Abstract
Attachment inhibitors (AI) are a novel class of HIV-1 antivirals, with little information available on clinical resistance. BMS-488043 is an orally bioavailable AI that binds to gp120 of HIV-1 and abrogates its binding to CD4+ lymphocytes. A clinical proof-of-concept study of the AI BMS-488043, administered as monotherapy for 8 days, demonstrated significant viral load reductions. In order to examine the effects of AI monotherapy on HIV-1 sensitivity, phenotypic sensitivity assessment of baseline and postdosing (day 8) samples was performed. These analyses revealed that four subjects had emergent phenotypic resistance (a 50% effective concentration [EC50] >10-fold greater than the baseline value) and four had high baseline EC50s (>200 nM). Population sequencing and sequence determination of cloned envelope genes uncovered five gp120 mutations at four loci (V68A, L116I, S375I/N, and M426L) associated with BMS-488043 resistance. Substitution at the 375 locus, located near the CD4 binding pocket, was the most common (maintained in 5/8 subjects at day 8). The five substitutions were evaluated for their effects on AI sensitivity through reverse genetics in functional envelopes, confirming their role in decreasing sensitivity to the drug. Additional analyses revealed that these substitutions did not alter sensitivity to other HIV-1 entry inhibitors. Thus, our studies demonstrate that although the majority of the subjects' viruses maintained sensitivity to BMS-488043, substitutions can be selected that decrease HIV-1 susceptibility to the AI. Most importantly, the substitutions described here are not associated with resistance to other approved antiretrovirals, and therefore, attachment inhibitors could complement the current arsenal of anti-HIV agents.
HIV-1 entry is a multistep process and presents numerous opportunities by which compounds can contain virus spread (18, 29). This process is initiated when the gp120 viral envelope protein binds to a well-defined locus on the CD4 protein of lymphocytes, leading to a conformational change from a pre-CD4-bound state to a CD4-bound state. This interaction induces additional conformational changes in the viral envelope, resulting in coreceptor binding site access (24). While a number of lymphocyte surface proteins can serve as HIV-1 coreceptors, the most commonly utilized ones are CCR5 and CXCR4 (2, 3, 9). Coreceptor binding leads to further conformational changes, exposing gp41 and initiating a virus-cell fusion process that results in delivery of the HIV-1 core to the cell cytoplasm (7). Molecules that target any of these defined steps have been designated entry inhibitors (18).
Attachment inhibitors (AIs) target the initial entry step, gp120 binding to the CD4 receptor (1, 11, 14, 16, 22). Since they act upstream of coreceptor binding, AIs are active against CCR5- and/or CXCR4-utilizing virus, as well as strains resistant to nucleoside and nonnucleoside reverse transcriptase, protease, and integrase inhibitors (22). The mechanism underlying AI antientry activity likely involves interaction with gp120 in a pre-CD4-bound conformation and blockage of conformational changes in gp120 that prevent gp120-CD4 receptor contact and subsequent entry events (14).
As with other classes of antiretrovirals, the capacity of HIV-1 to evolve leads to the emergence of resistance to AIs (6, 10, 21, 22, 25). Amino acid substitutions that confer decreased susceptibility to an earlier AI (BMS-378806) in cell culture mapped primarily to the envelope surface protein gp120, although changes within the envelope transmembrane domain gp41 can also be observed (22, 25, 34). However, BMS-378806 failed to achieve targeted plasma exposures in humans. Therefore, a related compound, BMS-488043, with improved in vitro antiviral activity and pharmacokinetic properties was advanced into clinical development (1, 23, 30, 33). Taken with a high-fat meal, BMS-488043 achieved a 10-fold higher concentration in the plasma of human subjects than was observed for BMS-378806. This favorable profile has led to the analysis of this compound in a proof-of-concept study with HIV-1-infected subjects in which in vivo activity was demonstrated (13). This report describes the identity and effect of the genotypic correlates for preexisting and emergent resistance to BMS-488043 and the ramifications of these findings for the potential clinical impact of AIs.
MATERIALS AND METHODS
Clinical study design and subjects.
A randomized, double-blind, multiple-dose, sequential dose escalation monotherapy study was conducted with 30 HIV-1-infected subjects (15 subjects/dose level) at four sites in the United States (13). Inclusion criteria included no antiretroviral therapy for ≥16 weeks, CD4+ T-cell counts of ≥250 cells/μl, and plasma HIV-1 RNA levels between 5,000 and 500,000 copies/ml (Roche Amplicor Assay, version 1.5). Subjects were randomized to receive BMS-488043 (800 or 1,800 mg twice a day [BID]) or a matching placebo administered every 12 h orally with a daily high-fat meal on days 1 through 7 and on the morning of day 8. For each study arm (800 or 1,800 mg BID), the ratio of subjects receiving BMS-488043 to those receiving the placebo was 4:1. A total of 12 subjects received 800 mg BMS-488043 BID, 12 received 1,800 mg BMS-488043 BID, and 6 received the placebo BID. Blood was drawn prior to entry (on days −3 to −1) and prior to dosing each morning on days 1 through 10, 12, and 14 (the day of discharge) and served as the source of HIV-1 RNA and for use as the template for the amplification of HIV-1 envelope sequences. Blood samples from the baseline (prior to any BMS-488043 administration) and day 8 were used for assessment of viral susceptibility to BMS-488043 for all of the subjects; for select subjects, blood samples from other study days were also assessed. The pharmacokinetics and safety of BMS-488043 in this study are described elsewhere (13).
Genotyping of HIV-1 isolates.
HIV-1 RNA was isolated, and initial cDNA synthesis was achieved with random hexamer primers as described elsewhere (23). In order to amplify the envelope gene via PCR, the primer pair HX5860F (5′-ATGGAGCCAGTAGATCCTAGACTAGAGCCCT-3′) and HX9068R (5′-TAGCCCTTCCAGTCCCCCCTTTTCTTTTA-3′) was used. Uncloned envelope cDNA was subjected to population-based sequencing using a library of envelope-specific primers. As expected, changes, including insertions and deletions, were detected throughout the envelope gene, resulting in low-level mixtures at many sites. Therefore, only the most prevalent base at each position was identified, allowing a consensus sequence to be determined. Amino acid positions were numbered according to the HXB2 sequence. In certain cases, individual envelope clones were isolated, sequenced, and examined for functionality in a cell fusion assay.
Drug susceptibility assay.
The amplified envelope gene populations were used to directly express envelope protein using the TOPO tool kit (Invitrogen), or the cloned envelope genes were placed into a pTRE2 vector for analysis (Clontech). Both the TOPO-constructed envelope population and the cloned constructs were examined in a cell fusion assay as previously described (23). Briefly, two populations of HeLa cells, designated effector and target cells, were used in a cell fusion assay. The effector cells were prepared by cotransfecting cells with TOPO-constructed envelopes and the pTET-Off plasmid (Clontech). HeLa cells expressing CD4, CXCR4, and CCR5 and containing an integrated copy of an inducible luciferase reporter gene were used as target cells. The effector and target cells were trypsinized, mixed at a ratio of 1:2, and seeded into a 96-well plate in the presence of the test compound. After 18 h of incubation, luciferase fluorescence served to quantify cell fusion and, hence, drug susceptibility.
Allele-specific PCR.
In order to quantify the proportion of sequences expressing the 375N substitution within a subject, reverse transcription-PCR products of the HIV-1 envelope from subjects were subjected to two separate amplifications. One was nonselective amplification for all viral envelopes, in order to quantitate the total amount of envelope genes in the mixture. The other was the preferential amplification of genes containing the S375N mutation. Specific primers were designed for preferentially amplifying the 375N mutation based on the principles described by Hance et al. (12). Specific primers had to be identified for each subject, based upon the HIV-1 envelope sequences. For subject 1, the nonselective amplification used primers FN1 (5′-GACCCAGAAATTGTAATGCACA) and R1 (5′-GAGGGGCATACATTGCTTTT) and probe (FAM-TTGTGGAGGGGAATTTTTCTATTGTAAT-BHQ [FAM, 6-carboxyfluorescein; BHQ, black hole quencher]); for preferential amplification conditions, the FN1 primer was replaced with the FS1 primer (5′-GACCCAGAAATTGTAATGCACGA). For subject 21, the nonselective amplification used primers F21 (5′-TACAAGACCCAACAACAATACA) and RN1 (5′-CAAGTACTATTAAACAGTTGTGTTG) and probe (FAM-ATTACAATTTCTGGGTCCCCTCCTGA-BHQ); for preferential amplification conditions, the RN21 primer was replaced with the RS21 primer (5′-ATTCCCCTCCACAATTAAAGT). For subject 25, the nonselective amplification used primers FN25 (5′-GACCCAGAAATTGTAATGCATA) and R1 (5′-GAGGGGCATACATTGCTTTT) and probe (FAM-TGTTTAATAGCACTTGGAATAGTACTGCA-BHQ); for preferential amplification conditions, the FN25 primer was replaced with the FS25 primer (5′-GACCCAGAAATTGTAATGCATGA). Samples were evaluated by real-time PCR using an ABI 9700 sequence detection system (Applied Biosystems) using the following cycling parameters: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 50°C for 1 min. Wild-type LAI strain gp160 and a 375N-containing clone were amplified by PCR. The quantitated PCR product of the mutant envelope was serially diluted with known amounts of the wild-type PCR product and used as standards for real-time PCR. The same DNA standard concentration (quantified with nonselective PCR) was used for both the nonselective and S375N-selective amplifications of clinical samples. The number of cycles required to reach threshold fluorescence (threshold cycle) of the subject samples was determined, and the quantity of sequences initially present was calculated by extrapolation onto the standard curve. The percentage of viral sequences containing 375N was calculated as follows: % mutated sequences = (quantity of mutated sequences in the sample)/(quantity of total sequences in the sample) × 100. Nonselective and selective amplifications were always performed at the same time. All reactions were performed in duplicate, and the mean of the two values was used for calculations.
BMS-488043/pre-CD4-bound HIV-1 gp120 model.
The pre-CD4-bound HIV-1 gp120 homology model was constructed from the simian immunodeficiency virus structure with the V3 loop modeled in 2bf1.pdb (5) using the Quanta Protein Design Module (QUANTA Modeling Environment release 2006; Accelrys Software Inc., San Diego, CA). BMS-488043 was manually docked into the structure and then minimized using CHARMM (4).
Nucleotide sequence accession numbers.
Population sequences have been deposited in the Los Alamos National Laboratory (LANL) database under accession numbers HM234442 to HM234501.
RESULTS
BMS-488043 sensitivity of HIV-1 envelope populations before and during administration of BMS-488043.
In order to determine the sensitivity of the clinical sample envelopes to BMS-488043, gp160 sequences were amplified and the gene population was analyzed in a fusion-based assay (22). The sensitivities of the baseline (prior to any BMS-488043 administration) and day 8 samples were analyzed, and the data are shown in Table 1. For subject 7, blood samples were not available on days 7 to 10, so the earliest posttreatment sample available was from day 12. Since up to approximately 6-fold variability in BMS-488043 sensitivity was observed with placebo samples in this study (e.g., subject 24), susceptibility changes of >10-fold the baseline sensitivity were considered significant for emergent resistance development in the clinical study. Thus, four subjects (1, 4, 21, and 25) were deemed to have emergent resistance development (13). In addition, there were three subjects randomized to BMS-488043 (10, 19, and 27) and one subject randomized to the placebo (subject 30) who exhibited high baseline EC50s (>200 nM) that did not change substantially during the study. At the initiation of treatment, these subjects presumably maintained genotypes that encode a preexisting marker that decreases sensitivity to BMS-488043. Consistent with this assumption, none of the three subjects who received BMS-488043 responded well to dosing (maximum plasma HIV-1 RNA decreases of 0.36, 0.31, and 0.24 log10 for subjects 10, 19, and 27, respectively).
TABLE 1.
BMS-488043 sensitivities of uncloned HIV-1 envelopes from subjects before and during BMS-488043 administration
| Subject | BMS-488043 dose (mg) | EC50 (nM) |
Max decrease in HIV-1 RNA [log10 copies/ml (day)] | |
|---|---|---|---|---|
| Baselined | Day 8e | |||
| 1 | 800 | 7 | 244a | 0.49 (6) |
| 2 | 800 | 3 | 20 | 1.17 (6) |
| 3 | Placebo | 1 | 2 | 0.38 (6) |
| 4 | 800 | 10 | 1,792a | 0.64 (7) |
| 5 | 800 | 66 | 358 | 0.33 (5) |
| 6 | Placebo | 7 | 12 | 0.22 (2) |
| 7 | 800 | 4 | 6b | 0.87 (12)b |
| 8 | 800 | 29 | 29 | 0.98 (7) |
| 9 | 800 | 5 | 3 | 1.13 (9) |
| 10 | 800 | >10,000 | >10,000 | 0.36 (5) |
| 11 | 800 | 70 | 358 | 0.50 (5) |
| 12 | 800 | 6 | 9 | 1.53 (10) |
| 13 | 800 | 10 | 10 | 1.37 (8) |
| 14 | 800 | 3 | 5 | 1.51 (10) |
| 15 | Placebo | 10 | 13 | 0.30 (9) |
| 16 | Placebo | 4 | 14 | 0.17 (6) |
| 17 | 1,800 | 78 | 80 | 2.05 (9) |
| 18 | 1,800 | 2 | 6 | 1.88 (9) |
| 19 | 1,800 | 373 | 251 | 0.31 (5) |
| 20 | 1,800 | 1 | 1 | 1.70 (10) |
| 21 | 1,800 | 3 | 895a | 0.81 (8) |
| 22 | 1,800 | 7 | 5 | 1.14 (5) |
| 23 | 1,800 | 6 | 2 | 1.69 (9) |
| 24 | Placebo | 5 | 28 | 0.11 (8) |
| 25 | 1,800 | 5 | 416a | 0.38 (5) |
| 26 | 1,800 | 5 | 4 | 1.32 (9) |
| 27 | 1,800 | 813 | 864 | 0.24 (7) |
| 28 | 1,800 | 60 | 19 | 1.06 (8) |
| 29 | 1,800 | 6 | 4 | 1.33 (9) |
| 30 | Placebo | >10,000 | >10,000 | NDc |
Subject whose virus, by definition (>10-fold increase in EC50), developed resistance.
Samples for days 7 to 10 not available for this subject; day 12 sample used.
ND, no decrease in HIV-1 RNA was noted for this subject during the study.
Median, 6.
Median, 19.
Genotypic analysis of HIV-1 envelope populations before and during administration of BMS-488043.
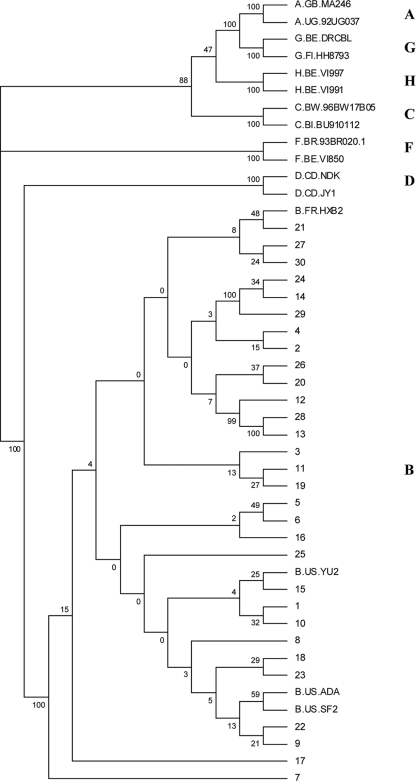
In order to correlate a BMS-488043-resistant phenotype with specific genotypes, the sequences of uncloned virus envelopes were determined. Phylogenetic analyses of these sequences revealed that all of the viruses were most closely aligned with HIV-1 subtype B sequences (Fig. 1), as expected given that this trial took place in the United States, where subtype B predominates (for subsequent analyses, subject sequences were compared with subtype B consensus sequences) (8). In order to map changes that underlie BMS-488043 resistance, the amino acid sequences of day 8 samples were compared with baseline sequences in the four subjects (subjects 1, 4, 21, and 25) thought to have emergent resistance to BMS-488043 (shown compared to the HXB2 sequence in Fig. S1 in the supplemental material). In addition, both baseline and day 8 sequences from subjects thought to possess preexisting resistance markers (subjects 10, 19, 27, and 30) were analyzed in this manner. As a guide, specific loci were examined that were previously shown to be associated with development of in vitro resistance to BMS-488043, which include amino acid substitutions V68A, M426L, M434I, S440R, and M475I in gp120 (23). Of these substitutions, only one subject treated with 800 mg BMS-488043 (subject 1, Table 2) exhibited an emergent V68A substitution at day 8, while two subjects (subjects 27 and 30, Table 2) suspected to contain preexisting genotypic markers that encode resistance contained the M426L change at both the baseline and day 8. No other subjects exhibited any of the other substitutions associated with decreased BMS-488043 sensitivity that were prominent in cell culture BMS-488043 selections (23). Further analysis of the day 8 samples from the other subjects with emerging resistance (subjects 4, 21, and 25) showed that these three subjects, along with subject 1 (who also had an emergent V68A change), all exhibited an emergent S375N substitution at day 8 (Table 2). A distinct substitution at this locus (S375I) was observed in a subject (subject 10) who exhibited a preexisting resistance phenotype. In addition, subject 19 contained a substitution unique to this subject population (L116I), which, in the absence of additional signature substitutions (see Fig. S1 in the supplemental material), was suspected to underlie preexisting BMS-488043 resistance (see below).
FIG. 1.
Phylogenetic analysis of the baseline HIV-1 envelopes from the 30 subjects in the clinical study. Envelope sequences from representative viruses of subtypes A, B, C, D, F, G, and H are included and labeled.
TABLE 2.
Substitutions unique to viruses displaying BMS-488043 resistance
| Subject and sample | Substitution at: |
|||
|---|---|---|---|---|
| V68 | L116 | S375 | M426 | |
| Virus with emerging loss of susceptibility to BMS-488043 | ||||
| 1 | ||||
| Baseline | —a | — | — | — |
| Day 8 | A | — | N | — |
| 4 | ||||
| Baseline | — | — | — | — |
| Day 8 | — | — | N | — |
| 21 | ||||
| Baseline | — | — | — | — |
| Day 8 | — | — | N | — |
| 25 | ||||
| Baseline | — | — | — | — |
| Day 8 | — | — | N | — |
| Virus with preexisting resistance to BMS-488043 | ||||
| 10 | ||||
| Baseline | — | — | I | — |
| Day 8 | — | — | I | — |
| 19 | ||||
| Baseline | — | I | — | — |
| Day 8 | — | I | — | — |
| 27 | ||||
| Baseline | — | — | — | L |
| Day 8 | — | — | — | L |
| 30 | ||||
| Baseline | — | — | — | L |
| Day 8 | — | — | — | L |
—, no change from consensus sequence.
S375N is a major marker of BMS-488043 resistance.
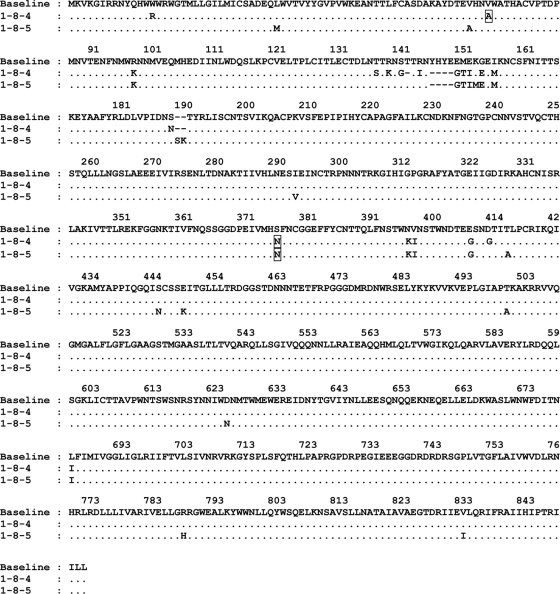
As S375N was prominently associated with emergent resistance to BMS-488043 in vivo, its specific effect on sensitivity to the AI was subjected to further investigation. For these studies, envelope samples from subject 1 (baseline and day 8) were cloned and functional envelopes from day 8 were sequenced. Two such clones (1-8-4 and 1-8-5) were further evaluated. Among the 26 amino acid changes compared to the baseline consensus sequence, clone 1-8-5 contained an S375N substitution (Fig. 2) and displayed a 38-fold decrease in sensitivity to BMS-488043 compared to that of the consensus population (Table 3). Clone 1-8-4 contained 25 such amino acid changes, including an S375N substitution, as well as a V68A substitution, and displayed a 499-fold decrease in sensitivity (Table 3). Site-directed mutagenesis was employed on each of these clones to evaluate the effects of 68A and 375N on resistance in each background. For clone 1-8-4, back mutation to 68V increased susceptibility 10-fold, reducing the EC50 from 3,600 to 386 nM, while back mutating both positions to the consensus brought the sensitivity back to wild-type levels (EC50 = 4.8 nM, Table 3). Mutating position 375N back to Ser, so that only the V68A change was present in the 1-8-4 background, brought the EC50 down to 56 nM (Table 3). Moreover, introduction of a V68A change into clone 1-8-5 increased the EC50 to over 10 μM, while back mutating residue 375N to Ser reduced the EC50 >10-fold to 21 nM (Table 3). These analyses strongly suggest that substitutions V68A and S375N contributed to decreased viral susceptibility to BMS-488043 in this subject.
FIG. 2.
Amino acid changes in subject 1 day 8 HIV-1 envelope clones 1-8-4 and 1-8-5 from the subject 1 baseline consensus sequence using the HXB2 numbering system. Changes thought to be associated with resistance to BMS-488043 are boxed.
TABLE 3.
Effect of gp120 68V/375N on BMS-488043 sensitivitya
| Background | Amino acid at position: |
EC50 (nM) | Fold change in sensitivity compared to baseline | |
|---|---|---|---|---|
| 68 | 375 | |||
| 1-8-4 | A | N | 3,645 | 499 |
| V | N | 386 | 53 | |
| A | S | 56 | 8 | |
| V | S | 4.8 | 0.7 | |
| 1-8-5 | V | N | 278 | 38 |
| A | N | >10,000 | >1,370 | |
| V | S | 21 | 3 | |
In the context of the envelope gene from subject 1.
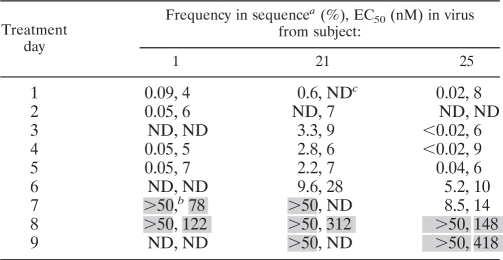
The contribution of the S375N mutation to the emergence of resistance to BMS-488043 was further analyzed by examining its appearance and correlation with sensitivity to the AI over time. A quantitative real-time PCR assay was designed to measure the percentage of the population that contained this substitution. Plasma samples from the baseline through day 9 from three subjects with emerging S375N were used (subjects 1, 21, and 25), and the total PCR population on each day was analyzed for phenotypic sensitivity to BMS-488043 (Table 4). For subject 1, the frequency was low on day 5 but on day 7 had reached the upper limit of the assay (>50%). The S375N percentage in subject 21 stayed relatively low up to day 6, when it more than quadrupled to 9.6%, and reached the upper limit on day 7. Subject 25 had a similar pattern, with a substantial increase (>100-fold) on day 6 and a smaller increase on day 7 to 8.5%, reaching the upper limit by day 8. In all cases, once S375N reached the assay ceiling, virus sensitivity greatly decreased (Table 4), leading to the conclusion that this change was largely responsible for BMS-488043 resistance development in this subject, and likely represents a sentinel of resistance to the AI in vivo, although it was not observed in vitro (23).
TABLE 4.
Emergence of S375N in viruses from subjects treated with BMS-488043
Values indicate percentages of S375N within the population.
A shaded value indicates either a breakthrough in mutant prevalence or a >10-fold increase in resistance compared with the baseline value.
ND, not done.
Effect of envelope substitution in subject baseline viral backgrounds on BMS-488043 sensitivity.
The data suggested that all of the emerging or preexisting phenotypic resistance observed, except for that of subject 19, could be explained through changes in only three amino acids in gp120, V68A, S375N/I, and M426L. In order to support this observation, day 8 functional clones from subjects 4, 10, 25, 27, and 30 were back mutated to remove these changes and the original and mutated clones were examined for susceptibility to BMS-488043 (Table 5). In each case, susceptibility was substantially increased (>32- to 791-fold), showing that these substitutions (including S375I) are the signature resistance substitutions in these clinical isolates.
TABLE 5.
Effects of signature envelope substitutions on BMS-488043 sensitivity
| Type of sensitivity change, mutagenesis, and subject | Fold change with: |
||||
|---|---|---|---|---|---|
| V68A | L116I | S375I | S375N | M426L | |
| Increase, mutant to wild type | |||||
| 19 | 52 | ||||
| 10 | >32 | ||||
| 25 | 229 | ||||
| 4 | 176 | ||||
| 27 | 791 | ||||
| 30 | >74 | ||||
| Decrease, wild type to mutant | |||||
| 15 | 28 | 20 | 39 | 155 | 1,308 |
| 22 | 6 | 3 | 166 | 61 | 259 |
| 23 | 11 | 67 | 120 | 204 | 829 |
For subject 19, we analyzed the sequence by looking for positions that were selected in vitro to encode resistance to one of a number of other attachment inhibitors (unpublished observations). From this analysis, one such possible substitution was identified as L116I. Back mutating this position in a functional day 8 clone increased susceptibility 52-fold down to single-digit nanomolar levels, suggesting that this is indeed the major resistance substitution for the virus in this subject.
In addition, baseline functional clones from three subjects (subjects 15, 22, and 23) who maintained susceptibility to BMS-488043 throughout the course of the study were used to insert each of the five individual signature substitutions into its respective envelope protein (Table 5). These recombinant envelopes were tested for BMS-488043 susceptibility and demonstrated that all of the signature substitutions decreased susceptibility to the compound in the various genetic backbones. Changes at amino acid residues 375 and 426 had the greatest effect on AI potency, decreasing susceptibility from 39- to >1,000-fold. Changes at the 68 and 116 loci had lesser but still substantial effects in the context of the envelope gene from subjects 15 and 23 but only minor effects in the context of the envelope gene in subject 22 (Table 5). Thus, the data show that in the clinic, preexisting or emerging phenotypic resistance to BMS-488043 typically occurs through changes at one of at least four different amino acid positions in the gp120 protein (Table 5) and that context can have an effect on the level of resistance (23).
Sensitivity of BMS-488043-resistant envelopes to other HIV-1 entry inhibitors.
In order to examine whether the resistance observed for the HIV-1 envelopes from these subjects was specific for AIs and not other entry inhibitors, we determined the sensitivities of eight clones containing the AI resistance signature mutations to the fusion inhibitor enfuvirtide, the CCR5 antagonist Sch C, and the CXCR4 antagonist AMD-3100 (17, 27, 28). As shown in Table 6, all subject HIV-1 envelopes maintained single-digit nanomolar sensitivity to enfuvirtide and Sch C. None of the isolates were inhibited by AMD-3100 (not shown), consistent with the expectation that these isolates used CCR5 as a coreceptor. These studies demonstrate that the effect of these substitutions in gp120 was specific for BMS-488043 and not other classes of inhibitors in these CCR5-utilizing envelopes.
TABLE 6.
Sensitivities of uncloned HIV-1 envelopes from subjects before and during BMS-488043 administration to HIV-1 entry inhibitors
| Subject | EC50, nM (n-fold change)a |
|||
|---|---|---|---|---|
| Baseline |
Day 8 |
|||
| Enfuvirtide | Sch C | Enfuvirtide | Sch C | |
| 2 | 2.2 | 3.1 | 1.0 (<1) | 1.2 (<1) |
| 4 | 3.0 | 1.4 | 6.6 (2) | 2.0 (1) |
| 10 | 2.4 | 1.4 | 1.3 (<1) | 1.4 (1) |
| 19 | 4.3 | 2.0 | 4.0 (1) | 1.0 (1) |
| 21 | 5.0 | 2.5 | 5.6 (1) | 1.4 (1) |
| 25 | 1.7 | 1.0 | 1.6 (1) | 1.1 (1) |
| 27 | 4.6 | 1.5 | 11.8 (3) | 1.7 (1) |
| 30 | 5.2 | 1.7 | 5.7 (1) | 1.4 (1) |
The n-fold change is the difference between the sensitivities of the baseline and day 8 envelopes to each inhibitor.
Modeling of BMS-488043 resistance.
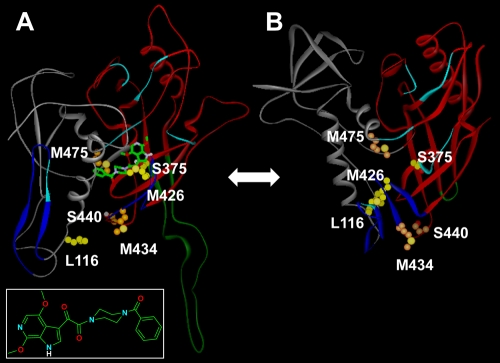
For most antiretrovirals, resistance maps at or near the docking site of the drug (15). The S375N/I signature substitutions described in this report, along with M475I (selected in vitro [23]), are located near the CD4 binding pocket and suggest a model in which the drug docks deep within this cavity (Fig. 3). In this model, BMS-488043 binds to the pre-CD4-bound conformation of gp120 and locks it so it cannot adopt a conformation that allows binding to CD4 (Fig. 3B). Such a model is supported by previous observations showing that AI can affect soluble CD4 binding to the envelope (14). The model predicts that S375 hydrogen bonds to the piperazine carbonyl of the oximide moiety, while M475 packs against the benzamide ring of the AI. Meanwhile, M426 is predicted to pack against W427, which π stacks with the azaindole ring of BMS-488043. L116, M434, and S440 (selected in vitro [23]) are outside the AI binding site, and therefore their effects on AI binding would be indirect in this model. Since the model begins at amino acid 83, it was not possible to ascertain the potential effect of the V68A mutation on BMS-488043 binding.
FIG. 3.
(A) Homology model of the BMS-488043/pre-CD4-bound gp120 complex. BMS-488043 is also shown in the box. (B) X-ray structure of CD4-bound gp120 extracted from 1g9m.pdb (19). Carbon atoms for amino acid changes observed in this study that induce resistance to BMS-488043 (L116, S375, and M426) are yellow, while changes observed during in vitro selection (23) (M434, S440, and M475.) are orange. The gp120 inner domain is gray, and the outer domain is red. The V3 loop (A) or V3 stem (B) is dark green, the bridging β sheets are dark blue, and the CD4 contact residues are light blue. BMS-488043 (inset), when bound to the pre-CD4-bound gp120 (A) structure, blocks the formation of the CD4 binding site (B).
DISCUSSION
AIs represent a novel class of HIV-1 entry inhibitors. Even though BMS-488043 is not a candidate for further development, a proof-of-concept study showed that, with monotherapy, this compound can be effective at reducing viral loads (13). Although the viruses that infected the majority of the subjects studied remained sensitive to BMS-488043 during the course of dosing with BMS-488043, emergent resistant variants associated with a viral load rebound were observed, along with clinical isolates with preexisting resistance at the baseline. The focus of this study was on the 8 of the 30 subjects in the clinical trial whose viruses showed emergent or preexisting resistance. Genotyping analyses of baseline and day 8 samples were completed and examined for established BMS-488043 resistance substitutions observed in vitro (23). Surprisingly, only three of the eight subjects' viruses had such changes. The virus from subject 1 (dosed with 800 mg BMS-488043) had an emerging V68A substitution, while those of subjects 27 (dosed with 1,800 mg BMS-488043) and 30 (placebo) both had a preexisting M426L substitution (Table 2). These analyses also revealed the presence of three novel substitutions at two positions (S375N/I and L116I) associated with decreased susceptibility to BMS-488043 in vivo. These substitutions were shown to be key residues for resistance through reverse genetics, as reversion to the consensus sequence at each position within the context of the virus envelope greatly increased susceptibility and their introduction into three highly susceptible envelope backgrounds decreased the susceptibility to BMS-488043 in each case (Table 5). Moreover, having these substitutions led to a mitigated response to the AI in the clinical study (Table 1). Thus, the data reveal five signature substitutions at four amino acid residues capable of conferring resistance to BMS-488043 in vivo (Table 5).
Previous studies have demonstrated that a tryptophan substitution for serine 375, which contacts the Phe 43 cavity, fills the cavity and predisposes gp120 to assume a CD4-bound conformation (32). In viruses with either the HXBc2 or the YU2 HIV-1 envelope glycoprotein, the S375W change resulted in dramatic levels of resistance to the earlier AI BMS-378806 (25). For the eight subjects with resistant variants, the most prevalent amino acid changes occurred at position 375, which changed from Ser to Asn in the viruses from four subjects and to Ile in one subject (Table 2). One additional subject (subject 5, who received 800 mg BMS-488043) harbored HIV-1 with S375N at both the baseline and day 8 (see Fig. S1 in the supplemental material). The EC50s for the baseline and day 8 samples from this subject in the phenotyping assay were 66 and 358 nM, respectively (Table 1), which did not meet our criteria for emergent or preexisting resistance. However, the maximum observed viral load decrease in this subject was only 0.33 log10 at day 5 of the study. In the absence of other genetic markers (see Fig. S1 in the supplemental material), we attribute the lack of an antiviral response in this subject to the S375N signature mutation at the baseline. The prevalence of resistance substitutions at the 375 locus and the magnitude of their effect on antiviral activity (Table 5) may be ascribed to its location near the CD4 binding pocket (Fig. 3). In the model presented in which BMS-488043 binds within this cavity (Fig. 3), S375 would make direct contact with the inhibitor and, consequently, a change to Asn or Ile could directly affect AI-gp120 binding characteristics. Furthermore, it seems clear that only certain substitutions can have an effect on BMS-488043 activity. The viruses from two subjects (12 and 16) had an emerging S375T substitution on day 8 (see Fig. S1 in the supplemental material). Subject 16 was in the placebo group (Table 1), but subject 12 was in the 800-mg dose group and had a maximum viral load drop of 1.53 log10 at day 10 (Table 1). Moreover, in the phenotypic sensitivity assay, the baseline (containing S375) and day 8 (containing S375T) envelopes from both subjects retained full sensitivity to BMS-488043 (Table 1), suggesting that this substitution does not induce resistance to the AI.
An analogous situation seems to operate at the 426 locus in which only the specific M426L change substantially affects BMS-488043 sensitivity and antiviral response. Subject 23 (1,800 mg BMS-488043), who responded well to the compound (maximum decrease in viral load from the baseline of 1.69 log10 copies/ml on day 9) and whose viral envelope maintained a highly sensitive phenotype throughout the study (Table 1), harbored a virus with an M426R mutation in both the baseline and day 8 samples (see Fig. S1 in the supplemental material). This change is relatively common among 2008 subtype B isolates (18.4%, http://www.hiv.lanl.gov/). Since M426 packs against W427 in the AI docking model, it seems that the M426L substitution is required to affect W427 positioning and resultant interactions with BMS-488043.
In contrast to S375 and M426, which are located at or near the AI binding residue, L116 is located outside the AI binding site, and therefore, the effect of the L116I substitution on AI binding/activity is likely to be indirect. This could explain why its effect on BMS-488043 was less robust than that observed for mutations at the other two loci (Table 5). L116 is situated at the terminus of the first α helix, proximal to the base of the V1/V2 stem. Consequently, this residue may influence the proper positioning of the V1/V2 loop, thereby facilitating inhibitor binding. Two mutations selected in vitro, M434 and S440 (23), also located outside the AI binding site (Fig. 3), may play an indirect role in neutralizing BMS-488043, in this case by stabilizing the CD4-bound conformation of gp120, as they are part of the bridging β sheet in the CD4-bound structure that is disrupted in the pre-CD4-bound gp120 model (Fig. 3).
Interestingly, only two of five substitutions selected in vitro (V68A and M426L) were selected in the clinical trial. Among the possible explanations for this disparity is the fact that the laboratory-adapted CXCR4-utilizing envelopes LAI and NL4-3 were utilized for in vitro selections. Based on the data obtained with entry inhibitors (Table 6), it seems clear that the HIV-1 harbored by trial subjects with AI resistance markers utilized CCR5 as a coreceptor. Moreover, there is substantial variability among the subject consensus envelope sequences (see Fig. S1 in the supplemental material). We suspect, therefore, that the introduction of certain changes could adversely affect viral fitness in the context of different envelope backgrounds, which could constrain the development of particular AI resistance markers. Another potential influence on AI resistance development is humoral immunity. The envelope is the primary HIV-1 target for neutralizing antibodies, and we have previously documented that AI resistance development can influence envelope immunogenicity (34). It is well documented that escape from neutralizing antibodies can affect envelope evolution (20, 26, 31), thereby potentially constraining patterns of AI resistance development. Thus, the markers reported here more realistically model BMS-488043 resistance. The rapid appearance of these mutations suggests that they existed at a low level (undetectable by conventional sequence analyses) in the virus pool of some of the patients prior to AI administration.
In summary, we have presented antiviral and resistance data on BMS-488043, a prototype AI that is the first one to be tested for antiviral activity in HIV-infected subjects. We believe that the data show that inhibition of viral attachment is a viable mechanism of action for the development of new antiviral agents. A potential shortcoming for BMS-488043 is that resistance can preexist. However, this circumstance was observed in only 4 of 30 subjects, which is a frequency consistent with the prevalence of the signature resistance markers among the 583 subtype B isolates currently in the LANL database (5 for 116I, 9 for 375I, and 47 for 426L; http://www.hiv.lanl.gov/). The fact that four subjects had emergent phenotypic/genotypic resistance after 8 days of treatment may be an indication of the suboptimal nature of this particular AI. Newer molecules with improved potency have been developed, and a prodrug of one, BMS-663068, is currently in early clinical development (ClinicalTrials.gov no. NCT01009814). The signature resistance mutations that emerged with BMS-488043 monotherapy, 68A and 375N, are also uncommon, present in only 2 and 15 of the 583 isolates, respectively, suggesting a barrier to their acquisition in the absence of a drug. These signature mutations are less common among non-subtype B, group M isolates (http://www.hiv.lanl.gov/). Most importantly, none of these changes affected sensitivity to other entry inhibitors (Table 6) and have not been associated with resistance to any other class of antiretroviral (http://hivdb.stanford.edu/). Thus, AIs could provide a novel mechanism to complement the current arsenal of antiretrovirals currently in use.
Supplementary Material
Footnotes
Published ahead of print on 15 November 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Alexander, L., et al. 2009. Inhibition of envelope-mediated CD4+-T-cell depletion by human immunodeficiency virus attachment inhibitors. Antimicrob. Agents Chemother. 53:4726-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazan, H. A., G. Alkhatib, C. C. Broder, and E. A. Berger. 1998. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J. Virol. 72:4485-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, B. R., et al. 2009. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30:1545-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, B., et al. 2005. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature 433:834-841. [DOI] [PubMed] [Google Scholar]
- 6.Colonno, R., et al. 2004. Identification of I50L as the signature atazanavir (ATV)-resistance mutation in treatment-naive HIV-1-infected patients receiving ATV-containing regimens. J. Infect. Dis. 189:1802-1810. [DOI] [PubMed] [Google Scholar]
- 7.Doms, R. W., and J. P. Moore. 2000. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 151:F9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essex, M. 1999. Human immunodeficiency viruses in the developing world. Adv. Virus Res. 53:71-88. [DOI] [PubMed] [Google Scholar]
- 9.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 10.Gong, Y. F., et al. 2000. In vitro resistance profile of the human immunodeficiency virus type 1 protease inhibitor BMS-232632. Antimicrob. Agents Chemother. 44:2319-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo, Q., et al. 2003. Biochemical and genetic characterizations of a novel human immunodeficiency virus type 1 inhibitor that blocks gp120-CD4 interactions. J. Virol. 77:10528-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hance, A. J., et al. 2001. Changes in human immunodeficiency virus type 1 populations after treatment interruption in patients failing antiretroviral therapy. J. Virol. 75:6410-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna, G. J., et al. 2011. Antiviral activity, pharmacokinetics, and safety of BMS-488043, a novel oral small-molecule HIV-1 attachment inhibitor, in HIV-1-infected subjects. Antimicrob. Agents Chemother. 55:722-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho, H. T., et al. 2006. Envelope conformational changes induced by human immunodeficiency virus type 1 attachment inhibitors prevent CD4 binding and downstream entry events. J. Virol. 80:4017-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, V. A., et al. 2009. Update of the drug resistance mutations in HIV-1: December 2009. Top. HIV Med. 17:138-145. [PubMed] [Google Scholar]
- 16.Kadow, J., H. G. Wang, and P. F. Lin. 2006. Small-molecule HIV-1 gp120 inhibitors to prevent HIV-1 entry: an emerging opportunity for drug development. Curr. Opin. Investig. Drugs 7:721-726. [PubMed] [Google Scholar]
- 17.Khan, A., J. Greenman, and S. J. Archibald. 2007. Small molecule CXCR4 chemokine receptor antagonists: developing drug candidates. Curr. Med. Chem. 14:2257-2277. [DOI] [PubMed] [Google Scholar]
- 18.Kuritzkes, D. R. 2009. HIV-1 entry inhibitors: an overview. Curr. Opin. HIV AIDS 4:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwong, P. D., et al. 2000. Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Structure 8:1329-1339. [DOI] [PubMed] [Google Scholar]
- 20.Kwong, P. D., et al. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larder, B. A., S. D. Kemp, and P. R. Harrigan. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696-699. [DOI] [PubMed] [Google Scholar]
- 22.Lin, P. F., et al. 2003. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc. Natl. Acad. Sci. U. S. A. 100:11013-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, P. F., et al. 2004. Characterization of a small molecule HIV-1 attachment inhibitor BMS-488043: virology, resistance, and mechanism of action, abstr. 534, p. 256. Program Abstr. 11th Conf. Retrovir. Oppor. Infect. 2004, San Francisco, CA.
- 24.Liu, J., A. Bartesaghi, M. J. Borgnia, G. Sapiro, and S. Subramaniam. 2008. Molecular architecture of native HIV-1 gp120 trimers. Nature 455:109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madani, N., et al. 2004. Localized changes in the gp120 envelope glycoprotein confer resistance to human immunodeficiency virus entry inhibitors BMS-806 and #155. J. Virol. 78:3742-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13(Suppl. A):S137-S162. [PubMed] [Google Scholar]
- 27.Schols, D., et al. 1997. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 186:1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starr-Spires, L. D., and R. G. Collman. 2002. HIV-1 entry and entry inhibitors as therapeutic agents. Clin. Lab. Med. 22:681-701. [DOI] [PubMed] [Google Scholar]
- 29.Tilton, J. C., and R. W. Doms. 2010. Entry inhibitors in the treatment of HIV-1 infection. Antiviral Res. 85:91-100. [DOI] [PubMed] [Google Scholar]
- 30.Wang, T., et al. 2009. Inhibitors of human immunodeficiency virus type 1 (HIV-1) attachment. 5. An evolution from indole to azaindoles leading to the discovery of 1-(4-benzoylpiperazin-1-yl)-2-(4,7-dimethoxy-1H-pyrrolo[2,3-c]pyridin-3-yl)ethane-1,2-dione (BMS-488043), a drug candidate that demonstrates antiviral activity in HIV-1-infected subjects. J. Med. Chem. 52:7778-7787. [DOI] [PubMed] [Google Scholar]
- 31.Watkins, B. A., et al. 1996. Resistance of human immunodeficiency virus type 1 to neutralization by natural antisera occurs through single amino acid substitutions that cause changes in antibody binding at multiple sites. J. Virol. 70:8431-8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang, S. H., et al. 2002. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 76:9888-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, S., et al. 2010. Protection of HIV envelope-induced neuronal cell destruction by HIV attachment inhibitors. Arch. Virol. 155:777-781. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, N., et al. 2010. Increased sensitivity of HIV variants selected by attachment inhibitors to broadly neutralizing antibodies. Virology 402:256-261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.