Abstract
We have selected for resistance to etravirine (ETR) and efavirenz (EFV) in tissue culture using three subtype B, three subtype C, and two CRF02_AG clinical isolates, grown in cord blood mononuclear cells. Genotypic analysis was performed at baseline and at various weeks of selection. Phenotypic resistance in regard to ETR, EFV, and nevirapine (NVP) was evaluated at weeks 25 to 30 for all ETR-selected viruses and in viral clones that contained specific resistance mutations that were inserted by site-directed mutagenesis into pNL-4.3 and AG plasmids. The results show that ETR selected mutations at positions V90I, K101Q, E138K, V179D/E/F, Y181C, V189I, G190E, H221H/Y, and M230L and that E138K was the first of these to emerge in most instances. The time to the emergence of resistance was longer in the case of ETR (18 weeks) compared to EFV (11 weeks), and no differences in the patterns of emergent mutations could be documented between the B and non-B subtypes. Viral clones containing E138K displayed low-level phenotypic resistance to ETR (3.8-fold) and modestly impaired replication capacity (2-fold) compared to wild-type virus. ETR-selected virus showed a high degree of cross-resistance to NVP but not to EFV. We identified K101Q, E138K, V179E, V189I, G190E, and H221Y as mutations not included among the 17 currently recognized resistance-associated mutations for ETR.
HIV-1 reverse transcriptase (RT) is responsible for the conversion of the single-stranded RNA genome into double-stranded DNA and is therefore an important target of anti-HIV-1 therapy (16). Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are noncompetitive inhibitors of HIV-1 RT that bind to the polymerase active site and disrupt enzyme function. NNRTIs represent an important component of standard antiretroviral therapy in HIV-1-infected patients. NNRTIs can also exert additive effects when combined with nucleoside reverse transcriptase inhibitors (NRTIs) (5) and have potency comparable to that of protease inhibitors (PIs) (39, 41). However, the efficacy of narrow-spectrum NNRTIs such as nevirapine (NVP) and efavirenz (EFV) is often hindered by their low genetic barrier for resistance, since only a single amino acid substitution in the NNRTI binding pocket can often lead to high-level resistance against these drugs (3, 25). Furthermore, mutations associated with NNRTI resistance can be transmitted to others, and this has been documented in both developed and developing countries as occurring in between 10 and 20% of newly infected individuals (1, 19).
Etravirine (ETR) is an expanded-spectrum NNRTI with potent antiviral activity against both wild-type (wt) HIV-1 subtypes and against some viruses resistant to narrow-spectrum NNRTIs (2, 47). Clinical studies have shown that ETR plus optimized background regimens consisting of NRTIs, as well as integrase and protease inhibitors, significantly decreased viral loads in patients with resistance against narrow-spectrum NNRTIs and some PIs (9, 27, 33).
The structure of ETR allows it to bind to the RT enzyme in more than one distinct mode through conformational adaptations based on changes in the NNRTI-binding pocket. This allows ETR to reorient itself and provides alternative binding conformation when mutations in the binding pocket occur (12). In vitro selection studies and both the DUET-1 and the DUET-2 clinical trials identified 17 ETR resistance-associated mutations (RAMs) (V90I, A98G, L100I, K101E/H/P, V106I, E138A, V179D/F/T, Y181C/I/V, G190A/S, and M230L) and have permitted the assignment of a weighted score for each mutation (48). In general, three or more ETR RAMs are required for a diminished responsiveness to ETR to occur.
Non-subtype B infections represent >90% of the global HIV problem. Non-B viruses predominate in sub-Saharan Africa and are becoming more prevalent even in areas dominated by subtype B, such as North America and western Europe (18). Although some studies have established that subtype polymorphisms can play a role in the development of drug resistance, based on specific mutations that are selected by antiviral pressure (6, 7, 11, 21), most have focused on narrow-spectrum antiretrovirals. It is known, however, that naturally occurring polymorphisms at RT sites that correspond to ETR RAMs can vary among subtypes (24, 25, 28, 36). We sought to investigate the possible importance of such polymorphisms in the development of ETR resistance, even though ETR seems to possess similar antiviral activity against various HIV subtypes (A to H) and some circulating recombinant forms (CRFs).
(This study was performed by E.L.A. in partial fulfillment of the requirements of a Ph.D. degree for the Département de Microbiologie et d'Immunologie, Université de Montréal, Montréal, Quebec, Canada.)
MATERIALS AND METHODS
Virus isolates, cells, drugs, and plasmids.
Three subtype B (BK132, 5326, and 5331), three subtype C (Mole 03, BG 05, and 10680) and two CRF02_AG (6383 and 6399) wt clinical isolates were studied (Fig. 1). BK132 was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. Isolates 5326, 5331, 10680, 6383, and 6399 were obtained with informed consent from drug-naive individuals at our clinics in Montreal, Canada. The two subtype C clinical samples, Mole 03 and BG 05, originated in Botswana and were obtained courtesy of Max Essex of Harvard University, Boston, MA.
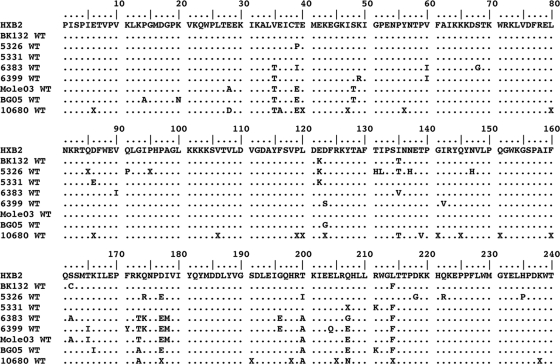
FIG. 1.
Baseline polymorphisms of HIV-1, as shown in an amino acid alignment (residues 1 to 240) of RT for all clinical isolates used in the present study. Subtype B: BK132, 5326, and 5331; CRF02_AG, 6383 and 6399; subtype C, Mole 03, BG 05, and 10680. HXB2 was used as a reference sequence. All isolates were sequenced at baseline and passaged simultaneously in the presence or absence of drugs. The sequence of the wild-type RT at baseline was the same as that of isolates passaged without drugs. The dots represent identical positions, while letters indicate variations in amino acids relative to HXB2. Isolate 6383 at baseline harbored the NNRTI resistance mutation V90I that is known to decrease susceptibility to ETR.
ETR was a gift from Tibotec, Inc., while lopinavir (LPV), NVP, and EFV were obtained from Abbott Laboratories (North Chicago, IL), Boehringer Ingelheim, Inc., and the NIH AIDS Research and Reference Reagent Program, respectively.
Cord blood mononuclear cells (CBMCs) were obtained through the Department of Obstetrics, Jewish General Hospital, Montreal, Canada. The HEK293T cell line was obtained from the American Type Culture Collection. The AG plasmid (p97GH-AG2) was kindly provided by Masashi Tatsumi, National Institute of Infectious Diseases, Tokyo, Japan. The infectious molecular clone pNL-4.3 and TZM-bl cells were obtained through the NIH AIDS Research and Reference Reagent Program, courtesy of Malcolm Martin and John C. Kappes, respectively.
Selection of resistance mutations in CBMCs.
Phytohemagglutinin (PHA)-stimulated CBMCs were infected with viruses (multiplicity of infection [MOI] of 0.1) for 2 h, incubated at 37°C, and subsequently washed with RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum and seeded into a 24-well plate at a density of 2.5 × 106 cells per well (14). Selection for resistance in CBMCs was performed with increasing concentrations of drugs (ETR and EFV) at starting concentrations below the 50% effective concentration (EC50) of the drugs (34). As controls, all viruses were simultaneously passaged without drugs. RT assays were performed weekly as described to monitor viral replication (26, 35). Based on the ratio of the RT value in culture fluids of control wells to wells with drug at the previous round of replication, drug concentrations were increased at subsequent passages. Selection at a particular drug concentration was considered to be complete when repeated passage revealed that RT levels in culture fluids had peaked at the same time as that of a control well that did not contain drugs. Virus-containing culture fluids were harvested and kept at −80°C for subsequent genotypic analysis at the same time that drug concentrations were increased. Selections for resistance were performed over 25 to 30 weeks.
Nucleic acid extraction, amplification, and sequencing analysis.
Viral RNA was extracted from culture supernatants by using a Qiagen QIAamp viral extraction kit (Mississauga, Ontario, Canada). Viral RNA amplification was performed by reverse transcription-PCR and nested PCR using a previously published protocol (Virco BVBA, Mechelen, Belgium). The resulting PCR-amplified DNA fragment was purified by using a QIAquick PCR purification kit (Mississauga, Ontario, Canada), as specified by the manufacturer. The presence of the 1.5-kb PR and reverse transcription-PCR product was confirmed by running 5 μl of each product on a 1% agarose gel with Sybersafe (Invitrogen).
Genotyping was performed by a published protocol (Virco BVBA), based on sequencing of a 1,200-bp fragment of the HIV-1 pol gene encompassing up to 400 amino acids in the RT region, using Virco primers with a BigDye terminator sequencing kit (version 1.1; Applied Biosystems, Foster City, CA) and an automated sequencer (ABI Prism 3130 genetic analyzer; Applied Biosystems). The data were analyzed using SeqScape software version 2.5.
Site-directed mutagenesis and virus production.
The E138K mutation was introduced into pNL-4.3 and the p97GH-AG2 plasmid by site-directed mutagenesis using a QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). For site-directed mutagenesis of E138K in pNL-4.3, the forward primer 5′-GCATTTACCATACCTAGTATAAACAATAAGACACCAGGGATTA-3′ and the reverse primer 5′-TAATCCCTGGTGTCTTATTGTTTATACTAGGTATGGTAAATGC-3′ were used, while for E138K in p97GH-AG2 the forward primer was 5′-GCATTCACTATACCTAGTGTAAACAATAAGACACCAGGGATT-3′ and the reverse primer was 5′-AATCCCTGGTGTCTTATTGTTTACACTAGGTATAGTGAATGC-3′. The underlined codons denote sites at which single nucleotide substitutions were introduced into the plasmids. This was confirmed by sequencing, and DNA was ultimately transformed into DH5α cells (Invitrogen) for a high yield of plasmid. Viruses were produced by transfection of 16 μg of pNL-4.3 wt (pNL-4.3wt), pNL-4.3E138K, p97GH-AG2 wt (AGwt), and AGE138K plasmids into HEK293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocols. Two days after transfection, supernatants of transfected cells were clarified by centrifugation at 1,500 rpm for 5 min to remove cellular debris, filtered through a 0.45-μm-pore-size filter, and stored in aliquots at −80°C. Virus production was confirmed by measurement of the RT activity and p24 production.
Phenotypic drug susceptibility.
Drug susceptibility to NNRTIs (i.e., ETR, EFV, and NVP) was measured in cell-culture-based phenotypic assays as previously described (26, 40). In brief, CBMCs were infected for 2 h with either wt virus, drug-selected variants, or recombinant viruses; washed to remove unbound virus; and plated into 96-well plates containing drugs or not. After 3 or 4 days of incubation at 37°C, the cells were fed with fresh medium containing appropriate drug dilutions, and RT assays were performed at day 7. The data were analyzed by using Prism software (version 5.0; GraphPad, Inc.) to determine the EC50 for each drug tested. Resistance to a given drug was determined based on the published lower clinical cutoff (CCO), which determines the cutoff fold change value for the sensitivity of NNRTIs (46, 48). These values are 3.0 for ETR, 3.4 for EFV, and 5.5 for NVP (46, 48).
Determination of relative replication capacity in TZM-bl cells.
The replicative capacities of competent clonal wt and E138K-containing HIV-1 strains were evaluated in a noncompetitive infectivity assay using TZM-bl cells (51). Twenty-thousand cells per well were added into a 96-well culture plate in 100 μl of Dulbecco modified Eagle medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (Gibco), 1% penicillin-streptomycin, and 1% l-glutamine (Invitrogen). Viral stocks for both wt and mutant viruses were normalized by p24 and recombinant viruses from AG and pNL-4.3 plasmids were serially diluted 2- and 4-fold, respectively, from viral stock suspensions. After 4 h, 50 μl of DMEM was removed from the wells and replaced by 50 μl of virus dilution; a control well did not contain virus. Viruses and cells were cocultured for 48 h, after which 100 μl of Bright-Glo reagent was added, and the luciferase activity measured in a luminometer as described previously (Promega). The viral replication level was expressed as a percentage of relative light units with reference to wt virus for each virus studied.
Viral growth kinetics in CBMCs.
PHA-stimulated CBMCs were infected with viruses at an MOI of 0.1 as described above for the selection of resistance. Culture fluids were collected at various times to determine the levels of RT activity.
Statistical analysis.
A two-way analysis of variance (ANOVA) was performed on group means using Prism software (version 5.0; GraphPad, Inc.). A P value of <0.05 was considered to represent a significant difference.
RESULTS
Baseline genotyping of clinical isolates.
The first 240 amino acids in the RTs of all of the isolates (BK132, 5326, 5331, 6383, 6399, Mole 03, BG 05, and 10680) were sequenced at baseline and compared to the HIV-1 subtype B reference strain HXB2 (Fig. 1). Amino acid changes were identified in 37 of 240 positions in RT and were more frequent in non-subtype B than in subtype B isolates. Only one of these substitutions, i.e., V90I, present at baseline in the 6383 isolate, is known to be implicated in HIV-1 resistance to NNRTIs with a weighted factor of 1.0 for ETR resistance (48).
Time to development of resistance to ETR.
ETR resistance was selected in culture using CBMCs as described above (Table 1). Despite attainment of high ETR concentrations at weeks 11 to 13, only the BK132 isolate selected the E138E/K mutation, a finding consistent with previous results on the difficulty of selecting resistance to this compound (47). Subsequently, all isolates showed the accumulation of other mutations at weeks 18 to 20 (V90I, K101Q, E138K, V179D/E/F, Y181C, V189I, G190E, H221Y, and M230L). E138K emerged as the first mutation in all cases except for isolate 5331, in which it was detected between weeks 25 and 30. All selected mutations were maintained in culture under drug pressure as other mutations accumulated. A strong association was observed between the presence of E138K and Y181C and between Y181C and substitutions at position 179 for all isolates. Isolate 6399 selected for E138K alone. No differences in the patterns of mutations for ETR were observed between subtype B and non-B viruses. Novel amino acid substitutions not included in lists of published RAMs for ETR (48) were K101Q, E138K, V179E, V189I, G190E, and H221Y.
TABLE 1.
Evolution of viral variants selected with ETR in tissue culture
| Wks | Variant(s) in: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Subtype B |
CRF02_A/G |
Subtype C |
||||||
| BK132 | 5326 | 5331 | 6383 | 6399 | Mole 03 | BG 05 | 10680 | |
| 11-13 | E138E/K | NDa | ND | V90Ib | wt | wt | wt | ND |
| 18-20 | E138K, G190G/E, H221H/Y | E138E/K, V179D, Y181C | K101Q, V189I | V90I, E138E/K, V179V/E, Y181C, M230M/L | E138E/K | V90I, E138K, V179D, Y181Y/C | E138K, Y181C, M230L | E138K, K238K/N |
| 25-30 | E138K, G190E, H221H/Y | E138K, V179D, Y181C | V90I/V, K101Q, E138K, V189I | V90I, E138K, V179E, Y181C, M230L | E138K | V90I, E138K, V179D, Y181Y/C | E138K, V179F, Y181C, M230L | E138K, G190E/G, K238K/N |
ND, not determined.
V90I is a baseline polymorphism in isolate 6383.
Time to development of resistance to EFV.
As expected, all isolates had selected for mutations K103N/Q, V106I/M, V179D/V, Y188C/Y, and/or M230L/M that contribute to high-level EFV resistance by weeks 11 to 13 (Table 2). Additional mutations (L100I, V108I/V, K101E/K, and N348N/T) were observed at subsequent passages. This demonstrates the low genetic barrier of EFV. V106I and V106M were selected in subtypes B and C, respectively, due to a well-described polymorphism at this position (6). Other mutations selected by EFV included L100I, K103N/Q, V108V/I, V179D/V, Y188C/L, G190A, H221Y, and N348I.
TABLE 2.
Evolution of viral variants selected with EFV in tissue culture
| Wks | Variant(s) in: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Subtype B |
CRF02_A/G |
Subtype C |
||||||
| BK132 | 5326 | 5331 | 6383 | 6399 | Mole 03 | BG 05 | 10680 | |
| 11-13 | K103Q, V106I, Y188C | NDa | ND | V90Ib, K103N | K103N | V106M, Y188C/Y, M230L/M | V106M, V179D/V, Y188C | Y188C |
| 18-20 | K103Q, V106I, Y188C | K101E/K, V108I/V, Y188C/Y | L100I, N348I/N | V90I, L100I, K103N | N348N/T, K103N | V106M, Y188C | V106M, V179D/V, Y188C | V106M, Y188C |
| 25-30 | K103Q, V106I, Y188C | L100I, Y188L | L100I, V108V/I, H221Y, N348I | V90I, L100I, K103N, G190A | K103N | V106M, Y188C | V106M, V179D/V, Y188C | V106M, Y188C |
ND, not determined.
V90I is a baseline polymorphism in isolate 6383.
Resistance profile of ETR-selected variants.
Table 3 shows the phenotypic susceptibility of viruses containing ETR RAMs. At baseline, the mean EC50s for ETR ranged between 1 and 4.5 nM, while those for EFV ranged between 1.2 and 3.5 nM. Isolate 6383 that harbored V90I at baseline had the highest EC50 of 4.5 nM for ETR. Isolate 6399 that selected for E138K alone after 25 to 30 weeks in culture displayed low-level resistance to ETR, EFV, and NVP, with fold resistances of 5.1, 3.7, and 6.7, respectively. Isolate BK132 (subtype B) variants selected at weeks 25 to 30 possessed E138K, G190E, and H221H/Y and displayed moderate and low-level resistance to ETR and EFV, i.e., 8.7- and 3.8-fold, respectively (Table 3). ETR-resistant variants selected by three other non-B isolates at weeks 25 to 30 contained mutations as follows: 6383 (V90I, E138K, V179E Y181C, and M230L), Mole 03 (V90I, E138K, V179D, and Y181Y/C), and BG 05 (E138K, V179F, Y181C, and M230L). These viruses all displayed very high-level resistance to ETR and NVP (>95-fold) and low-to-moderate resistance (<11-fold) to EFV. The presence of Y181C in combination with other RAMs may have contributed to high-level ETR resistance in these situations. As a control, we used the isolate BG05 (E138K, V179F, Y181C, and M230L) and the protease inhibitor LPV. The EC50s of BG05 wt and the BG05-selected variant (E138K, V179F, Y181C, and M230L) were 5.5 ± 2.1 and 6.0 ± 2.0 nM, respectively (fold change [FC] = 1.1).
TABLE 3.
In vitro antiviral activity of NNRTIs against ETR-selected variants
| Isolatea | Subtype | NNRTI mutation(s) | Mean EC50 ± SDb (fold change) |
||
|---|---|---|---|---|---|
| ETR (nM) | EFV (nM) | NVP (μM) | |||
| BK132 | B | wt | 3.7 ± 0.9 | 3.2 ± 0.4 | 0.04 ± 0.02 |
| BK132* | B | E138K, G190E, H221H/Y | 32 ± 3 (8.7) | 12 ± 4 (3.8) | NDc |
| 6383 | A/G | wt | 4.5 ± 0.7 | 3.5 ± 0.7 | 0.041 ± 0.012 |
| 6383* | A/G | V90I, E138K, V179E, Y181C, M230L. | 430 ± 20 (95.6) | 40 ± 10 (11.4) | >5.0 (>121.9) |
| 6399 | A/G | wt | 1 ± 0.2 | 1.2 ± 0.4 | 0.031 ± 0.001 |
| 6399* | A/G | E138K | 5.1 ± 0.2 (5.1) | 4.4 ± 0.1 (3.7) | 0.21 ± 0.01 (6.7) |
| Mole 03 | C | wt | 1.3 ± 0.4 | 1.5 ± 0.1 | 0.038 ± 0.01 |
| Mole 03* | C | V90I, E138K, V179D, Y181Y/C | 140 ± 20 (107.7) | 4.7 ± 2 (3.1) | >10.0 (>263) |
| BG 05 | C | wt | 3.5 ± 0.1 | 1.5 ± 0.2 | 0.012 ± 0.002 |
| BG 05* | C | E138K, V179F, Y181C, M230L | 490 ± 40 (140) | 16 ± 1 (10.7) | >5.0 (>416) |
Asterisks (*) indicate an ETR-selected variant at weeks 25 to 30. As stated in the text, isolate 6383 contained the V90I mutation at the baseline.
The values represent means of two or three independent experiments, each performed in duplicate. Drug susceptibility was expressed as fold change in EC50, determined by calculating the ratio of EC50s for selected variants and wt viruses (values in parentheses).
ND, not determined.
Impact of E138K in the pNL-4.3 and AG plasmids.
We introduced E138K by site-directed mutagenesis into the pNL-4.3 and AG plasmids to determine its impact on resistance to ETR, EFV, and NVP in CBMCs. The EC50s of pNL-4.3wt and pNL-4.3E138K were 1.6 ± 0.25 nM and 6.2 ± 1.2 nM for ETR (FC = 3.8), 1.73 ± 0.25 nM and 4.8 ± 0.69 nM for EFV (FC = 2.7), and 26 ± 6 nM and 38 ± 10 nM for NVP (FC = 1.4), respectively. Similarly, the EC50s of AGwt and AGE138K were 1.3 ± 0.15 nM and 5.1 ± 0.17 nM for ETR (FC = 3.9), 1.46 ± 0.15 nM and 3.2 ± 0.42 nM for EFV (FC = 2.2), and 40.0 ± 7 nM and 75.0 ± 2 nM for NVP (FC = 1.8), respectively. Decreases in susceptibility were modest for ETR, i.e., 3.8- and 3.9-fold for pNL-4.3E138K and AGE138K, respectively (Fig. 2). EFV and NVP both retained relative sensitivity to the pNL-4.3E138K and AGE138K viruses containing E138K, i.e., FCs of 2.7 and 2.2 for EFV and FCs of 1.4 and 1.8 for NVP, respectively. The EC50s of a control drug, LPV, for pNL-4.3wt and pNL-4.3E138K were 9.5 ± 0.7 nM and 10.0 ± 0.02 nM (FC = 1.05), respectively.
FIG. 2.
Comparative phenotypes of recombinant viruses derived from pNL-4.3E138K and AGE138K and tested for susceptibilities to ETR, EFV, and NVP in CBMCs. Recombinant clones containing E138K were tested for their susceptibilities to ETR, EFV, and NVP in cell culture assays. The mean EC50s of mutated variants were compared to those of wt pNL-4.3 or AG plasmids. The results shown are means of two or three independent experiments. Bars in the figure represent the mean fold change, while error bars represent ± the standard deviation (SD). Bars with dots, fold change (FC) to ETR; bars with slashes, fold change to EFV; open bars, fold change to NVP.
Viral replication capacity.
We also studied the relative replication capacity of wt virus and viruses carrying the E138K mutation by infecting TZM-bl cells with serially diluted viral stocks normalized for p24 production. The infectiousness of the wt and E138K viruses was determined by measuring the luciferase activity at 48 h postinfection. At a p24 input of 10,000 pg/ml, the relative replication of E138K compared to wt virus was diminished after 48 h by 2- and 3-fold for pNL-4.3E138K and AGE138K, respectively (P < 0.01 and P < 0.001) (Fig. 3 A and B). In a multiple round infection in CBMCs, viral growth experiments in the absence of drug confirmed that the E138K mutation conferred a 2-fold drop in replication rates for pNL-4.3E138K compared to pNL-4.3wt by both 6 and 8 days after infection (Fig. 3C) (P < 0.001).
FIG. 3.
Effect of E138K on viral replication capacity. Viral stocks of both wt and E138K-containing virus were normalized for p24 and used to infect TZM-bl cells. (A and B) The luciferase activity was measured at 48 h postinfection as an indication of viral replication. The relative infectivity of the wt compared to E138K-containing virus is shown on the y axis, while the x axis denotes the input of p24. Statistical analysis using a two-way ANOVA shows that replication capacity was decreased by 2 -and 3-fold compared to the wt for pNL-4.3E138K and AGE138K, respectively, after 48 h. A significant difference was observed after a p24 input greater than 10,000 pg/ml. The points denoted by asterisks (***) indicate a significant difference. CBMCs were also infected as described in Materials and Methods, and viral growth was measured by determining the RT activity in culture supernatants at different times (C). Significant differences observed at the peak of infection (days 6 and 8) are indicated by asterisks (***). In the figure, a P value of <0.01 is represented by “**”, while a P value of <0.001 is represented by “***”. Values are means of at least two independent experiments ± the SD. Error bars represent the SD.
DISCUSSION
Although ETR is currently approved for NNRTI-experienced patients, a number of studies have suggested that its use in patients with NNRTI mutations at baseline might lead to poor virological response (VR) (22, 29, 30, 37). In most of these studies, the presence in the background regimen of newer drugs such as enfuvirtide, darunavir, or raltegravir was positively associated with VR, whereas previous exposure to NVP and the presence of baseline mutations such as E138A, V179I, and Y181C were negatively associated with VR. We provide here a comprehensive in vitro analysis of the resistance pattern of ETR in wild-type clinical isolates of subtype B, subtype C, and CRF02_AG grown over 25 to 30 weeks in CBMCs in increasing concentrations of ETR compared to EFV. Our data show that ETR RAMs emerged after 18 weeks of drug pressure except for one isolate (BK132) that selected for E138E/K at weeks 11 to 13. The E138K mutation was selected in all isolates and was almost always the first mutation to emerge.
Although E138K has been reported in a few cases of ETR treatment failure (44, 49), its clinical significance has not yet been elucidated. Site-directed mutagenesis of E138K revealed low-level resistance to ETR in both pNL-4.3 and AG plasmids, with a fold change slightly above the clinical cutoff (CCO). Others have reported a median ETR FC of 2.6-fold for E138K with first- and third-quartile values of 2.0- and 4.1-fold, respectively, data that are consistent with the site-directed mutagenesis results reported here (4). Although E138K cannot on its own exclude ETR as a potentially useful drug, its presence might facilitate the accumulation of other resistance mutations or may result in an increased FC for resistance for ETR. In an analysis of the DUET studies, two different groups showed that mutations at position 138 were among the most frequent with E138K emerging in three patients (44, 49). Furthermore, all patients with E138 mutations demonstrated an increased FC for ETR over the upper CCO for resistance for ETR (>13), documenting the role of mutations at position 138 in ETR resistance. Of course, the emergence of resistance in the treatment-experienced DUET patients may not be comparable to in vitro selections beginning with wt viruses, especially because several of the NRTI mutations that patients possessed in the DUET studies are known to hypersensitize them to ETR. Clinical studies with ETR in NNRTI-naive patients will be required to validate the present results.
In contrast, an FC for EFV below the CCO was observed with the site-directed mutants of E138K. This is consistent with the fact that E138K is not selected in vitro by EFV and was not observed in EFV-treated patients. One study reported the emergence of E138K after in vitro passage of viruses containing amino acid substitutions at position 135 at baseline (15). These authors demonstrated FCs for EFV of 2 and 7 for E138K and I135T/E138K, respectively.
The ETR RAMs observed here include V90I, K101Q, E138K, V179D/E/F, Y181C, V189I, G190E, H221Y, and M230L, a pattern similar to that observed by others who also observed a high frequency of L100I if viruses contained some NNRTI mutations at baseline (47). These authors also selected E138G if they began with a virus containing the K103N substitution.
We did not observe any difference in the pathway of ETR resistance in B and non-B subtypes. The Y181C mutation was associated with either E138K or V179D/E/F. Both Y181C and V179F were also observed to co-emerge in the DUET studies (44, 48), a finding that is possibly due to the fact that the combination of either E138K and Y181C or of V179D/E/F and Y181C improves viral replication capacity compared to the presence of only a single mutation. In a recent analysis of genotypic and phenotypic changes of rebounders in the DUET studies, it was shown that V179 (n = 35), E138 (n = 17), Y181 (n = 15), and K101 (n = 13) (44) were the positions at which new mutations were most frequently observed. In contrast, EFV selected for classic mutations, including V106I and V106M in subtypes B and C, respectively, at weeks 11 to 13, as previously described (6). The specific selection here of G190A and G190E by EFV and ETR, respectively, confirms the nonassociation of G190A with ETR (50). However, G190E has been shown to be selected by both ETR and another novel NNRTI, rilpivirine (RIL) (4, 47).
Our phenotypic data show that accumulation of several ETR RAMs especially Y181C and M230L, resulted in high level ETR resistance. Viruses possessing high-level resistance to ETR also displayed moderate and high-level resistance to EFV and NVP, respectively. This confirms cross-resistance between ETR and NVP and is also consistent with studies showing that the prior use of NVP is associated with resistance to ETR (23, 31, 42). The low and moderate resistance to ETR displayed by isolates 6399 (E138K) and BK132 (E138K, G190E, and H221H/Y) are probably due to the low weight of these mutations on resistance to ETR.
In general, NNRTI mutations that emerge early in treatment-experienced patients, e.g., K103N, do not impair viral replication capacity to significant extend (10). We found that E138K in both pNL-4.3 and AG exerted only modest impact on replication capacity, a finding that helps to explain the frequency of this mutation in our selection, as well as the fact that E138K was usually the first mutation to emerge. In contrast, M230L impairs viral replication capacity by ∼8-fold (51). This is consistent with the selection of E138K in all eight isolates, whereas M230L was selected in only two of eight isolates in the present study.
E138K has previously been selected in vitro by other broad-spectrum NNRTIs (4, 8, 13, 17, 20). However, it has low prevalence (0.5%) in clinical samples (43) and only little impact on susceptibility to narrow-spectrum NNRTIs. E138K (GAG→AAG) is found in the p51 subunit of RT as part of the NNRTI binding pocket. The K101 side chain can normally form a salt bridge with E138, but the presence of E138K causes residues to move away from the NNRTI pocket due to the juxtaposition of similar charges that result from the mutation (38), potentially preventing the specific binding of drug. In addition, interactions of Y318 and E138 in the presence of ETR have been described as unusual for NNRTI-RT complexes (45). The emergence of mutations at residues 138 and 318 could disrupt drug binding ability (4, 47). The identification of a new E138R mutation selected by RIL reinforces the importance of this position (4). E138K and other mutations (K101Q, V179E, V189I, G190E, and H221Y) selected in our study are not yet included among the list of ETR RAMs. Although G190E was previously selected by ETR and RIL (4, 47), its role, alone or in combination with other mutations, remains unknown. Both V179E and H221Y have also been previously shown to confer a degree of resistance to ETR (28, 31, 32). Additional studies in our laboratory will evaluate whether the mutations K101Q, V179E, V189I, G190E, and H221Y either alone or in combination with E138K might affect susceptibility to ETR and other NNRIs.
A recent study analyzed the weights of 17 ETR RAMs, based on the DUET-1 and -2 studies (48). Others have also assigned weighted scores for ETR mutations based on different work (32); the assigned scores were 2, 3, 1, and 1 for E138K, V179E, G190E, and H221Y, respectively. As new results of ETR resistance are released, there will be a need to improve algorithms to also take into account the partial resistance that is associated with certain mutations and mutational patterns.
Our in vitro results confirm that ETR is a potent antiviral drug with a higher genetic barrier for resistance than narrow-spectrum NNRTIs. Although the data also suggest that virological response to ETR in NNRTI-naive patients might be superior than in experienced individuals, clinical studies with patient samples will be needed to provide further information on this topic, as well as on the clinical significance of the E138K substitution in cases of ETR treatment failure.
Acknowledgments
This research was supported by grants from the Canadian Institutes of Health Research. E.L.A. is the recipient of a Departmental Scholarship from the Département de Microbiologie et d'Immunologie, Université de Montréal.
The AG plasmid (p97GH-AG2) was kindly provided by Masashi Tatsumi, National Institute of Infectious Diseases, Tokyo, Japan. The Mole 03 and BG 05 clinical isolates were of Botswana origin and were obtained courtesy of Max Essex, Harvard University, Boston, MA.
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Aghokeng, A. F., et al. 2009. Evaluation of transmitted HIV drug resistance among recently infected antenatal clinic attendees in four Central African countries. Antivir. Ther. 14:401-411. [DOI] [PubMed] [Google Scholar]
- 2.Andries, K., et al. 2004. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:4680-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antinori, A., et al. 2002. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res. Hum. Retrovir. 18:835-838. [DOI] [PubMed] [Google Scholar]
- 4.Azijn, H., et al. 2010. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob. Agents Chemother. 54:718-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basavapathruni, A., C. M. Bailey, and K. S. Anderson. 2004. Defining a molecular mechanism of synergy between nucleoside and nonnucleoside AIDS drugs. J. Biol. Chem. 279:6221-6224. [DOI] [PubMed] [Google Scholar]
- 6.Brenner, B., et al. 2003. A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS 17:F1-F5. [DOI] [PubMed] [Google Scholar]
- 7.Brenner, B. G., et al. 2006. HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture. AIDS 20:F9-F13. [DOI] [PubMed] [Google Scholar]
- 8.Brillant, J. E., K. K. Swallow, S. Cammack, N., and G. Heilek-Snyder. 2004. In vitro resistance development for a second-generation NNRTI: TMC125. Antivir. Ther. 9(Suppl. 1):S20. [Google Scholar]
- 9.Briz, V., et al. 2009. Raltegravir and etravirine are active against HIV type 1 group O. AIDS Res. Hum. Retroviruses. 25:225-227. [DOI] [PubMed] [Google Scholar]
- 10.Collins, J. A., et al. 2004. Competitive fitness of nevirapine-resistant human immunodeficiency virus type 1 mutants. J. Virol. 78:603-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutsinos, D., et al. 2009. Template usage is responsible for the preferential acquisition of the K65R reverse transcriptase mutation in subtype C variants of human immunodeficiency virus type 1. J. Virol. 83:2029-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das, K., et al. 2004. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J. Med. Chem. 47:2550-2560. [DOI] [PubMed] [Google Scholar]
- 13.Ferris, R. G., et al. 2005. Antiviral activity of GW678248, a novel benzophenone nonnucleoside reverse transcriptase inhibitor. Antimicrob. Agents Chemother. 49:4046-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao, Q., et al. 1993. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine confers high-level resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 37:1390-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatanaga, H., et al. 2010. Impact of human leukocyte antigen-B*51-restricted cytotoxic T-lymphocyte pressure on mutation patterns of nonnucleoside reverse transcriptase inhibitor resistance. AIDS 24:F15-F22. [DOI] [PubMed] [Google Scholar]
- 16.Gotte, M., X. Li, and M. A. Wainberg. 1999. HIV-1 reverse transcription: a brief overview focused on structure-function relationships among molecules involved in initiation of the reaction. Arch. Biochem. Biophys. 365:199-210. [DOI] [PubMed] [Google Scholar]
- 17.Guoping Su, Y. L., et al. 2007. In vitro selection and characterization of viruses resistant to R1206, a novel nonnucleoside reverse transcriptase inhibitor. Antivir. Ther. 12:S35. [Google Scholar]
- 18.Hemelaar, J., E. Gouws, P. D. Ghys, and S. Osmanov. 2006. Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS 20:W13-W23. [DOI] [PubMed] [Google Scholar]
- 19.Hurt, C. B., et al. 2009. Transmitted antiretroviral drug resistance among acute and recent HIV infections in North Carolina from 1998 to 2007. Antivir. Ther. 14:673-678. [PMC free article] [PubMed] [Google Scholar]
- 20.Javanbakht, H., et al. 2010. In vitro resistance development for RO-0335, a novel diphenylether nonnucleoside reverse transcriptase inhibitor. Antiviral Res. 86:212-219. [DOI] [PubMed] [Google Scholar]
- 21.Kantor, R., et al. 2005. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med. 2:e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kekitiinwa, A., F. D. Coakley, E. Lie, and Y. Frank Granziano. 2010. Profiling etravirine resistance in Ugandan children with extended failure of a NNRTI-inclusive regimen as first-line ART, abstr. 891. CRIO, San Francisco, CA.
- 23.Kiertiburanakul, S., S. Wiboonchutikul, C. Sukasem, W. Chantratita, and S. Sungkanuparph. 2010. Using of nevirapine is associated with intermediate and reduced response to etravirine among HIV-infected patients who experienced virologic failure in a resource-limited setting. J. Clin. Virol. 47:330-334. [DOI] [PubMed] [Google Scholar]
- 24.Lapadula, G., et al. 2008. Prevalence and risk factors for etravirine resistance among patients failing on non-nucleoside reverse transcriptase inhibitors. Antivir. Ther. 13:601-605. [PubMed] [Google Scholar]
- 25.Llibre, J. M., et al. 2008. Prevalence of etravirine-associated mutations in clinical samples with resistance to nevirapine and efavirenz. J. Antimicrob. Chemother. 62:909-913. [DOI] [PubMed] [Google Scholar]
- 26.Loemba, H., et al. 2002. Genetic divergence of human immunodeficiency virus type 1 Ethiopian clade C reverse transcriptase (RT) and rapid development of resistance against nonnucleoside inhibitors of RT. Antimicrob. Agents Chemother. 46:2087-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madruga, J. V., et al. 2007. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 370:29-38. [DOI] [PubMed] [Google Scholar]
- 28.Maiga, A. I., et al. Resistance-associated mutations to etravirine (TMC-125) in antiretroviral-naive patients infected with non-B HIV-1 subtypes. Antimicrob. Agents Chemother. 54:728-733. [DOI] [PMC free article] [PubMed]
- 29.Manosuthi, W., et al. 2010. Patients infected with HIV type 1 subtype CRF01_AE and failing first-line nevirapine- and efavirenz-based regimens demonstrate considerable cross-resistance to etravirine. AIDS Res. Hum. Retroviruses. 26:609-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcelin, A. G., et al. 2010. Mutations selected in patients displaying treatment failure under an etravirine-containing regimen. Antivir. Ther. 15(Suppl. 2):A64. [DOI] [PubMed] [Google Scholar]
- 31.Marcelin, A. G., et al. Factors associated with virological response to etravirine in nonnucleoside reverse transcriptase inhibitor-experienced HIV-1-infected patients. Antimicrob. Agents Chemother. 54:72-77. [DOI] [PMC free article] [PubMed]
- 32.Mojgan Haddad, E. S., J. Benhamida, and E. Coakley. 2010. Improved genotypic algorith for predicting etravirine susceptibility: comprehensive list of mutations identified through correlation with matched phenotype, abstr. 574. CROI, San Francisco, CA.
- 33.Nadler, J. P., et al. 2007. Efficacy and safety of etravirine (TMC125) in patients with highly resistant HIV-1: primary 24-week analysis. AIDS 21:F1-F10. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira, M., B. G. Brenner, and M. A. Wainberg. 2009. Isolation of drug-resistant mutant HIV variants using tissue culture drug selection. Methods Mol. Biol. 485:427-433. [DOI] [PubMed] [Google Scholar]
- 35.Petrella, M., et al. 2004. Differential maintenance of the M184V substitution in the reverse transcriptase of human immunodeficiency virus type 1 by various nucleoside antiretroviral agents in tissue culture. Antimicrob. Agents Chemother. 48:4189-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poveda, E., et al. 2010. Etravirine resistance associated mutations in HIV-infected patients failing efavirenz or nevirapine in the Spanish antiretroviral resistance database. AIDS 24:469-471. [DOI] [PubMed] [Google Scholar]
- 37.Poveda, E., et al. 2008. Phenotypic impact of resistance mutations on etravirine susceptibility in HIV patients with prior failure to nonnucleoside analogues. AIDS 22:2395-2398. [DOI] [PubMed] [Google Scholar]
- 38.Ren, J., et al. 2008. Structural basis for the improved drug resistance profile of new generation benzophenone non-nucleoside HIV-1 reverse transcriptase inhibitors. J. Med. Chem. 51:5000-5008. [DOI] [PubMed] [Google Scholar]
- 39.Riddler, S. A., et al. 2008. Class-sparing regimens for initial treatment of HIV-1 infection. N. Engl. J. Med. 358:2095-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomon, H., et al. 1994. Comparison of cord blood and peripheral blood mononuclear cells as targets for viral isolation and drug sensitivity studies involving human immunodeficiency virus type 1. J. Clin. Microbiol. 32:2000-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shafer, R. W., et al. 2003. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N. Engl. J. Med. 349:2304-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taiwo, B., et al. 2010. Suboptimal etravirine activity is common during failure of nevirapine-based combination antiretroviral therapy in a cohort infected with non-B subtype HIV-1. Curr. HIV Res. 8:194-198. [DOI] [PubMed] [Google Scholar]
- 43.Tambuyzer, L., et al. 2009. Compilation and prevalence of mutations associated with resistance to non-nucleoside reverse transcriptase inhibitors. Antivir. Ther. 14:103-109. [PubMed] [Google Scholar]
- 44.Tambuyzer, L., et al. 2010. Characterization of genotypic and phenotypic changes in HIV-1-infected patients with virologic failure on an etravirine-containing regimen in the DUET-1 and DUET-2 clinical studies. AIDS Res. Hum. Retroviruses. 26:1197-1205. [DOI] [PubMed] [Google Scholar]
- 45.Udier-Blagovic, M., J. Tirado-Rives, and W. L. Jorgensen. 2003. Validation of a model for the complex of HIV-1 reverse transcriptase with nonnucleoside inhibitor TMC125. J. Am. Chem. Soc. 125:6016-6017. [DOI] [PubMed] [Google Scholar]
- 46.Van Houtte, M., et al. 2009. A comparison of HIV-1 drug susceptibility as provided by conventional phenotyping and by a phenotype prediction tool based on viral genotype. J. Med. Virol. 81:1702-1709. [DOI] [PubMed] [Google Scholar]
- 47.Vingerhoets, J., et al. 2005. TMC125 displays a high genetic barrier to the development of resistance: evidence from in vitro selection experiments. J. Virol. 79:12773-12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vingerhoets, J., et al. 2010. Resistance profile of etravirine: combined analysis of baseline genotypic and phenotypic data from the randomized, controlled phase III clinical studies. AIDS 24:503-514. [DOI] [PubMed] [Google Scholar]
- 49.Vingerhoets, J., T. L. Azijn, H. Nijs, and S. G. Picchio. 2010. Effect of Mutations at position E138 of HIV-1 reverse transcriptase on phenotypic susceptibility and virological response to etravirine. Antivir. Ther. 15(Suppl. 2):A125. [DOI] [PubMed] [Google Scholar]
- 50.Xu, H., et al. 2009. Human immunodeficiency virus type 1 recombinant reverse transcriptase enzymes containing the G190A and Y181C resistance mutations remain sensitive to etravirine. Antimicrob. Agents Chemother. 53:4667-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu, H. T., et al. 2010. The M230L nonnucleoside reverse transcriptase inhibitor resistance mutation in HIV-1 reverse transcriptase impairs enzymatic function and viral replicative capacity. Antimicrob. Agents Chemother. 54:2401-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]