Abstract
Tigecycline is a broad-spectrum glycylcycline antibiotic with potent in vitro activity against Clostridium difficile. We used a mouse model to test the hypothesis that tigecycline has a low propensity to promote colonization and toxin production by C. difficile due to inhibitory activity in the colon. Mice (5 to 8 per group) received subcutaneous injections of tigecycline (low and high doses) alone or in combination with clindamycin for 6 days. Growth of and toxin production by 3 strains of C. difficile (tigecycline MICs ≤ 0.012 μg/ml) were measured in cecal contents collected 6 h or 3 days after the final antibiotic dose. Antibiotic concentrations were measured using a bioassay, and concentrations of total anaerobes and Bacteroides spp. were measured. The effects of tigecycline on rendering mice susceptible to colonization with and reducing the burden of C. difficile were also examined. In comparison to saline controls, clindamycin promoted the growth of C. difficile (P < 0.001) in cecal contents, whereas tigecycline did not. Tigecycline did not suppress total anaerobes or Bacteroides spp. in comparison to saline controls. Concurrent administration of tigecycline prevented clindamycin-induced promotion of C. difficile in cecal contents collected 6 h or 3 days (high dose only) after the final antibiotic dose. Tigecycline did not promote the establishment of colonization in mice, yet it did not reduce concentrations of C. difficile in animals with established colonization. In summary, tigecycline did not promote the growth of or toxin production by C. difficile, probably due to inhibitory activity against C. difficile and relative sparing of indigenous anaerobic microflora.
Antimicrobial therapy plays a central role in the pathogenesis of Clostridium difficile infection (CDI). The presumed mechanism by which antibiotics induce CDI is through disruption of the indigenous microflora of the colon, thereby allowing C. difficile to grow to high concentrations with production of toxin (13). Although nearly all classes of antibiotics have been associated with CDI, clindamycin, broad-spectrum cephalosporins, and penicillins have traditionally been considered the agents that pose the greatest risk (13). With the emergence of the North American pulsed-field gel electrophoresis type 1 (NAP1) epidemic strain of C. difficile that exhibits increased resistance to fluoroquinolone antibiotics, fluoroquinolones have also been associated with CDI in multiple studies (1, 9, 13). However, there remains some uncertainty regarding the relative importance of fluoroquinolones as a risk factor for CDI because these agents cause only minor disruption of intestinal anaerobes (1, 13). Some recent studies suggest that antibiotics with inhibitory activity against C. difficile may be less likely to promote CDI (1-3, 7, 13-15, 17-19). For example, piperacillin-tazobactam, a beta-lactam/beta-lactamase inhibitor combination, not only disrupts the indigenous intestinal microflora but also has potent activity against C. difficile and has been infrequently associated with CDI in clinical studies (15, 19). In mice, piperacillin-tazobactam inhibited growth of C. difficile in the colon during treatment but facilitated growth and toxin production when exposure occurred after treatment during the period of recovery of the indigenous microflora (14).
Tigecycline, a broad-spectrum glycylcycline antibiotic, also has potent activity against C. difficile and is excreted in significant concentrations in bile (median fecal concentration in human volunteers, 5.6 mg/kg of body weight on day 8 of administration) (12). In a chemostat model of the human intestinal microflora, tigecycline markedly decreased concentrations of bacteroides and bifidobacteria but did not induce proliferation or toxin production by C. difficile (3). Wilcox (17) has noted that tigecycline has infrequently been associated with CDI in clinical studies. Moreover, Herpers et al. recently reported successful use of intravenous tigecycline as salvage therapy for 4 patients with refractory CDI (7). These data suggest that tigecycline may achieve sufficient concentrations in the intestinal tract to inhibit the growth of C. difficile. In this study, we used a mouse model to examine the effect of tigecycline on the growth of and toxin production by C. difficile. We hypothesized that the effect of tigecycline on C. difficile in the colon is similar to the effect of piperacillin-tazobactam: inhibition of growth and toxin production during tigecycline treatment but promotion of growth when exposure occurs after treatment during the period of recovery of the indigenous microflora.
MATERIALS AND METHODS
C. difficile strains.
Three strains of C. difficile were studied. ATCC 43593 is a nontoxigenic strain from the American Type Culture Collection (ATCC). The other strains were cultured from patients with CDI in Cleveland, OH. VA 17 is an epidemic North American pulsed-field gel electrophoresis type 1 (NAP1) strain. VA 11 is a restriction endonuclease analysis (REA) J-type strain. For all three strains, the MICs of tigecycline, piperacillin-tazobactam, and clindamycin were ≤0.012 μg/ml, ≤1 μg/ml, and ≥256 μg/ml, respectively, as determined by broth dilution (10).
Bioassay for tigecycline concentrations.
The concentration of tigecycline in stool samples on day 5 of treatment was determined using an agar diffusion assay, as described previously by Nord et al. (12), but Escherichia coli, rather than Bacillus cereus, was used as the indicator strain. The limit of detection was 1 μg/gm of feces.
Mouse model of in vitro colonization resistance to C. difficile.
The in vitro mouse model was adapted from the hamster model of colonization resistance to CDI, developed by Borriello et al. (5). These investigators demonstrated that antibiotics that promoted in vitro growth of and toxin production by C. difficile in cecal emulsions of hamsters also caused CDI in hamsters, whereas antibiotics that did not promote in vitro growth and toxin production did not cause disease (5). We have previously found that this model yields similar results in mice (1, 13). The Animal Care Committee of the Cleveland Veterans Affairs Medical Center approved the experimental protocol.
Female CF-1 mice weighing 25 to 30 g (Harlan Sprague-Dawley, Indianapolis, IN) were housed in individual cages. Mice received daily subcutaneous injections (total volume, 0.2 ml) of saline, tigecycline (0.05 mg/day), clindamycin (1.4 mg/day), or piperacillin-tazobactam (8 mg/day) for 4 days. In some experiments, mice received daily injections of tigecycline and clindamycin. The antibiotic doses were equal to the usual human doses administered over a 24-hour period (milligrams of antibiotic per gram of body weight). Because tigecycline did not promote overgrowth of C. difficile, the effect of a higher daily dose of 12 times the initial dosage was also measured.
Mice (8 per group) were killed by CO2 asphyxiation 6 h or 3 days after the final antibiotic dose. The ceca were removed and opened longitudinally, and the contents were collected and transferred to an anaerobic chamber (Coy Laboratories, Grass Lake, MI) within 5 min. The cecal contents were diluted 3-fold (volume/volume) with sterile prereduced phosphate-buffered saline (PBS). A final concentration of 104 CFU/ml of each C. difficile strain was added to separate aliquots of cecal contents from individual mice. The C. difficile strains were prepared for inoculation by serially diluting 72-hour broth cultures in sterile, prereduced PBS. To quantify the C. difficile spores, after incubation for 24 h, samples were diluted in sterile PBS and plated on prereduced cycloserine-cefoxitin-brucella agar containing 0.1% taurocholic acid and 5 mg/ml lysozyme (C. difficile brucella agar [CDBA]) (11). To determine toxin production in cecal contents, the C. difficile Tox A/B II (Wampole Laboratories, Princeton, NJ) test kit was used, according to the manufacturer's instructions.
Effect of antibiotic treatment on concentrations of total anaerobes and Bacteroides spp.
Six hours after completion of the final dose of 4 days of subcutaneous antibiotic treatment, fresh cecal contents were collected and transferred to the anaerobic chamber. The contents were serially diluted in prereduced PBS and plated onto brucella agar and Bacteroides bile-esculin agar (Becton Dickinson) to measure the concentrations of total anaerobes and Bacteroides spp., respectively. The lower limit of detection was ∼4 log10CFU/g of stool.
Mouse model of in vivo C. difficile colonization.
To assess the effect of tigecycline on the establishment of in vivo colonization with C. difficile, mice (6 per group) were given subcutaneous saline, tigecycline, clindamycin, or both antibiotics for 5 days. On day 2 of antibiotic treatment, mice were administered 104 CFU of C. difficile spores in 0.5 ml of PBS by oral gavage. To quantify the burden of C. difficile, fresh stool samples were collected on days 1, 3, and 7 after administration of C. difficile. Stool samples were emulsified with 5-fold (weight/volume) prereduced PBS, serially diluted, and inoculated onto CDBA plates.
To assess whether tigecycline treatment results in suppression of C. difficile after colonization has been established, mice (5 per group) received subcutaneous injections of clindamycin for 2 days, followed by administration of 4 log10CFU of C. difficile spores. Two days after administration of spores, the mice received daily subcutaneous saline, subcutaneous tigecycline (0.6 mg/day), subcutaneous piperacillin-tazobactam (8 mg/day), or oral vancomycin (0.25 mg/day) for 5 days. Piperacillin-tazobactam and oral vancomycin were included for comparison because these agents also have potent inhibitory activity against C. difficile; the dose of oral vancomycin was equivalent (in mg/kg) to the human dose used for treatment of CDI.
Statistical analysis.
Data analyses were performed using R software (version 2.10.1, 14 December 2009). A one-way analysis of variance (ANOVA) (Fig. 1 and 2) or multivariate ANOVA (MANOVA) (Fig. 3 and 4) was performed to compare the groups, with P values adjusted for multiple comparisons using Tukey's honestly significant difference test.
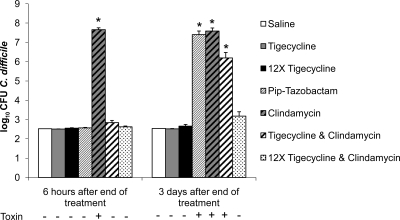
FIG. 1.
Mouse model of in vitro colonization resistance to C. difficile. Mice (n = 8 per group) received daily subcutaneous antibiotics for 4 days. Six hours or 3 days after the final antibiotic dose, cecal contents were collected, and aliquots were inoculated with 104 CFU/ml of C. difficile. Samples were incubated anaerobically for 24 h and then plated onto selective media to quantify C. difficile (lower limit of detection, 2.5 log10CFU/ml). Data for three strains of C. difficile were pooled. Error bars represent standard errors of the means. Pip = piperacillin. Detection of C. difficile toxin by C. difficile Tox A/B II (Wampole Laboratories) is indicated below the x axis. *, P < 0.001 versus saline.
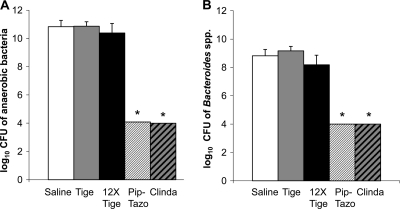
FIG. 2.
Effect of antibiotic treatment on concentrations of total anaerobes and Bacteroides spp. Mice (n = 6) received daily subcutaneous antibiotic treatment for 4 days. Six hours after the final dose, cecal contents were collected and plated anaerobically onto prereduced brucella agar to quantify total anaerobes (A) and onto Bacteroides bile-esculin agar to quantify Bacteroides spp. (B). The lower limit of detection was 4 logs. Error bars represent standard errors of the means. Tige = tigecycline; Pip-Tazo = piperacillin-tazobactam; Clinda = clindamycin. *, P < 0.001 versus saline.
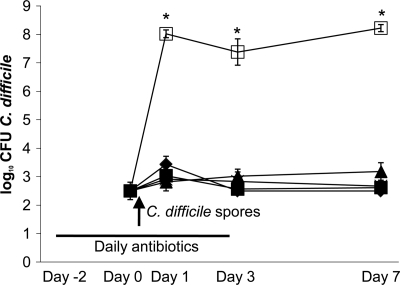
FIG. 3.
Mouse model of in vivo C. difficile colonization. Mice (n = 6) received subcutaneous antibiotic treatment daily for 5 days. On day 0, 104 CFU of C. difficile spores were administered by oral gavage (arrow). Stool was collected, and dilutions were plated onto selective media to quantify C. difficile. The lower limit of detection was ∼3 log10CFU/g. Saline, ♦; tigecycline, ▪; 12× tigecycline, ▴; clindamycin, □; tigecycline and clindamycin, •. *, P < 0.001 versus saline.
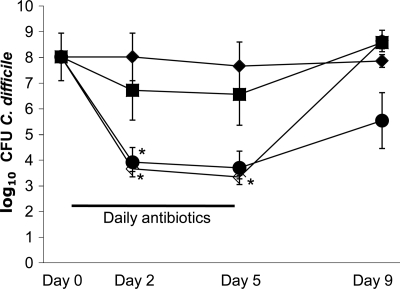
FIG. 4.
Impact of antibiotic treatment on C. difficile levels in colonized mice. Mice (n = 5) were colonized with C. difficile and then administered saline, piperacillin-tazobactam, or tigecycline by subcutaneous injection or vancomycin by oral gavage for 5 days. Stool was collected, and dilutions were plated onto selective media to quantify C. difficile spores. The lower limit of detection was ∼4 logs. Saline, ♦; tigecycline, ▪; piperacillin-tazobactam, □; oral vancomycin, •. *, P < 0.05 versus saline.
RESULTS
Mouse model of in vitro colonization resistance to C. difficile.
Figure 1 shows the effects of antibiotic treatment on in vitro growth of C. difficile in cecal contents collected 6 h or 3 days after the final antibiotic dose. In comparison to saline controls, clindamycin promoted overgrowth of C. difficile at both time points (P < 0.001), whereas piperacillin-tazobactam treatment prevented overgrowth in contents collected at 6 h but promoted overgrowth in samples collected 3 days after the final antibiotic dose (P < 0.001). Tigecycline treatment at both doses did not promote overgrowth of C. difficile in cecal contents collected at either time point. When administered in combination with clindamycin, tigecycline prevented clindamycin-induced overgrowth of C. difficile in cecal contents collected 6 h (both doses of tigecycline) and 3 days (high-dose tigecycline only) after antibiotics were withdrawn. By bioassay, levels of tigecycline in cecal contents collected 6 h after the final dose on day 4 were 2.8 ± 0.4 and 9.0 ± 3.4 μg/ml (± standard errors of the means [SEM]) at the human-equivalent (mg/kg) and the 12-times-higher doses, respectively. Tigecycline was not detected in the cecal contents of mice receiving either dose when collected 3 days after the final dose. C. difficile toxin was detected in all cecal content samples obtained from clindamycin-treated mice, whereas none of the mice in the other groups had detectable levels of toxin in cecal contents.
Figure 2 shows the effects of antibiotic treatment on concentrations of total anaerobes and Bacteroides spp. in cecal contents. Tigecycline did not suppress the levels of total anaerobes or Bacteroides spp., whereas piperacillin-tazobactam and clindamycin did (P < 0.001).
Mouse model of in vivo C. difficile colonization.
Figure 3 shows the effect of antibiotic treatment on the establishment of colonization with the nontoxigenic C. difficile strain ATCC 43593. In comparison to saline controls, clindamycin-treated mice developed high concentrations of C. difficile in stool (P < 0.001), whereas mice receiving either the human-equivalent dose or the 12-times-higher dose of tigecycline did not.
Figure 4 shows the assessment of whether antibiotic treatment results in suppression of C. difficile levels after colonization has been established. Subcutaneous injection of piperacillin-tazobactam resulted in a significant decrease in C. difficile concentrations in stool during treatment (P of <0.05 on days 2 and 5). Oral vancomycin also caused a decrease in stool concentrations of C. difficile that was significant on day 2 (P < 0.05) and approached significance on day 5 (P = 0.058). After treatment discontinuation, C. difficile levels in the groups of mice rapidly rebounded to pretreatment levels. Tigecycline treatment did not reduce C. difficile concentrations in stool in comparison to saline controls.
DISCUSSION
We found that tigecycline did not promote in vitro growth of or toxin production by C. difficile in cecal contents collected from mice during or 3 days after completion of treatment. As in previous studies, piperacillin-tazobactam inhibited growth of C. difficile during treatment but promoted overgrowth in cecal contents collected 3 days after completion of treatment (14). Clindamycin promoted overgrowth of clindamycin-resistant C. difficile in cecal contents during and after treatment. In previous studies, clindamycin inhibited the growth of clindamycin-susceptible strains during, but not after, treatment (1). Piperacillin-tazobactam and clindamycin treatment reduced concentrations of total anaerobes and Bacteroides spp. in cecal contents, whereas tigecycline treatment did not. For each of the antibiotics studied, the levels detected in mouse stool or cecal contents are comparable to concentrations detected in stool samples of healthy human volunteers or patients receiving the same antibiotics (6, 8, 11, 18). Our findings suggest that tigecycline might have a relatively low propensity to promote CDI due to inhibitory activity against C. difficile and relative sparing of indigenous anaerobic microflora.
Our results are consistent with some findings of previous studies in human volunteers and using a chemostat model of human intestinal microflora (3, 12). As in mice, tigecycline did not inhibit Bacteroides spp. in healthy human adults (12). Bifidobacteria were significantly reduced in human volunteers (12), but levels of bifidobacteria were not measured in the current study. In the chemostat model, instillation of tigecycline resulted in a 3- to 4-log decrease in total anaerobes and marked suppression of bacteroides, which failed to recover during the 14-day postantibiotic period (3). Despite the reduction in anaerobic microflora in the chemostat model, instillation of tigecycline was not associated with germination or outgrowth of C. difficile spores, suggesting that growth was inhibited (3).
Tigecycline treatment did not promote C. difficile colonization in mice, yet it did not reduce C. difficile levels in animals with preestablished colonization. In contrast, piperacillin- tazobactam and oral vancomycin suppressed levels of C. difficile in mice with preestablished colonization. The concentration of the antibiotics in the intestinal tract may explain the differing effects of the 3 agents. Compared to piperacillin-tazobactam and vancomycin levels determined in previous mouse model studies (piperacillin-tazobactam, 31.2 ± 9.7 μg and 2.0 ± 0.8 μg/ml of cecal contents at 6 h and 3 days after discontinuation of treatment, respectively; vancomycin, >500 μg/ml of cecal contents at both time points) (13; our unpublished data), the peak fecal concentration of tigecycline was much lower (2.8 ± 0.4 μg/gm), and no drug was detectable 3 days after discontinuation of treatment. Although tigecycline achieves peak concentrations in stool several times above the typical MICs of C. difficile isolates, factors such as rapid clearance from the intestinal tract, an inoculum effect (i.e., intestinal concentrations above the 105 inoculum used for MIC testing), or binding to fecal matter could potentially reduce its in vivo effects in the intestinal tract (13).
In conclusion, tigecycline did not promote growth of or toxin production by C. difficile, probably due to inhibitory activity against C. difficile and relative sparing of indigenous anaerobic microflora.
Acknowledgments
This work was supported by a Merit Review grant from the Department of Veterans Affairs to C.J.D.
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Adams, D. A., M. M. Riggs, and C. J. Donskey. 2007. Effect of fluoroquinolone treatment on growth of and toxin production by epidemic and nonepidemic Clostridium difficile strains in the cecal contents of mice. Antimicrob. Agents Chemother. 51:2674-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines, S. D., J. Freeman, and M. H. Wilcox. 2005. Effects of piperacillin/tazobactam on Clostridium difficile growth and toxin production in a human gut model. J. Antimicrob. Chemother. 55:974-982. [DOI] [PubMed] [Google Scholar]
- 3.Baines, S. D., K. Saxton, J. Freeman, and M. H. Wilcox. 2006. Tigecycline does not induce proliferation or cytotoxin production by epidemic Clostridium difficile strains in a human gut model. J. Antimicrob. Chemother. 58:1062-1065. [DOI] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Borriello, S. P., F. E. Barclay, and A. R. Welch. 1988. Evaluation of the predictive capability of an in-vitro model of colonisation resistance to Clostridium difficile infection. Microb. Ecol. Health Dis. 1:61-64. [Google Scholar]
- 6.Edlund, C., L. Barkholt, B. Olsson-Liljequist, and C. E. Nord. 1997. Effect of vancomycin on intestinal flora of patients who previously received antimicrobial therapy. Clin. Infect. Dis. 25:729-732. [DOI] [PubMed] [Google Scholar]
- 7.Herpers, B. L., et al. 2009. Intravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infection. Clin. Infect. Dis. 48:1732-1735. [DOI] [PubMed] [Google Scholar]
- 8.Kager, L., L. Liljeqvist, A. S. Malmborg, and C. E. Nord. 1981. Effect of clindamycin prophylaxis on the colonic microflora in patients undergoing colorectal surgery. Antimicrob. Agents Chemother. 20:736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loo, V. G., et al. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow anaerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 11.Nerandzic, M. M., and C. J. Donskey. 2009. Effective and reduced-cost modified selective medium for isolation of Clostridium difficile. J. Clin. Microbiol. 47:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nord, C. E., E. Sillerstrom, and E. Wahlund. 2006. Effect of tigecycline on normal oropharyngeal and intestinal microflora. Antimicrob. Agents Chemother. 50:3375-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owens, J., C. Robert, C. J. Donskey, R. P. Gaynes, V. G. Loo, and C. A. Muto. 2008. Antimicrobial associated risk factors for Clostridium difficile infection. Clin. Infect. Dis. 46:S19-S31. [DOI] [PubMed] [Google Scholar]
- 14.Pultz, N. J., and C. J. Donskey. 2005. Effect of antibiotic treatment on growth of and toxin production by Clostridium difficile in the cecal contents of mice. Antimicrob. Agents Chemother. 49:3529-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Settle, C. D., M. H. Wilcox, W. N. Fawley, O. J. Corrado, and P. M. Hawkey. 1998. Prospective study of the risk of Clostridium difficile diarrhoea in elderly patients following treatment with cefotaxime or piperacillin-tazobactam. Aliment Pharmacol. Ther. 12:1217-1223. [DOI] [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.Wilcox, M. H. 2007. Evidence for low risk of Clostridium difficile infection associated with tigecycline. Clin. Microbiol. Infect. 13:949-952. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox, M. H., A. Brown, and J. Freeman. 2001. Faecal concentrations of piperacillin and tazobactam in elderly patients. J. Antimicrob. Chemother. 48:155-156. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox, M. H., et al. 2004. Long-term surveillance of cefotaxime and piperacillin-tazobactam prescribing and incidence of Clostridium difficile diarrhoea. J. Antimicrob. Chemother. 54:168-172. [DOI] [PubMed] [Google Scholar]