Abstract
Infection is an important medical problem associated with the use of bone allografts. To retard bacterial colonization, we have recently reported on the modification of bone allografts with the antibiotic vancomycin (VAN). In this report, we examine the ability of this antibiotic-modified allograft to resist bacterial colonization and biofilm formation. When antibiotic was coupled to the allograft, a uniform distribution of the antibiotic was apparent. Following challenges with Staphylococcus aureus for 6 h, the covalently bonded VAN decreased colonization as a function of inoculum, ranging from 0.8 to 2.0 log10 CFU. Furthermore, the VAN-modified surface resisted biofilm formation, even in topographical niches that provide a protected environment for bacterial adhesion. Attachment of the antibiotic to the allograft surface was robust, and the bonded VAN was stable whether incubated in aqueous media or in air, maintaining levels of 75 to 100% of initial levels over 60 days. While the VAN-modified allograft inhibited the Gram-positive S. aureus colonization, in keeping with VAN′s spectrum of activity, the VAN-modified allograft was readily colonized by the Gram-negative Escherichia coli. Finally, initial toxicity measures indicated that the VAN-modified allograft did not influence osteoblast colonization or viability. Since the covalently tethered antibiotic is stable, is active, retains its specificity, and does not exhibit toxicity, it is concluded that this modified allograft holds great promise for decreasing bone graft-associated infections.
Bacterial adhesion to surfaces is a common phenomenon that, in a permissive environment, initiates formation of biofilm, a thick polymeric matrix that engulfs the resident organisms, and protects them from environmental challenges (16). Biofilm formation on medical devices and transplanted tissues is especially problematic, as it allows resident bacteria to evade immune surveillance and greatly attenuates antibiotic effectiveness (6). While high concentrations of antibiotics from controlled-release systems can effectively combat acute infections, as time progresses and antibiotic elution wanes, bacterial colonization and biofilms can be reestablished (5).
Bacterial adhesion, infection, and subsequent rejection are important medical problems associated with use of bone allografts (2). When implanted, the allograft matrix provides an ideal substrate for bacterial adhesion and biofilm formation. The highly porous nature of the bone further enhances bacterial colonization by making grafts inaccessible to immune surveillance and local cellular defense mechanisms (17). Moreover, since penetration of antibiotics into the graft is poor, bacterial colonization/infection is difficult to treat. Indeed, because of the high prevalence of infection (as high as 14 to 16% [3, 11]), high levels of antibiotics are commonly adsorbed to the allograft prior to implantation (4), a procedure that can slow incorporation of the graft into the surrounding bone tissue due to elution of toxic levels of antibiotics (7). In this paper we describe a noneluting permanent system that may circumvent the toxicity associated with high levels of antibiotics (1).
To overcome infection-related problems linked to the use of bone allografts, we have recently reported modification of the graft with the antibiotic vancomycin (VAN) (9), where this modification is stable for at least 60 days (10). In this study, we examined the ability of this modified allograft to resist bacterial colonization. We show that the covalently bonded vancomycin inhibits colonization by Staphylococcus aureus. Moreover, the vancomycin surface resists biofilm formation while maintaining biocompatibility.
MATERIALS AND METHODS
Experimental design.
VAN was covalently bonded to allograft bone (referred to as VAN-allograft), and the extent of bonding was visualized by immunohistochemistry; this bonding was stable under various conditions for at least 7 days. To evaluate its biocidal activity, the allograft was exposed to S. aureus and inhibition of bacterial adhesion was determined as a function of initial inoculum and incubation time. In these experiments, bacterial surface colonization was measured by determining the numbers of bacterial CFU adherent to the treated allograft bone. In parallel, adherent bacteria were visualized by treatment with a vital stain and by scanning electron microscopy (SEM). The antimicrobial specificity of bonded VAN was next determined by incubation of the VAN-allograft with the Gram-negative organism Escherichia coli, an organism that is insensitive to this antibiotic. Also, because bacteria that are adherent to surfaces acquire a “resilient” phenotype, the effects of solution antibiotics on survival of bacteria present on the VAN-allograft were determined. Finally, the effects of the VAN-allograft on osteoblastic cell adhesion, proliferation, and cell viability (measured by comparison of free with cellular lactate dehydrogenase activity) were determined as an initial assessment of biocompatibility.
Procedure for covalently bonding antibiotic to the bone allograft (Fig. 1 a).
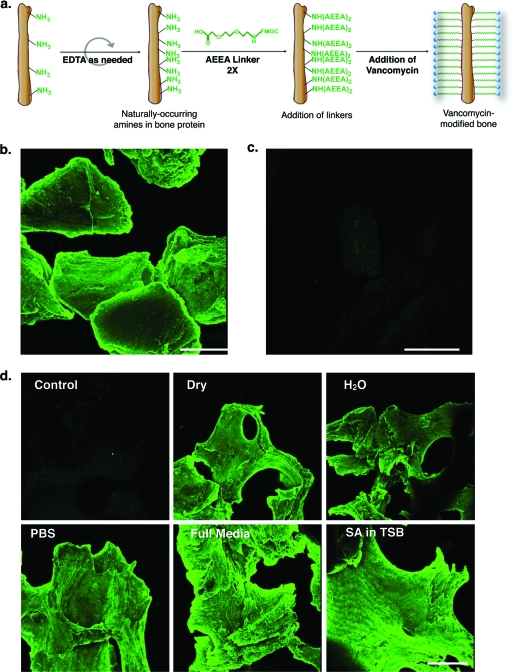
FIG. 1.
VAN attachment to and stability on allograft bone. (a) Cartoon showing the steps in the synthesis of the VAN-allograft. Human morselized allograft bone was first partially demineralized by EDTA treatment to increase the number of exposed primary amines. The free amines were then coupled to two AEEA linkers. VAN was then coupled to these end-to-end linkers to yield vancomycin-modified bone (VAN-allograft). (b) The allograft-tethered VAN was detected with an anti-VAN antibody. Note the extent and homogeneity of fluorescence on the surface of the bone. (c) The control bone was also incubated with the anti-VAN antibody. Only a low, diffuse level of fluorescence was apparent. Bar, 400 μm. (d) Amounts of tethered VAN remaining on the VAN-allograft after 7 days of incubation in air (dry), H2O, PBS, or DMEM with 10% FBS (full medium) or with S. aureus in TSB (SA in TSB) were detected by staining with the anti-VAN antibody. Note that under all conditions, VAN fluorescence remains high, indicating retention of the tethered antibiotic. Bar, 400 μm.
VAN was covalently coupled to cancellous/cortical human bone granules (0.5 to 2.0 mm, a gift of the Musculoskeletal Transplant Foundation [Edison, NJ]), as previously described (9). Briefly, the granules were washed, sonicated with distilled water (dH2O) until eluates were clear, and incubated in 12.5% EDTA at pH 7 and room temperature (RT) for 3 days with shaking. Prior to coupling, samples were washed with dH2O and sonicated twice for 30 min in dimethylformamide (DMF) (Acros Organics, Morris Plains, NJ). The washed allograft was reacted with 10 mg/ml 9-fluorenylmethoxy carbonyl-[2-(2-amino-ethoxy)-ethoxy]-acetic acid (Fmoc-AEEA) (Peptides International, Louisville, KY) in DMF-diisopropylethylamine (DIEA) (100:1; Fisher Scientific, Pittsburgh, PA) in the presence of O-(7-azabenzo-triazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU) (2 mg/ml; Sigma-Aldrich, St. Louis, MO), followed by deprotection with 20% piperidine (Alfa Aesar, Ward Hill, MA) in DMF; a total of two linker additions were performed. The two linkers on a single stem were then coupled with 10 mg/ml clinical grade VAN (American Pharmaceutical Partners, Inc., Schaumburg, IL) in DMF-DIEA in the presence of HATU. Allograft bone modified with VAN (VAN-allograft) was washed extensively with DMF and dH2O to elute any VAN adsorbed (rather than bonded) during the synthesis. Washing was deemed complete when the eluent no longer caused inhibition of S. aureus growth as tested by a disk diffusion assay. In this assay, the eluent is spotted onto a standard filter paper disk which is laid onto an agar plate uniformly seeded with S. aureus; the clear zone around the filter paper is roughly indicative of antibiotic concentration. Washed allograft was dried and used for the experiments described below.
Immunohistochemical detection of VAN bonded to the allograft.
The VAN-allograft was washed three times with phosphate-buffered saline (PBS), blocked with 10% fetal bovine serum (FBS) in PBS (blocking buffer) for 1 h, and incubated with rabbit anti-VAN IgG (1:500; U.S. Biological, Swampscott, MA) in blocking buffer and 4°C for 12 h. Samples were washed three times with PBS and incubated with an Alexa Fluor 488-goat anti-rabbit IgG secondary antibody (1:1,000; Invitrogen, Carlsbad, CA) at RT for 1 h. Following three PBS washes, samples were visualized by epifluorescence.
Biocidal activity of the allograft.
Ten milligrams of allograft was sterilized with 70% ethanol for 15 min, followed by washing three times with PBS and three times with Trypticase soy broth (TSB). The allograft was challenged with S. aureus (Xen36 derived from ATCC 49525 [Caliper Life Science, Hopkinton, MA]; Xen36 is a methicillin-sensitive strain of S. aureus that shows normal growth on mannitol salts agar [Caliper Life Science]) or E. coli (DH5α) and cultured in TSB at 250 rpm and 37°C for 12 to 14 h (overnight culture). Using a 0.5 McFarland standard (a turbidity standard where an A600 of 0.10 yields ∼1 × 108 CFU/ml), 1 ml of 1 × 104 CFU/ml bacteria was incubated with the sterilized allograft in TSB at 37°C without agitation. Upon harvesting, samples were washed three times with PBS to remove nonadherent bacteria and either (i) stained with the Live/Dead BacLight kit (Invitrogen; 20 min, RT) and visualized or (ii) washed three more times, sonicated for 5 min in 0.3% Tween 80 in PBS to suspend the adherent bacteria, and, after serial dilution, plated on 3M Petrifilms (3M Corp, St. Paul, MN). Films were scanned, and the colonies were counted using Adobe Photoshop CS3.
Microscopy of the allograft. (i) Confocal laser scanning microscopy.
Fluorescently stained allograft morsels or cubes were visualized using optical sectioning in the Z plane, followed by reconstruction using a confocal laser scanning microscope (Olympus Fluoview 300).
(ii) Scanning electron microscopy.
Allograft morsels were fixed with 4% paraformaldehyde for 1 h and dehydrated with an ethanol series. After vacuum drying overnight, samples were sputter coated with gold and visualized using a Hitachi TM-1000 SEM. For each assessment, several separate morsels were examined.
Bacterial growth/adhesion in the presence of exogenous antibiotic.
To evaluate the antibiotic susceptibility of bacteria adherent to the allograft, control allograft or VAN-allograft was incubated with S. aureus (1 ml of 104 CFU/ml) in TSB for 12 h with or without 10 μg/ml VAN. After incubation, samples were washed three times with PBS, moved to a new well, washed three more times, and incubated in TSB for 4 h. Alternately, control allograft or VAN-allograft was incubated with 1 ml of S. aureus in 1 ml of TSB for 12 h, washed three times with PBS, moved to a new well, washed three more times, and incubated in 1 ml of TSB with or without 10 μg/ml VAN for 4 h. At the end of the 16 h of incubation, all allograft was washed as described above and bacterial colonization was assessed by direct counts.
Stability of the tethered antibiotic.
Samples (∼10 mg) of control allograft or VAN-allograft were incubated in 1 ml of (i) H2O, (ii) PBS, (iii) Dulbecco modified Eagle medium (DMEM)-F-12 with 10% FBS, or (iv) S. aureus in TSB for 7 days at 37°C. At the end of 1 week, samples exposed to bacteria were sonicated in 1% SDS for 30 min to remove adherent bacteria. All samples were then washed, stained for VAN by immunohistochemistry, and visualized.
Cell culture.
Larger (1 by 1 cm), flat samples of cortical control or VAN-allograft were sterilized with 70% ethanol, rinsed three times with PBS and three times with DMEM-F-12 containing 10% FBS (complete medium), and exposed to UV radiation for 10 min. Sterilized samples were incubated with 1 ml of 50,000 cells/ml of a temperature-sensitive, immortalized human fetal osteoblastic cell line (hFOBs) (8) in complete medium for up to 3 days. After 48 h, cells were harvested for SEM or staining for actin.
Actin cytoskeletal visualization.
hFOB cells were fixed in 4% paraformaldehyde for 10 min at RT, permeabilized with 0.1% Triton X-100 in PBS for 5 min at RT, and, after incubation with blocking buffer for 30 min, stained with Alexa 488-phalloidin (1:1,000; Invitrogen) and propidium iodide (1:100; Invitrogen) for 30 min at RT. Stained cells were then visualized using confocal laser scanning microscopy.
Toxicity assessment.
hFOB cells were plated on the flat cortical control or VAN-allograft samples and, after 1 or 3 days, lysed by incubation with M-PER lysis buffer (Thermo Scientific/Pierce Biotechnology, Rockford, IL) for 30 min with shaking. Total protein content was determined with a Bradford protein assay (Thermo Scientific) (15). In parallel, released lactate dehydrogenase (LDH) activity was measured using the Tox-7 kit (Sigma-Aldrich), per the manufacturer's instructions (14).
Statistical analysis.
Experiments were performed at least three times, and data are presented as means ± standard errors. Statistical analyses were performed on normal equally variant data, unless indicated otherwise in the figure legends. Differences were determined using a one- or two-way analysis of variance (ANOVA) with a Tukey multiple-comparison procedure. An alpha value of 0.05 was considered significant.
RESULTS
VAN-allograft shows uniform coverage with VAN that is stable after incubation for 7 days.
We first determined if coupling of two AEEA linkers followed by VAN onto the surface of the partially demineralized bone allograft resulted in detectable VAN coverage. After antibody staining, the VAN-allograft elicited a bright, uniform fluorescence over the surface of the bone particles (Fig. 1b), suggesting successful covalent attachment of VAN to bone. In contrast, the control samples showed weak, diffuse fluorescence (Fig. 1c). When the stability of the antibiotic attached to the allograft was evaluated, immunofluorescent staining for VAN remained undiminished after 7 days in air, H2O, PBS, or complete medium (DMEM) or even after incubation with S. aureus in TSB; control bone showed no fluorescence (Fig. 1d). Thus, the antibiotic appeared to remain stably attached to the allograft when exposed to media likely to be encountered both in vitro and in vivo.
VAN-allograft resists biofilm formation.
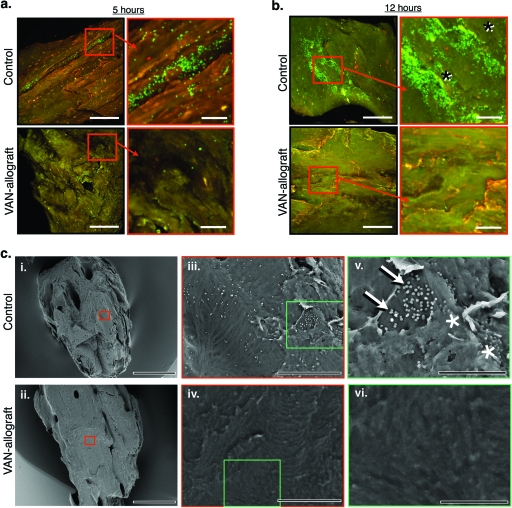
To determine VAN-allograft activity, S. aureus was cultured on either control allograft or VAN-allograft for 5 h or 12 h. When adherent bacteria were stained with the Live/Dead dye and examined using confocal laser scanning microscopy (viable bacteria fluoresce bright green), S. aureus microcolonies were apparent on the control bone allograft by 5 h (Fig. 2 a, top row) and displayed a predilection for natural topographical niches. By 12 h, control bone exhibited bacterial aggregates and patchy stain uptake, features that are consistent with biofilm formation (Fig. 2b, top row; aggregates are noted by asterisks in the high-magnification picture). Under the same conditions, the VAN-allograft remained largely free of bacterial colonies (5 h, Fig. 2a, bottom row; 12 h, Fig. 2b, bottom row). To better characterize biofilm formation, the VAN-allograft was evaluated by SEM following a 6-h bacterial challenge. The low-magnification micrographs show that the sizes and geometries of the VAN-coupled and control allografts were very similar (Fig. 2C, i and ii). At higher magnification (×1,500), the control allograft exhibited small, uniformly distributed, spherical structures (Fig. 2C, iii) that were absent from the VAN-allograft (Fig. 2C, iv). At the highest magnification (×4,000), the spherical structures (diameter, ∼1 μm) on the control surface were identifiable as bacteria aggregated into grape-like clusters characteristic of S. aureus. A thin layer of glycocalyx matrix covering the microorganisms was apparent (Fig. 2C, v [arrows, adherent bacteria; asterisks, bacteria encased in biofilm]). In contrast, the surface of the VAN-allograft appeared to be colony free (Fig. 2C, vi) aside from an occasional bacterium,
FIG. 2.
VAN-allograft resists S. aureus attachment over time. (a) After a 5-h challenge, S. aureus adhered to control allograft or VAN-allograft, as visualized using the Live/Dead stain and confocal laser scanning microscopy. Low-magnification pictures are shown on the left, with areas of interest boxed and shown at higher magnification on the right. Note the abundant bright green (viable) S. aureus colonies on the control allograft that are absent on the VAN-allograft. (b) S. aureus adhered to control or VAN-allograft after a 12-h S. aureus challenge, as visualized by Live/Dead staining and fluorescence microscopy. Low-magnification pictures are shown on the left, with higher magnification of the boxed area of interest on the right. The control allograft shows areas of intense fluorescence, which upon high magnification appear to be indicative of biofilm formation (asterisks). In contrast, the VAN-allograft does not exhibit green staining at either low or high magnification. Bars, 200 μm for low magnification and 50 μm for high magnification. (c) Bacterial colonization of control allograft or VAN-allograft after challenge with S. aureus for 6 h as visualized by SEM. (i and ii) Control bone morsels (i) and VAN-allograft morsels (ii) were of similar size and morphology. (iii) At higher magnification (×1,500), small, uniformly distributed spherical structures consistent with S. aureus colonies are evident on the control bone. (iv) At the same magnification, no equivalent spherical structures are apparent on the VAN-allograft. (v) At even higher magnification (×4,000), control bone can be seen to be populated by bacteria (∼1 μm in diameter), which aggregate in clusters characteristic of S. aureus (arrows). Also note the thin layer of biofilm covering some of the bacteria in the cluster (asterisks). (vi) At the same magnification, the VAN-allograft surface appears to be free of bacterial colonization. Bars in panel c, 500 μm (i and ii); 50 μm (iii and iv); and 20 μm (v and vi).
VAN-allograft causes a dose-dependent reduction in bacterial numbers.
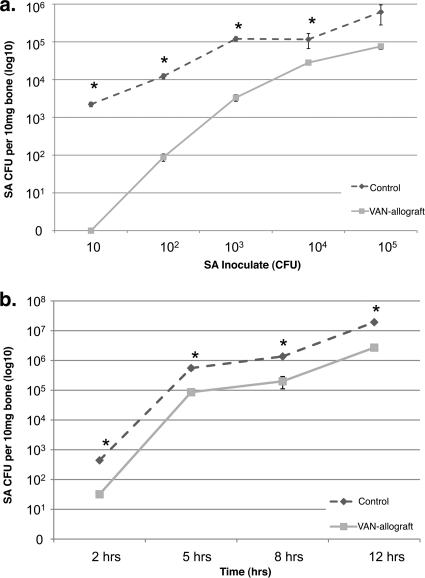
We next asked how well the VAN-allograft resisted colonization (6 h) by bacteria (Ci [initial concentration of inoculate] = 10 to 105 CFU S. aureus) (Fig. 3 a). Compared to control bone, significantly fewer adherent bacteria were recovered from the VAN-allograft at most bacterial loads; at starting inocula of 105 and above, there was no statistically significant reduction. Using 104 CFU S. aureus as a starting inoculate, the control allograft and VAN-allograft were challenged for 2 to 12 h and bacterial numbers determined (Fig. 3b). The VAN-allograft was able to resist colonization over time, with adherent bacterial counts approximately 8 to 18% of the control levels.
FIG. 3.
VAN-allograft resists colonization by S. aureus. (a) Allografts were challenged with 10 to 105 CFU for 6 h and adherent S. aureus collected. Colonization of control bone and VAN-allograft increased as a function of inoculum, with VAN-allograft values consistently lower than those measured for control allograft. *, P < 0.05. (b) Control allograft or VAN-allograft was challenged with S. aureus (Ci = 104 CFU/ml) for 2, 5, 8, or 12 h, and numbers of bacteria adherent to the surface of the bone were assessed by direct colony counts, shown as a percentage of the control value for each time period. At all times, numbers of adherent bacteria recovered from the VAN-allograft were smaller than those from the control. Values shown are mean ± SEM for three separate determinations in one representative experiment. *, P < 0.05.
VAN bonded to the bone allograft retains its spectrum of activity.
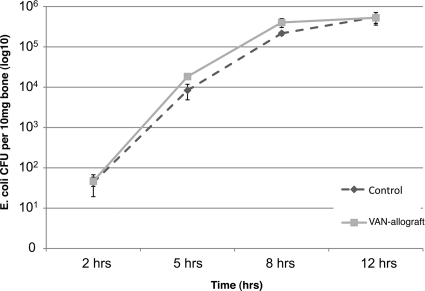
VAN is active predominately against Gram-positive bacteria (12) and should not decrease colonization by Gram-negative bacteria such as E. coli. To learn if the tethered VAN retains this specificity, VAN-allografts were challenged with E. coli. The Gram-negative E. coli colonized the VAN-allograft to the same extent as it colonized control bone, with population size increasing with incubation time (Fig. 4). Thus, the VAN-allograft prevented colonization by Gram-positive S. aureus but not Gram-negative E. coli, suggesting that surface-tethered VAN retained the specificity and activity of the untethered antibiotic.
FIG. 4.
VAN-allograft does not inhibit adherence of the Gram-negative E. coli. VAN affects Gram-positive but not Gram-negative bacteria. A control allograft or VAN-allograft was challenged with the Gram-negative organism E. coli DH5α (Ci = 104 CFU/ml), for 2, 5, 8, or 12 h. After extensive rinsing, numbers of adherent bacteria were determined by colony counting. It is noteworthy that there was no statistical difference in numbers of organisms adherent to the surfaces at each time interval.
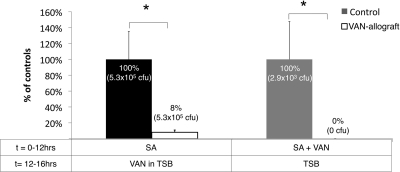
VAN-allograft retards infection more effectively than solution antibiotics.
We then evaluated VAN-allograft activity in systems that modeled the use of either systemic antibiotic therapy or antibiotic prophylaxis (Fig. 5). As already noted, compared to controls exposed to S. aureus for 12 h, the VAN-allograft exhibited a significant decrease in bacterial colonization. When the VAN-allograft was incubated with S. aureus for 12 h, followed by 4 h with 10 μg/ml VAN (modeling systemic antibiotic therapy), colonization of control surfaces was reduced (data not shown). Importantly, the combination of solution antibiotic and VAN-allograft lowered S. aureus counts to 8% of those in the control group. When 10 μg/ml VAN was added concomitantly with S. aureus during the 12 h of incubation (modeling prophylaxis), colonization, albeit reduced, of control surfaces still occurred. Importantly, colonization of the VAN-allograft was not measurable.
FIG. 5.
VAN-allograft complements solution antibiotics. Control or VAN-allograft was incubated with S. aureus (SA) (Ci = 104 CFU/ml) for 12 h, followed by an additional 4 h in the presence of 10 μg/ml VAN (left pair of histograms) or with 10 μg/ml VAN that was added concurrently with the inoculum (right pair of histograms). After suspension and plating of adherent bacteria, recovered S. aureus was represented as a percentage of the control value per condition. After the establishment of S. aureus colonization, treatment with solution antibiotics maintained the same relative colonization levels described for the previous figures, with VAN-allograft values at ∼8% of control values (log10 CFU = 0.08). When the control allograft and VAN-allograft were incubated with both bacteria and antibiotics, bacterial colonization of the VAN-allograft surface was undetectable. *, P < 0.05.
VAN-allograft does not alter cell morphology or viability.
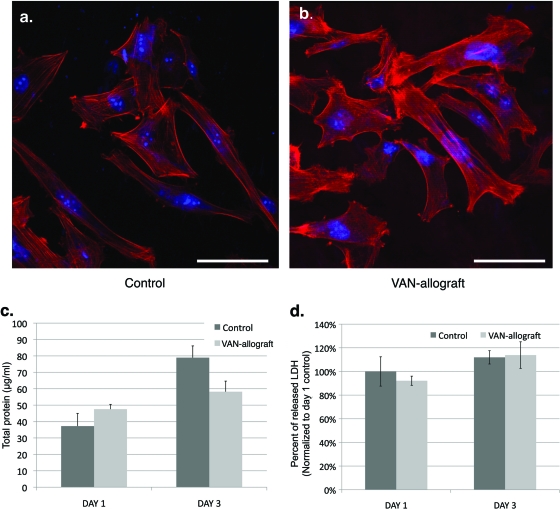
Biocompatibility of the VAN-allograft was then assessed using human fetal osteoblast-like cells (hFOBs) (8). After 48 h in culture, hFOBs grown on the control bone appeared to have either an elongated or a trapezoidal morphology; both shapes are characteristic of adherent osteoblastic cells (Fig. 6 a) (13). Both cortical actin staining and actin stress filaments were apparent in these cells, as were brightly staining areas at the cell edges. In some cells, a finer meshwork of actin filaments was present, as were some short actin bundles. On the VAN-allograft (Fig. 6b), hFOBs appeared to be similar in size and shape to those described for control bone, although there appeared to be greater numbers of cells with intensely staining actin bundles. Overall actin cytoskeletal architecture appeared to be similar to that described for the control samples. Normal nuclear size and number, as detected by propidium iodide staining, were present in both fields.
FIG. 6.
Human fetal osteoblast-like cell adhesion and proliferation on VAN-allograft are similar to control values. (a and b) Human fetal osteoblast-like cells (hFOBs) were cultured on cortical control (a) or VAN-allograft bone (b) for 48 h. Alexa Fluor 488-phalloidin (red) staining for actin and SYTO9 (blue) staining of nuclei are shown to assess morphology and cytoskeletal architecture. Note the normal actin cytoskeletal (red) morphology, with stress filaments, short actin bundles, and adhesion complexes evident on both control and VAN-allograft surfaces. Bars, 100 μm. (c) Protein content was measured as a reflection of cellular adhesion and proliferation. hFOBs were cultured on cortical control or VAN-allograft for 1 and 3 days and total protein content measured. No statistically significant differences between the control and VAN-allograft were noted at either time point. (d) Lactate dehydrogenase activity was measured to assess cell toxicity. hFOBs were cultured on cortical control or VAN-allograft for 1 and 3 days. Membrane damage was determined by measuring the amount of lactate dehydrogenase (LDH) released in the medium. Data are expressed as a percentage of the released LDH over the total LDH content of the cells and normalized to day 1 controls. No statistically significant differences were noted at either time point.
The number of adherent cells, as measured by total protein content, was measured after 1 and 3 days in culture (Fig. 6c). While VAN-allograft protein content trended upward in comparison to that in control bone after 1 day and downward after 3 days, no significant differences were observed between the two samples. Importantly, the increase in protein content observed with time indicated that the proliferative ability of the hFOBs had not been impaired by plating on the VAN-allograft surface. Finally, measurement of release of lactate dehydrogenase (LDH) into the bathing medium as an indicator of cell lysis, and hence toxicity (Fig. 6d), indicated no significant differences between control and VAN-allograft surfaces at either 1 or 3 days. These results suggest that the control and VAN-bone surfaces served as essentially equivalent substrates for the osteoblastic-cell population.
DISCUSSION
The goal of this investigation was to characterize the effects of the VAN-modified allograft on bacterial adhesion, biofilm formation, and cellular adhesion and toxicity. When coupled to the allograft, the antibiotic was uniformly distributed over the bone surface and was stable (9). The VAN-allograft retained its antimicrobial activity and was able to retard bacterial colonization; moreover, the decrease in bacterial colonization was maintained over time. Furthermore, the grafted VAN remained stably attached on the allograft following incubation in physiologically relevant media over 7 days. Importantly, in the presence of the grafted VAN, biofilm formation did not occur, even in topographical niches that provide a protected environment for bacterial adhesion. The tethered VAN was active against the Gram-positive organism S. aureus and exhibited no biocidal activity against the Gram-negative E. coli. It is noteworthy that the combination of VAN-allograft with bathing VAN resulted in significant reductions in numbers of adherent bacteria, suggesting that, clinically, use of the VAN-allograft along with systemic or locally released antibiotics would prevent allograft infection. Finally, compared to the untreated surface, no marked differences in osteoblast-like cell morphology or number were evident, nor was there any evidence of increased cell injury.
Based on our previous studies, we reasoned that modification of allograft surfaces with antibiotics would decrease bacterial colonization and, by implication, biofilm formation. The topography of the morselized bone was a cause for concern: it is nonplanar, irregular, and at the microscopic level naturally inhomogenous. Moreover, demineralization generates an enormous number of sites for bacterial attachment. All of these factors add to the difficulties in preventing and subsequently eradicating biofilm formation on the allograft surface. To address this problem, it was critical that the allograft was completely covered by the tethered antibiotic. This task was facilitated by the plethora of free amine groups on the demineralized bone surface. Indeed, little trouble was experienced in linking the VAN to the bone morsel surfaces; moreover, when the allograft was treated with the VAN antibody, VAN surface coverage appeared to be uniform. In other studies, we have attempted to measure amounts of antibiotic on the surface and calculated a lower limit of ∼26 ng/mg of bone for this method (9).
Because infection is established over the course of days, it was important that the VAN-allograft was stable in physiological media that would model physiological electrolytes. Immunohistochemical staining indicated that VAN remained bonded to the allograft over the 7-day incubation period in the bathing medium. In terms of activity, we challenged the surface with 102 to 105 CFU S. aureus (with concentrations reaching >107 CFU by 24 h), for 2 to 12 h. While all levels of inocula caused abundant colonization of the control allograft surfaces, the VAN-allograft was significantly more resistant to the bacterial challenge. At initial inocula of above 104 to 105 CFU, the modified surfaces decreased colonization 80 to 90%; similarly, the VAN-allograft surface was able to maintain a significant inhibition of bacterial colonization with time. Moreover, while biofilm was apparent on the control surfaces, few if any bacterial colonies were evident on VAN-allograft surfaces, where biofilm did not appear to be synthesized.
Inhibition of bacterial colonization could occur either through the biocidal activity of the VAN or through the creation of a surface that nonspecifically prevented bacterial adhesion. We therefore reasoned that if VAN was acting as a Gram-positive antibiotic, Gram-negative bacteria should readily colonize the surface. In fact, our experiments showed that the Gram negative bacterium, E. coli, colonized both control and VAN-allograft surfaces to the same extent. This result strongly implied that the VAN-allograft surface showed specificity for the defined target of vancomycin action, the Gram-positive bacterial cell wall. Based on the data presented, if the surface is repellent, it would account for only a minor aspect of its total biocidal activity. However, for S. aureus specifically, this question could best be answered by testing with a vancomycin-resistant S. aureus (VRSA) strain.
Since few of the bacteria still adhered to the VAN-allograft surface, we determined if the nonadherent planktonic organisms would be susceptible to antibiotics present in the medium. Not surprisingly, while the VAN-allograft alone caused a significant reduction in the number of adherent S. aureus bacteria compared to control allograft, when the two surfaces were incubated in medium that contained soluble VAN, the control graft was still colonized, whereas colonization of the VAN-allograft was below measurable levels. From a clinical perspective, these finding indicate that if the VAN-tethered allograft were to be used clinically, the presence of systemic antibiotics would add to the effectiveness of the allograft in maintaining a biofilm-free surface, even when there is an overwhelming number of infective organisms.
Finally, while the VAN-allograft surface appears to retard bacterial colonization, its utility is linked to its ability to serve as a bone substitute, i.e., to provide a matrix that facilitates new bone formation. As a first step in determining the ability of the VAN-allograft to support bone ingrowth, we examined bone cell morphology, and membrane integrity. We found that despite the presence of an antibiotic layer on the allograft surface, there was little difference in the behavior of cells on the VAN-allograft and the control allograft. These findings were in keeping with previous studies by us and by others in which VAN toxicity occurred only at concentrations far greater than those present on the bone surface. (1). More-detailed studies are in progress to evaluate the effect of low concentrations of VAN on the most sensitive aspects of bone cell function.
In sum, we have described the ability of a bone allograft modified with vancomycin to resist bacterial colonization and biofilm formation while retaining its ability to foster osteoblastic cell adhesion and proliferation. From a clinical viewpoint, inhibition of biofilm formation should enhance osteogenesis and favor retention and integration of the implanted material in vivo. Based on these new findings, we suggest that this new chimeric construct may represent a new substrate for use in a plethora of applications in medicine and surgery.
Acknowledgments
We thank the Musculoskeletal Transplant Foundation for providing samples for this work as well as for their generous support for these studies.
We thank the NIH (grants DE-13319, DE-10875, AR-051303, DE019901, and HD061053) and the Department of Defense (grant DAMD17-03-1-0713) for funding for this study.
The results presented are not the statement or policy of the funding agencies. No animals or human subjects were used in this study. C.K., C.S.A., I.M.S., J.P., and N.J.H. have filed an invention disclosure on this process.
Footnotes
Published ahead of print on 22 November 2010.
REFERENCES
- 1.Antoci, V., Jr., C. S. Adams, N. J. Hickok, I. M. Shapiro, and J. Parvizi. 2007. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin. Orthop. Rel. Res. 462:200-206. [DOI] [PubMed] [Google Scholar]
- 2.Boyce, T., J. Edwards, and N. Scarborough. 1999. Allograft bone. The influence of processing on safety and performance. Orthop. Clin. North Am. 30:571-581. [DOI] [PubMed] [Google Scholar]
- 3.Bullens, P., et al. 2009. Survival of massive allografts in segmental oncological bone defect reconstructions. Int. Orthop. 33:757-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buttaro, M., F. Comba, and F. Piccaluga. 2007. Vancomycin-supplemented cancellous bone allografts in hip revision surgery. Clin. Orthop. Rel. Res. 461:74-80. [DOI] [PubMed] [Google Scholar]
- 5.Costerton, J. W. 2005. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin. Orthop. Rel. Res. 437:7-11. [DOI] [PubMed] [Google Scholar]
- 6.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 7.Edin, M., T. Miclau, G. Lester, R. Lindsey, and L. Dahners. 1996. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin. Orthop. Rel. Res. 454:245-251. [PubMed] [Google Scholar]
- 8.Harris, S. A., R. J. Enger, B. L. Riggs, and T. C. Spelsberg. 1995. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J. Bone Min. Res. 10:178-186. [DOI] [PubMed] [Google Scholar]
- 9.Ketonis, C., et al. 2010. Antibiotic modification of native grafts: improving upon nature's scaffolds. Tiss. Eng. A 16:2041-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ketonis, C., et al. 28 October 2010. Antibacterial activity of bone allografts: comparison of a new vancomycin-tethered allograft with allograft loaded with adsorbed vancomycin. Bone [Epub ahead of print.] doi: 10.1016/j.bone.2010.10.171. [DOI] [PMC free article] [PubMed]
- 11.Lord, C., M. Gebhardt, W. Tomford, and H. Mankin. 1988. Infection in bone allografts. Incidence, nature, and treatment. J. Bone Joint Surg. Am. 70:369-376. [PubMed] [Google Scholar]
- 12.Lundstrom, T., and J. Sobel. 2004. Antibiotics for gram-positive bacterial infections: vancomycin, quinupristin-dalfopristin, linezolid, and daptomycin. Infect. Dis. Clin. North Am. 18:651-668. [DOI] [PubMed] [Google Scholar]
- 13.Shah, A. K., R. K. Sinha, N. J. Hickok, and R. S. Tuan. 1999. High-resolution morphometric analysis of human osteoblastic cell adhesion on clinically relevant orthopedic alloys. Bone 24:499-506. [DOI] [PubMed] [Google Scholar]
- 14.Sigma-Aldrich. 2010. TOX7 technical bulletin. Sigma-Aldrich, St. Louis, MO.
- 15.Thermo Scientific. 2010. Overview of protein assay methods. Thermo Scientific, Rockford, IL.
- 16.Trampuz, A., et al. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 16:654-663. [DOI] [PubMed] [Google Scholar]
- 17.Witso, E., L. Persen, P. Benum, and K. Bergh. 2005. Cortical allograft as a vehicle for antibiotic delivery. Acta Orthop. 76:481-486. [DOI] [PubMed] [Google Scholar]