Abstract
The aim of this study was to investigate the factors limiting the blood-brain barrier (BBB) transport of colistin in healthy mice and to assess the impact of systemic inflammation on the transport of this antibiotic across the BBB. Colistin sulfate (40 mg/kg) was administered subcutaneously to Swiss outbred mice as single and multiple doses to determine any relationship between brain uptake and plasma concentrations of colistin. To assess the effect of P-glycoprotein (P-gp) on BBB transport, colistin sulfate (5 mg/kg) was concomitantly administered intravenously with PSC833 or GF120918 (10 mg/kg). Systemic inflammation was induced by three intraperitoneal injections of lipopolysaccharide (LPS; 3 mg/kg), and BBB transport of colistin was subsequently measured following subcutaneous administration and by an in situ brain perfusion. The brain uptake of colistin was low following single and multiple subcutaneous doses, with brain-to-plasma concentration ratios ranging between 0.021 and 0.037, and this was not significantly enhanced by coadministration of GF120918 or PSC833 (P > 0.05). LPS significantly increased the brain uptake of subcutaneously administered colistin with area under the brain concentration time curve (AUCbrain) values of 11.7 ± 2.7 μg·h/g and 4.0 ± 0.3 μg·h/g for LPS- and saline-treated mice, respectively (mean ± standard deviation). Similarly, in situ perfusion of colistin led to higher antibiotic brain concentrations in LPS-treated animals than in saline-treated animals, with colistin brain-to-perfusate concentration ratios of 0.019 ± 0.001 and 0.014 ± 0.001, respectively. This study demonstrates that the BBB transport of colistin is negligible in healthy mice; however, brain concentrations of colistin can be significantly enhanced during systemic inflammation, as might be observed in infected patients.
Colistin (polymyxin E) is one of two polymyxins clinically used to treat infections caused by Gram-negative bacteria such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae (12). Although the clinical use of colistin waned in the 1970s due to concerns related to its adverse effects (10), colistin has reemerged as a last-line therapy for Gram-negative bacterial infections and is increasingly employed against multidrug-resistant Gram-negative bacteria (9). One of the adverse effects of colistin that has attracted the attention of clinicians and scientists is neurotoxicity (6, 8); however, it remains unknown as to whether this toxicity is centrally or peripherally mediated. If colistin were to exert any centrally mediated toxicity, it would need to permeate the blood-brain barrier (BBB), the endothelial lining separating the brain parenchyma from the blood. It has been clinically demonstrated that intravenous administration of colistin methanesulfonate, an inactive prodrug of colistin (5), leads to detectable levels of colistin in cerebrospinal fluid (CSF) (1, 15, 21); however, this is an indicator of blood-CSF barrier penetration and not BBB penetration. We have previously demonstrated that the brain uptake of colistin following a single intravenous dose to mice was negligible (16), suggesting minimal penetration across an intact BBB. However, the mechanisms governing the low BBB penetration of colistin remain unknown, and, furthermore, it is unclear as to whether brain accumulation of colistin occurs with repeated doses (as is used clinically), given that our previous study assessed colistin brain penetration after only a single intravenous dose (16).
It is generally accepted that only small molecules with low molecular mass (<450 Da) and high lipid solubility permeate the healthy BBB by a passive transcellular process (13). The tight junctions of the interendothelial domains restrict the passage of large hydrophilic molecules through the paracellular route (14), and this is expected to be the main reason for the low BBB penetration of colistin observed (given a molecular mass of 1,163 Da) (19). However, even for compounds that possess the appropriate physicochemical properties for transcellular permeation, efficient BBB transport is not always guaranteed due to the presence of active efflux proteins expressed at the luminal surface of brain endothelial cells (29). P-glycoprotein (P-gp) is one such efflux transporter and is responsible for restricting the brain penetration of a wide range of substrates, including anticancer drugs, analgesics, and antibiotics (27). Many of these substrates share some common physicochemical properties such as a positive charge at physiological pH and a molecular mass of >400 Da (7). Given that the primary amines of colistin possess a pKa value of approximately 10 and that colistin has a high molecular weight (19), it is plausible that the low brain uptake of colistin observed in our previous study (16) may also be due, in part, to P-gp-mediated efflux.
While it is important to understand the mechanisms limiting colistin brain uptake in healthy subjects, it is crucial to identify whether colistin has a higher propensity to access the central nervous system (CNS) during bacterial infections, where the integrity of the BBB may be compromised (26). Indeed, other investigators have detected colistin in the CSF of infected patients following systemic administration (1, 15, 21), whereas we have previously observed limited brain uptake in healthy mice although this could also be attributed to species differences and/or differences between BBB and blood-CSF barrier penetration profiles. While the impact of infection on BBB transport may be investigated by inducing bacteremia in mice, bacterial lipopolysaccharide (LPS) is often used to mimic the infective state as it results in the release of proinflammatory cytokines (25, 31). Several studies have shown that BBB disruption can be induced by bacterial LPS, leading to increased brain uptake of compounds that normally exhibit limited access into the CNS (3, 22). Whether a similar enhancement in BBB penetration would occur with colistin remains unknown; however, it is of importance to assess this phenomenon as this would better reflect the scenario encountered in infected patients. Therefore, the aims of the current study were as follows: (i) to identify whether the brain uptake of colistin increases with repeated administration, under conditions where sustained colistin plasma concentrations may be achieved; (ii) to determine the impact of coadministration of P-gp inhibitors on the brain uptake of colistin; and (iii) to examine if the BBB penetration of colistin is enhanced following administration of LPS.
MATERIALS AND METHODS
Chemicals and reagents.
Colistin sulfate was purchased from Zhejiang Shenghua Biok Biology Co., Ltd. (EP5 grade; Zhejiang, China). PSC833 (Valspodar) was a gift from Novartis (Basel, Switzerland) and GF120918 (Elacridar) was a gift from GlaxoSmithKline (Middlesex, United Kingdom). [3H]digoxin, Ultima Gold scintillation fluid, and Solvable were purchased from Perkin Elmer (Boston, MA), and [14C]inulin and [14C]sucrose were obtained from American Radiolabeled Chemicals (St. Louis, MO). Lipopolysaccharide from Salmonella enterica serotype Typhimurium was obtained from Sigma-Aldrich (Castle Hill, New South Wales, Australia). Solid-phase extraction (SPE) cartridges (C18 Sep-Pak; 100 mg) were purchased from Waters (Milford, MA). All other reagents were of analytical and/or high-performance liquid chromatography (HPLC) grade, and water was obtained from a Millipore purification system (Billerica, MA).
Animal studies.
Animal experiments were approved by the Monash Institute of Pharmaceutical Sciences Animal Ethics Committee and were performed in accordance with the Australian National Health and Medical Research Council (NHMRC) guidelines for the care and use of animals for scientific purposes. Male Swiss outbred mice (6 to 8 weeks of age; 25 to 30 g) were used in all studies. Mice had free access to food and water during all experimental periods.
Brain uptake of colistin following subcutaneous administration.
An aliquot (200 μl) of a sterile-filtered solution of colistin sulfate (equivalent to 40 mg/kg of body weight in saline) was administered to mice subcutaneously over the interscapular region. At 0.5, 1, 2, and 4 h, mice (n = 4 at each time point) were anesthetized with an intraperitoneal dose of 133 mg/kg of body weight of ketamine and 10 mg/kg xylazine, blood was collected by cardiac puncture, and the whole brain was removed following cervical dislocation. Plasma and brain samples were stored at −20°C until the day of analysis. To determine whether the brain uptake of colistin increased with repeated administration, subcutaneous injections of colistin sulfate were administered (at 0, 6, and 12 h) at the same dose and using the same method described above. Two hours after the last dose, plasma and brain samples were collected and stored at −20°C until analysis. Concentrations of colistin in brain homogenate and in plasma samples were measured using HPLC (16), and a brain-to-plasma concentration (B/P) ratio of colistin was calculated at each postdose time point.
Impact of P-gp inhibitors on brain uptake of colistin.
An aliquot (50 μl) of a colistin sulfate solution was administered to mice by tail vein injection (5 mg/kg of body weight) immediately after a 50-μl intravenous injection of PSC833 (10 mg/kg), GF120918 (10 mg/kg), or blank vehicle (n = 6 for each pretreatment). The blank vehicle consisted of 20% (vol/vol) ethanol, 60% (vol/vol) polyethylene glycol 400 (PEG 400), and 20% (wt/vol) glucose. Mice were anesthetized (as detailed above), and plasma and brain samples were harvested 5 min after administration of colistin sulfate. Samples were analyzed for colistin (16), and B/P ratios were calculated.
To ensure that these doses of PSC833 and GF120918 were effective in inhibiting P-gp function at the BBB, the brain uptake of the P-gp substrate digoxin was assessed in the presence and absence of the inhibitors. An aliquot (50 μl) of a [3H]digoxin solution (40 μCi/ml) was administered to mice by tail vein injection in a vehicle consisting of 20% (vol/vol) ethanol, 60% (vol/vol) PEG 400, and 20% (wt/vol) glucose with or without PSC833 or GF120918 (10 mg/kg) (n = 6 mice for each group). Plasma and brain samples were collected at 5 min postdose, and radioactivity in plasma and brain was determined using liquid scintillation counting. Briefly, 50 μl of plasma was placed into a 5-ml scintillation vial, and 2 ml of Ultima Gold scintillation fluid was added, followed by brief vortex mixing. Brain samples were placed into 20-ml scintillation vials containing 2 ml of Solvable and were maintained at 50°C overnight. The next day, 200 μl of hydrogen peroxide (30%, vol/vol) was added to the digested brains to bleach the samples, and they were allowed to sit for 30 min at 50°C. An aliquot (10 ml) of Ultima Gold scintillation fluid was then added to each vial containing brain digest, followed by brief vortex mixing. Radioactivity in both brain digest and plasma samples was then measured by liquid scintillation counting (Tri-carb 2800 TR; Perkin Elmer, Boston, MA) and a B/P ratio was calculated by the following formula: B/P = (number of disintegrations per minute [dpm]/g of brain)/(dpm/ml of plasma).
Effect of LPS on brain uptake of colistin.
Mice were administered intraperitoneal injections (200 μl) of 0.9% (wt/vol) saline (control) or LPS (3 mg/kg of body weight in saline) at 0, 6, and 24 h. An aliquot (200 μl) of a colistin sulfate solution (40 mg/kg in saline) was then administered subcutaneously to mice over the interscapular region 4 h after the third LPS or saline dose. At various time points after administration of colistin (0.5, 1, 2, and 4 h), plasma and brain samples (n = 4 mice at each time point) were harvested, and concentrations of colistin in brain homogenate and plasma were determined by HPLC to obtain B/P ratios at each time point. The area under the plasma concentration-time curve from 0 to 4 h (AUCplasma, 0-4), the area under the brain concentration-time curve from 0 to 4 h (AUCbrain, 0-4), and their associated variances were also determined using Bailer's approach (2).
It is possible that any enhancement in colistin brain uptake following LPS administration could have been due to a direct effect of LPS on BBB integrity or an indirect effect (e.g., an alteration to plasma protein binding and unbound fraction of colistin). To delineate these effects, the BBB transport of colistin was measured in saline- and LPS-treated animals using a modified in situ perfusion technique (4). The perfusion fluid used was carbonated Krebs buffer consisting of the following components (per liter): 128.0 mmol of NaCl, 24.0 mmol of NaHCO3, 4.2 mmol of KCl, 2.4 mmol of NaH2PO4, 1.5 mmol of CaCl2, 0.9 mmol of MgSO4, and 9.0 mmol of d-glucose. The buffer was carbonated with 5% CO2-95% O2, the pH was adjusted to 7.4, and the buffer was warmed to 37°C before the experiment. Mice were pretreated with LPS or saline, as described above, and 4 h after the last administration, mice were anesthetized. After the thorax was opened, the heart was exposed, and the descending thoracic aorta was clamped, followed by severing of both the left and right jugular veins. A 21-gauge butterfly needle (attached to polyethylene tubing and perfusion solution) was inserted into the left ventricle of the heart. To mimic the plasma concentrations obtained after multiple subcutaneous administrations of colistin, 40 μg/ml of colistin was infused transcardially at a rate of 2 ml/min for 4 min. At the completion of the perfusion, brains were removed and stored at −20°C until the day of analysis. Three aliquots of the perfusion fluid eluting from the polyethylene tubing were also collected and stored at −20°C until the day of analysis. The concentrations of colistin in brain homogenate were then determined by an HPLC method described previously (16). The concentration of colistin in the perfusate solution was also determined using the above assay with minor modifications. Briefly, 50 μl of acetonitrile was added to 50 μl of perfusion fluid, and the mixture was vortex mixed for 1 min and centrifuged at 16,100 × g for 10 min before being loaded onto an SPE cartridge. All other derivatization and analytical parameters were identical to those reported previously (16). The calibration curve relating chromatographic peak area to perfusate colistin concentration exhibited good linearity (r2 of >0.993) over the range of 0.31 to 10.0 μg/ml. The coefficient of variation for quality control samples (n = 6) prepared at 0.63 μg/ml and 10.0 μg/ml was less than 4.4%, and the accuracy values of these replicates ranged from 108.8% to 110.5% of the nominal concentrations.
Effect of LPS on BBB integrity.
To ensure that the LPS regimen used in the studies above was affecting the integrity of the BBB, the brain uptake and BBB transport of impermeable markers were determined following intravenous administration and in situ perfusion, respectively. At 4 h after the last saline or LPS administration, mice were intravenously administered a 50-μl solution of [14C]inulin (2 μCi in saline) or [14C]sucrose (2 μCi in saline) (n = 6 per group). Plasma and brain samples were obtained 5 min following intravenous administration, and samples were measured for radioactivity, as described previously. Similarly, 4 h after the last pretreatment with saline or LPS, the BBB penetration of [14C]sucrose or [14C]inulin was determined following perfusion of each compound (0.25 μCi/ml) at a rate of 2 ml/min for 4 min (n = 3 per group). Brain and perfusate were collected, and samples were prepared for liquid scintillation counting, as described previously. The brain-to-plasma concentration or brain-to-perfusate concentration ratio of [14C]sucrose and [14C]inulin (as a measure of BBB integrity) was calculated by comparing the concentration of compound in brain (dpm/g) to that in plasma or perfusate (dpm/ml).
Data analysis.
All data are presented as means ± standard deviations (SD) unless otherwise stated. For comparisons of the brain-to-plasma concentration (B/P) or brain-to-perfusate concentration ratios between saline-treated (control) and LPS-treated animals, Student's t test was used, whereas B/P ratios between vehicle-, PSC833-, and GF120918-treated animals were compared using a one-way analysis of variance followed by a Newman-Keuls multiple comparisons test (PASW Statistics for Windows, version 17.0; Chicago, Illinois). A P value of <0.05 was considered to be a significant difference. For comparison of the AUCplasma or AUCbrain between saline- and LPS-treated groups using Bailer's approach, a z test was used to determine significant differences. A z value of >1.96 was considered to be a significant difference between two groups.
RESULTS
Brain uptake of colistin after single and repeated subcutaneous administrations.
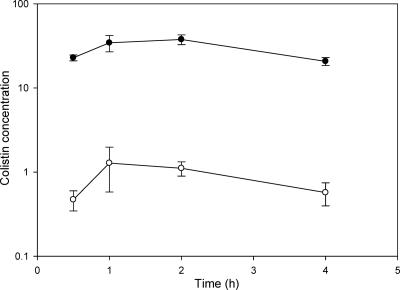
Mice tolerated a subcutaneous dose of 40 mg/kg of body weight of colistin sulfate with no observed toxicity over the experimental period. Subcutaneous administration of colistin sulfate at this dose resulted in sustained plasma concentrations of colistin over the 4-h sampling period, with maximum plasma concentrations at 1 to 2 h postdose, as shown in Fig. 1. Despite these high and sustained plasma concentrations, the resulting concentration of colistin in brain was very low at all four postdose time points, with B/P ratios of 0.021 ± 0.009 (0.5 h), 0.037 ± 0.014 (1 h), 0.029 ± 0.005 (2 h), and 0.028 ± 0.007 (4 h). Similar results were also obtained following multiple subcutaneous dosing to mice. Two hours after the last of three subcutaneous doses of colistin sulfate, the plasma concentration of colistin was 32.0 ± 3.5 μg/ml, yet the brain colistin concentration at this time was 0.8 ± 0.1 μg/g, leading to a B/P ratio of 0.024 ± 0.002 (n = 4). It should be noted that in all studies, the amount of colistin remaining in the cerebral vasculature was not subtracted for calculation of B/P ratios as this resulted in ratios close to zero (or negative).
FIG. 1.
Plasma (•; μg/ml) and brain (○; μg/g) concentrations of colistin following a subcutaneous dose of colistin sulfate (40 mg/kg) to Swiss outbred mice. Data are presented as means ± standard errors of the means (n = 4).
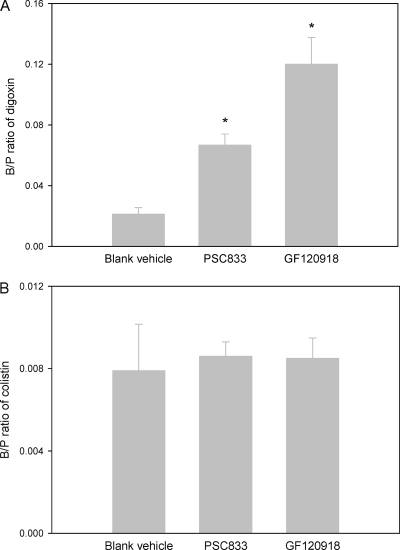
Impact of P-gp inhibitors on brain uptake of colistin.
The B/P ratios of [3H]digoxin in the presence and absence of P-gp inhibitors are shown in Fig. 2 A. Both of these inhibitors significantly (P < 0.05) enhanced the brain uptake of [3H]digoxin, demonstrating that the dose of inhibitors (10 mg/kg) was valid for assessing the involvement of P-gp in colistin brain uptake. However, the average B/P ratio of colistin following administration of the same dose of P-gp inhibitors was still negligible (Fig. 2B); there was no significant difference in the B/P ratios of colistin between mice treated with vehicle, PSC833, or GF120918 (P > 0.05).
FIG. 2.
(A) B/P ratios of [3H]digoxin 5 min after intravenous administration (2 μCi) to Swiss outbred mice with and without coadministration of PSC833 or GF120918 (10 mg/kg). Data are presented as means ± standard errors of the means (n = 6; *, P < 0.05 between PSC833 or GF120918 and vehicle group using a one-way analysis of variance and Newman-Keuls multiple comparison test). (B) B/P ratios of colistin 5 min after intravenous administration of colistin sulfate (5 mg/kg) to Swiss outbred mice with and without coadministration of PSC833 or GF120918 (10 mg/kg). Data are presented as means ± standard errors of the means (n = 6).
Brain uptake of colistin after pretreatment with LPS.
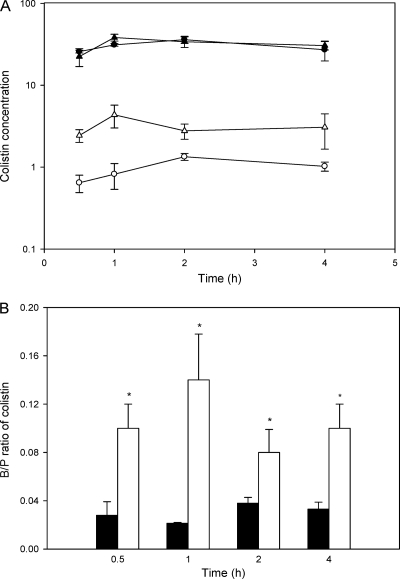
The plasma and brain concentrations of colistin after subcutaneous administration of colistin sulfate to saline- and LPS-treated mice are shown in Fig. 3 A. The plasma concentrations of colistin were not affected by administration of LPS at any postdose time points, with AUCplasma values not significantly different between LPS- and saline-treated groups (values of 117.5 ± 9.2 μg·h/ml and 121.4 ± 8.2 μg·h/ml, respectively; z = 0.33). Even though plasma concentrations were unaffected by LPS treatment, the brain concentrations of colistin in LPS-treated mice were significantly higher than in saline-treated mice at all postdose time points, as shown in Fig. 3A. The average B/P ratios of colistin at the designated time points in saline- and LPS-treated animals are shown in Fig. 3B. In addition, the overall brain exposure to colistin was significantly enhanced following LPS pretreatment, with AUCbrain values of 11.7 ± 2.7 μg·h/g and 4.0 ± 0.3 μg·h/g for LPS- and saline-treated mice, respectively (z = 2.81). To ensure that LPS did not interfere with the HPLC analysis of colistin and that the increased peak areas observed in brain homogenate were indeed due to increased brain concentration of colistin, the HPLC methods were also validated using brain and plasma from LPS-treated mice not exposed to colistin. No changes in colistin chromatographic peak areas or retention times were observed in the presence of LPS (data not shown), indicating that the increased peak areas detected in brain homogenates following LPS pretreatment were indeed reflective of increased brain concentrations of colistin.
FIG. 3.
(A) Plasma (μg/ml) and brain (μg/g) concentrations of colistin after subcutaneous administration of colistin sulfate (40 mg/kg) to Swiss outbred mice 4 h after the final dose of a pretreatment regimen (comprising administration at 0, 6, and 24 h) with intraperitoneal LPS (3 mg/kg) (▴, plasma; ▵, brain) or saline (•, plasma; ○, brain). Data are presented as means ± standard errors of the means (n = 4). (B) Corresponding B/P ratios of colistin from data depicted in panel A after intraperitoneal LPS (3 mg/kg) (□) or saline (▪) administration. Data are presented as means ± standard errors of the means (n = 4; *, P < 0.05 between saline- and LPS-treated animals using Student's t test).
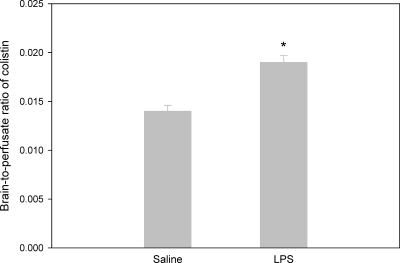
Using the in situ perfusion technique, the BBB permeability of colistin was shown to be significantly (P < 0.01) enhanced in LPS-treated mice (Fig. 4), with brain-to-perfusate colistin concentration ratios of 0.019 ± 0.001 and 0.014 ± 0.001 for LPS- and saline-treated mice, respectively. However, the increase in the brain uptake of colistin mediated by LPS as measured by the in situ perfusion (36% increase) was not as large as the effect measured following subcutaneous administration of colistin (average 286% increase). These findings suggested that either (i) the effect of LPS may have been due to an event imparted in the systemic circulation (e.g., altered plasma protein binding of colistin) or (ii) that the in situ perfusion method is a less sensitive technique than the in vivo brain uptake technique for detecting changes to BBB permeability for poorly penetrating compounds. Therefore, the effect of LPS on the integrity of the BBB was compared following in vivo administration and in situ perfusion of the BBB integrity markers [14C]sucrose and [14C]inulin. The B/P ratios of [14C]sucrose and [14C]inulin following intravenous administration in saline- and LPS-treated mice are shown in Table 1. The average B/P ratios of [14C]sucrose and [14C]inulin were 0.035 to 0.038 in saline-treated animals, whereas pretreatment with LPS resulted in average apparent B/P ratios of 0.065 to 0.069. This approximate doubling in the B/P ratios of [14C]sucrose and [14C]inulin following administration of LPS is suggestive of a significant disturbance in the integrity of the BBB. However, when assessed using the in situ perfusion technique, the extent of BBB damage (as measured by the brain-to-perfusate concentration ratio of [14C]sucrose and [14C]inulin) did not appear to be as marked as that observed following intravenous administration of these BBB markers (Table 2). Compared with the LPS-induced doubling in the apparent B/P ratio of the intravenously administered BBB markers, the in situ perfusion technique detected a 20 to 32% increase in the brain-to-perfusate concentration ratio of the BBB markers following LPS pretreatment, albeit this increase was still significant (P < 0.05).
FIG. 4.
Brain-to-perfusate concentration ratios following in situ perfusion of colistin (40 μg/ml) at a rate of 2 ml/min for 4 min in Swiss outbred mice pretreated with LPS (3 mg/kg) or saline (0, 6 and 24 h). Data are presented as means ± standard errors of the means (n = 4; *, P < 0.01 using Student's t test).
TABLE 1.
B/P ratios of [14C]inulin and [14C]sucrose following intravenous administration to saline- and LPS-pretreated Swiss outbred mice
| Pretreatment | B/P ratioa |
|
|---|---|---|
| [14C]inulin | [14C]sucrose | |
| Saline | 0.038 ± 0.004 | 0.035 ± 0.007 |
| LPS | 0.065 ± 0.016* | 0.069 ± 0.007* |
[14C]inulin and [14C]sucrose were administered in a dose of 2 μCi. Data are presented as means ± SD (n = 6). *, P < 0.05 compared to saline pretreatment using Student's t test.
TABLE 2.
Brain-to-perfusate concentration ratios of [14C]inulin and [14C]sucrose following in situ perfusion to saline- and LPS-pretreated Swiss outbred mice
| Pretreatment | Brain-to-perfusate concentration ratioa |
|
|---|---|---|
| [14C]inulin | [14C]sucrose | |
| Saline | 0.019 ± 0.001 | 0.024 ± 0.002 |
| LPS | 0.025 ± 0.002* | 0.029 ± 0.003* |
Perfusion was conduced at a rate of 0.25 μCi/ml/min for 4 min. Data are presented as means ± SD (n = 3). *, P < 0.05 compared to saline pretreatment using Student's t test.
DISCUSSION
The relentless increase in resistance to almost all currently available antibiotics in Gram-negative bacteria, in particular, P. aeruginosa, A. baumannii, and K. pneumoniae, together with the shortage of new antibiotics with activity against these pathogens, has caused substantial worldwide concern (17). Consequently, the relatively old polymyxin antibiotic colistin is being increasingly used as a salvage therapy (20, 24). While there are a number of clinical cases and reports on the use of colistin (1, 15, 18), there is little information available on the BBB transport of this antibiotic, which may assist in understanding the general CNS disposition of this increasingly important antibiotic.
Consistent with the physicochemical properties of colistin, our previous study demonstrated that the brain penetration of colistin was negligible in healthy mice after a single intravenous dose (16). It is possible that the low brain uptake of colistin observed following this dosage regimen is not representative of that which may occur under circumstances where the colistin plasma concentrations are sustained at a relatively high level. Therefore, in the current studies, colistin sulfate was administered subcutaneously, and plasma colistin concentrations were maintained at high levels over the 4-h experimental period and were approximately double the concentration detected following a single intravenous dose at 5 mg/kg (15.8 ± 2.8 μg/ml) (16). Despite these higher and prolonged plasma concentrations, the mean B/P ratio of colistin was no higher than that observed in the previous single-dose intravenous study (16). Similar results were also obtained after colistin was administered in a multiple-dose regimen, more reflective of the scenario encountered clinically. Therefore, it is likely that in patients with an intact BBB, penetration of colistin into the brain would be minimal, even if this antibiotic was dosed multiple times to allow for sustained plasma concentrations. This negligible entry of colistin across a healthy BBB is most likely to be a result of the tight junctions sealing the paracellular route, hindering the penetration of this, and other, high-molecular-weight compounds.
While the interendothelial tight junctions minimize paracellular penetration of compounds, the transcellular absorption of many compounds can be limited by efflux transporters, including P-gp (27). Given that colistin exhibits some of the characteristics possessed by known P-gp substrates (cationic charge at physiological pH and high molecular weight) (19), we investigated whether efflux by P-gp was also contributing to the low brain uptake of colistin. In this study, the brain uptake of [3H]digoxin, a commonly used P-gp substrate (28), significantly increased when it was coadministered with PSC833 or GF120918, which indicated that the function of P-gp could be effectively blocked with the dose of inhibitors used. However, when these inhibitors were administered at the same dose, they had no effect on the brain uptake of colistin. These studies suggested that P-gp does not contribute to the low brain uptake of colistin and that it is likely that the existence of the tight junctions sealing the paracellular route is the main contributor to the low BBB penetration of colistin.
To further confirm that tight junctions were the major hindrance to colistin BBB penetration, we intentionally disturbed the BBB paracellular integrity by systemic administration of LPS. The administration of LPS not only causes dysfunction of the tight junctions and decreased resistance of the paracellular route (32) but has also been shown to cause a downregulation of efflux transporters, such as P-gp (11). However, given that we have demonstrated that the BBB transport of colistin does not appear to be limited by the action of P-gp, any increase in the brain uptake of colistin following LPS administration is likely to be a result of increased paracellular diffusion. Administration of LPS also served another important purpose in this study in that its effects can mirror the infected state in which colistin is normally clinically used (25). The reduced BBB integrity during the infected state may be beneficial as it may lead to increased antibiotic concentrations in the brain. This may be an advantage in treating brain infections or a disadvantage if the site of infection is outside the CNS and if the antibiotic causes centrally mediated adverse effects such as neurotoxicity. Indeed, the brain uptake of colistin was significantly enhanced after administration of LPS, and this increase was similar in extent to that observed for [14C]sucrose and [14C]inulin, suggesting that the enhanced colistin brain uptake was likely a result of increased BBB paracellular permeability, as might occur during the infected state (32). Moreover, this conclusion was supported by the results from the in situ brain perfusion technique showing a significant increase in the brain uptake of colistin and the BBB markers following LPS pretreatment. Interestingly, the effect of LPS on the brain uptake of colistin, [14C]sucrose, and [14C]inulin, as measured by in situ perfusion, was not as marked as that observed following in vivo administration of these compounds. Such observations have also been made by others for BBB-impermeable compounds (23, 30), suggesting that the in situ perfusion technique may be less sensitive for detecting LPS-induced changes to the BBB transport of poorly penetrating, high-molecular-weight compounds. Nevertheless, it has been demonstrated that the BBB transport of colistin is significantly enhanced during systemic inflammation, which may ultimately lead to higher CNS concentrations of colistin in infected patients. Further studies clarifying whether a similar increase in BBB transport of colistin occurs during an actual bacterial infection remain to be undertaken. Such studies may provide some insight into the disposition of this antibiotic in the clinical setting.
In summary, this study demonstrated that colistin exhibits minimal BBB penetration in healthy mice, regardless of the plasma concentrations present, and that this low brain uptake is not attributed to efflux by P-gp. Additionally, this study has provided evidence for the first time that the brain uptake of colistin is significantly increased in mice when the BBB is perturbed by employing LPS, which may resemble the scenario encountered in infected patients.
Footnotes
Published ahead of print on 29 November 2010.
REFERENCES
- 1.Antachopoulos, C., et al. 2010. Serum and cerebrospinal fluid levels of colistin in pediatric patients. Antimicrob. Agents Chemother. 54:3985-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailer, A. J. 1988. Testing for the equality of area under the curves when using destructive measurement techniques. J. Pharmacokinet. Biopharm. 16:303-309. [DOI] [PubMed] [Google Scholar]
- 3.Banks, W. A., et al. 2008. Nitric oxide isoenzymes regulate lipopolysaccharide-enhanced insulin transport across the blood-brain barrier. Endocrinology 149:1514-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks, W. A., M. Goulet, J. R. Rusche, M. L. Niehoff, and R. Boismenu. 2002. Differential transport of a secretin analog across the blood-brain and blood-cerebrospinal fluid barriers of the mouse. J. Pharmacol. Exp. Ther. 302:1062-1069. [DOI] [PubMed] [Google Scholar]
- 5.Bergen, P. J., J. Li, C. R. Rayner, and R. L. Nation. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosso, J. A., C. A. Liptak, D. K. Seilheimer, and G. M. Harrison. 1991. Toxicity of colistin in cystic fibrosis patients. DICP 25:1168-1170. [DOI] [PubMed] [Google Scholar]
- 7.Didziapetris, R., P. Japertas, A. Avdeef, and A. Petrauskas. 2003. Classification analysis of P-glycoprotein substrate specificity. J. Drug Target. 11:391-406. [DOI] [PubMed] [Google Scholar]
- 8.Evans, M. E., D. J. Feola, and R. P. Rapp. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 33:960-967. [DOI] [PubMed] [Google Scholar]
- 9.Falagas, M. E., and S. K. Kasiakou. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 40:1333-1341. [DOI] [PubMed] [Google Scholar]
- 10.Falagas, M. E., and S. K. Kasiakou. 2006. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit. Care 10:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartz, A. M., B. Bauer, G. Fricker, and D. S. Miller. 2006. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol. Pharmacol. 69:462-470. [DOI] [PubMed] [Google Scholar]
- 12.Hermsen, E. D., C. J. Sullivan, and J. C. Rotschafer. 2003. Polymyxins: pharmacology, pharmacokinetics, pharmacodynamics, and clinical applications. Infect. Dis. Clin. North Am. 17:545-562. [DOI] [PubMed] [Google Scholar]
- 13.Hitchcock, S. A. 2008. Blood-brain barrier permeability considerations for CNS-targeted compound library design. Curr. Opin. Chem. Biol. 12:318-323. [DOI] [PubMed] [Google Scholar]
- 14.Huber, J. D., R. D. Egleton, and T. P. Davis. 2001. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 24:719-725. [DOI] [PubMed] [Google Scholar]
- 15.Jiménez-Mejías, M. E., et al. 2002. Cerebrospinal fluid penetration and pharmacokinetic/pharmacodynamic parameters of intravenously administered colistin in a case of multidrug-resistant Acinetobacter baumannii meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 21:212-214. [DOI] [PubMed] [Google Scholar]
- 16.Jin, L., J. Li, R. L. Nation, and J. A. Nicolazzo. 2009. Brain penetration of colistin in mice assessed by a novel high-performance liquid chromatographic technique. Antimicrob. Agents Chemother. 53:4247-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landman, D., C. Georgescu, D. A. Martin, and J. Quale. 2008. Polymyxins revisited. Clin. Microbiol. Rev. 21:449-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, S. Y., et al. 2008. Multidrug-resistant Acinetobacter meningitis in a 3-year-old boy treated with i.v. colistin. Pediatr. Int. 50:584-585. [DOI] [PubMed] [Google Scholar]
- 19.Li, J., R. L. Nation, R. W. Milne, J. D. Turnidge, and K. Coulthard. 2005. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 25:11-25. [DOI] [PubMed] [Google Scholar]
- 20.Li, J., et al. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589-601. [DOI] [PubMed] [Google Scholar]
- 21.Markantonis, S. L., et al. 2009. Penetration of colistin into cerebrospinal fluid. Antimicrob. Agents Chemother. 53:4907-4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minami, T., J. Okazaki, A. Kawabata, R. Kuroda, and Y. Okazaki. 1998. Penetration of cisplatin into mouse brain by lipopolysaccharide. Toxicology 130:107-113. [DOI] [PubMed] [Google Scholar]
- 23.Nonaka, N., S. Shioda, and W. A. Banks. 2005. Effect of lipopolysaccharide on the transport of pituitary adenylate cyclase activating polypeptide across the blood-brain barrier. Exp. Neurol. 191:137-144. [DOI] [PubMed] [Google Scholar]
- 24.Payne, D. J., M. N. Gwynn, D. J. Holmes, and D. L. Pompliano. 2007. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6:29-40. [DOI] [PubMed] [Google Scholar]
- 25.Post, L. O., D. E. Farrell, C. V. Cope, J. D. Baker, and M. J. Myers. 2003. The effect of endotoxin and dexamethasone on enrofloxacin pharmacokinetic parameters in swine. J. Pharmacol. Exp. Ther. 304:889-895. [DOI] [PubMed] [Google Scholar]
- 26.Quagliarello, V., and W. M. Scheld. 1992. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N. Engl. J. Med. 327:864-872. [DOI] [PubMed] [Google Scholar]
- 27.Schinkel, A. H. 1999. P-glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 36:179-194. [DOI] [PubMed] [Google Scholar]
- 28.Schinkel, A. H., E. Wagenaar, L. van Deemter, C. A. Mol, and P. Borst. 1995. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J. Clin. Invest. 96:1698-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen, S., and W. Zhang. 2010. ABC transporters and drug efflux at the blood-brain barrier. Rev. Neurosci. 21:29-53. [DOI] [PubMed] [Google Scholar]
- 30.Takasato, Y., S. I. Rapoport, and Q. R. Smith. 1984. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am. J. Physiol. 247:H484-H493. [DOI] [PubMed] [Google Scholar]
- 31.Uchiumi, D., M. Kobayashi, T. Tachikawa, and K. Hasegawa. 2004. Subcutaneous and continuous administration of lipopolysaccharide increases serum levels of triglyceride and monocyte chemoattractant protein-1 in rats. J. Periodontal Res. 39:120-128. [DOI] [PubMed] [Google Scholar]
- 32.Wispelwey, B., A. J. Lesse, E. J. Hansen, and W. M. Scheld. 1988. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J. Clin. Invest. 82:1339-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]