Abstract
Herpes simplex viruses (HSV) type 1 and type 2 are responsible for recurrent orolabial and genital infections. The standard therapy for the management of HSV infections includes acyclovir (ACV) and penciclovir (PCV) with their respective prodrugs valacyclovir and famciclovir. These compounds are phosphorylated by the viral thymidine kinase (TK) and then by cellular kinases. The triphosphate forms selectively inhibit the viral DNA polymerase (DNA pol) activity. Drug-resistant HSV isolates are frequently recovered from immunocompromised patients but rarely found in immunocompetent subjects. The gold standard phenotypic method for evaluating the susceptibility of HSV isolates to antiviral drugs is the plaque reduction assay. Plaque autoradiography allows the associated phenotype to be distinguished (TK-wild-type, TK-negative, TK-low-producer, or TK-altered viruses or mixtures of wild-type and mutant viruses). Genotypic characterization of drug-resistant isolates can reveal mutations located in the viral TK and/or in the DNA pol genes. Recombinant HSV mutants can be generated to analyze the contribution of each specific mutation with regard to the drug resistance phenotype. Most ACV-resistant mutants exhibit some reduction in their capacity to establish latency and to reactivate, as well as in their degree of neurovirulence in animal models of HSV infection. For instance, TK-negative HSV mutants establish latency with a lower efficiency than wild-type strains and reactivate poorly. DNA pol HSV mutants exhibit different degrees of attenuation of neurovirulence. The management of ACV- or PCV-resistant HSV infections includes the use of the pyrophosphate analogue foscarnet and the nucleotide analogue cidofovir. There is a need to develop new antiherpetic compounds with different mechanisms of action.
Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) are responsible for recurrent orolabial and genital infections. In the United States, the seroprevalence of HSV-1 and HSV-2 infections has been estimated to be approximately 50% and 20%, respectively (243). Transmission occurs by contact with secretions from an infected person with either overt infection or asymptomatic excretion of virus. After primary infection, HSV establishes long-term latency in the ganglia of sensory nerves, from which it can reactivate episodically. These reactivations may be accompanied by symptoms or may be clinically silent. The most common clinical manifestations of HSV infections are vesicular lesions affecting the mucous membranes principally of the mouth, nose, or eyes for HSV-1 and the anogenital region for HSV-2. However, an increasing proportion of genital infections are caused by HSV-1 (243). The symptoms associated with both viruses are usually self-limiting, and the goals of treatment are to accelerate lesion healing and to prevent transmission in immunocompetent individuals (61). Both HSV-1 and HSV-2 can also cause infrequent but serious diseases, such as encephalitis and disseminated neonatal infections. HSV infections may be severe in immunocompromised patients, particularly those with defects in cell-mediated immunity. In patients with HIV infection and in recipients of solid organ or bone marrow transplants, herpetic lesions can be extensive, tend to persist for longer periods, and have the potential to disseminate. Recurrences tend to be more frequent and may be atypical in appearance (140). In addition to persistent mucocutaneous lesions, HSV can also lead to disseminated visceral infections, such as esophagitis, hepatitis, or pneumonia, as well as meningoencephalitis, in immunocompromised hosts (94, 99, 174).
ANTIVIRAL AGENTS FOR HSV INFECTIONS
Mechanism of action of nucleoside analogues.
Since its introduction in the 1980s, acyclovir (ACV) and its derivatives have become the first line drugs for prophylaxis and treatment of HSV infections. ACV [9-(2-hydroxyethoxymethyl) guanine] is a guanosine analogue which must be triphosphorylated to be potent. Upon its entry into infected cells, the first phosphorylation step is performed mainly by the virus-encoded thymidine kinase (TK). This step is followed by two additional phosphorylations carried out successively by host cellular GMP kinase and nucleoside diphosphate kinase (148, 149). ACV-triphosphate (ACV-TP) can serve as a substrate of the viral DNA polymerase (DNA pol) reaction and, hence, be incorporated into DNA at its 3′ terminus (80). The binding of the next required deoxynucleoside 5′-triphosphate to the primer-template leads to the formation of a dead-end complex (166). It has also been suggested that as the 3′-terminal ACV-monophosphate residues cannot be excised by the DNA pol-associated 3′→5′ exonuclease (71), they prevent further chain elongation. ACV-TP does not possess a hydroxyl group in the 3′ position and is thus an obligate DNA chain terminator (166). In vitro, ACV is most potent against HSV-1, approximately half as potent against HSV-2, a 10th as potent against varicella-zoster virus (VZV) and Epstein-Barr virus (EBV), and least potent against human cytomegalovirus (HCMV) (Table 1). ACV has a favorable safety profile in the clinic (228, 233). Its major limitation resides in its relatively poor oral bioavailability (i.e., 15 to 35%), which led to the development of valacyclovir (VACV), an l-valyl-ester prodrug of acyclovir (13, 22). The absolute bioavailability of ACV following oral administration of VACV is 54% (236). After oral administration, VACV is carried out by the human intestinal peptide transporter and then rapidly converted to ACV by ester hydrolysis in the small intestine (164).
TABLE 1.
In vitro inhibitory concentrations of antiherpetic drugs in different cell culture systems and assays
| Virus | IC50 value or range (μg/ml); reference |
||||
|---|---|---|---|---|---|
| Acyclovir | Penciclovir | Foscarnet | Cidofovir | Adefovir | |
| HSV-1 | 0.02-0.9; 38 | 0.04-1.8; 38 | 24-60; 38 | 3.5-8.9; 177 | 7; 154 |
| HSV-2 | 0.03-2.2; 38 | 0.06-4.4; 38 | 24-60; 38 | 3.5-8.9; 177 | 7; 154 |
| VZV | 0.8-4.0; 38 | 1.6-8.0; 38 | 24-60; 38 | 0.22; 177 | 10; 154 |
| HCMV | >20; 38 | 51; 38 | 30-90; 38 | 0.1-0.8; 177 | 25; 154 |
| EBV | 1.6-2.8; 8 | 1.5-3.1; 8 | 0.6-1.0; 69 | 0.008; 177 | 0.3; 154 |
Penciclovir [PCV; 9-(4-hydroxy-3-hydroxymethylbut-1-yl) guanine], an acyclic guanine derivative (which is not commercially available as an oral agent), has a spectrum of activity and a mechanism of action similar to those of ACV (34). However, by virtue of a 3′ hydroxyl group on its acyclic side chain, PCV-triphosphate allows limited chain elongation (short-chain terminator). The in vitro inhibitory concentrations of PCV depend on cell type but are usually 2-fold those of ACV for HSV and VZV (Table 1). PCV is very poorly absorbed when given orally, which led to the development of the oral prodrug famciclovir (FCV), which is a diacetylester of 6-deoxypenciclovir (230). The absolute bioavailability of PCV following oral administration of FCV is 77% (230). After oral administration, FCV is rapidly absorbed and efficiently converted to PCV. The first acetate group of FCV is cleaved by esterases found in the intestinal wall, and the second group is removed on the first pass through the liver (119). Thereafter, the conversion of 6-deoxypenciclovir to PCV is catalyzed by the aldehyde oxidase in the liver (53).
Clinical indications for nucleoside analogues.
Clinical indications for oral ACV, VACV, and FCV include treatment of primary and recurrent HSV infections, particularly genital HSV (39, 168, 172, 206, 209), as well as chronic suppressive therapy to prevent genital herpes recurrences (72, 169, 216, 234). Oral ACV, VACV, and FCV are also frequently used for prophylaxis and treatment of HSV infections in immunocompromised patients. An intravenous formulation of ACV is used for severe HSV infections (including encephalitis and neonatal herpes), as well as those occurring in immunocompromised patients. Topical formulations of ACV and PCV are available for the management of recurrent herpes labialis (207, 208). An ophthalmic formulation of ACV is also available for the management of HSV corneal diseases (107).
PREVALENCE OF DRUG-RESISTANT HSV
Long-term prophylaxis and treatment with ACV, VACV, or FCV can result in the development of resistance, especially in immunocompromised patients. The prevalence of ACV- or PCV-resistant HSV isolates differs greatly for immunocompetent and immunocompromised patients. This difference is most likely due to two reasons (86). First, virus replication may be prolonged in immunocompromised patients, having a tendency toward true persistence. Second, and most important, the host responses are impaired and less pathogenic viruses that would not survive in the face of normal host responses may continue to replicate. Thus, even if some resistant viruses are less pathogenic, they are capable of producing overt diseases in these patients.
Immunocompetent patients.
A low prevalence of HSV resistance to ACV was reported in immunocompetent patients during extensive screening surveys in the United Kingdom (0.7%) and the United States (0.3%) between 1980 and 1992 (59). Most studies since then did not report an increase in the prevalence of resistance (ranging from 0.1 to 0.7%) (7, 9, 33, 51, 68, 171, 212). In general, in this population, drug resistance was not associated with an adverse clinical outcome, due to immune competence. Cases of HSV resistance reported in immunocompetent patients were associated with diagnosis of recurrent genital herpes (81, 109, 125, 126, 153, 219), keratitis (156, 188), disseminated HSV infection (66), and encephalitis (197). Recently, a report documented a relatively high (6.4%) prevalence of ACV-resistant HSV-1 isolates in immunocompetent patients with herpes keratitis, and some of these cases were clinically refractory to ACV therapy (77). Similarly, a survey for HSV resistance to PCV reported a low prevalence of 0.19% in immunocompetent patients (186). Isolates from this study were also included in a larger survey, including 11 worldwide clinical trials. PCV-resistant HSV strains were isolated from a similar proportion (0.22%) of immunocompetent patients (187). In another study, a prevalence of less than 0.3% was reported in persons who used a topical PCV formulation to treat recurrent herpes labialis (200).
Immunocompromised patients.
The prevalence of HSV infections with reduced susceptibility to ACV in immunocompromised patients is higher and varies from 3.5% to 10% (9, 51, 82, 156, 212). The prevalence of ACV resistance has ranged from 3.5% to 7% in HIV-positive patients (68, 82, 137, 141, 171, 248) and from 2.5% to 10% in solid organ transplant recipients (51, 68). However, the highest prevalence rates have been reported in recipients of hematopoietic stem cells, with a range of 4.1% to 10.9% (43, 47, 68, 83, 94, 150, 232, 240). An even higher frequency (36%) of ACV resistance in that population has also been reported (131). Patients receiving either autologous or allogeneic bone marrow transplants had a similar incidence, i.e., 9%, of HSV infection, but resistance occurred only in allogeneic transplants, reaching a prevalence of 30% (151). ACV-resistant HSV isolates were associated with significant morbidity in immunocompromised hosts, including persistent and/or disseminated diseases refractory to antiviral therapy. Most infections with ACV-resistant viruses were detected among AIDS patients and were associated with extensive mucocutaneous lesions but usually not with severe generalized infections. However, a few cases of lethal disseminated visceral HSV infections caused by ACV-resistant mutants were reported in bone marrow transplant recipients (140), and a case of meningoencephalitis was described in an AIDS patient (97). In a survey including 11 worldwide clinical trials, the prevalence of PCV-resistant HSV isolates among immunocompromised patients was 2.1% and this was not associated with treatment failure (187).
MECHANISM OF RESISTANCE TO NUCLEOSIDE ANALOGUES
In agreement with ACV's mechanism of action, viral mutations (deletions/additions or substitutions of nucleotides) conferring resistance to ACV have been found either in the UL23 gene, which encodes the activating/phosphorylating TK enzyme, and/or in the UL30 gene, which encodes the viral target DNA pol enzyme (57, 196). Herpes simplex viruses require a functional DNA pol to replicate, whereas the viral TK is dispensable in cultured cells and certain mammalian tissues. Consequently, there is a higher probability of inducing a viable ACV-resistant virus by a mutation in the UL23 gene than by a mutation in the UL30 gene. In this respect, UL23 gene mutations have been reported in 95% of clinical isolates exhibiting ACV resistance (98, 111, 150). HSV resistance to PCV generally maps to mutations in the UL23 gene, with almost inevitable cross-resistance between the two nucleoside analogues (34, 186, 187). The mutations in UL23 and UL30 genes conferring resistance to nucleoside analogues have been extensively described in several reviews (96, 103, 151).
Thymidine kinase mutations.
Three different phenotypes of ACV-resistant TK mutants have been identified: TK-negative mutants which lack TK activity, TK-low-producer mutants which express reduced levels of enzymatic activity, and TK-altered isolates which are substrate-specific mutants, i.e., they phosphorylate thymidine but not ACV and/or PCV. Approximately 95% of ACV-resistant HSV clinical isolates are TK-negative and TK-low-producer mutants, whereas a minority consists of TK-altered mutants (165). Moreover, mixtures of wild-type and mutant viruses are also observed.
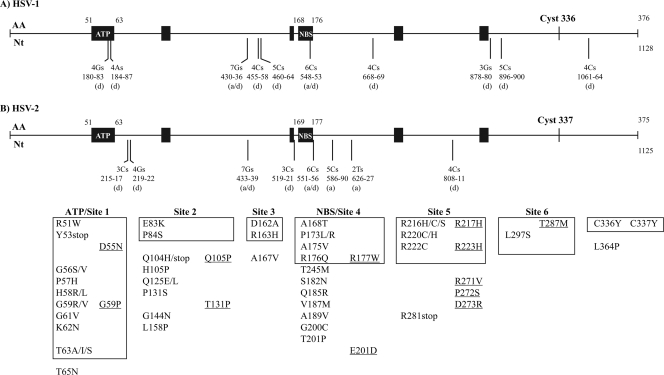
The HSV-1 thymidine kinase is a 376-amino-acid (aa) protein encoded by the UL23 gene (147). The most important sites involved in the enzyme activity are the ATP-binding site (aa 51 to 63), the nucleoside-binding site (aa 168 to 176), and the cysteine at codon 336 which maintains the three-dimensional structure of the active site (85). Moreover, six highly conserved regions have been identified among Herpesviridae TK (10). These regions are located at amino acids 56 to 62 (site 1), 83 to 88 (site 2), 162 to 164 (site 3), 171 to 173 (site 4), 216 to 222 (site 5), and 284 to 289 (site 6). The HSV-2 TK consists of a 375-amino-acid polypeptide with the six highly conserved domains located at positions 56 to 62, 83 to 88, 163 to 165, 172 to 174, 217 to 223, and 285 to 290.
The structure of the viral TK consists of an αβ structure made up of 15 α-helices and 7 β-sheets (36). Five-stranded parallel β-sheets form part of the core of the protein, which contains the active site. The active site consists of the substrate nucleoside-binding pocket, the DRH motif (aa 162 to 164), and the ATP/nucleotide-binding loop and provides the residues that are responsible for coordinating the magnesium counter ion. The ATP molecule binds in the nucleotide-binding site as a complex with Mg2+, which is coordinated by the aspartic acid located at position 162. The LID domain (aa 216 to 222), a region rich in arginine and lysine residues, forms a lid enclosing the active site. For the catalytic reaction, the γ-phosphate of ATP and the 5′-OH deoxyribose need to be activated. Clusters of positive charges from the LID domain and the Mg2+ make the phosphorus atom amenable for a nucleophilic attack of the 5′-OH of deoxyribose which is polarized by the two glutamines localized at positions 225 and 83 (239). The substrate binding site is composed of amino acid side chains derived primarily from a set of roughly parallel helices (85). These helices are localized at the N-terminal half of the polypeptide following the glycine-rich loop involved in the binding of the phosphate moiety of ATP. The cysteine at position 336 is located close to the ATP-binding and nucleoside-binding sites and is involved in local domain consolidation.
Genotypic characterization of HSV clinical isolates revealed a high degree of polymorphism in the UL23 gene. Such mutations were not associated with resistance and were located throughout the gene but mainly outside the active site of the enzyme (40, 48, 95, 127, 135, 150, 193). This fact emphasizes the importance of obtaining pretreatment isolates and/or generating recombinant virus to be sure that mutations actually confer resistance.
Half of the cases of ACV resistance are due to additions or deletions of nucleotides in the UL23 gene, particularly in homopolymer repeats of guanines (G) or cytosines (C), which are considered resistance hot spots (Fig. 1) (98, 150). These additions or deletions can lead to a frameshift reading, resulting in the synthesis of a nonfunctional truncated enzyme (158). The two longest homopolymers, one composed of 7 Gs and one of 6 Cs, are the sites of the most frequently reported mutations in ACV-resistant clinical isolates (98, 122, 150, 190, 192, 193, 195). Other reports of UL23 gene sequences in TK-negative clinical isolates have identified strains containing C deletion in strings of 3 Cs (122, 159) or G deletion from a 3-G stretch (48). An HSV-1 isolate had a deletion of one A in a string of 4 As that were part of the ATP-binding site (98). HSV-2 resistance to ACV was also mediated by a loss of TK enzyme activity due to a T deletion at nucleotide 927 of the UL23 gene (158). This mutation caused a phenylalanine-to-leucine change at position 309 and early termination at amino acid 348. A frameshift mutation resulting from a single C deletion from the homopolymer stretch of 4 C residues in the open reading frame (nucleotides 1061 to 1064) resulted in a TK polypeptide with a longer amino acid sequence (407 aa) (183).
FIG. 1.
Mutations identified in the UL23 gene of HSV-1 (A) and HSV-2 (B) isolates resistant to ACV. The ATP-binding site (ATP), the nucleoside-binding site (NBS), and the six regions of the UL23 gene that are conserved among Herpesviridae are shown by the black boxes. The six highly conserved regions are located at amino acids (AA) 56 to 62 (site 1), 83 to 88 (site 2), 162 to 164 (site 3), 171 to 173 (site 4), 216 to 222 (site 5), and 284 to 289 (site 6) for HSV-1 and 56 to 62, 83 to 88, 163 to 165, 172 to 174, 217 to 223, and 285 to 290 for HSV-2. The additions (a), deletions (d), or both additions and deletions (a/d) reported in homopolymer runs, as well as the nucleotides (Nt) involved, are indicated below vertical bars. Substitutions of amino acids reported in the UL23 gene that are included in the boxes correspond to those identified in conserved regions, and those outside the boxes are located in nonconserved regions. Underlined mutations correspond to the HSV-2 mutations.
The remaining ACV resistance cases are due to nucleotide substitutions that are usually in the conserved sites of the UL23 gene (Fig. 1). Several amino acid changes have been mapped in the ATP-binding site (48, 78, 98, 150, 182, 195, 214), the nucleoside-binding site (98, 125, 150, 182), the DRH motif (48, 213), and the LID domain (78, 98, 122, 214) of HSV TK clinical isolates.
A modification of the cysteine at position 336 of HSV-1 or of position 337 of HSV-2 by a tyrosine was reported in clinical isolates resistant to ACV (78, 98, 192). This mutation induced a TK-low-producer/TK-altered phenotype (98). The C336Y mutation primarily affected the ATP-binding site and led to alterations in the binding affinity of nucleoside analogues (167). The mutation seemed to disrupt the three-dimensional structure of the whole active site by shifting the LID domain, resulting in a more open catalytic active conformation (129). Mutations were also located in conserved site 2 (78, 150, 182) and site 6 (66, 98, 113). In addition, several mutations were also shown to confer resistance to ACV despite their location outside highly conserved regions of the UL23 gene (28, 44, 78, 98, 125, 150, 214, 223).
Two studies reported conflicting results on the mechanism of PCV-selected resistance in HSV strains. In the first study, mutations identified in PCV-selected mutants consisted of nonconservative amino acid changes distributed throughout the UL23 gene and were never found within homopolymeric G and C nucleotide stretches (190), whereas the second study reported the opposite (218). The nature of the discrepancy between these two studies may be related to the size of the viral inoculum used during serial passages. Indeed, in the first study, a high viral inoculum (7 × 104 PFU), which may contain spontaneous preexisting mutants, may be at the origin of the accumulated mutations developed after serial passages and attributed to PCV resistance. In contrast, in the second study, only a low viral inoculum was used as a starting material. Mutations in the UL23 gene from PCV mutants consisted of 4% single nucleotide substitutions and 96% frameshift mutations (218).
DNA polymerase mutations.
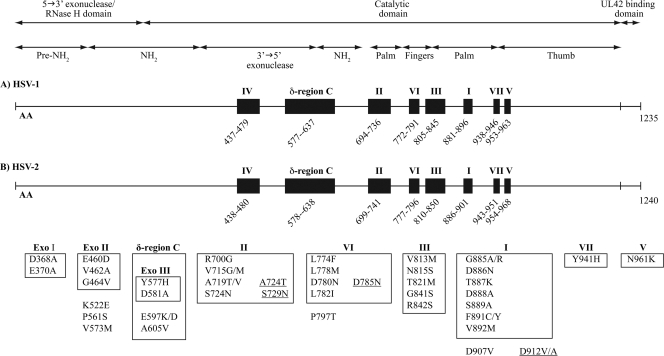
Although less frequently encountered in clinical isolates, mutants with altered DNA pol have also been identified (20, 60, 95, 115, 185, 195, 213). DNA pol is a heterodimer composed of the UL30 and UL42 gene products. The UL30 gene encodes the catalytic subunit, while the UL42 gene encodes a phosphoprotein that possesses double-stranded DNA-binding activity. DNA pol is a multifunctional enzyme (1,235 and 1,240 amino acids for HSV-1 and HSV-2, respectively [227]) which possesses a polymerase activity for the extension of DNA primer chains (136), an intrinsic 3′→5′ exonuclease proofreading activity (123), and an RNase H activity that could be involved in the removal of RNA primers that initiate the synthesis of Okazaki fragments at a replication fork during replication (64). In addition, the carboxyl-terminal region of the catalytic UL30 subunit interacts with the accessory UL42 subunit which acts as a processivity factor (73, 106). HSV DNA pol belongs to the family of α-like DNA polymerases (241) and is also closely related to DNA polymerases δ (247). The α-like DNA polymerase family shares significant amino acid sequence homology in the carboxyl-terminal half of the catalytic subunit. These regions are numbered I to VII on the basis of their degree of conservation among the DNA polymerase genes, with region I being the most conserved. Moreover, HSV DNA pol also contains a δ-region C, which is shared by polymerases related to eukaryotic DNA polymerases δ (247). The relative order of these regions in the polypeptide is IV, δ-region C, II, VI, III, I, VII, and V (Fig. 2). Based on sequence conservation in the α-like polymerase family, the 3′→5′ exonuclease domain of HSV pol contains three highly conserved sequence motifs (Exo I, Exo II, and Exo III) which map to the N-terminal half of the polypeptide (15). The Exo I, Exo II, and Exo III motifs are located, respectively, between positions 363 and 373, in region IV, and in δ-region C.
FIG. 2.
Mutations identified in the UL30 gene of HSV-1 (A) and HSV-2 (B) isolates resistant to ACV. Regions conserved among Herpesviridae genes are shown by the black boxes. The roman numbers (I to VII and δ-region C) corresponding to each of these regions are indicated above the boxes. Amino acid (AA) locations are noted below each of these regions for HSV-1 and HSV-2. Substitutions reported in the UL30 gene that are included in the boxes correspond to those identified in conserved regions, and those outside the boxes are located in nonconserved regions. Underlined mutations correspond to the HSV-2 mutations. Mutations E460D, G464V, K522E, and P561S in and outside Exo II are lethal to the virus; mutations Y577H and D581A in the Exo III motif in δ-region C are associated with hypersusceptibility to ACV; and none of the mutations in region I are spontaneously induced.
HSV DNA pol is formed by 6 structural domains, namely, a pre-NH2 domain, an NH2 domain, polymerase palm, fingers, and thumb domains, and a 3′→5′ exonuclease domain (Fig. 2) (138). Regions I, II, and VII are parts of the polymerase palm subdomain, and region V is the base of the thumb subdomain. These four regions appear to flank the catalytic site in the palm subdomain and contain the catalytic triad of aspartic acid residues (at positions 717, 886, and 888) that are essential for polymerase activity. Regions III and VI are located at the base of the fingers subdomain and may play a role in positioning the template and primer strands.
In clinical isolates, the mutations conferring resistance to nucleoside analogues are single amino acid substitutions located in regions II (95, 185, 195, 213), III (60, 195), VI (195), and VII (115) which are directly or indirectly involved in the recognition and binding of nucleotides or pyrophosphate, as well as in catalysis (Fig. 2). The greatest clusters of mutations in the DNA pol enzyme have been found in conserved regions II and III. Mutations within conserved regions II (95, 185, 195, 213) and VII (115) were frequently associated with cross-resistance to ACV and foscarnet (FOS). Only a few drug-resistant mutations were described within the other conserved regions or outside such regions (20, 115, 195). In this respect, the change of the aspartic acid to valine at position 907, within a nonconserved gene region, induced a low level of resistance to ACV (19).
In laboratory mutant viruses, some mutations in δ-region C (E597K/D and A605V) conferred resistance to ACV (101, 128, 132, 184). The 3′→5′ exonuclease activities associated with replicative polymerases have proofreading functions to improve replication fidelity. Mutant polymerases with defective exonuclease activity can increase the mutation frequency by up to 3 orders of magnitude (199). Laboratory strains containing mutated residues (Y577H and D581A) within the conserved Exo III motif of the δ-region C of the polymerase gene were shown to be defective in 3′→5′ exonuclease activity and exhibited extremely high mutation frequencies (116). These mutants also demonstrated higher resistance to phosphonoacetic acid and greater sensitivity to aphidicolin, ACV, and ganciclovir than wild-type virus (117). These results suggest that the conserved Exo III motif of HSV DNA pol may play an important role in maintaining the proper structure of the catalytic site for polymerase activity, in addition to its role in exonuclease activity.
LABORATORY DIAGNOSIS OF ANTIVIRAL DRUG RESISTANCE
The persistence of lesions for more than 1 week after the beginning of therapy without appreciable decrease in size, an atypical appearance of the lesions, or the emergence of new satellite lesions despite antiviral administration is suggestive of treatment failure. After healing of an infection caused by a TK-negative drug-resistant HSV strain and stopping antiviral therapy, recurrences are most often associated with a drug-sensitive strain, although some cases of spontaneous recurrences of ACV-resistant viruses have been reported (1, 78, 100, 113, 125, 126, 142, 146, 152, 153, 178, 201, 205, 232, 246). Laboratory diagnosis of ACV resistance is required to guide clinicians toward different treatment options in cases of therapy failure. ACV resistance can be diagnosed by testing a virus against antiviral agents (phenotypic assays) or by the identification of a specific mutation conferring resistance to antiviral drugs (genotypic assays) (2).
Phenotypic methods.
The common basis of phenotypic assays is the measurement of virus growth inhibition in the presence of the antiviral drug. After appropriate periods of incubation with the antiviral, viral replication is measured based on the reduction of virus-induced cytopathic effect or plaque formation, which is evaluated either colorimetrically or microscopically (62).
The gold standard phenotypic method for the evaluation of HSV susceptibility to antiviral drugs is the plaque reduction assay (180). This assay has been the object of most clinical correlation and standardization studies (235) and is approved as a standard protocol by the Clinical and Laboratory Standards Institute (220). In this assay, a controlled viral inoculum (50 to 100 PFU) is inoculated onto a permissive monolayer of Vero cells (African green monkey kidney cells, CCL 81; American Type Culture Collection, Manassas, VA) to allow adsorption. Infected cells are incubated in the presence of serial drug concentrations for 2 to 3 days. Cells are fixed and stained, and the number of plaques is counted. The concentration of drug that reduces the plaque number by 50% (IC50) compared to the number on the virus-infected, no-drug control is then calculated. However, this assay is time consuming and is based on a somewhat subjective read-out.
Virus multiplication can also be evaluated by measurement of antigen expression in cell culture based on enzyme-linked immunosorbent assays (ELISAs), including the sandwich ELISA (181) and the microplate in situ ELISA (134). Other currently used antiviral assays include DNA hybridization (210), flow cytometric analysis (161), and transgenic HSV-inducible reporter cell lines (224). A recombinant HSV-1 expressing the green fluorescent protein has also been described (222). More recently, antiviral drug susceptibility assays of HSV have been developed using real-time PCR quantification of DNA in cell culture supernatants (215) or of intracellular viral DNA load (226). These techniques are both sensitive and accurate. However, the readout must be done early during the exponential phase of virus growth because the long persistence of DNA in cell culture may jeopardize the interpretation of inhibition results at a late stage of infection (32).
In vitro resistance can be defined by an IC50 greater than a cutoff value or by showing a greater than 3- to 5-fold increase in the IC50 compared to that of a baseline isolate from the same patient. Breakpoint values of ≥2 μg/ml (8.8 μM) for ACV and of ≥100 μg/ml (333.3 μM) for FOS in the plaque reduction assay are widely accepted (55, 58, 89, 176, 179). There is no suggested consensus breakpoint value for PCV.
Laboratory strains and clinical isolates of HSV that are resistant to ACV naturally constitute a heterogeneous population which contains mixtures of wild-type and resistant virus (81, 156, 160, 174). The proportion of naturally occurring ACV- and PCV-resistant mutants within a virus preparation is in the same order of magnitude (189). It has been estimated that the proportion of ACV-resistant HSV must exceed 20% of the viral population in order to shift the IC50 to more than 2 μg/ml (199). The plaque reduction assay measures the overall sensitivity of the viral population. Therefore, an isolate could be considered sensitive based on its IC50, although it may contain a significant proportion of resistant viruses (70, 87). To eliminate this possibility, the mutant virus isolate may be analyzed for evidence of TK heterogeneity by plaque autoradiography. The amount of TK activity in the mutant virus isolate relative to the amount in a wild-type laboratory strain is quantified in situ using thymidine labeled to high specific activity with [3H] and storage phosphor technology (108, 113). Infected 143B cells (a human osteosarcoma cell line which is TK negative; American Type Culture Collection) are incubated with a medium containing a semisolid overlay at 37°C. Once plaques reach a reasonable size, the overlay is removed and the cells are incubated with a medium containing [3H]-thymidine for 14 h. Cells are stained, washed, and air dried. Plates are exposed for 6 days to a tritium plate from a phosphorimager and then scanned to obtain images and quantitative data.
Genotypic methods.
The objective of genotypic assays is the search for specific mutations in the viral genes encoding the activating/phosphorylating TK enzyme (UL23) and the target DNA pol enzyme (UL30). The gene of interest is amplified by PCR, and the PCR products are then sequenced. The mutations detected in the gene of interest are then interpreted by comparison with the whole panels of mutations described in the literature. For the mutations that have not been characterized previously, some predictions can be made from the knowledge of the conserved regions implicated in the structural integrity of the enzyme or in its catalytic functions. However, in order for genotypic methods to be helpful in clinical practice, it is essential to be able to discriminate between random variations (polymorphism) and true drug resistance mutations. The generation of recombinant mutant viruses allows the formal assessment of the role of nonconfirmed mutations in antiviral drug resistance.
Methods for generating recombinant mutants.
Traditionally, putative resistance mutations have been confirmed by marker transfer experiments. A specific mutation is transferred to a wild-type virus background by homologous recombination (49, 115, 116, 133, 144, 174). The intact viral genomic DNA from a reference strain is cotransfected with a mutated gene in permissive cells, and the resistant recombinant virus is then selected with an antiviral. However, this method is fastidious and inefficient and generally relies on the selection of recombinant mutants through drug pressure, which frequently leads to additional undesired mutations.
The generation of recombinant mutants following cotransfection of overlapping cosmids and plasmids into permissive cells represents an important improvement (19, 52, 65, 198). This procedure leads to the reconstitution of mutant virus only and circumvents the tedious selection procedure against the parental virus. However, it still relies on several recombination events in eukaryotic cells that cannot be controlled. Moreover, this system is inefficient for generating mutations in two or more viral genes.
Recently, several laboratories reported the cloning of HSV-1 genomes into a plasmid as bacterial artificial chromosomes (BACs) (102, 175, 211, 221). This technique allows the stable maintenance of HSV genomes as BACs in Escherichia coli and mutagenesis of the viral genome by using the bacterial recombination machinery. The reconstitution of infectious virus is achieved by transfection of the BAC plasmid into permissive mammalian cells. This approach has several advantages. The powerful methods of bacterial genetics allow the introduction of any kind of mutation (deletion, insertion, or point mutation) into the cloned viral genome. Manipulated genomes can be characterized prior to the reconstitution of virus. The transfection of the mutated genomes into permissive cells results in the reconstitution only of viral mutants that are free of contamination with parental virus.
PATHOGENESIS OF DRUG-RESISTANT HSV STRAINS IN ANIMAL MODELS/HUMANS
Among the unique properties of HSV strains are the establishment of latency followed by intermittent reactivations, as well as their neurovirulence potential (14, 121, 237, 245).
HSV TK mutants.
The persistence and virulence of a drug-resistant HSV strain depends on its ability to replicate relative to other genetic variants (so-called viral fitness). Although TK is not essential for growth in cell culture, it is important for viral pathogenesis, particularly for reactivation from latently infected trigeminal ganglia in animal models. Depending on the phenotype of the TK mutant, viral replication, establishment of latency, and reactivation can be unaltered or decreased in comparison with wild-type strains. TK-low-producer mutants show some reduction in pathogenicity compared with wild-type strains but are generally able to reactivate from latency (16, 54). In contrast, TK-negative mutants have impaired pathogenicity, establish latency in sensory ganglia with a lower efficiency than wild-type strains, and reactivate poorly in mice (46, 56, 79, 110, 111, 118, 152, 225), in guinea pigs (16), and in rabbits (29, 42, 105, 217, 244). The vast majority of the latter mutants are attenuated for neurovirulence and demonstrate defective replication in the brain and, often, in the peripheral nervous system compared with wild-type strains (30, 50, 74). However, reactivation of some TK-negative HSV clinical isolates has been reported (110, 113, 114, 152, 178, 191). Some of these isolates have been shown to express TK activity at ultra-low levels, demonstrating that the amounts of TK activity required for reactivation are far lower than previously appreciated (18, 54). It has been proposed that mutant viruses with a single G insertion into the 7-G string generated low levels of full-length active TK (less than 1% of wild-type activity) by a net +1 ribosomal frameshift during translation that is sufficient for reactivation (108, 114). In addition, heterogeneous populations (TK competent/TK negative) may coexist in HSV isolates from immunocompromised patients (191).
HSV DNA pol mutants.
Much less is known concerning the neurovirulence and potential to establish latency of clinical DNA pol mutants. Viral DNA pol has a crucial role in the replicative cycle, and it is expected that DNA pol mutants would have decreased neurovirulence. It has been shown that HSV DNA pol mutants exhibit different degrees of attenuation of neurovirulence in mice (88, 89, 91, 162, 163, 174, 204). Some HSV DNA pol mutants are clearly attenuated, while others are able to replicate in the central nervous system and cause encephalitis. The degrees of neurovirulence of DNA pol mutants resistant to ACV and/or FOS do not correlate with the in vitro levels of resistance to these compounds or with the polypeptide subdomain where the amino acid substitutions are mapped (4). Neuroinvasiveness is expected to correlate with viral fitness, so that highly replicating viruses would be more neurovirulent. Recently, it has been shown that the degree of neurovirulence of several DNA pol mutants could be generally predicted by their in vitro replicative capacity (67). However, Pelosi et al. (163) reported a DNA pol mutant (R842S) that could replicate in the central nervous system but, paradoxically, exhibited attenuated neurovirulence. The reason for such discrepancy remains unclear.
MANAGEMENT OF NUCLEOSIDE ANALOGUE-RESISTANT HSV INFECTIONS
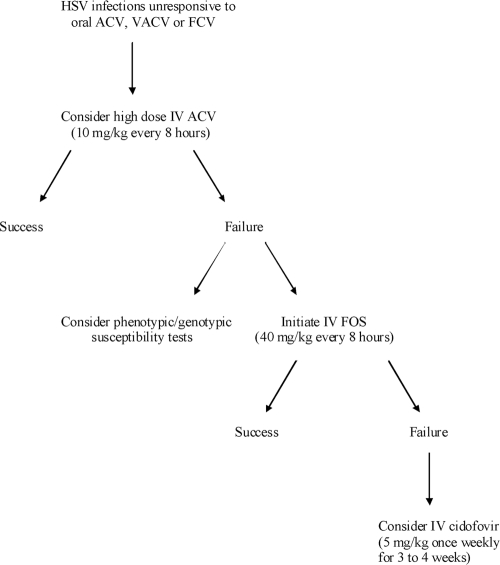
When treatment failure is suspected, isolates from the lesions should be submitted for phenotypic or genotypic testing, and a change of therapy should be considered as shown in the algorithm proposed in Fig. 3. It is very unlikely that a patient failing to respond to ACV or VACV therapy will respond to FCV because there will most probably be cross-resistance between ACV and PCV. An initial step in case of treatment failure with oral drugs is to switch to high doses of intravenous ACV (10 mg/kg of body weight every 8 h adjusted for renal function). Most successful treatment of refractory ACV-resistant HSV infections has been achieved with the use of FOS. Lesions that fail to respond to FOS could be treated with cidofovir (5 mg/kg once a week for 3 to 4 weeks).
FIG. 3.
Management of HSV infections that are unresponsive to nucleoside analogues. IV, intravenous.
Foscarnet.
FOS (phosphonoformic acid) is a pyrophosphate analogue that inhibits the viral DNA pol by mimicking the structure of pyrophosphate produced during the elongation of DNA (157). FOS, which does not require phosphorylation by viral and cellular kinases, binds to the pyrophosphate binding site on the viral DNA pol and blocks the release of pyrophosphate from the terminal nucleoside triphosphate added onto the growing DNA chain. FOS acts as a noncompetitive inhibitor of DNA pol activity. FOS is potent against all herpesviruses (157) (Table 1). Its principal indication is for HCMV infections, including strains resistant to ganciclovir, but it is also potent against most ACV-resistant HSV and VZV strains. The oral bioavailability of FOS is poor and, thus, it is only available as an intravenous formulation. Foscarnet is nephrotoxic and induces electrolyte disorders. Foscarnet is indicated as second-line therapy for HSV infections that are refractory to nucleosidic drugs. Foscarnet treatment has shown some antiviral efficacy against ACV-resistant infections in AIDS patients (3, 45, 84, 176) and bone marrow transplant patients (170, 229). Few data are available regarding the isolation of FOS-resistant HSV strains in the clinic, mainly from AIDS patients failing therapy (20, 43, 68, 115, 131, 174, 179, 180, 185, 195, 213). Most FOS-resistant clinical HSV isolates contain single base substitutions in conserved regions II, III, VI, or VII and in a nonconserved region (between I and VII) of the DNA pol gene (20, 95, 115, 185, 195, 213). Some of these isolates retained susceptibility or, at the most, borderline levels of susceptibility to ACV and cidofovir (20, 195). However, some mutations, in particular in regions II (95, 185, 195, 213) and VII (115) of the DNA pol, can confer resistance to both ACV and FOS. In this respect, mutations V715G and S724N/S729N (HSV-1/HSV-2) located in region II (185, 195) and Y941H located in region VII (115) have been described in ACV- and FOS-resistant clinical isolates. Mutants with alterations in both HSV TK and DNA pol can also occur, resulting in double resistance to both ACV and FOS.
Cidofovir and adefovir.
Acyclic nucleoside phosphonates (ANPs), including (S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl) derivatives of cytosine (cidofovir) and adenine (adefovir), are converted into active ANP-diphosphates by cellular kinases. Thus, they circumvent the need for activation by the virus-encoded TK and so retain their activity against TK-negative or TK-altered strains of HSV (112). The active forms are competitive inhibitors of the viral DNA pol, and they may act as chain terminators (242). The principal indication for cidofovir is for HCMV infections, including ganciclovir-resistant strains with mutations in the UL97 gene (112) (Table 1). It is also potent against HSV and VZV strains, including most of the isolates resistant to ACV and/or FOS. Cidofovir is approved for treatment of HCMV retinitis in AIDS patients and is available as an intravenous formulation. It has a particularly long intracellular half-life, and infrequent dosing is possible. Because of its nephrotoxicity, cidofovir is routinely administered with probenecid and requires intravenous hydration. Cidofovir has proven to be effective in the treatment of progressive mucocutaneous infections due to ACV- and/or FOS-resistant HSV in immunocompromised patients (41, 124, 130, 143, 202, 204), but it is not approved for this indication. The safety and efficacy of single topical applications of 1, 3, and 5% cidofovir gels have been evaluated for the treatment of early, lesional, recurrent genital herpes (173), but they are not commercially available. In addition, oral formulations of ether lipid ester prodrugs of cidofovir are under development (23). Mutations associated with cidofovir resistance mapped in DNA pol region II (R700M), region III (G841C and G850I), region VI (L773M), and region VII (Y941H) and in the Exo III motif in δ-region C (V573M) (5). Laboratory-derived HSV strains resistant to cidofovir also contained mutations in nonconserved regions (L1007M and I1028T) of the DNA pol gene (5). Adefovir is effective against hepatitis B virus and also has activity against HSV strains, including most of the isolates resistant to ACV (155) (Table 1). Its oral prodrug adefovir dipivoxil is used for the treatment of chronic active hepatitis B virus infections. Although not approved for that indication, adefovir could be used in the treatment of HSV infections unresponsive to ACV and FOS. The dosage is not clearly established, and the renal function must be monitored. Resistance to adefovir may occur following selection of HSV DNA pol mutants in the laboratory (93, 231). FOS-resistant isolates with DNA pol mutations in regions II and VI and between regions I and VII were also associated with an important decrease in susceptibility to adefovir, suggesting that adefovir-resistant HSV could be selected in vivo by FOS therapy (20). In addition, the mutations S724N and L778M which conferred cross-resistance to ACV and FOS also caused reduced susceptibility to cidofovir and adefovir (19).
Other viral or host targets.
The helicase-primase complex is composed of 3 proteins encoded by the UL5, UL8, and UL52 genes. This complex unwinds double-stranded viral DNA and generates primers for DNA synthesis by the viral DNA pol. Therefore, the helicase-primase complex plays an essential role in HSV DNA replication and thus constitutes an excellent target for antiviral therapy. Helicase-primase inhibitors are nonnucleosidic/nonnucleotidic inhibitors that are extremely potent against HSV in cell culture. The candidate BAY 57-1293 (Bayer) has potent anti-HSV activity in vitro and is effective against ACV-resistant mutants. This compound had a greater efficacy than ACV against genital herpes in a guinea pig model (12), as well as against ocular and lethal infections in both mouse and rabbit models (21, 24, 120). Resistant viruses were rapidly selected by serial passages in cell cultures (25-27). Another helicase-primase inhibitor candidate, BILS 45 BS (Boehringer Ingelheim), was much more potent than ACV against wild-type laboratory and clinical HSV-1 strains, as well as ACV-resistant isolates. Oral administration of BILS 45 BS was very effective against cutaneous infection in nude mice infected with ACV-resistant isolates due to mutations in the TK or the DNA pol gene (63, 76).
BILD 1633 SE (Boehringer Ingelheim) is a peptidomimetic inhibitor of HSV ribonucleotide reductase which catalyzes the synthesis of the deoxyribonucleotide precursors required for DNA synthesis (75, 139). BILD 1633 SE prevents the association between the two subunits that form the protein and thus acts as a nonsubstrate-based antiviral agent. In vitro, this compound was more potent than ACV against several wild-type strains and ACV-resistant HSV mutants. This compound was effective in a murine ocular model of HSV-1-induced keratitis.
A novel class of nonnucleosidic inhibitors belonging to the 4-hydroxyquinoline-3-carboxamide derivatives is specific for a broad range of viral DNA pol, including that of HSV-1 and HSV-2. The PNU-183792 derivative has anti-HSV activity in cell culture (35). It also maintains potent antiviral activity against ACV-resistant mutants, suggesting that its mechanism of action is different.
RNA interference (RNAi) is a natural mechanism of posttranscriptional gene silencing that is widely conserved in multicellular organisms. The molecular mediators of RNAi are double-stranded RNAs 21 to 23 nucleotides in length that induce the sequence-specific degradation of homologous RNAs. Synthetic small interfering RNAs (siRNAs) against essential gene products of HCMV could trigger RNAi in infected cells and inhibit viral replication (238), opening the way to a new class of antiviral agents.
A new class of topical drugs has recently been developed that serve as immune response modifiers. These drugs upregulate the natural immune response to infectious organisms, such response being regulated primarily through induced production of interferon and cytokines from activated lymphocytes at the site of application. The Toll-like receptor (TLR)-7/8 agonists imiquimod and resiquimod stimulate the production of cytokines that promote an antigen-specific T helper type 1 (Th1) acquired immune response. A topical formulation of imiquimod is licensed for the treatment of genital and perianal warts caused by human papillomavirus (90). Topical application of imiquimod and resiquimod showed contradictory results in clinical trials evaluating their efficacy in the treatment of herpes labialis (17) and genital herpes (11, 37, 92, 104, 145, 194, 203). The TLR-3 agonist poly(I:C) induced innate-immune-mediated protection against genital HSV-2 infection in mice when administered intravaginally but not when administered systemically (6). In another study, a single intraperitoneal or intranasal pretreatment of mice with poly(I:C) reduced the mortality rate after intranasal challenge with HSV-1, suggesting that this TLR-3 agonist may increase the innate immune mechanism of neuroprotection against HSV (31).
CONCLUSIONS
ACV and PCV, as well as their respective prodrugs VACV and FCV, are the gold standard antiviral agents for the prevention and treatment of HSV-1 and HSV-2 infections. The prevalence of resistance to these drugs is low in immunocompetent individuals. However, immunocompromised patients may develop chronic infections which are more prone to become resistant to currently available drugs. In this respect, the prevalence of resistance in immunocompromised patients reaches levels varying from 3.5% to 10%. With the increased number of transplant and cancer patients, an immunocompromised status is no longer a rare event. Therefore, the problem of drug-resistant HSV infections is not expected to fade. On the other hand, the reduced viral fitness and pathogenicity of most drug-resistant HSV mutants seen in animal models may contribute to the limited impact of resistance in the clinical setting. There is a need to develop new antiherpetic compounds with different mechanisms of action which will be safe and effective against ACV- and/or FOS-resistant HSV isolates.
Acknowledgments
The authors have no conflict of interest to declare.
Footnotes
Published ahead of print on 15 November 2010.
REFERENCES
- 1.About, I., J. Capdeville, P. Bernard, and P. Massip. 1994. Chronic herpes resistant to acyclovir in a patient with AIDS. Ann. Dermatol. Venereol. 121:810-813. [PubMed] [Google Scholar]
- 2.Agut, H., D. Boutolleau, C. Deback, P. Bonnafous, and A. Gautheret-Dejean. 2009. Testing the susceptibility of human herpesviruses to antivirals. Future Microbiol. 4:1111-1123. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-McLeod, A., J. Havlik, and K. E. Drew. 1999. Foscarnet treatment of genital infection due to acyclovir-resistant herpes simplex virus type 2 in a pregnant patient with AIDS: case report. Clin. Infect. Dis. 29:937-938. [DOI] [PubMed] [Google Scholar]
- 4.Andrei, G., et al. 2007. DNA polymerase mutations in drug-resistant herpes simplex virus mutants determine in vivo neurovirulence and drug-enzyme interactions. Antivir. Ther. 12:719-732. [PubMed] [Google Scholar]
- 5.Andrei, G., et al. 2000. Resistance of herpes simplex virus type 1 against different phosphonylmethoxyalkyl derivatives of purines and pyrimidines due to specific mutations in the viral DNA polymerase gene. J. Gen. Virol. 81:639-648. [DOI] [PubMed] [Google Scholar]
- 6.Ashkar, A. A., et al. 2004. Toll-like receptor (TLR)-3, but not TLR4, agonist protects against genital herpes infection in the absence of inflammation seen with CpG DNA. J. Infect. Dis. 190:1841-1849. [DOI] [PubMed] [Google Scholar]
- 7.Bacon, T. H., R. J. Boon, M. Schultz, and C. Hodges-Savola. 2002. Surveillance for antiviral-agent-resistant herpes simplex virus in the general population with recurrent herpes labialis. Antimicrob. Agents Chemother. 46:3042-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacon, T. H., and M. R. Boyd. 1995. Activity of penciclovir against Epstein-Barr virus. Antimicrob. Agents Chemother. 39:1599-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacon, T. H., M. J. Levin, J. J. Leary, R. T. Sarisky, and D. Sutton. 2003. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 16:114-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balasubramaniam, N. K., V. Veerisetty, and G. A. Gentry. 1990. Herpesviral deoxythymidine kinases contain a site analogous to the phosphoryl-binding arginine-rich region of porcine adenylate kinase; comparison of secondary structure predictions and conservation. J. Gen. Virol. 71(Pt. 12):2979-2987. [DOI] [PubMed] [Google Scholar]
- 11.Bangsgaard, N., and L. Skov. 2008. Chronic genital ulceration due to herpes simplex infection treated successfully with imiquimod. Acta Derm. Venereol. 88:202-203. [DOI] [PubMed] [Google Scholar]
- 12.Baumeister, J., et al. 2007. Superior efficacy of helicase-primase inhibitor BAY 57-1293 for herpes infection and latency in the guinea pig model of human genital herpes disease. Antivir. Chem. Chemother. 18:35-48. [DOI] [PubMed] [Google Scholar]
- 13.Beauchamp, L. M., G. F. Orr, P. de Miranda, T. Burnette, and T. A. Krenitsky. 1992. Amino acid ester prodrugs of acyclovir. Antiviral Chem. Chemother. 3:157-164. [Google Scholar]
- 14.Berger, J. R., and S. Houff. 2008. Neurological complications of herpes simplex virus type 2 infection. Arch. Neurol. 65:596-600. [DOI] [PubMed] [Google Scholar]
- 15.Bernad, A., L. Blanco, J. M. Lazaro, G. Martin, and M. Salas. 1989. A conserved 3′—-5′ exonuclease active site in prokaryotic and eukaryotic DNA polymerases. Cell 59:219-228. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein, D. I., J. Ireland, and N. Bourne. 2000. Pathogenesis of acyclovir-resistant herpes simplex type 2 isolates in animal models of genital herpes: models for antiviral evaluations. Antiviral Res. 47:159-169. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein, D. I., S. L. Spruance, S. S. Arora, J. L. Schroeder, and T. C. Meng. 2005. Evaluation of imiquimod 5% cream to modify the natural history of herpes labialis: a pilot study. Clin. Infect. Dis. 41:808-814. [DOI] [PubMed] [Google Scholar]
- 18.Besecker, M. I., C. L. Furness, D. M. Coen, and A. Griffiths. 2007. Expression of extremely low levels of thymidine kinase from an acyclovir-resistant herpes simplex virus mutant supports reactivation from latently infected mouse trigeminal ganglia. J. Virol. 81:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bestman-Smith, J., and G. Boivin. 2003. Drug resistance patterns of recombinant herpes simplex virus DNA polymerase mutants generated with a set of overlapping cosmids and plasmids. J. Virol. 77:7820-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bestman-Smith, J., and G. Boivin. 2002. Herpes simplex virus isolates with reduced adefovir susceptibility selected in vivo by foscarnet therapy. J. Med. Virol. 67:88-91. [DOI] [PubMed] [Google Scholar]
- 21.Betz, U. A., R. Fischer, G. Kleymann, M. Hendrix, and H. Rubsamen-Waigmann. 2002. Potent in vivo antiviral activity of the herpes simplex virus primase-helicase inhibitor BAY 57-1293. Antimicrob. Agents Chemother. 46:1766-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beutner, K. R. 1995. Valacyclovir: a review of its antiviral activity, pharmacokinetic properties, and clinical efficacy. Antiviral Res. 28:281-290. [DOI] [PubMed] [Google Scholar]
- 23.Bidanset, D. J., J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2004. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection. J. Infect. Dis. 190:499-503. [DOI] [PubMed] [Google Scholar]
- 24.Biswas, S., L. Jennens, and H. J. Field. 2007. The helicase primase inhibitor, BAY 57-1293 shows potent therapeutic antiviral activity superior to famciclovir in BALB/c mice infected with herpes simplex virus type 1. Antiviral Res. 75:30-35. [DOI] [PubMed] [Google Scholar]
- 25.Biswas, S., L. Jennens, and H. J. Field. 2007. Single amino acid substitutions in the HSV-1 helicase protein that confer resistance to the helicase-primase inhibitor BAY 57-1293 are associated with increased or decreased virus growth characteristics in tissue culture. Arch. Virol. 152:1489-1500. [DOI] [PubMed] [Google Scholar]
- 26.Biswas, S., et al. 2008. A single drug-resistance mutation in HSV-1 UL52 primase points to a difference between two helicase-primase inhibitors in their mode of interaction with the antiviral target. J. Antimicrob. Chemother. 61:1044-1047. [DOI] [PubMed] [Google Scholar]
- 27.Biswas, S., L. S. Tiley, H. Zimmermann, A. Birkmann, and H. J. Field. 2008. Mutations close to functional motif IV in HSV-1 UL5 helicase that confer resistance to HSV helicase-primase inhibitors, variously affect virus growth rate and pathogenicity. Antiviral Res. 80:81-85. [DOI] [PubMed] [Google Scholar]
- 28.Bodaghi, B., et al. 2000. Acyclovir-resistant bilateral keratitis associated with mutations in the HSV-1 thymidine kinase gene. Exp. Eye Res. 71:353-359. [DOI] [PubMed] [Google Scholar]
- 29.Boisjoly, H. M., N. H. Park, D. Pavan-Langston, and E. De Clercq. 1983. Herpes simplex acyclovir-resistant mutant in experimental keratouveitis. Arch. Ophthalmol. 101:1782-1786. [DOI] [PubMed] [Google Scholar]
- 30.Boivin, G., Z. Coulombe, and S. Rivest. 2002. Intranasal herpes simplex virus type 2 inoculation causes a profound thymidine kinase dependent cerebral inflammatory response in the mouse hindbrain. Eur. J. Neurosci. 16:29-43. [DOI] [PubMed] [Google Scholar]
- 31.Boivin, N., Y. Sergerie, S. Rivest, and G. Boivin. 2008. Effect of pretreatment with toll-like receptor agonists in a mouse model of herpes simplex virus type 1 encephalitis. J. Infect. Dis. 198:664-672. [DOI] [PubMed] [Google Scholar]
- 32.Bonnafous, P., A. Gautheret-Dejean, D. Boutolleau, D. Caiola, and H. Agut. 2005. Persistence of DNA in cell cultures may jeopardize the analysis of human herpesvirus 6 dynamics by means of real-time PCR. J. Virol. Methods 125:95-98. [DOI] [PubMed] [Google Scholar]
- 33.Boon, R. J., et al. 2000. Antiviral susceptibilities of herpes simplex virus from immunocompetent subjects with recurrent herpes labialis: a UK-based survey. J. Antimicrob. Chemother. 46:1051. [DOI] [PubMed] [Google Scholar]
- 34.Boyd, M. R., S. Safrin, and E. R. Kern. 1993. Penciclovir: a review of its spectrum of activity, selectivity, and cross-resistance pattern. Antiviral Chem. Chemother. 4:3-11. [Google Scholar]
- 35.Brideau, R. J., et al. 2002. Broad-spectrum antiviral activity of PNU-183792, a 4-oxo-dihydroquinoline, against human and animal herpesviruses. Antiviral Res. 54:19-28. [DOI] [PubMed] [Google Scholar]
- 36.Brown, D. G., et al. 1995. Crystal structures of the thymidine kinase from herpes simplex virus type-1 in complex with deoxythymidine and ganciclovir. Nat. Struct. Biol. 2:876-881. [DOI] [PubMed] [Google Scholar]
- 37.Brummitt, C. F. 2006. Imiquimod 5% cream for the treatment of recurrent, acyclovir-resistant genital herpes. Clin. Infect. Dis. 42:575. [DOI] [PubMed] [Google Scholar]
- 38.Brunton, L. L., J. S. Lazo, and K. L. Parker. 2006. Goodman and Gilman's the pharmacological basis of therapeutics, 11th ed. McGraw-Hill, New York, NY.
- 39.Bryson, Y., et al. 1985. Treatment of first episode genital HSV with oral acyclovir: long term follow-up of recurrences. A preliminary report. Scand. J. Infect. Dis. Suppl. 47:70-75. [PubMed] [Google Scholar]
- 40.Burrel, S., C. Deback, H. Agut, and D. Boutolleau. 2010. Genotypic characterization of UL23 thymidine kinase and UL30 DNA polymerase of clinical isolates of herpes simplex virus: natural polymorphism and mutations associated with resistance to antivirals. Antimicrob. Agents Chemother. 54:4833-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castelo-Soccio, L., R. Bernardin, J. Stern, S. A. Goldstein, and C. Kovarik. 2010. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch. Dermatol. 146:124-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caudill, J. W., E. Romanowski, T. Araullo-Cruz, and Y. J. Gordon. 1986. Recovery of a latent HSV-1 thymidine kinase negative strain following iontophoresis and co-cultivation in the ocularly-infected rabbit model. Curr. Eye Res. 5:41-45. [DOI] [PubMed] [Google Scholar]
- 43.Chakrabarti, S., et al. 2000. Resistance to antiviral drugs in herpes simplex virus infections among allogeneic stem cell transplant recipients: risk factors and prognostic significance. J. Infect. Dis. 181:2055-2058. [DOI] [PubMed] [Google Scholar]
- 44.Chatis, P. A., and C. S. Crumpacker. 1991. Analysis of the thymidine kinase gene from clinically isolated acyclovir-resistant herpes simplex viruses. Virology 180:793-797. [DOI] [PubMed] [Google Scholar]
- 45.Chatis, P. A., C. H. Miller, L. E. Schrager, and C. S. Crumpacker. 1989. Successful treatment with foscarnet of an acyclovir-resistant mucocutaneous infection with herpes simplex virus in a patient with acquired immunodeficiency syndrome. N. Engl. J. Med. 320:297-300. [DOI] [PubMed] [Google Scholar]
- 46.Chen, S. H., A. Pearson, D. M. Coen, and S. H. Chen. 2004. Failure of thymidine kinase-negative herpes simplex virus to reactivate from latency following efficient establishment. J. Virol. 78:520-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen, Y., et al. 2000. Resistant herpes simplex virus type 1 infection: an emerging concern after allogeneic stem cell transplantation. Clin. Infect. Dis. 31:927-935. [DOI] [PubMed] [Google Scholar]
- 48.Chibo, D., J. Druce, J. Sasadeusz, and C. Birch. 2004. Molecular analysis of clinical isolates of acyclovir resistant herpes simplex virus. Antiviral Res. 61:83-91. [DOI] [PubMed] [Google Scholar]
- 49.Chiou, H. C., D. Kumura, A. Hu, K. M. Kerns, and D. M. Coen. 1995. Penciclovir-resistance mutations in the herpes simplex virus DNA polymerase gene. Antivir. Chem. Chemother. 6:281-288. [Google Scholar]
- 50.Chrisp, C. E., J. C. Sunstrum, D. R. Averill, Jr., M. Levine, and J. C. Glorioso. 1989. Characterization of encephalitis in adult mice induced by intracerebral inoculation of herpes simplex virus type 1 (KOS) and comparison with mutants showing decreased virulence. Lab. Invest. 60:822-830. [PubMed] [Google Scholar]
- 51.Christophers, J., et al. 1998. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob. Agents Chemother. 42:868-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cihlar, T., M. D. Fuller, and J. M. Cherrington. 1998. Characterization of drug resistance-associated mutations in the human cytomegalovirus DNA polymerase gene by using recombinant mutant viruses generated from overlapping DNA fragments. J. Virol. 72:5927-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clarke, S. E., A. W. Harrell, and R. J. Chenery. 1995. Role of aldehyde oxidase in the in vitro conversion of famciclovir to penciclovir in human liver. Drug Metab. Dispos. 23:251-254. [PubMed] [Google Scholar]
- 54.Coen, D. M. 1994. Acyclovir-resistant, pathogenic herpesviruses. Trends Microbiol. 2:481-485. [DOI] [PubMed] [Google Scholar]
- 55.Coen, D. M., H. E. Fleming, Jr., L. K. Leslie, and M. J. Retondo. 1985. Sensitivity of arabinosyladenine-resistant mutants of herpes simplex virus to other antiviral drugs and mapping of drug hypersensitivity mutations to the DNA polymerase locus. J. Virol. 53:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coen, D. M., et al. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. U. S. A. 86:4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coen, D. M., and P. A. Schaffer. 1980. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc. Natl. Acad. Sci. U. S. A. 77:2265-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collins, P., G. Appleyard, and N. M. Oliver. 1982. Sensitivity of herpes virus isolates from acyclovir clinical trials. Am. J. Med. 73:380-382. [DOI] [PubMed] [Google Scholar]
- 59.Collins, P., and M. N. Ellis. 1993. Sensitivity monitoring of clinical isolates of herpes simplex virus to acyclovir. J. Med. Virol. Suppl. 1:58-66. [DOI] [PubMed] [Google Scholar]
- 60.Collins, P., et al. 1989. Characterization of a DNA polymerase mutant of herpes simplex virus from a severely immunocompromised patient receiving acyclovir. J. Gen. Virol. 70(Pt. 2):375-382. [DOI] [PubMed] [Google Scholar]
- 61.Corey, L., and P. G. Spear. 1986. Infections with herpes simplex viruses (1). N. Engl. J. Med. 314:686-691. [DOI] [PubMed] [Google Scholar]
- 62.Cotarelo, M., et al. 1999. Cytopathic effect inhibition assay for determining the in-vitro susceptibility of herpes simplex virus to antiviral agents. J. Antimicrob. Chemother. 44:705-708. [DOI] [PubMed] [Google Scholar]
- 63.Crute, J. J., et al. 2002. Herpes simplex virus helicase-primase inhibitors are active in animal models of human disease. Nat. Med. 8:386-391. [DOI] [PubMed] [Google Scholar]
- 64.Crute, J. J., and I. R. Lehman. 1989. Herpes simplex-1 DNA polymerase. Identification of an intrinsic 5′—-3′ exonuclease with ribonuclease H activity. J. Biol. Chem. 264:19266-19270. [PubMed] [Google Scholar]
- 65.Cunningham, C., and A. J. Davison. 1993. A cosmid-based system for constructing mutants of herpes simplex virus type 1. Virology 197:116-124. [DOI] [PubMed] [Google Scholar]
- 66.Czartoski, T., et al. 2006. Fulminant, acyclovir-resistant, herpes simplex virus type 2 hepatitis in an immunocompetent woman. J. Clin. Microbiol. 44:1584-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dambrosi, S., et al. 2010. Neurovirulence and latency of drug-resistant clinical herpes simplex viruses in animal models. J. Med. Virol. 82:1000-1006. [DOI] [PubMed] [Google Scholar]
- 68.Danve-Szatanek, C., et al. 2004. Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J. Clin. Microbiol. 42:242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Datta, A. K., and R. E. Hood. 1981. Mechanism of inhibition of Epstein-Barr virus replication by phosphonoformic acid. Virology 114:52-59. [DOI] [PubMed] [Google Scholar]
- 70.Dekker, C., et al. 1983. Virus resistance in clinical practice. J. Antimicrob. Chemother. 12(Suppl. B):137-152. [DOI] [PubMed] [Google Scholar]
- 71.Derse, D., Y. C. Cheng, P. A. Furman, M. H. St. Clair, and G. B. Elion. 1981. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J. Biol. Chem. 256:11447-11451. [PubMed] [Google Scholar]
- 72.Diaz-Mitoma, F., R. G. Sibbald, S. D. Shafran, R. Boon, and R. L. Saltzman. 1998. Oral famciclovir for the suppression of recurrent genital herpes: a randomized controlled trial. Collaborative Famciclovir Genital Herpes Research Group. JAMA 280:887-892. [DOI] [PubMed] [Google Scholar]
- 73.Digard, P., W. R. Bebrin, K. Weisshart, and D. M. Coen. 1993. The extreme C terminus of herpes simplex virus DNA polymerase is crucial for functional interaction with processivity factor UL42 and for viral replication. J. Virol. 67:398-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dix, R. D., R. R. McKendall, and J. R. Baringer. 1983. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect. Immun. 40:103-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duan, J., et al. 1998. Antiviral activity of a selective ribonucleotide reductase inhibitor against acyclovir-resistant herpes simplex virus type 1 in vivo. Antimicrob. Agents Chemother. 42:1629-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duan, J., et al. 2003. Oral bioavailability and in vivo efficacy of the helicase-primase inhibitor BILS 45 BS against acyclovir-resistant herpes simplex virus type 1. Antimicrob. Agents Chemother. 47:1798-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duan, R., R. D. de Vries, A. D. Osterhaus, L. Remeijer, and G. M. Verjans. 2008. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J. Infect. Dis. 198:659-663. [DOI] [PubMed] [Google Scholar]
- 78.Duan, R., et al. 2009. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J. Infect. Dis. 200:1402-1414. [DOI] [PubMed] [Google Scholar]
- 79.Efstathiou, S., S. Kemp, G. Darby, and A. C. Minson. 1989. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J. Gen. Virol. 70(Pt. 4):869-879. [DOI] [PubMed] [Google Scholar]
- 80.Elion, G. B. 1993. Acyclovir: discovery, mechanism of action, and selectivity. J. Med. Virol. Suppl 1:2-6. [DOI] [PubMed] [Google Scholar]
- 81.Ellis, M. N., et al. 1987. Clinical isolate of herpes simplex virus type 2 that induces a thymidine kinase with altered substrate specificity. Antimicrob. Agents Chemother. 31:1117-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Englund, J. A., et al. 1990. Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann. Intern. Med. 112:416-422. [DOI] [PubMed] [Google Scholar]
- 83.Erard, V., A. Wald, L. Corey, W. M. Leisenring, and M. Boeckh. 2007. Use of long-term suppressive acyclovir after hematopoietic stem-cell transplantation: impact on herpes simplex virus (HSV) disease and drug-resistant HSV disease. J. Infect. Dis. 196:266-270. [DOI] [PubMed] [Google Scholar]
- 84.Erlich, K. S., et al. 1989. Foscarnet therapy for severe acyclovir-resistant herpes simplex virus type-2 infections in patients with the acquired immunodeficiency syndrome (AIDS). An uncontrolled trial. Ann. Intern. Med. 110:710-713. [DOI] [PubMed] [Google Scholar]
- 85.Evans, J. S., et al. 1998. Herpesviral thymidine kinases: laxity and resistance by design. J. Gen. Virol. 79(Pt. 9):2083-2092. [DOI] [PubMed] [Google Scholar]
- 86.Field, H. J. 1989. Persistent herpes simplex virus infection and mechanisms of virus drug resistance. Eur. J. Clin. Microbiol. Infect. Dis. 8:671-680. [DOI] [PubMed] [Google Scholar]
- 87.Field, H. J. 1983. A perspective on resistance to acyclovir in herpes simplex virus. J. Antimicrob. Chemother. 12(Suppl. B):129-135. [DOI] [PubMed] [Google Scholar]
- 88.Field, H. J., and D. M. Coen. 1986. Pathogenicity of herpes simplex virus mutants containing drug resistance mutations in the viral DNA polymerase gene. J. Virol. 60:286-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Field, H. J., and G. Darby. 1980. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob. Agents Chemother. 17:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Field, H. J., and E. De Clercq. 2008. Antiviral Chemistry & Chemotherapy's current antiviral agents FactFile (2nd edition): DNA viruses. Antivir. Chem. Chemother. 19:51-62. [DOI] [PubMed] [Google Scholar]
- 91.Field, H. J., and E. Lay. 1984. Characterization of latent infections in mice inoculated with herpes simplex virus which is clinically resistant to acyclovir. Antiviral Res. 4:43-52. [DOI] [PubMed] [Google Scholar]
- 92.Fife, K. H., T. C. Meng, D. G. Ferris, and P. Liu. 2008. Effect of resiquimod 0.01% gel on lesion healing and viral shedding when applied to genital herpes lesions. Antimicrob. Agents Chemother. 52:477-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foster, S. A., J. Cerny, and Y. C. Cheng. 1991. Herpes simplex virus-specified DNA polymerase is the target for the antiviral action of 9-(2-phosphonylmethoxyethyl) adenine. J. Biol. Chem. 266:238-244. [PubMed] [Google Scholar]
- 94.Frangoul, H., M. Wills, C. Crossno, M. Engel, and J. Domm. 2007. Acyclovir-resistant herpes simplex virus pneumonia post-unrelated stem cell transplantation: a word of caution. Pediatr. Transplant. 11:942-944. [DOI] [PubMed] [Google Scholar]
- 95.Frobert, E., et al. 2008. Genotypic detection of acyclovir-resistant HSV-1: characterization of 67 ACV-sensitive and 14 ACV-resistant viruses. Antiviral Res. 79:28-36. [DOI] [PubMed] [Google Scholar]
- 96.Frobert, E., D. Thouvenot, B. Lina, and F. Morfin. 2007. Genotyping diagnosis of acyclovir resistant herpes simplex virus. Pathol. Biol. (Paris) 55:504-511. (In French.) [DOI] [PubMed] [Google Scholar]
- 97.Gateley, A., et al. 1990. Herpes simplex virus type 2 meningoencephalitis resistant to acyclovir in a patient with AIDS. J. Infect. Dis. 161:711-715. [DOI] [PubMed] [Google Scholar]
- 98.Gaudreau, A., E. Hill, H. H. Balfour, Jr., A. Erice, and G. Boivin. 1998. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J. Infect. Dis. 178:297-303. [DOI] [PubMed] [Google Scholar]
- 99.Genereau, T., et al. 1996. Herpes simplex esophagitis in patients with AIDS: report of 34 cases. The Cooperative Study Group on Herpetic Esophagitis in HIV Infection. Clin. Infect. Dis. 22:926-931. [DOI] [PubMed] [Google Scholar]
- 100.Ghislanzoni, M., M. Cusini, R. Zerboni, and E. Alessi. 2006. Chronic hypertrophic acyclovir-resistant genital herpes treated with topical cidofovir and with topical foscarnet at recurrence in an HIV-positive man. J. Eur. Acad. Dermatol. Venereol. 20:887-889. [DOI] [PubMed] [Google Scholar]
- 101.Gibbs, J. S., H. C. Chiou, K. F. Bastow, Y. C. Cheng, and D. M. Coen. 1988. Identification of amino acids in herpes simplex virus DNA polymerase involved in substrate and drug recognition. Proc. Natl. Acad. Sci. U. S. A. 85:6672-6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gierasch, W. W., et al. 2006. Construction and characterization of bacterial artificial chromosomes containing HSV-1 strains 17 and KOS. J. Virol. Methods 135:197-206. [DOI] [PubMed] [Google Scholar]
- 103.Gilbert, C., J. Bestman-Smith, and G. Boivin. 2002. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist. Updat. 5:88-114. [DOI] [PubMed] [Google Scholar]
- 104.Gilbert, J., M. M. Drehs, and J. M. Weinberg. 2001. Topical imiquimod for acyclovir-unresponsive herpes simplex virus 2 infection. Arch. Dermatol. 137:1015-1017. [PubMed] [Google Scholar]
- 105.Gordon, Y. J., J. W. Caudill, E. Romanowski, and T. Araullo-Cruz. 1987. HSV-1 latency: thymidine kinase requirement and the round-trip theory. Curr. Eye Res. 6:611-616. [DOI] [PubMed] [Google Scholar]
- 106.Gottlieb, J., A. I. Marcy, D. M. Coen, and M. D. Challberg. 1990. The herpes simplex virus type 1 UL42 gene product: a subunit of DNA polymerase that functions to increase processivity. J. Virol. 64:5976-5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grant, D. M. 1987. Acyclovir (Zovirax) ophthalmic ointment: a review of clinical tolerance. Curr. Eye Res. 6:231-235. [DOI] [PubMed] [Google Scholar]
- 108.Griffiths, A., S. H. Chen, B. C. Horsburgh, and D. M. Coen. 2003. Translational compensation of a frameshift mutation affecting herpes simplex virus thymidine kinase is sufficient to permit reactivation from latency. J. Virol. 77:4703-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gupta, R., et al. 2005. Acyclovir sensitivity of sequential herpes simplex virus type 2 isolates from the genital mucosa of immunocompetent women. J. Infect. Dis. 192:1102-1107. [DOI] [PubMed] [Google Scholar]
- 110.Harris, W., et al. 2003. Phenotypic and genotypic characterization of clinical isolates of herpes simplex virus resistant to aciclovir. J. Gen. Virol. 84:1393-1401. [DOI] [PubMed] [Google Scholar]
- 111.Hill, E. L., G. A. Hunter, and M. N. Ellis. 1991. In vitro and in vivo characterization of herpes simplex virus clinical isolates recovered from patients infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 35:2322-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hitchcock, M. J. M., H. S. Jaffe, J. C. Martin, and R. J. Stagg. 1996. Cidofovir, a new agent with potent anti-herpesvirus activity. Antiviral Chem. Chemother. 7:115-127. [Google Scholar]
- 113.Horsburgh, B. C., et al. 1998. Recurrent acyclovir-resistant herpes simplex in an immunocompromised patient: can strain differences compensate for loss of thymidine kinase in pathogenesis? J. Infect. Dis. 178:618-625. [DOI] [PubMed] [Google Scholar]
- 114.Hwang, C. B., et al. 1994. A net +1 frameshift permits synthesis of thymidine kinase from a drug-resistant herpes simplex virus mutant. Proc. Natl. Acad. Sci. U. S. A. 91:5461-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hwang, C. B., K. L. Ruffner, and D. M. Coen. 1992. A point mutation within a distinct conserved region of the herpes simplex virus DNA polymerase gene confers drug resistance. J. Virol. 66:1774-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hwang, Y. T., B. Y. Liu, D. M. Coen, and C. B. Hwang. 1997. Effects of mutations in the Exo III motif of the herpes simplex virus DNA polymerase gene on enzyme activities, viral replication, and replication fidelity. J. Virol. 71:7791-7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hwang, Y. T., J. F. Smith, L. Gao, and C. B. Hwang. 1998. Mutations in the Exo III motif of the herpes simplex virus DNA polymerase gene can confer altered drug sensitivities. Virology 246:298-305. [DOI] [PubMed] [Google Scholar]
- 118.Jacobson, J. G., et al. 1993. Herpes simplex virus thymidine kinase and specific stages of latency in murine trigeminal ganglia. J. Virol. 67:6903-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jarvest, R. L., D. Sutton, and R. A. Vere Hodge. 1998. Famciclovir. Discovery and development of a novel antiherpesvirus agent. Pharm. Biotechnol. 11:313-343. [PubMed] [Google Scholar]
- 120.Kaufman, H. E., et al. 2008. Efficacy of a helicase-primase inhibitor in animal models of ocular herpes simplex virus type 1 infection. J. Ocul. Pharmacol. Ther. 24:34-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kennedy, P. G. 2005. Viral encephalitis. J. Neurol. 252:268-272. [DOI] [PubMed] [Google Scholar]
- 122.Kit, S., et al. 1987. Nucleotide sequence changes in thymidine kinase gene of herpes simplex virus type 2 clones from an isolate of a patient treated with acyclovir. Antimicrob. Agents Chemother. 31:1483-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Knopf, K. W. 1979. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur. J. Biochem. 98:231-244. [DOI] [PubMed] [Google Scholar]
- 124.Kopp, T., A. Geusau, A. Rieger, and G. Stingl. 2002. Successful treatment of an aciclovir-resistant herpes simplex type 2 infection with cidofovir in an AIDS patient. Br. J. Dermatol. 147:134-138. [DOI] [PubMed] [Google Scholar]
- 125.Kost, R. G., E. L. Hill, M. Tigges, and S. E. Straus. 1993. Brief report: recurrent acyclovir-resistant genital herpes in an immunocompetent patient. N. Engl. J. Med. 329:1777-1782. [DOI] [PubMed] [Google Scholar]
- 126.Kriesel, J. D., S. L. Spruance, M. Prichard, J. N. Parker, and E. R. Kern. 2005. Recurrent antiviral-resistant genital herpes in an immunocompetent patient. J. Infect. Dis. 192:156-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kudo, E., H. Shiota, T. Naito, K. Satake, and M. Itakura. 1998. Polymorphisms of thymidine kinase gene in herpes simplex virus type 1: analysis of clinical isolates from herpetic keratitis patients and laboratory strains. J. Med. Virol. 56:151-158. [DOI] [PubMed] [Google Scholar]
- 128.Kuhn, F. J., and C. W. Knopf. 1996. Herpes simplex virus type 1 DNA polymerase. Mutational analysis of the 3′-5′-exonuclease domain. J. Biol. Chem. 271:29245-29254. [DOI] [PubMed] [Google Scholar]
- 129.Kussmann-Gerber, S., O. Kuonen, G. Folkers, B. D. Pilger, and L. Scapozza. 1998. Drug resistance of herpes simplex virus type 1—structural considerations at the molecular level of the thymidine kinase. Eur. J. Biochem. 255:472-481. [DOI] [PubMed] [Google Scholar]
- 130.Lalezari, J., et al. 1997. A randomized, double-blind, placebo-controlled trial of cidofovir gel for the treatment of acyclovir-unresponsive mucocutaneous herpes simplex virus infection in patients with AIDS. J. Infect. Dis. 176:892-898. [DOI] [PubMed] [Google Scholar]
- 131.Langston, A. A., et al. 2002. Development of drug-resistant herpes simplex virus infection after haploidentical hematopoietic progenitor cell transplantation. Blood 99:1085-1088. [DOI] [PubMed] [Google Scholar]
- 132.Larder, B. A., S. D. Kemp, and G. Darby. 1987. Related functional domains in virus DNA polymerases. EMBO J. 6:169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Larder, B. A., J. J. Lisle, and G. Darby. 1986. Restoration of wild-type pathogenicity to an attenuated DNA polymerase mutant of herpes simplex virus type 1. J. Gen. Virol. 67(Pt. 11):2501-2506. [DOI] [PubMed] [Google Scholar]
- 134.Leahy, B. J., K. J. Christiansen, and G. Shellam. 1994. Standardisation of a microplate in situ ELISA (MISE-test) for the susceptibility testing of herpes simplex virus to acyclovir. J. Virol. Methods 48:93-108. [DOI] [PubMed] [Google Scholar]
- 135.Lee, N. Y., et al. 1999. Role of genotypic analysis of the thymidine kinase gene of herpes simplex virus for determination of neurovirulence and resistance to acyclovir. J. Clin. Microbiol. 37:3171-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lehman, I. R., and P. E. Boehmer. 1999. Replication of herpes simplex virus DNA. J. Biol. Chem. 274:28059-28062. [DOI] [PubMed] [Google Scholar]
- 137.Levin, M. J., T. H. Bacon, and J. J. Leary. 2004. Resistance of herpes simplex virus infections to nucleoside analogues in HIV-infected patients. Clin. Infect. Dis. 39(Suppl. 5):S248-S257. [DOI] [PubMed] [Google Scholar]
- 138.Liu, S., et al. 2006. Crystal structure of the herpes simplex virus 1 DNA polymerase. J. Biol. Chem. 281:18193-18200. [DOI] [PubMed] [Google Scholar]
- 139.Liuzzi, M., et al. 1994. A potent peptidomimetic inhibitor of HSV ribonucleotide reductase with antiviral activity in vivo. Nature 372:695-698. [DOI] [PubMed] [Google Scholar]
- 140.Ljungman, P., M. N. Ellis, R. C. Hackman, D. H. Shepp, and J. D. Meyers. 1990. Acyclovir-resistant herpes simplex virus causing pneumonia after marrow transplantation. J. Infect. Dis. 162:244-248. [DOI] [PubMed] [Google Scholar]
- 141.Lolis, M. S., L. Gonzalez, P. J. Cohen, and R. A. Schwartz. 2008. Drug-resistant herpes simplex virus in HIV infected patients. Acta Dermatovenerol. Croat. 16:204-208. [PubMed] [Google Scholar]
- 142.Longerich, T., et al. 2005. Recurrent herpes simplex virus hepatitis after liver retransplantation despite acyclovir therapy. Liver Transpl. 11:1289-1294. [DOI] [PubMed] [Google Scholar]
- 143.LoPresti, A. E., J. F. Levine, G. B. Munk, C. Y. Tai, and D. B. Mendel. 1998. Successful treatment of an acyclovir- and foscarnet-resistant herpes simplex virus type 1 lesion with intravenous cidofovir. Clin. Infect. Dis. 26:512-513. [DOI] [PubMed] [Google Scholar]
- 144.Marcy, A. I., C. B. Hwang, K. L. Ruffner, and D. M. Coen. 1990. Engineered herpes simplex virus DNA polymerase point mutants: the most highly conserved region shared among alpha-like DNA polymerases is involved in substrate recognition. J. Virol. 64:5883-5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mark, K. E., et al. 2007. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J. Infect. Dis. 195:1324-1331. [DOI] [PubMed] [Google Scholar]
- 146.Martinez, V., J. M. Molina, C. Scieux, P. Ribaud, and F. Morfin. 2006. Topical imiquimod for recurrent acyclovir-resistant HSV infection. Am. J. Med. 119:e9-e11. [DOI] [PubMed] [Google Scholar]
- 147.McKnight, S. L. 1980. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 8:5949-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miller, W. H., and R. L. Miller. 1980. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J. Biol. Chem. 255:7204-7207. [PubMed] [Google Scholar]
- 149.Miller, W. H., and R. L. Miller. 1982. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem. Pharmacol. 31:3879-3884. [DOI] [PubMed] [Google Scholar]
- 150.Morfin, F., et al. 2000. Genetic characterization of thymidine kinase from acyclovir-resistant and -susceptible herpes simplex virus type 1 isolated from bone marrow transplant recipients. J. Infect. Dis. 182:290-293. [DOI] [PubMed] [Google Scholar]
- 151.Morfin, F., and D. Thouvenot. 2003. Herpes simplex virus resistance to antiviral drugs. J. Clin. Virol. 26:29-37. [DOI] [PubMed] [Google Scholar]
- 152.Morfin, F., D. Thouvenot, M. Aymard, and G. Souillet. 2000. Reactivation of acyclovir-resistant thymidine kinase-deficient herpes simplex virus harbouring single base insertion within a 7 Gs homopolymer repeat of the thymidine kinase gene. J. Med. Virol. 62:247-250. [PubMed] [Google Scholar]
- 153.Mouly, F., et al. 1995. Chronic recurrent acyclovir-resistant genital herpes in an immunocompetent patient. Dermatology 190:177. [DOI] [PubMed] [Google Scholar]
- 154.Naesens, L., J. Balzarini, and E. de Clercq. 1994. Therapeutic potential of PMEA as an antiviral drug. Rev. Med. Virol. 4:147-159. [Google Scholar]
- 155.Naesens, L., et al. 1997. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 8:1-23. [Google Scholar]
- 156.Nugier, F., J. N. Colin, M. Aymard, and M. Langlois. 1992. Occurrence and characterization of acyclovir-resistant herpes simplex virus isolates: report on a two-year sensitivity screening survey. J. Med. Virol. 36:1-12. [DOI] [PubMed] [Google Scholar]
- 157.Oberg, B. 1989. Antiviral effects of phosphonoformate (PFA, foscarnet sodium). Pharmacol. Ther. 40:213-285. [DOI] [PubMed] [Google Scholar]
- 158.Oram, R. J., et al. 2000. Characterization of an acyclovir-resistant herpes simplex virus type 2 strain isolated from a premature neonate. J. Infect. Dis. 181:1458-1461. [DOI] [PubMed] [Google Scholar]
- 159.Palu, G., G. Gerna, F. Bevilacqua, and A. Marcello. 1992. A point mutation in the thymidine kinase gene is responsible for acyclovir-resistance in herpes simplex virus type 2 sequential isolates. Virus Res. 25:133-144. [DOI] [PubMed] [Google Scholar]
- 160.Parris, D. S., and J. E. Harrington. 1982. Herpes simplex virus variants restraint to high concentrations of acyclovir exist in clinical isolates. Antimicrob. Agents Chemother. 22:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pavic, I., et al. 1997. Flow cytometric analysis of herpes simplex virus type 1 susceptibility to acyclovir, ganciclovir, and foscarnet. Antimicrob. Agents Chemother. 41:2686-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pelosi, E., G. B. Mulamba, and D. M. Coen. 1998. Penciclovir and pathogenesis phenotypes of drug-resistant herpes simplex virus mutants. Antiviral Res. 37:17-28. [DOI] [PubMed] [Google Scholar]
- 163.Pelosi, E., F. Rozenberg, D. M. Coen, and K. L. Tyler. 1998. A herpes simplex virus DNA polymerase mutation that specifically attenuates neurovirulence in mice. Virology 252:364-372. [DOI] [PubMed] [Google Scholar]
- 164.Perry, C. M., and D. Faulds. 1996. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs 52:754-772. [DOI] [PubMed] [Google Scholar]
- 165.Pottage, J. C., Jr., and H. A. Kessler. 1995. Herpes simplex virus resistance to acyclovir: clinical relevance. Infect. Agents Dis. 4:115-124. [PubMed] [Google Scholar]
- 166.Reardon, J. E., and T. Spector. 1989. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J. Biol. Chem. 264:7405-7411. [PubMed] [Google Scholar]
- 167.Rechtin, T. M., M. E. Black, F. Mao, M. L. Lewis, and R. R. Drake. 1995. Purification and photoaffinity labeling of herpes simplex virus type-1 thymidine kinase. J. Biol. Chem. 270:7055-7060. [DOI] [PubMed] [Google Scholar]
- 168.Reichman, R. C., et al. 1984. Treatment of recurrent genital herpes simplex infections with oral acyclovir. A controlled trial. JAMA 251:2103-2107. [PubMed] [Google Scholar]
- 169.Reitano, M., et al. 1998. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J. Infect. Dis. 178:603-610. [DOI] [PubMed] [Google Scholar]
- 170.Reusser, P., et al. 1996. European survey of herpesvirus resistance to antiviral drugs in bone marrow transplant recipients. Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 17:813-817. [PubMed] [Google Scholar]
- 171.Reyes, M., et al. 2003. Acyclovir-resistant genital herpes among persons attending sexually transmitted disease and human immunodeficiency virus clinics. Arch. Intern. Med. 163:76-80. [DOI] [PubMed] [Google Scholar]
- 172.Sacks, S. L., F. Y. Aoki, A. Y. Martel, S. D. Shafran, and M. Lassonde. 2005. Clinic-initiated, twice-daily oral famciclovir for treatment of recurrent genital herpes: a randomized, double-blind, controlled trial. Clin. Infect. Dis. 41:1097-1104. [DOI] [PubMed] [Google Scholar]
- 173.Sacks, S. L., et al. 1998. A multicenter phase I/II dose escalation study of single-dose cidofovir gel for treatment of recurrent genital herpes. Antimicrob. Agents Chemother. 42:2996-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sacks, S. L., et al. 1989. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann. Intern. Med. 111:893-899. [DOI] [PubMed] [Google Scholar]
- 175.Saeki, Y., et al. 1998. Herpes simplex virus type 1 DNA amplified as bacterial artificial chromosome in Escherichia coli: rescue of replication-competent virus progeny and packaging of amplicon vectors. Hum. Gene Ther. 9:2787-2794. [DOI] [PubMed] [Google Scholar]
- 176.Safrin, S., T. Assaykeen, S. Follansbee, and J. Mills. 1990. Foscarnet therapy for acyclovir-resistant mucocutaneous herpes simplex virus infection in 26 AIDS patients: preliminary data. J. Infect. Dis. 161:1078-1084. [DOI] [PubMed] [Google Scholar]
- 177.Safrin, S., J. Cherrington, and H. S. Jaffe. 1999. Cidofovir. Review of current and potential clinical uses. Adv. Exp. Med. Biol. 458:111-120. [PubMed] [Google Scholar]
- 178.Safrin, S., et al. 1991. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N. Engl. J. Med. 325:551-555. [DOI] [PubMed] [Google Scholar]
- 179.Safrin, S., et al. 1994. Correlation between response to acyclovir and foscarnet therapy and in vitro susceptibility result for isolates of herpes simplex virus from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 38:1246-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Safrin, S., et al. 1994. Foscarnet-resistant herpes simplex virus infection in patients with AIDS. J. Infect. Dis. 169:193-196. [DOI] [PubMed] [Google Scholar]
- 181.Safrin, S., E. Palacios, and B. J. Leahy. 1996. Comparative evaluation of microplate enzyme-linked immunosorbent assay versus plaque reduction assay for antiviral susceptibility testing of herpes simplex virus isolates. Antimicrob. Agents Chemother. 40:1017-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Saijo, M., et al. 2002. Genotypic and phenotypic characterization of the thymidine kinase of ACV-resistant HSV-1 derived from an acyclovir-sensitive herpes simplex virus type 1 strain. Antiviral Res. 56:253-262. [DOI] [PubMed] [Google Scholar]
- 183.Saijo, M., et al. 1999. Nucleotide sequence of thymidine kinase gene of sequential acyclovir-resistant herpes simplex virus type 1 isolates recovered from a child with Wiskott-Aldrich syndrome: evidence for reactivation of acyclovir-resistant herpes simplex virus. J. Med. Virol. 58:387-393. [PubMed] [Google Scholar]
- 184.Saijo, M., T. Suzutani, S. Morikawa, and I. Kurane. 2005. Genotypic characterization of the DNA polymerase and sensitivity to antiviral compounds of foscarnet-resistant herpes simplex virus type 1 (HSV-1) derived from a foscarnet-sensitive HSV-1 strain. Antimicrob. Agents Chemother. 49:606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Saijo, M., et al. 2002. Bone marrow transplantation in a child with Wiskott-Aldrich syndrome latently infected with acyclovir-resistant (ACV(r)) herpes simplex virus type 1: emergence of foscarnet-resistant virus originating from the ACV(r) virus. J. Med. Virol. 68:99-104. [DOI] [PubMed] [Google Scholar]
- 186.Sarisky, R. T., et al. 2002. Penciclovir susceptibilities of herpes simplex virus isolates from patients using penciclovir cream for treatment of recurrent herpes labialis. Antimicrob. Agents Chemother. 46:2848-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sarisky, R. T., et al. 2003. Profiling penciclovir susceptibility and prevalence of resistance of herpes simplex virus isolates across eleven clinical trials. Arch. Virol. 148:1757-1769. [DOI] [PubMed] [Google Scholar]
- 188.Sarisky, R. T., et al. 2001. Biochemical characterization of a virus isolate, recovered from a patient with herpes keratitis, that was clinically resistant to acyclovir. Clin. Infect. Dis. 33:2034-2039. [DOI] [PubMed] [Google Scholar]
- 189.Sarisky, R. T., T. T. Nguyen, K. E. Duffy, R. J. Wittrock, and J. J. Leary. 2000. Difference in incidence of spontaneous mutations between Herpes simplex virus types 1 and 2. Antimicrob. Agents Chemother. 44:1524-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sarisky, R. T., et al. 2001. Characterization of herpes simplex viruses selected in culture for resistance to penciclovir or acyclovir. J. Virol. 75:1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sasadeusz, J. J., and S. L. Sacks. 1996. Spontaneous reactivation of thymidine kinase-deficient, acyclovir-resistant type-2 herpes simplex virus: masked heterogeneity or reversion? J. Infect. Dis. 174:476-482. [DOI] [PubMed] [Google Scholar]
- 192.Sasadeusz, J. J., et al. 1997. Homopolymer mutational hot spots mediate herpes simplex virus resistance to acyclovir. J. Virol. 71:3872-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sauerbrei, A., S. Deinhardt, R. Zell, and P. Wutzler. 2010. Phenotypic and genotypic characterization of acyclovir-resistant clinical isolates of herpes simplex virus. Antiviral Res. 86:246-252. [DOI] [PubMed] [Google Scholar]
- 194.Schacker, T. W., et al. 2002. Imiquimod 5-percent cream does not alter the natural history of recurrent herpes genitalis: a phase II, randomized, double-blind, placebo-controlled study. Antimicrob. Agents Chemother. 46:3243-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schmit, I., and G. Boivin. 1999. Characterization of the DNA polymerase and thymidine kinase genes of herpes simplex virus isolates from AIDS patients in whom acyclovir and foscarnet therapy sequentially failed. J. Infect. Dis. 180:487-490. [DOI] [PubMed] [Google Scholar]
- 196.Schnipper, L. E., and C. S. Crumpacker. 1980. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc. Natl. Acad. Sci. U. S. A. 77:2270-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schulte, E. C., et al. 2010. Acyclovir resistance in herpes simplex encephalitis. Ann. Neurol. 67:830-833. [DOI] [PubMed] [Google Scholar]
- 198.Sergerie, Y., and G. Boivin. 2006. Thymidine kinase mutations conferring acyclovir resistance in herpes simplex type 1 recombinant viruses. Antimicrob. Agents Chemother. 50:3889-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shin, Y. K., G. Y. Cai, A. Weinberg, J. J. Leary, and M. J. Levin. 2001. Frequency of acyclovir-resistant herpes simplex virus in clinical specimens and laboratory isolates. J. Clin. Microbiol. 39:913-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shin, Y. K., et al. 2003. Susceptibility of herpes simplex virus isolates to nucleoside analogues and the proportion of nucleoside-resistant variants after repeated topical application of penciclovir to recurrent herpes labialis. J. Infect. Dis. 187:1241-1245. [DOI] [PubMed] [Google Scholar]
- 201.Shupack, J., M. Stiller, I. Davis, C. Kenny, and L. Jondreau. 1992. Topical alpha-interferon ointment with dimethyl sulfoxide in the treatment of recurrent genital herpes simplex. Dermatology 184:40-44. [DOI] [PubMed] [Google Scholar]
- 202.Sims, C. R., et al. 2007. Oral topical cidofovir: novel route of drug delivery in a severely immunosuppressed patient with refractory multidrug-resistant herpes simplex virus infection. Transpl. Infect. Dis. 9:256-259. [DOI] [PubMed] [Google Scholar]
- 203.Slade, H. B., T. Schacker, M. Conant, and C. Thoming. 2002. Imiquimod and genital herpes. Arch. Dermatol. 138:534. [DOI] [PubMed] [Google Scholar]
- 204.Snoeck, R., et al. 1994. Successful treatment of progressive mucocutaneous infection due to acyclovir- and foscarnet-resistant herpes simplex virus with (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC). Clin. Infect. Dis. 18:570-578. [DOI] [PubMed] [Google Scholar]
- 205.Soriano, J. M., J. Funk, P. Janknecht, and B. Matz. 1992. Recurrent herpetic keratitis during topical acyclovir application. Eur. J. Ophthalmol. 2:155-157. [DOI] [PubMed] [Google Scholar]
- 206.Spruance, S. L., and J. Hill. 2004. Clinical significance of antiviral therapy for episodic treatment of herpes labialis: exploratory analyses of the combined data from two valaciclovir trials. J. Antimicrob. Chemother. 53:703-707. [DOI] [PubMed] [Google Scholar]
- 207.Spruance, S. L., et al. 2002. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob. Agents Chemother. 46:2238-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Spruance, S. L., et al. 1997. Penciclovir cream for the treatment of herpes simplex labialis. A randomized, multicenter, double-blind, placebo-controlled trial. Topical Penciclovir Collaborative Study Group. JAMA 277:1374-1379. [PubMed] [Google Scholar]
- 209.Spruance, S. L., et al. 1999. Peroral famciclovir in the treatment of experimental ultraviolet radiation-induced herpes simplex labialis: a double-blind, dose-ranging, placebo-controlled, multicenter trial. J. Infect. Dis. 179:303-310. [DOI] [PubMed] [Google Scholar]
- 210.Standring-Cox, R., T. H. Bacon, and B. A. Howard. 1996. Comparison of a DNA probe assay with the plaque reduction assay for measuring the sensitivity of herpes simplex virus and varicella-zoster virus to penciclovir and acyclovir. J. Virol. Methods 56:3-11. [DOI] [PubMed] [Google Scholar]
- 211.Stavropoulos, T. A., and C. A. Strathdee. 1998. An enhanced packaging system for helper-dependent herpes simplex virus vectors. J. Virol. 72:7137-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Stranska, R., et al. 2005. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: prevalence and characterization. J. Clin. Virol. 32:7-18. [DOI] [PubMed] [Google Scholar]
- 213.Stranska, R., et al. 2004. Sequential switching of DNA polymerase and thymidine kinase-mediated HSV-1 drug resistance in an immunocompromised child. Antivir. Ther. 9:97-104. [PubMed] [Google Scholar]
- 214.Stranska, R., et al. 2004. Genotypic and phenotypic characterization of acyclovir-resistant herpes simplex viruses isolated from haematopoietic stem cell transplant recipients. Antivir. Ther. 9:565-575. [PubMed] [Google Scholar]
- 215.Stranska, R., A. M. van Loon, M. Polman, and R. Schuurman. 2002. Application of real-time PCR for determination of antiviral drug susceptibility of herpes simplex virus. Antimicrob. Agents Chemother. 46:2943-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Straus, S. E., et al. 1984. Suppression of frequently recurring genital herpes. A placebo-controlled double-blind trial of oral acyclovir. N. Engl. J. Med. 310:1545-1550. [DOI] [PubMed] [Google Scholar]
- 217.Stroop, W. G., M. C. Banks, H. Qavi, J. Chodosh, and S. M. Brown. 1994. A thymidine kinase deficient HSV-2 strain causes acute keratitis and establishes trigeminal ganglionic latency, but poorly reactivates in vivo. J. Med. Virol. 43:297-309. [DOI] [PubMed] [Google Scholar]
- 218.Suzutani, T., et al. 2003. Differential mutation patterns in thymidine kinase and DNA polymerase genes of herpes simplex virus type 1 clones passaged in the presence of acyclovir or penciclovir. Antimicrob. Agents Chemother. 47:1707-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Swetter, S. M., et al. 1998. Chronic vulvar ulceration in an immunocompetent woman due to acyclovir-resistant, thymidine kinase-deficient herpes simplex virus. J. Infect. Dis. 177:543-550. [DOI] [PubMed] [Google Scholar]
- 220.Swierkosz, E. M., et al. 2004. Antiviral susceptibility testing: herpes simplex virus by plaque reduction assay. Approved standard, vol. 24. Clinical and Laboratory Standards Institute, Wayne, PA.
- 221.Tanaka, M., H. Kagawa, Y. Yamanashi, T. Sata, and Y. Kawaguchi. 2003. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J. Virol. 77:1382-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tanaka, M., H. Kodaira, Y. Nishiyama, T. Sata, and Y. Kawaguchi. 2004. Construction of recombinant herpes simplex virus type I expressing green fluorescent protein without loss of any viral genes. Microbes Infect. 6:485-493. [DOI] [PubMed] [Google Scholar]
- 223.Tanaka, S., Y. Toh, and R. Mori. 1993. Molecular analysis of a neurovirulent herpes simplex virus type 2 strain with reduced thymidine kinase activity. Arch. Virol. 131:61-73. [DOI] [PubMed] [Google Scholar]
- 224.Tebas, P., E. C. Stabell, and P. D. Olivo. 1995. Antiviral susceptibility testing with a cell line which expresses beta-galactosidase after infection with herpes simplex virus. Antimicrob. Agents Chemother. 39:1287-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tenser, R. B., and W. A. Edris. 1987. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus after in vivo complementation. J. Virol. 61:2171-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Thi, T. N., et al. 2006. Rapid determination of antiviral drug susceptibility of herpes simplex virus types 1 and 2 by real-time PCR. Antiviral Res. 69:152-157. [DOI] [PubMed] [Google Scholar]
- 227.Tsurumi, T., K. Maeno, and Y. Nishiyama. 1987. Nucleotide sequence of the DNA polymerase gene of herpes simplex virus type 2 and comparison with the type 1 counterpart. Gene 52:129-137. [DOI] [PubMed] [Google Scholar]
- 228.Tyring, S. K., D. Baker, and W. Snowden. 2002. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years' experience with acyclovir. J. Infect. Dis. 186(Suppl. 1):S40-S46. [DOI] [PubMed] [Google Scholar]
- 229.Verdonck, L. F., et al. 1993. Successful foscarnet therapy for acyclovir-resistant mucocutaneous infection with herpes simplex virus in a recipient of allogeneic BMT. Bone Marrow Transplant. 11:177-179. [PubMed] [Google Scholar]
- 230.Vere Hodge, R. A., D. Sutton, M. R. Boyd, M. R. Harnden, and R. L. Jarvest. 1989. Selection of an oral prodrug (BRL 42810; famciclovir) for the antiherpesvirus agent BRL 39123 [9-(4-hydroxy-3-hydroxymethylbut-l-yl)guanine; penciclovir]. Antimicrob. Agents Chemother. 33:1765-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Vonka, V., et al. 1990. Properties of a 9-(2-phosphonylmethoxyethyl) adenine (PMEA)-resistant herpes simplex virus type 1 virus mutant. Antiviral Res. 14:117-121. [DOI] [PubMed] [Google Scholar]
- 232.Wade, J. C., C. McLaren, and J. D. Meyers. 1983. Frequency and significance of acyclovir-resistant herpes simplex virus isolated from marrow transplant patients receiving multiple courses of treatment with acyclovir. J. Infect. Dis. 148:1077-1082. [DOI] [PubMed] [Google Scholar]
- 233.Wagstaff, A. J., D. Faulds, and K. L. Goa. 1994. Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 47:153-205. [DOI] [PubMed] [Google Scholar]
- 234.Wald, A., et al. 2006. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex. Transm. Dis. 33:529-533. [DOI] [PubMed] [Google Scholar]
- 235.Weinberg, A., J. J. Leary, R. T. Sarisky, and M. J. Levin. 2007. Factors that affect in vitro measurement of the susceptibility of herpes simplex virus to nucleoside analogues. J. Clin. Virol. 38:139-145. [DOI] [PubMed] [Google Scholar]
- 236.Weller, S., et al. 1993. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin. Pharmacol. Ther. 54:595-605. [DOI] [PubMed] [Google Scholar]
- 237.Whitley, R. J. 2006. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 71:141-148. [DOI] [PubMed] [Google Scholar]
- 238.Wiebusch, L., M. Truss, and C. Hagemeier. 2004. Inhibition of human cytomegalovirus replication by small interfering RNAs. J. Gen. Virol. 85:179-184. [DOI] [PubMed] [Google Scholar]
- 239.Wild, K., T. Bohner, G. Folkers, and G. E. Schulz. 1997. The structures of thymidine kinase from herpes simplex virus type 1 in complex with substrates and a substrate analogue. Protein Sci. 6:2097-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Williamson, E. C., et al. 1999. Infections in adults undergoing unrelated donor bone marrow transplantation. Br. J. Haematol. 104:560-568. [DOI] [PubMed] [Google Scholar]
- 241.Wong, S. W., et al. 1988. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 7:37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xiong, X., J. L. Smith, and M. S. Chen. 1997. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob. Agents Chemother. 41:594-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Xu, F., et al. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964-973. [DOI] [PubMed] [Google Scholar]
- 244.Yao, Y. F., et al. 1996. Clinical characteristics of acyclovir-resistant herpetic keratitis and experimental studies of isolates. Graefes Arch. Clin. Exp. Ophthalmol. 234(Suppl. 1):S126-S132. [DOI] [PubMed] [Google Scholar]
- 245.Yeung-Yue, K. A., M. H. Brentjens, P. C. Lee, and S. K. Tyring. 2002. Herpes simplex viruses 1 and 2. Dermatol. Clin. 20:249-266. [DOI] [PubMed] [Google Scholar]
- 246.Youle, M. M., et al. 1988. Acyclovir-resistant herpes in AIDS treated with foscarnet. Lancet ii:341-342. [DOI] [PubMed] [Google Scholar]
- 247.Zhang, J., et al. 1991. Primary structure of the catalytic subunit of calf thymus DNA polymerase delta: sequence similarities with other DNA polymerases. Biochemistry 30:11742-11750. [DOI] [PubMed] [Google Scholar]
- 248.Ziyaeyan, M., et al. 2007. Frequency of acyclovir-resistant herpes simplex viruses isolated from the general immunocompetent population and patients with acquired immunodeficiency syndrome. Int. J. Dermatol. 46:1263-1266. [DOI] [PubMed] [Google Scholar]