular parasites were exposed to either normal culture medium or culture medium plus 15 ng salinomycin/ml for 24 h. DNA content was measured using Sytox green. Representative histograms are shown. Each histogram represents 10,000 total events. Percentage ± standard deviation of parasites in G1 or S/M phases determined by gating for three separate experiments is indicated in the accompanying bar graph. (B) Real-time quantitative PCR (RT-qPCR) showing changes in transcript abundance for the canonical histone subunit H2A and the variant subunits H2AX and H2AZ after exposure to salinomycin (15 ng/ml, 24 h) in both RH and Tgmsh-1 mutant strains. Each bar represents the mean value for three independent replicates. Error bar = 1 standard deviation.
TgMSH-1-deficient mutants are also resistant to the DNA alkylating agent methylnitrourea (MNU). Thus, we analyzed the cell cycle distribution for RH and Tgmsh-1 mutant strains by flow cytometry to see if MNU also caused a TgMSH-1-dependent accumulation of parasites in late S phase (Fig. 5 A). Asynchronously dividing populations of the RH strain showed a distribution of 65% ± 3% of parasites in G1 and 35% ± 3% in S/M phases, while asynchronously dividing populations of the Tgmsh-1 mutant similarly showed 66% ± 1% in G1 and 34% ± 1% in S/M. After MNU exposure of RH strain parasites, we observed statistically significant changes in the cell cycle distribution (t test, P ≤ 0.05), with 31% ± 8% of parasites in G1 phase and 69% ± 8% in S/M phases. In contrast, in Tgmsh-1 mutant parasites exposed to MNU, there were significantly smaller changes in the cell cycle distribution of the parasites, with 59% ± 1% in G1 and 41% ± 1% in S/M, showing that the effect of MNU on the parasite cell cycle is also TgMSH-1 dependent. Furthermore, transcription of the histone subunits H2A, H2AX, and H2AZ in response to MNU was similar to the responses to monensin and salinomycin, with a significant (t test, P ≤ 0.05) increase in transcription of H2A and H2AX that was TgMSH-1 dependent (Fig. 5B). Thus, the anticoccidian ionophores monensin and salinomycin affect the T. gondii cell cycle in the same manner as a known DNA-damaging agent.
FIG. 5.
Effect of MNU on T. gondii cell cycle distribution and histone transcription. (A) Flow cytometry analysis of RH and Tgmsh-1 mutant strains in response to MNU (1 mM, 24 h). Percentage ± standard deviation of parasites in G1 or S/M phases determined by gating for three separate experiments is indicated in the accompanying bar graph. (B) RT-qPCR showing effects of MNU (1 mM, 24 h) on transcription of histone subunits H2A, H2AX, and H2AZ. Each bar represents the mean value for three independent replicates. Error bar = 1 standard deviation.
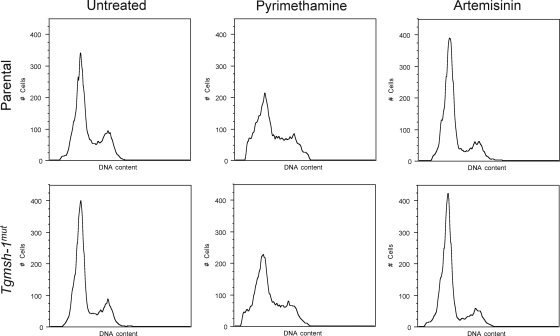
To determine the specificity of monensin's effect on the parasite's cell cycle, we examined the effects of two other antiparasitic drugs with different modes of action, pyrimethamine and artemisinin. Pyrimethamine inhibits the parasite's dihydrofolate reductase enzyme (11), while artemisinin appears to perturb calcium homeostasis in the parasite (27). Tgmsh-1 mutant parasites are not resistant to either of these drugs (E. M. Garrison and G. Arrizabalaga, unpublished observations). Parental and Tgmsh-1 mutant parasites were thus exposed to 0.5 μM pyrimethamine or 10 μM artemisinin for 24 h and analyzed for DNA content by fluorescence-activated cell sorter (FACS). As shown in Fig. 6, neither pyrimethamine nor artemisinin caused an accumulation of T. gondii in late S phase. Pyrimethamine caused a loss of a distinct late-S-phase peak, potentially as a result of degradation of DNA in dying 1.8N parasites. Artemisinin caused a slight increase in the proportion of parasites in G1 relative to late S/M phase. In addition, Tgmsh-1 mutant parasites showed identical responses to wild-type parasites. Thus, the accumulation of parasites in late S phase, as well as TgMSH-1 dependence, is specific to the ionophores monensin and salinomycin and the DNA alkylating agent MNU and not a general response to stress or antiparasitic drugs.
FIG. 6.
Flow cytometry analysis of RH and Tgmsh-1 mutant strains in response to pyrimethamine and artemisinin. Intracellular parasites were exposed to either normal culture medium or culture medium plus pyrimethamine (0.5 μm) or artemisinin (10 μm) for 24 h. DNA content was measured using Sytox green. Representative histograms are shown. Each histogram represents 10,000 total events.
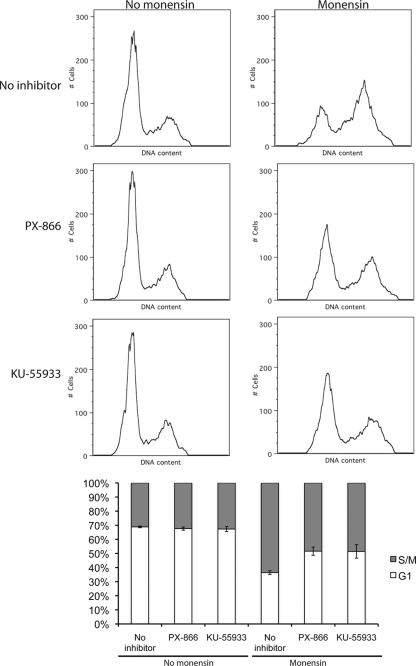
PX-866 and KU-55933 reduce the effects of monensin on parasite division and the cell cycle.
Because MutS homologues have been shown to mediate cell cycle arrest through the phosphoinositide-3-kinase (PI-3-kinase) family members ataxia-telangiectasia-mutated (ATM) kinase and ATM- and rad-3-related (ATR) kinase (28), we examined whether inhibitors of ATM/ATR function could abrogate the effect of monensin on T. gondii cell cycle distribution. PX-866 acts as a general inhibitor of PI-3-kinases, including ATM and ATR (19), while KU-55933 has been reported to specifically inhibit the function of ATM and thus serves to block or reduce cell cycle arrest caused by some DNA-damaging agents (18). To test for the effect of these compounds, RH strain parasites were allowed to invade and develop in HFF monolayers for 24 h. Growth medium was then replaced with DMEM plus 0.75 ng/ml monensin, or fresh DMEM alone (controls). After 6 h, PX-866 (final concentration of 5 μΜ) or KU-55933 (final concentration of 2 μM) was added to some of the samples. After a further 18-h incubation, parasites were isolated and analyzed by flow cytometry. Neither PX-866 nor KU-55933 alone affected T. gondii cell cycle distribution (Fig. 7). Control untreated samples showed 68.6% ± 0.6% of parasites in G1 and 31.4% ± 0.6% in S/M, while PX-866-exposed samples showed 67.6% ± 1.1% of parasites in G1 and 32.4% ± 1.1% in S/M, and KU-55933-exposed samples showed 67.3% ± 1.8% of parasites in G1 and 32.7% ± 1.8% in S/M. Thus, neither PX-866 nor KU-55933 alone significantly (t test, P ≤ 0.05) affected the parasite's cell cycle distribution. As with previous experiments, monensin alone caused a significant accumulation of cells in late S phase, with 36.4% ± 1.3% of parasites in G1 and 63.6% ± 1.3% in S/M (Fig. 7). In samples that were exposed to both monensin and PX-866 or KU-55933, this effect on the parasite's cell cycle was significantly reduced (t test, P ≤ 0.05) compared to monensin alone, with 51.6% ± 2.9% of parasites in G1 and 48.4% ± 2.9% in S/M for monensin plus PX-866 and 50.7% ± 5.4% in G1 and 49.3% ± 5.4% in S/M for KU-55933 (Fig. 7). Thus, PX-866 and KU-55933 cause a partial reduction in the monensin-dependent accumulation of parasites in late S phase, suggesting that this process may be mediated by a kinase with similarity to ATM.
FIG. 7.
Effect of PI-3-K family (PX-866) and ATM kinase (KU-55933) inhibitors on monensin-induced cell cycle redistribution. Intracellular parasites were exposed either to normal culture medium or culture medium plus 5μΜ PX-866 or 2μΜ KU-55933 for 18 h, 0.75 ng/ml monensin for 24 h, or 0.75 ng/ml monensin for 6 h, followed by 0.75 ng/ml monensin plus 5 μΜ PX-866 or 2 μΜ KU-55933 for 18 h. DNA content was measured using Sytox green and analyzed by flow cytometry. Representative histograms are shown. Each histogram represents 10,000 total events. Percentage ± standard deviation of parasites in G1 or S/M phases determined by gating for three separate experiments is indicated in the bar graph.
DISCUSSION
Previously, we demonstrated that monensin resistance in a mutated strain of T. gondii was due to disruption of a homologue of the MutS DNA mismatch repair enzyme (TgMSH-1) (13). MutS and MutS homologues have been demonstrated to mediate both mismatch repair and recognition of DNA damage and consequent cell cycle arrest (8, 28). In fact, mutations rendering MutS nonfunctional result in a phenotype of resistance to a number of DNA-damaging drugs, by preventing cell cycle arrest and consequent cell death (1). The mode of action of monensin has been understood primarily in terms of its function as a Na+/H+ antiporter and consequent potential disruption of ionic homeostasis, as well as a demonstrated inhibition of vesicular transport. However, the identification of TgMSH-1 as a mediator of monensin resistance (13) suggests that monensin may alter the cell cycle, perhaps as a consequence of inducing some type of DNA damage or some other cellular stress. Our results show TgMSH-1 transcriptional upregulation of the S-phase-dependent canonical histones in response to monensin, as well as TgMSH-1-dependent accumulation of cells with an approximately 1.8N DNA content, indicative of late S phase. We obtained similar results with a second coccidiostat ionophore, salinomycin, to which TgMSH-1-deficient mutants had previously been demonstrated resistant (13). Thus, TgMSH-1 appears to mediate coccidiostat ionophore susceptibility and resistance through its effect on the T. gondii cell cycle.
The fact that monensin resistance can result from the loss of TgMSH-1, which has significant homology to DNA damage repair enzymes, suggests that monensin may in part act on the parasite by directly or indirectly causing DNA damage. We have not yet directly demonstrated DNA damage as a result of exposure to monensin. However, several lines of evidence suggest that this could be the case. TgMSH-1-deficient parasites are resistant not only to monensin, but also to MNU, a drug with known DNA-damaging action. Parasites also accumulate in late S phase in a TgMSH-1-dependent manner in response to MNU, in a pattern very similar to that caused by monensin. Thus, it appears that monensin and the DNA-damaging agent MNU may have similar modes of action on T. gondii. Also, monensin exposure resulted in upregulation of not only the cell cycle-dependent canonical histones, but also one of the variant histones, H2AX. H2AX has been shown to mediate DNA damage repair (12). In addition, it has been shown that H2AX transcription is upregulated in T. gondii in response to oxidative DNA damage (10). Previously, Singh et al. (32) demonstrated that monensin caused significant oxidative stress and DNA fragmentation in rat testicular cells. Other genes that our microarrays indicated were transcriptionally upregulated in response to monensin include that which encodes the protein phosphatase 2A (TGME49 000400), which has been shown to mediate signal transduction in DNA damage response, resulting in cell cycle arrest (24).
Because of the subcellular localization of TgMSH-1 in the parasite's mitochondrion, we hypothesize that if monensin is causing DNA damage, it is targeting mitochondrial DNA. Most of our understanding of the function of MutS homologues is based on studies of nuclear proteins. However, mitochondrial MutS homologues, such as MSH1 from Saccharomyces cerevisiae, have been shown to function in base excision repair, protecting the mitochondria from oxidative damage (29). A role for mitochondrial MSHs in regulating the cell cycle akin to that of nuclear MSHs has not been established, but our data indicate that this could be the case in T. gondii. Thus, the mode of action of monensin against parasites such as T. gondii may be due to the mitochondrial DNA (mtDNA)-damaging properties of the drug, rather than a direct consequence of osmotic stress or energy depletion, as has been suggested (33). This DNA damage may be a general consequence of ionic disruption in the mitochondrion, as we have found that the Na+/Ca2+ ionophore salinomycin has a very similar TgMSH-1-dependent effect on the parasite's cell cycle. Further research will be required to determine if monensin does, in fact, cause mitochondrial DNA damage.
Because flow cytometric analysis of the T. gondii cell cycle reveals no accumulation of parasites with a duplicated genome (2N), it has been suggested that T. gondii does not possess a G2 phase (16, 30). Instead, T. gondii accumulates in late S phase (approximately 1.8N). However, it is not known if this represents a checkpoint analogous to the G2 phase of other organisms or is some other type of pause in or slowing of DNA synthesis. Interestingly, Gubbels et al. (15) isolated a temperature-sensitive T. gondii mutant that showed an accumulation of parasites in late S phase very similar to what we observed for T. gondii treated with monensin. This mutant appeared to be defective in an AAA ATPase, a class of enzymes whose loss of function can in some cases result in mitotic arrest. Although we do not hypothesize that this particular enzyme mediates the TgMSH-1-dependent response to monensin, these results do suggest that such an accumulation of parasites in late S phase may reflect a broader mechanism of cell cycle arrest in T. gondii. Our results also suggest that there are some functional similarities between this late-S-phase accumulation and the G2 checkpoint. As previously mentioned, MutS homologues from other organisms have been shown to mediate cell cycle arrest at G2 in response to DNA damage, and MSH-deficient cells are frequently resistant to DNA-damaging drugs. Thus, the Tg-MSH1-dependent accumulation of T. gondii in late S phase in response to monensin may reflect a cell cycle checkpoint arrest in response to damaged DNA. Our finding that PX-866 and KU-55933 reduce the monensin-induced accumulation of T. gondii in late S phase further indicates that this late-S-phase accumulation may be functioning as a cell cycle checkpoint, specifically under the control of an ATM- or ATR-like molecule. Vonlaufen et al. (36) have identified a putative ATM kinase from T. gondii whose expression is regulated by a MYST family acetyltransferase. However, neither PX-866 nor KU-55933 completely abrogated the effect of monensin on late-S-phase parasite accumulation, and further research is needed to identify which T. gondii kinase(s) may operate downstream of TgMSH-1. Also, any arrest of parasites in late S phase in response to drugs like monensin or MNU is not absolute. A proportion of the parasite population remains in G1 even after exposure to monensin. It is not clear whether this subpopulation is comprised of parasites that are able to eventually move through late S phase and complete the cell cycle or of parasites that remain in G1 (or G0) and never enter S phase.
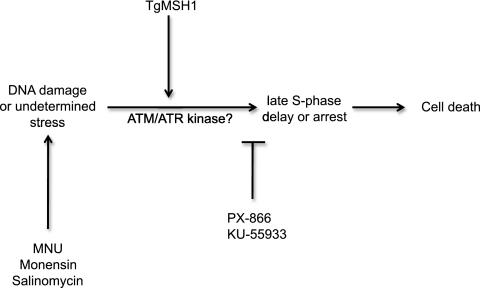
Based on these findings and the known roles of MutS homologues in other organisms, we propose a preliminary model in which monensin directly or indirectly causes mitochondrial DNA damage or some unrelated stress in the parasite (Fig. 8). TgMSH1 mediates detection of this damage or stress and induction of a signaling cascade that leads to delay or arrest in late S phase, potentially mediated by ATM, ATR, or a similar damage control checkpoint kinase, ultimately resulting in the death of the parasite by an unknown mechanism. TgMSH-1-deficient mutants are able to proceed through S phase at a much greater rate in the presence of monensin and thus show a resistance phenotype. Together, our results suggest that DNA repair and cell cycle disruption may be novel and important mediators of susceptibility and resistance to antiparasitic drugs in apicomplexans.
FIG. 8.
Model for TgMSH-1-dependent monensin action on T. gondii. In this model, monensin results directly or indirectly in damage to mitochondrial DNA or another undetermined stress. This results in a TgMSH-1-mediated signal transduction cascade, possibly involving ATM/ATR or similar damage control checkpoint kinases, which causes an arrest or delay of the parasite cell cycle in late S phase. Wild-type parasites with altered cell cycles then die by an unknown mechanism. TgMSH-1-deficient mutants can continue to divide in the presence of monensin and thus show a resistance phenotype.
Acknowledgments
This work was supported by NIH grant R01 AI 89808-01, NIH grant P20 RR15587 from the NCRR Center of Biomedical Research Centers, and a grant from the American Cancer Society, 115626-RSG-08-193-01-MBC.
We thank Matthew Settles for his assistance with the analysis of the microarray data.
Footnotes
Published ahead of print on 22 November 2010.
REFERENCES
- 1.Aebi, S., et al. 1997. Resistance to cytotoxic drugs in DNA mismatch repair-deficient cells. Clin. Cancer Res. 3:1763-1767. [PubMed] [Google Scholar]
- 2.Behnke, M. S., et al. 2010. Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PLoS One 5:e12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R Stat. Soc. B Stat. Methodol. 57:289-300. [Google Scholar]
- 4.Bolstad, B. M., R. A. Irizarry, M. Astrand, and T. P. Speed. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185-193. [DOI] [PubMed] [Google Scholar]
- 5.Chen, T., W. Zhang, J. Wang, H. Dong, and M. Wang. 2008. Eimeria tenella: analysis of differentially expressed genes in the monensin- and maduramicin-resistant lines using cDNA array. Exp. Parasitol. 119:264-271. [DOI] [PubMed] [Google Scholar]
- 6.Cleary, M. D., U. Singh, I. J. Blader, J. L. Brewer, and J. C. Boothroyd. 2002. Toxoplasma gondii asexual development: identification of developmentally regulated genes and distinct patterns of gene expression. Eukaryot. Cell 1:329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couzinet, S., J. F. Dubremetz, D. Buzoni-Gatel, G. Jeminet, and G. Prensier. 2000. In vitro activity of the polyether ionophorous antibiotic monensin against the cyst form of Toxoplasma gondii. Parasitology 121(4):359-365. [DOI] [PubMed] [Google Scholar]
- 8.Dalhus, B., J. K. Laerdahl, P. H. Backe, and M. Bjoras. 2009. DNA base repair—recognition and initiation of catalysis. FEMS Microbiol. Rev. 33:1044-1078. [DOI] [PubMed] [Google Scholar]
- 9.Dalmasso, M. C., et al. 2006. Toxoplasma gondii has two lineages of histones 2b (H2B) with different expression profiles. Mol. Biochem. Parasitol. 148:103-107. [DOI] [PubMed] [Google Scholar]
- 10.Dalmasso, M. C., D. O. Onyango, A. Naguleswaran, W. J. Sullivan, Jr., and S. O. Angel. 2009. Toxoplasma H2A variants reveal novel insights into nucleosome composition and functions for this histone family. J. Mol. Biol. 392:33-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derouin, F. 2001. Anti-toxoplasmosis drugs. Curr. Opin. Invest. Drugs 2:1368-1374. [PubMed] [Google Scholar]
- 12.Downs, J. A., N. F. Lowndes, and S. P. Jackson. 2000. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature 408:1001-1004. [DOI] [PubMed] [Google Scholar]
- 13.Garrison, E. M., and G. Arrizabalaga. 2009. Disruption of a mitochondrial MutS DNA repair enzyme homologue confers drug resistance in the parasite Toxoplasma gondii. Mol. Microbiol. 72:425-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentleman, R. C., et al. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubbels, M. J., et al. 2008. Forward genetic analysis of the apicomplexan cell division cycle in Toxoplasma gondii. PLoS Pathog. 4:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gubbels, M. J., M. White, and T. Szatanek. 2008. The cell cycle and Toxoplasma gondii cell division: tightly knit or loosely stitched? Int. J. Parasitol. 38:1343-1358. [DOI] [PubMed] [Google Scholar]
- 17.Haberkorn, A. 1996. Chemotherapy of human and animal coccidioses: state and perspectives. Parasitol. Res. 82:193-199. [DOI] [PubMed] [Google Scholar]
- 18.Hickson, I., et al. 2004. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64:9152-9159. [DOI] [PubMed] [Google Scholar]
- 19.Ihle, N. T., et al. 2004. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol. Cancer Ther. 3:763-772. [PubMed] [Google Scholar]
- 20.Irizarry, R. A., et al. 2003. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irizarry, R. A., et al. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249-264. [DOI] [PubMed] [Google Scholar]
- 22.Kim, K., and L. M. Weiss. 2004. Toxoplasma gondii: the model apicomplexan. Int. J. Parasitol. 34:423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence, K. 1988. Use of monensin sodium against Toxoplasma. Vet. Rec. 123:323. [DOI] [PubMed] [Google Scholar]
- 24.Li, H. H., X. Cai, G. P. Shouse, L. G. Piluso, and X. Liu. 2007. A specific PP2A regulatory subunit, B56gamma, mediates DNA damage-induced dephosphorylation of p53 at Thr55. EMBO J. 26:402-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmoudi, N., et al. 2006. Identification of new antimalarial drugs by linear discriminant analysis and topological virtual screening. J. Antimicrob. Chemother. 57:489-497. [DOI] [PubMed] [Google Scholar]
- 26.Marzluff, W. F., E. J. Wagner, and R. J. Duronio. 2008. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat. Rev. Genet. 9:843-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagamune, K., W. L. Beatty, and L. D. Sibley. 2007. Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot. Cell 6:2147-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien, V., and R. Brown. 2006. Signalling cell cycle arrest and cell death through the MMR System. Carcinogenesis 27:682-692. [DOI] [PubMed] [Google Scholar]
- 29.Pogorzala, L., S. Mookerjee, and E. A. Sia. 2009. Evidence that msh1p plays multiple roles in mitochondrial base excision repair. Genetics 182:699-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radke, J. R., et al. 2001. Defining the cell cycle for the tachyzoite stage of Toxoplasma gondii. Mol. Biochem. Parasitol. 115:165-175. [DOI] [PubMed] [Google Scholar]
- 31.Shirley, M. W., A. L. Smith, and D. P. Blake. 2007. Challenges in the successful control of the avian coccidia. Vaccine 25:5540-5547. [DOI] [PubMed] [Google Scholar]
- 32.Singh, M., N. R. Kalla, and S. N. Sanyal. 2006. Effect of monensin on the enzymes of oxidative stress, thiamine pyrophosphatase and DNA integrity in rat testicular cells in vitro. Exp. Toxicol. Pathol. 58:203-208. [DOI] [PubMed] [Google Scholar]
- 33.Smith, C. K., II, and R. B. Galloway. 1983. Influence of monensin on cation influx and glycolysis of Eimeria tenella sporozoites in vitro. J. Parasitol. 69:666-670. [PubMed] [Google Scholar]
- 34.Smyth, G. K. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:Article3. [DOI] [PubMed] [Google Scholar]
- 35.Tartakoff, A. M. 1983. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell 32:1026-1028. [DOI] [PubMed] [Google Scholar]
- 36.Vonlaufen, N., A. Naguleswaran, I. Coppens, and W. J. Sullivan, Jr. 2010. MYST family lysine acetyltransferase facilitates ataxia telangiectasia mutated (ATM) kinase-mediated DNA damage response in Toxoplasma gondii. J. Biol. Chem. 285:11154-11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, Z., J. Shen, X. Suo, S. Zhao, and X. Cao. 2006. Experimentally induced monensin-resistant Eimeria tenella and membrane fluidity of sporozoites. Vet. Parasitol. 138:186-193. [DOI] [PubMed] [Google Scholar]
- 38.Wang, Z., X. Suo, X. Xia, and J. Shen. 2006. Influence of monensin on cation influx and Na+-K+-ATPase activity of Eimeria tenella sporozoites in vitro. J. Parasitol. 92:1092-1096. [DOI] [PubMed] [Google Scholar]
- 39.Weiss, L. M., and K. Kim. 2000. The development and biology of bradyzoites of Toxoplasma gondii. Front. Biosci. 5:D391-D405. [DOI] [PMC free article] [PubMed] [Google Scholar]