Abstract
Staphylococcus aureus is the leading cause of invasive and superficial human infections, is increasingly antibiotic resistant, and is therefore the target for the development of new antimicrobials. Compounds (1835F03 and targocil) were recently shown to function as bacteriostatic inhibitors of wall teichoic acid (WTA) biosynthesis in S. aureus. To assess the value of targeting WTA biosynthesis in human infection, it was therefore of interest to verify the involvement of WTA in bacterial binding to human corneal epithelial cells (HCECs) and to assess the activities of inhibitors of WTA biosynthesis against clinical isolates of methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) from cases of human keratitis. The 1835F03 MIC90s were 8 μg/ml for MSSA keratitis isolates and >32 μg/ml for MRSA keratitis isolates. The MIC90 for the analog of 1835F03, targocil, was 2 μg/ml for both MRSA and MSSA. Targocil exhibited little toxicity at concentrations near the MIC, with increased toxicity occurring at higher concentrations and with longer exposure times. Targocil activity was moderately sensitive to the presence of serum, but it inhibited extracellular and intracellular bacteria in the presence of HCECs better than vancomycin. Targocil-resistant strains exhibited a significantly reduced ability to adhere to HCECs.
Staphylococcus aureus is a leading cause of community and hospital-acquired infections of the skin, soft tissue, bloodstream, and other sites (14, 37). Compounding the problem is antibiotic resistance, which initially developed in the hospital but which has since spread to the community, where rates of methicillin-resistant S. aureus (MRSA) are now approaching those of hospitals (14, 37). Keratitis is an infection of the cornea that occurs following injury or in association with contact lens wear, and S. aureus is a leading cause (1, 4, 31). Infectious keratitis can progress rapidly and can lead to corneal scarring and loss of vision. Fluoroquinolones and cephalosporins are often used empirically for the treatment or prevention of keratitis. However, along with increased levels of methicillin resistance among isolates, resistance to cephalosporins and fluoroquinolones is increasing in parallel (1, 13). Furthermore, S. aureus recently acquired resistance to vancomycin, a drug of last resort (6, 32, 34). Because of the advance of antibiotic resistance among S. aureus isolates from cases of keratitis, as well as other anatomical sites, it was of interest to explore the potential utility of a novel, recently described inhibitor of S. aureus wall teichoic acid (WTA) biosynthesis.
The WTA biosynthesis pathway constitutes a novel target for small-molecule inhibitors, and two inhibitory compounds have recently been reported (23, 36). WTAs are phosphate-rich, highly anionic polymers that are covalently linked to the peptidoglycan cell wall of most Gram-positive pathogens. They affect the cell envelope by contributing charge/cation binding, tensile strength, rigidity, and permeability (28). Furthermore, these polymers have been suggested to play key roles in S. aureus colonization of epithelial and endothelial cells (35, 38, 39, 40, 41) and contribute to virulence by promoting the establishment and spread of infection. Therefore, targeting WTA biosynthesis could result in antimicrobials that, in addition to inhibiting microbial growth, inhibit host colonization and tissue invasion.
The S. aureus WTA polymer is composed of repeating units of ribitol-phosphate, and its biosynthesis is carried out by enzymes encoded by the tar (teichoic acid-ribitol) pathway (28, 35). The primary S. aureus WTA polymer is synthesized on a bactoprenol carrier lipid embedded in the cytoplasmic membrane. WTA assembly begins with the addition of two sugars (by TarO and TarA), followed by two glycerol-3-phosphate units (by TarB and TarF) and, finally, the polyribitol-phosphate repeat (mediated by TarL). They are then exported through an ABC transporter (TarGH) to the external surface of the membrane, where the polymer is covalently linked to peptidoglycan through an unknown mechanism (27, 35).
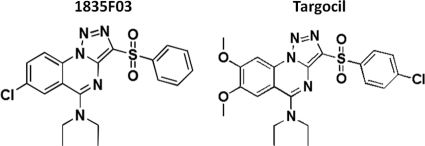
S. aureus tarO and tarA null mutants are viable in vitro; however, genes downstream of tarA in the WTA pathway cannot be deleted unless tarO (or tarA) is deleted first (10). This mixed gene dispensability pattern implies that blocking late-acting WTA biosynthetic enzymes after flux into the pathway has been initiated is deleterious to bacterial growth, likely through the accumulation of toxic intermediates (or the depletion of the peptidoglycan carrier lipid bactoprenol). A cell-based, pathway-specific high-throughput screen exploiting this mixed gene dispensability was used to identify the first small molecule to target WTA biosynthesis, 1835F03 (36) (Fig. 1). The target of 1835F03 was identified to be tarG, the transmembrane component of the ABC transporter that exports WTA to the cell surface (36). This compound inhibits the growth of select S. aureus strains, including MRSA (36). A structure-activity relationship study of 1835F03 led to the discovery of an analog, targocil, that is 10-fold more potent (23) (Fig. 1). In this study, the efficacies of these WTA inhibitors for a panel of MSSA and MRSA isolates from cases of bacterial keratitis, postantibiotic effect (PAE), and synergistic activities were assessed. The compounds were tested further to assess activity in the presence of serum and human corneal epithelial cells (HCECs) and for toxicity to corneal epithelial cells. It was found that targocil is more effective than vancomycin in blocking intracellular S. aureus growth. Moreover, resistant mutants that arise upon selection with these compounds are highly attenuated and show reduced epithelial cell binding and invasion.
FIG. 1.
Chemical structures of 1835F03 and targocil.
MATERIALS AND METHODS
Bacteria and agents.
Eighteen S. aureus keratitis isolates, including methicillin-sensitive and -resistant strains, were culled from the Massachusetts Eye and Ear Infirmary collection and tested. In addition to clinical isolates, the S. aureus strains listed in Table 1 were used for in vitro assays. S. aureus was grown in tryptic soy broth (TSB) at 37°C unless otherwise noted. Compounds 1835F03 and targocil were dissolved in 100% dimethyl sulfoxide (DMSO) and stored at −20°C. Final concentrations of DMSO were adjusted to 1% in all assays unless otherwise stated (vehicle controls consisted of 1% DMSO in the appropriate medium). Antibacterial agents used as controls were purchased from Sigma-Aldrich (Poole, United Kingdom), with the exception of gentamicin (EMD Chemicals, Darmstadt, Germany).
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype and/or phenotype | Reference or source |
|---|---|---|
| RN4220 | Mutant of NCTC 8325-4 that accepts foreign DNA partial agr defect | 22 |
| Newman | Clinical isolates, methicillin-susceptible strain | 11 |
| MW2 | Clinical isolates, methicillin-resistant strain | 5 |
| MG2375 | Keratitis isolates, methicillin-susceptible strain | This study |
| MG2389 | Keratitis isolates, methicillin-resistant strain | This study |
| RN4220 ΔtarO | RN4220 ΔtarO | 15 |
| Newman ΔtarO | Newman ΔtarO | 15 |
| Pspac tarO | RN4220 ΔtarO::tetL[geh::(pCL25int-PspactarO)Emr] | 35 |
Cell culture.
In this study, we used simian virus 40-immortalized HCECs. The cells were maintained in defined keratinocyte serum-free medium (Invitrogen, Carlsbad, CA) in a humidified 5% CO2 incubator at 37°C. Before bacterial infection or toxicity tests, HCECs (2 × 104 or 1 × 105 cells/well) were cultured in 24- or 96-well plates to 95% confluence.
MIC tests.
The in vitro susceptibility (MIC) tests were conducted using the Clinical and Laboratory Standards Institute (CLSI) broth microplate assay guidelines (7). To determine the effects of serum on antimicrobial properties in vitro, MIC determinations were performed in the presence of 25% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA).
Measurement of PAE.
Log-phase strains MG2375, MG2389, Newman, and MW2 were collected and adjusted to a concentration of 2 × 108 CFU/ml. After the treatment of bacterial cultures with targocil at 10× MIC for 1 h, the cells were diluted 1:1,000 in fresh medium and then incubated and plated at the appropriate time points for viability determination. The PAE was calculated by the standard equation T − C, where T is the time required for the CFU count in the test culture to increase 10-fold above the count observed immediately after drug removal, and C is the time required for the count of the untreated control to increase 10-fold under the same conditions (25).
Synergistic activities.
A standard checkerboard assay was performed by standard methods (25) to test for possible synergy with other antibiotics. The fractional inhibitory concentration (FIC) indexes for MRSA and MSSA strains MG2375, MG2389, Newman, and MW2 were calculated as described previously (25). An FIC index of less than or equal to 0.5 is evidence of synergism, an FIC index of between 0.5 and 2 is evidence of indifference, and an FIC index equal to or more than 2 reflects antagonism (25).
Corneal epithelial toxicity and hemolytic activity.
HCECs in 96-well culture plates were exposed to targocil at various concentrations (5, 10, 20, and 40 μg/ml) for 6, 15, and 25 h. Two separate experiments were performed on five individual samples. Cell culture medium controls with or without 1% DMSO were also examined. Viable epithelial cells were quantified by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt, assay (Promega, Madison, WI) and expressed as (cell viability in culture medium, including drug/cell viability in culture medium) × 100 (9). The hemolytic activity of targocil was determined using human red blood cells (RBCs). Serial dilutions (2-fold) of targocil were made in 0.9% NaCl. RBCs were then diluted (2% in 0.9% [vol/vol] NaCl), and aliquots of this suspension (150 μl) were distributed in 96-well microtiter plates. Then, 150 μl of medium containing targocil was added and the plates were incubated for 1 h at 37°C, followed by centrifugation at 13,000 × g for 5 min. Aliquots (100 μl) of the supernatant were then transferred to 96-well microtiter plates, and hemoglobin release was measured at 560 nm (GeNios; Tecan, Switzerland). Percent hemolysis was calculated as [(A560 of the targocil solution − A560 of 0.9% NaCl)/(A560 of 0.1% Triton X-100 − A560 of 0.9% NaCl)] × 100.
Effect of human corneal epithelial cells on antibiotic activity.
To test whether the metabolic activity of human corneal epithelial cells or their physical presence affected the activity of the compound, dilutions of S. aureus strains Newman and MG2375 (approximately 105 CFU of stationary-phase bacteria in 1 ml of medium) were aliquoted into 24-well culture plates with or without HCEC monolayers. The culture medium included 10 μg/ml targocil or 1% DMSO solvent control. Following incubation for 6 h, cells were detached with a trypsin-EDTA (0.25%)-Triton X-100 (0.025%) mixture, and viable bacteria in each well were measured by plating serial dilutions.
To check the efficacy of targocil against internalized bacteria, S. aureus Newman, MG2375, and MG2389 (approximately 108 CFU of stationary-phase bacteria in 1 ml of medium) were inoculated into wells containing HCEC monolayers. Following incubation for 1 h and washing three times with medium, monolayers were covered with 1 ml of medium containing 100 μg of gentamicin and incubated for an additional hour to kill residual extracellular bacteria. Monolayers were then washed three times, and 1 ml of medium containing 1% DMSO, 10 μg/ml targocil in 1% DMSO, or 10 μg/ml vancomycin was added. After 6 h of incubation, internalized bacteria were counted by serial dilution of cell lysates. Five individual wells were run for each group, and experiments were performed in duplicate.
Adhesion and internalization of S. aureus by human corneal epithelial cells.
Adhesion and internalization assays were performed essentially as described previously (24). In brief, for determination of adhesion, bacteria (mid-log phase or stationary phase) were washed twice with culture medium and were then applied to HCEC monolayers (approximately 108 CFU in 1 ml of medium). Following incubation for 1 h, epithelial cell cultures were washed and disaggregated, and adherent bacteria were counted by serial dilution. For determination of internalization, bacteria were added to cells as described above, and following incubation for 1 h, cells were washed, covered with medium containing 100 μg/ml gentamicin, and incubated for an additional hour to kill residual extracellular bacteria, prior to epithelial cell disruption and enumeration of bacteria. The following equations were used for estimation of adhesion and internalization: growth index = total number of bacteria at harvest/number of bacteria initially added; percent adhesion = (number of attached bacteria/number of bacteria initially added) × 100; relative adhesion = (percent experimental adhesion/percent adhesion in control); percent invasion = (number of bacteria internalized/number of bacteria initially added) × 100; corrected invasion = percent invasion/growth index; and relative invasion = (corrected invasion of experimental/corrected invasion of control) × 100. Five samples were run for each group, and experiments were performed in duplicate.
Selection of targocil survival mutants.
S. aureus strains Newman, MG2375, and MG2389 (approximately 105 CFU of stationary-phase bacteria in 10 ml of medium) were grown with shaking at 37°C in the presence of either vehicle alone (1% DMSO) or 5 μg/ml of targocil. After 36 h, the population surviving targocil treatment was inoculated into fresh liquid culture medium containing either vehicle or 5 μg/ml of targocil and was subsequently used in adhesion assays. Presumed mutants of MG2375 that grew despite the added targocil were plated on brain heart infusion agar, and three colonies were randomly picked and cultured. These purified presumptive resistant mutant cultures were then evaluated for adhesion, targocil sensitivity, and WTA production, and their tar operons were resequenced as described previously (36).
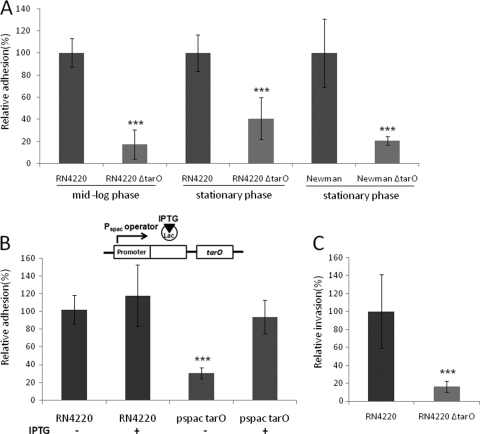
HCEC adhesion and invasion of tarO-null mutants.
To assess the extent to which wall teichoic acids contributed to human corneal epithelial cell binding, RN4220 ΔtarO or Newman ΔtarO (mid-log phase or stationary phase), along with parental strains, were applied to HCEC monolayers, and the ability to adhere to or invade HCECs was tested. Additionally, RN4220 PspactarO, a strain that expresses tarO under the control of an isopropyl-β-d-1-thiogalactopyranoside (IPTG)-inducible promoter (36), was included in the adhesion assay to prove the role of tarO. Dilutions of an overnight culture of S. aureus RN4220 or RN4220(PspactarO) were applied to HCECs with or without IPTG and tested for adhesion and invasion.
Statistical analysis.
Data were analyzed by Student's t test for significance. P values of <0.05 were considered significant.
RESULTS
In vitro antibacterial activity.
MICs of targocil against S. aureus strains Newman, MW2, MG2375, and MG2389 were 1 μg/ml for all strains. Targocil showed excellent activity against S. aureus isolates from suspected cases of bacterial keratitis, including both MSSA and MRSA isolates, with MICs that ranged from 1 to 2 μg/ml (Table 2). Targocil, a derivative of 1835F03 engineered for greater activity, exhibited better activity against all keratitis isolates than the original lead compound, 1835F03. As shown in Table 2, bovine serum exhibited a detectable but moderate inhibitory effect on the in vitro antimicrobial activities of both 1835F03 and targocil, increasing the MICs of both by 4- to 8-fold.
TABLE 2.
In vitro activities of wall teichoic acid inhibitors against keratitis isolates
| Organism (no. of isolates) and growth condition | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|
| 1835F03 |
Targocil |
|||||
| 50% | 90% | Range | 50% | 90% | Range | |
| S. aureus, methicillin susceptible (12) | ||||||
| CAMHBa | 8 | 8 | 8->32 | 1 | 2 | 1-2 |
| CAMHB + 25% bovine serum | >32 | >32 | >32 | 8 | 8 | 4-8 |
| S. aureus, methicillin resistant (6) | ||||||
| CAMHB | 8 | >32 | 4->32 | 1 | 2 | 1-2 |
| CAMHB + 25% bovine serum | >32 | >32 | >32 | 8 | 8 | 4-8 |
CAMHB, cation-adjusted Mueller-Hinton broth.
Postantibiotic effect.
The PAE of targocil against strains Newman, MW2, MG2375, and MG2389 was examined in vitro. Little PAE was seen using strains MW2, MG2375, and MG2389, but a short (0.5-h) PAE was observed with strain Newman.
Synergy.
A standard broth microplate checkerboard synergy assay was conducted to determine the extent to which targocil and antibiotics of other classes, including vancomycin, ciprofloxacin, methicillin, and gentamicin, interact as detected using S. aureus strains Newman, MW2, MG2375, and MG2389. Targocil showed synergistic activity with methicillin, with an FIC index of less than 0.4. No drug interactions were observed between targocil and representatives of the other classes of antibiotics tested, with FIC indices ranging from 1 to 2.
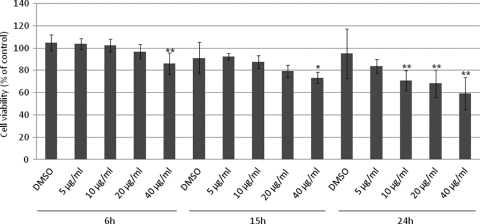
Toxicity.
Figure 2 shows the toxicity of targocil against corneal epithelial cells. Compared to the vehicle alone, targocil at 5 μg/ml exhibited little toxicity for HCECs, even after 24 h of exposure. However, 40 μg/ml targocil showed toxicity at all time points tested. Targocil exhibits detectable HCEC toxicity in a concentration- and exposure-time-dependent manner. Because modest toxicity was observed, the ability of targocil at 50 μg/ml (or 1% DMSO control) to lyse erythrocytes was tested by measuring hemoglobin release. Targocil treatment resulted in release of 3.96% of the amount of hemoglobin released by the control detergent, and DMSO induced release of 3.95%, indicating that the low-level toxicity of targocil does not result from membrane damage to the point of permeability for hemoglobin.
FIG. 2.
Toxicity of targocil in HCECs. Data represent means ± standard errors (n = 10). *, P < 0.05; **, P < 0.01 (in comparison with the results with 1% DMSO).
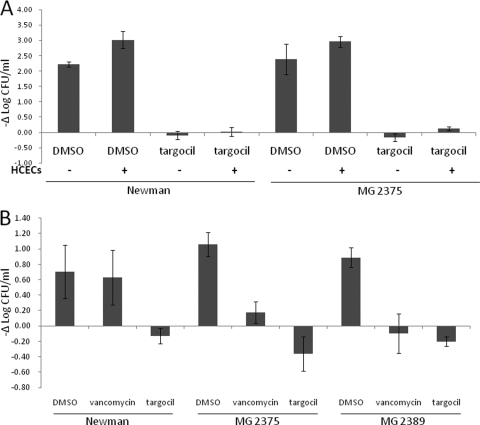
In vitro antibacterial activity in the presence of HCECs.
The antibacterial activity of targocil in the presence of HCECs was examined. Both strains Newman and MG2375 grew better in the presence of HCECs (Fig. 3 A). Targocil at levels equal to 10× MIC in vitro readily inhibited growth of Newman and MG2375 in the presence of HCECs (Fig. 3A). The efficacy of targocil against internalized bacteria was tested by measuring the number of viable internalized bacteria following treatment with 1% DMSO or 10× MIC of vancomycin or targocil. Figure 3B shows the change of log10 CFU/ml of internalized bacteria after 6 h of incubation. Strains Newman, MG2375, and MG2389 treated with medium containing 1% DMSO persisted within corneal epithelial cells. Vancomycin inhibited the intracellular growth of strains MG2375 and MG2389 but not that of strain Newman. In contrast, targocil reduced the number of viable bacteria inside the HCECs for all strains tested.
FIG. 3.
Efficacy of wall teichoic acid inhibitor in the presence of corneal epithelial cells. (A) Growth changes (−Δlog CFU/ml) of strains Newman and MG2375 after a 6-h incubation with 1% DMSO or 10 μg/ml targocil in the presence or absence of HCECs are shown. (B) Growth changes (−Δlog CFU/ml) of internalized bacteria (Newman, MG2375, and MG2389) which were treated with 1% DMSO, 10 μg/ml vancomycin, or 10 μg/ml targocil for 6 h are shown. Data represent mean values ± standard errors of the means (n = 10).
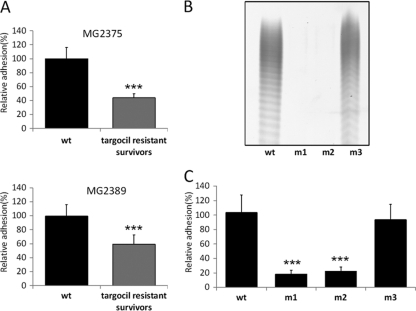
Adherence of targocil escape mutants to HCECs.
Since the first two genes in the WTA biosynthesis pathway are nonessential and inactivating them confers resistance to the WTA inhibitor, it seemed likely that a targocil-resistant mutant population would consist largely of mutations in tarO or tarA. It was also possible that mutations that would generate strains still capable of synthesizing WTA but perhaps at altered amounts or localized differently occurred elsewhere, such as in the molecular target of the WTA inhibitor (tarG) (35). To determine whether targocil-resistant S. aureus mutants exhibited altered HCEC adherence, approximately 105 CFU of two keratitis isolates (MG2375 and MG2389) was treated with 5 μg/ml of targocil to select for the development of resistance. The resistant population was then cultured and tested in adherence assays. Mixed populations of targocil-resistant mutants from both S. aureus strains were found to be greatly attenuated in their ability to attach to HCECs compared to the parent wild-type strains (Fig. 4 A). To determine whether targocil resistance resulted in a loss of WTA biosynthesis, we purified resistant colonies of MG2375 and tested them for targocil sensitivity, WTA production, and tar gene mutations. The MICs of targocil for all mutants were >32 μg/ml. Mutants 1 and 2 (m1 and m2, respectively) did not produce WTA, but mutant 3 (m3) was WTA positive (WTA+) (Fig, 4B). We observed that mutants 1 and 2 contained a unique point mutation in tarA (TarA R229C), while mutant 3 contained a unique point mutation in tarG (TarG S204T). Mutants having a point mutation in tarA (m1 and m2) exhibited a significant reduction in HCEC adherence. However, the mutant containing a point mutation in tarG (m3) behaved similarly to the original strain (Fig. 4C). The extent of reduction in the adherence of spontaneous tarA mutants was similar to that observed for the strains with specific deletions engineered into the tarO gene. Figure 5 A shows the adhesion of tarO-null mutants to HCECs. Both RN4220 and Newman ΔtarO mutants were attenuated in their ability to attach to HCECs compared to the parental strains, independent of growth phase. Using a strain with an IPTG-inducible promoter controlling the expression of tarO, we confirmed the role of WTA in adhesion. The adhesion of the wild-type strain was not influenced by the presence of IPTG. The Pspac strain without IPTG induction exhibited reduced binding, whereas treatment with IPTG (WTA+) restored S. aureus binding to HCECs (Fig. 5B). The RN4220 ΔtarO mutant showed a similar reduction in its ability to invade HCECs compared to that of RN4220 (Fig. 5C).
FIG. 4.
Adhesion of targocil-resistant bacteria to HCECs. (A) The levels of adhesion to HCECs of mixed populations of mutants that arise after prolonged incubation in targocil in both MG2375 and MG2389 are shown. (B) WTA PAGE analysis of the targocil-resistant mutants and wild type (wt). (C) The relative levels of adhesion of purified targocil-resistant mutants (generated from MG2375) to HCECs are shown. Data represent means ± standard errors (n = 10). (A and C) ***, P < 0.001 (in comparison with the results for the wild type).
FIG. 5.
Role of wall teichoic acids in adhesion to or invasion of corneal epithelial cells. (A) Adhesion of HCECs by WTA-deficient bacteria. (B) Cartoon depicting the regulation of tarO by lac repressor binding to the Pspac operator. Induction of tarO occurs upon addition of IPTG. Adhesion of RN4220 and the IPTG-inducible tarO strain in the absence and presence of IPTG to HCECs is shown. (C) Invasion of HCECs by RN4220 ΔtarO. Data represent means ± standard errors (n = 10). ***, P < 0.001 (in comparison with the results for the parental strain).
DISCUSSION
Methicillin resistance in S. aureus, a leading cause of skin, soft tissue, and invasive infections, is on the rise in the community in large part due to the proliferation of the infectious USA300 lineage (37). A population review conducted in three communities showed the annual incidence of community-acquired MRSA during 2001 and 2002 to be 18 to 25.7/100,000 population (14); most community-acquired MRSA isolates were associated with clinically relevant infections, and 23% of patients required hospitalization. This necessitates the development of new anti-MRSA therapies for both hospital and community-acquired infections.
Keratitis associated with contact lens wear or ocular injury is generally a community-acquired infection and reflects the growing antibiotic-resistant phenotypes observed in the community. Worldwide, 125 million people use contact lenses for either corrective, cosmetic, or therapeutic reasons (20). Additionally, keratoplasty and refractive surgery create surgical alterations to the cornea, mostly in the form of laser-assisted in situ keratomileusis (LASIK) surgery. S. aureus is a leading pathogen of infectious keratitis associated with contact lens wear, trauma, and corneal surgery, because it is a commensal of the adjacent skin and mucosa and can easily contaminate the surface of the eye (4). Topical antibiotics are sometimes used prophylactically in corneal surgery, but there is concern that this practice can lead to the appearance of drug-resistant S. aureus on the external surfaces, and infections with these strains are much more difficult to treat (18).
A new strategy for addressing the problem of antibiotic resistance is to develop antimicrobials that select for mutants that exhibit a profound attenuation. The biosynthesis of WTA in S. aureus offers an opportunity to explore this unconventional strategy (30, 38). WTA biosynthetic genes downstream of tarO and tarA are essential, except in a tarO or tarA mutant background (10, 27). The WTA biosynthesis inhibitors tested here target the conditionally essential WTA enzyme tarG (36). Using a limited number of strains, the in vitro sensitivity of WTA biosynthesis inhibitors against S. aureus was initially reported to be excellent. In this study, we examined the MICs of 1835F03 and targocil against 18 S. aureus isolates from suspected cases of keratitis using standard CLSI methods. Although the MICs for the lead compound 1835F03 are more than 8 μg/ml and in some strains exceed 32 μg/ml, the MICs of targocil, an analog of 1835F03, range from 1 to 2 μg/ml even against MRSA strains. However, the antimicrobial activities of both WTA inhibitors were reduced in the presence of 25% bovine serum. Since these compounds are hydrophobic molecules, they may have a tendency to bind to serum, thereby shifting their MICs (17).
Because of this promise, it was of interest to evaluate other characteristics of targocil. We tested both its PAE and its synergistic interactions with other antibiotics. Because there is little postantibiotic effect, it is likely that targocil does not extensively damage bacterial cells. PAE is thought to be related to the degree of cellular damage done by an antibiotic (25), such that the more damaged that the cell is, the longer the time needed for repair. Targocil must be in contact with the bacteria for an extended period of time to inhibit bacterial growth continuously. It has been suggested that targocil blocks WTA assembly and ties up bactoprenol (a limiting substrate in cells and the same carrier used for peptidoglycan biosynthesis) (36). This creates the prospect that other drugs that reduce the availability of this lipid carrier (bactoprenol) or decrease cell wall synthesis may exhibit synergy with targocil. We observed a synergistic effect with methicillin against the tested strains (MG2375, MG2389, Newman, and MW2). It was reported in 1994 that insertional inactivation of a gene of unknown function sensitized MRSA strains to methicillin (26). The gene was subsequently identified as tarO, which is responsible for the first step of WTA biosynthesis, and recent studies have established that WTA expression is necessary for high-level methicillin resistance (4a). It has been suggested that WTAs, which are highly abundant on the cell surface, may play a role in the stable assembly of the peptidoglycan biosynthetic machinery, and their absence may thus disrupt the function of the resistant transpeptidase. These data suggest that combination therapies consisting of a WTA inhibitor and a beta-lactam may be beneficial.
The toxicity of this new class of drugs had yet to be examined. In this study, toxicity of targocil against corneal epithelial cells was found to increase in a concentration- and exposure-time-dependent manner. Toxic effects were seen only at concentrations greater than 5 times above the MIC values. However, the concentration of 10 μg/ml significantly altered the viability of epithelial cells, and the MIC in serum was approximately 8 μg/ml. The margin between nontoxic and effective drug concentrations at 24 h is small, but it could still be clinically acceptable if these compounds are used topically in ocular infections.
Because the antimicrobial activity of targocil is reduced in the presence of serum, we sought to test whether viable epithelial cells affected activity, potentially through adsorption, uptake, or metabolism. No loss of activity was observed, and it was further noted that targocil effectively inhibited the growth of internalized bacteria. This indicates that the drug can penetrate into the eukaryotic cell by an undetermined mechanism without being inhibited by cytoplasmic proteins. Interestingly, the number of bacteria present following treatment with targocil (which is a bacteriostatic agent in vitro) decreases in HCECs. Shiratsuchi et al. (33) have demonstrated that the concentrations of superoxide, which can kill engulfed bacteria, were increased in macrophages incubated with WTA-deficient but not wild-type S. aureus. Thus, one explanation for the observed decrease in S. aureus viability may be that bacteria treated with targocil and inhibited in WTA production are weakened against superoxide produced in epithelial cells. In contrast, vancomycin, a drug of last resort for MRSA infection, did little to inhibit growth of the internalized S. aureus strain Newman, consistent with previous observations (3). Since bacterial invasion may contribute to persistence of bacteria at the site of infection and treatment failure, targocil may represent an option for treating infections, such as keratitis, that fail with other treatments less capable of penetrating the epithelial cell.
Mutations in the targets of many antibiotics lead to bacteria having reduced fitness (2). Nevertheless, resistant mutants can still give rise to chronic infections in a clinical setting, in part because compensatory mutations arise. Targocil treatment at least in part generates mutants that lack normal representation of WTA in the cell wall and that are highly attenuated. Since bacterial adhesion to the epithelial surface is a prerequisite for skin and soft tissue infections, including keratitis, and WTAs are necessary for the colonization of epithelial tissues in animal models (38), WTA biosynthesis, in addition to being a conditionally required activity, also represents a virulence target. We found that targocil-resistant populations adhere poorly to HCECs relative to control bacteria. Bacteria that survive following targocil treatment belong to one of at least two classes: mutants that have unique mutations in tarA or in tarG (36). The WTA-null tarA mutants show less of an ability to adhere to HCECs; however, the tarG mutants do not show reduced adhesion. Although little is known about the exact ratio of mutant populations in the targocil-resistant bacteria, the adhesion ability of mixed populations of mutant bacteria was also decreased. Although the mutation frequency of S. aureus for targocil resistance is higher than most antibiotics (23), this is most likely due to the fact that two classes of mutations can relieve the inhibitory effects of targocil. This study shows that once WTA-null mutants such as tarO and tarA mutants appear, they have limited ability to attach to or invade epithelial cells. Generally, S. aureus surface proteins such as fibronectin-binding proteins (FnBPs) or clumping factor A protein play critical roles in bacterial adhesion to or invasion of epithelial cells (12, 19, 29). Although truncation of FnBPs in the Newman strain leads to deficient adherence due to loss of the cell wall anchor function (16), the tarO mutant of Newman exhibits less adhesion to HCECs than the parental strain. One interpretation is that WTA could be involved in target cell binding along with surface proteins (8). Since WTAs are abundant, highly negatively charged, and embedded in peptidoglycan, an alternative explanation is that WTAs affect how surface proteins fold or are presented. Irrespective of the ultimate mechanism, S. aureus WTAs directly or indirectly play a critical role in bacterial adhesion and invasion, which are key to S. aureus infection and persistence. Thus, targocil treatment elicits mutants that lack a virulence factor critical for adhesion and invasion. Additionally, Kohler et al. (21) demonstrated that WTAs confer resistance to antimicrobial fatty acids. These fatty acids exist in tears and other secretions and may contribute to the efficacy of a WTA inhibitor as an anti-infective treatment for the ocular surface or other wet mucosal site.
Targocil represents an example of a new class of compounds that first inhibit bacterial growth and second inhibit bacterial virulence. This combination effectively channels the evolution of a pathogen toward a nonpathogenic strain. It is not known whether compensatory mutations that allow infection will arise in mutant strains that no longer express a critical virulence factor such as WTAs. This issue is of considerable theoretical interest since current arguments in favor of virulence factors as antimicrobial targets rest on the unproven idea that there will be minimal pressure for the development of resistant mutants that are pathogenic.
The work reported here evaluates the efficacy of novel compounds that target the WTA biosynthetic pathway in S. aureus isolates from keratitis. Because compounds that inhibit this pathway elicit mutants in which WTAs are not expressed, they can be regarded as functioning both as antibiotics and as anti-virulence factor agents. Although targocil itself is specific for S. aureus, targeting conditionally essential enzymes linked to virulence pathways represents a previously unexplored strategy for antibiotics that may help to combat the problem of bacterial resistance. Thus, use of these compounds could represent a viable strategy for treating S. aureus keratitis infections and other infections that involve S. aureus adherence and/or internalization. Further investigation will be necessary to determine the efficacies of these compounds in vivo and their potential for use in clinical cases.
Acknowledgments
This work was supported by PHHS grants EY017381 and EY008289 to M.S.G., Harvard-wide project AI083214, and GM078477 to S.W. Fellowship support included F3178727 to J.G.S. and F32AI084316 to J.C. and support from the Uehara Memorial Foundation and the Japanese Eye Bank Society to T.S.
We thank Timothy Meredith for the Pspac taro strain and helpful discussions. We also thank Irmgard Behlau for providing clinical isolates.
Footnotes
Published ahead of print on 22 November 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Alexandrakis, G., E. C. Alfonso, and D. Miller. 2000. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 107:1497-1502. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, D. I., and B. R. Levin. 1999. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2:489-493. [DOI] [PubMed] [Google Scholar]
- 3.Barcia-Macay, M., C. Seral, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourcier, T., F. Thomas, V. Borderie, C. Chaumeil, and L. Laroche. 2003. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 87:834-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Campbell, J., et al. 2010. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem. Biol. [Epub ahead of print.] doi: 10.1021/cb100269f. [DOI] [PMC free article] [PubMed]
- 5.Centers for Disease Control and Prevention. 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus: Minnesota and North Dakota, 1997-1999. MMWR Morb. Mortal. Wkly. Rep. 48:707-710. [PubMed] [Google Scholar]
- 6.Chang, S., et al. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. CLSI M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Corrigan, R. M., H. Miajlovic, and T. J. Foster. 2009. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cory, A. H., T. C. Owen, J. A. Barltrop, and J. G. Cory. 1991. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 3:207-212. [DOI] [PubMed] [Google Scholar]
- 10.D'Elia, M. A., et al. 2006. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J. Bacteriol. 188:4183-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase; mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 12.Foster, T. J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 13.Freidlin, J., et al. 2007. Spectrum of eye disease caused by methicillin-resistant Staphylococcus aureus. Am. J. Ophthalmol. 144:313-315. [DOI] [PubMed] [Google Scholar]
- 14.Fridkin, S. K., et al. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436-1444. [DOI] [PubMed] [Google Scholar]
- 15.Grundling, A., and O. Schneewind. 2006. Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus, J. Bacteriol. 188:2463-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundmeier, M., et al. 2004. Truncation of fibronectin-binding proteins in Staphylococcus aureus strain Newman leads to deficient adherence and host cell invasion due to loss of the cell wall anchor function. Infect. Immun. 72:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gualtieri, M., F. Baneres-Roquet, P. Villain-Guillot, M. Pugniere, and J. P. Leonetti. 2009. The antibiotics in the chemical space. Curr. Med. Chem. 16:390-393. [DOI] [PubMed] [Google Scholar]
- 18.Iihara, H., et al. 2006. Emerging multiple mutations and high-level fluoroquinolone resistance in methicillin-resistant Staphylococcus aureus isolated from ocular infections. Diagn. Microbiol. Infect. Dis. 56:297-303. [DOI] [PubMed] [Google Scholar]
- 19.Jett, B. D., and M. S. Gilmore. 2002. Internalization of Staphylococcus aureus by human corneal epithelial cells: role of bacterial fibronectin-binding protein and host cell factors. Infect. Immun. 70:4697-4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Key, J. E. 2007. Development of contact lenses and their worldwide use. Eye Contact Lens 33:343-345. [DOI] [PubMed] [Google Scholar]
- 21.Kohler, T., C. Weidenmaier, and A. Peschel. 2009. Wall teichoic acid protects Staphylococcus aureus against antimicrobial fatty acids from human skin. J. Bacteriol. 191:4482-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreiswirth, B. N., et al. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 23.Lee, K., J. Campbell, J. G. Swoboda, G. D. Cuny, and S. Walker. 2010. Development of improved inhibitors of wall teichoic acid biosynthesis with potent activity against Staphylococcus aureus. Bioorg. Med. Chem. Lett. 20:1767-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang, X., and Y. Ji. 2007. Comparative analysis of staphylococcal adhesion and internalization by epithelial cells. Methods Mol. Biol. 391:145-151. [DOI] [PubMed] [Google Scholar]
- 25.Lorian, V. 1996. Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, MD.
- 26.Maki, H., T. Yamaguchi, and K. Murakami. 1994. Cloning and characterization of a gene affecting the methicillin resistance level and the autolysis rate in Staphylococcus aureus. J. Bacteriol. 176:4993-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meredith, T. C., J. G. Swoboda, and S. Walker. 2008. Late-stage polyribitol phosphate wall teichoic acid biosynthesis in Staphylococcus aureus. J. Bacteriol. 190:3046-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien, L. M., E. J. Walsh, R. C. Massey, S. J. Peacock, and T. J. Foster. 2002. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell. Microbiol. 4:759-770. [DOI] [PubMed] [Google Scholar]
- 30.Ohlsen, K., and U. Lorenz. 2007. Novel targets for antibiotics in Staphylococcus aureus. Future Microbiol. 2:655-666. [DOI] [PubMed] [Google Scholar]
- 31.Ormerod, L. D., et al. 1987. Epidemiology of microbial keratitis in southern California. A multivariate analysis. Ophthalmology 94:1322-1333. [DOI] [PubMed] [Google Scholar]
- 32.Peterson, D. L. 1999. Vancomycin-resistant Staphylococcus aureus. Infect. Med. 16:235-238. [Google Scholar]
- 33.Shiratsuchi, A., et al. 2010. Auxiliary role for d-alanylated wall teichoic acid in Toll-like receptor 2-mediated survival of Staphylococcus aureus in macrophages. Immunology 129:268-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sievert, D. M., et al. 2008. Vancomycin-resistant Staphylococcus aureus in the United States, 2002-2006. Clin. Infect. Dis. 46:668-674. [DOI] [PubMed] [Google Scholar]
- 35.Swoboda, J. G., J. Campbell, T. C. Meredith, and S. Walker. 2010. Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem 11:35-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swoboda, J. G., et al. 2009. Discovery of a small molecule that blocks wall teichoic acid biosynthesis in Staphylococcus aureus. ACS Chem. Biol. 4:875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenover, F. C., and R. V. Goering. 2009. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J. Antimicrob. Chemother. 64:441-446. [DOI] [PubMed] [Google Scholar]
- 38.Weidenmaier, C., et al. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10:243-245. [DOI] [PubMed] [Google Scholar]
- 39.Weidenmaier, C., et al. 2008. Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int. J. Med. Microbiol. 298:505-513. [DOI] [PubMed] [Google Scholar]
- 40.Weidenmaier, C., and A. Peschel. 2008. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 6:276-287. [DOI] [PubMed] [Google Scholar]
- 41.Weidenmaier, C., et al. 2005. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 191:1771-1777. [DOI] [PubMed] [Google Scholar]