Abstract
Doripenem is a carbapenem with potent broad-spectrum activity against Gram-negative pathogens, including antibiotic-resistant Enterobacteriaceae. As the incidence of extended-spectrum β-lactamase (ESBL)-producing Gram-negative bacilli is increasing, it was of interest to examine the in vivo comparative efficacy of doripenem, imipenem, and meropenem against a Klebsiella pneumoniae isolate expressing the TEM-26 ESBL enzyme. In a murine lethal lower respiratory infection model, doripenem reduced the Klebsiella lung burden by 2 log10 CFU/g lung tissue over the first 48 h of the infection. Treatment of mice with meropenem or imipenem yielded reductions of approximately 1.5 log10 CFU/g during this time period. Seven days postinfection, Klebsiella titers in the lungs of treated mice decreased an additional 2 log10 CFU/g relative to those in the lungs of untreated control animals. Lipopolysaccharide (LPS) endotoxin release assays indicated that 6 h postinfection, meropenem- and imipenem-treated animals had 10-fold more endotoxin in lung homogenates and sera than doripenem-treated mice. Following doripenem treatment, the maximum endotoxin release postinfection (6 h) was 53,000 endotoxin units (EU)/ml, which was 2.7- and 6-fold lower than imipenem or meropenem-treated animals, respectively. While the levels of several proinflammatory cytokines increased in both the lungs and sera following intranasal K. pneumoniae inoculation, doripenem treatment, but not meropenem or imipenem treatment, resulted in significantly increased interleukin 6 levels in lung homogenates relative to those in lung homogenates of untreated controls, which may contribute to enhanced neutrophil killing of bacteria in the lung. Histological examination of tissue sections indicated less overall inflammation and tissue damage in doripenem-treated mice, consistent with improved antibacterial efficacy, reduced LPS endotoxin release, and the observed cytokine induction profile.
Bacterial infections of the lower respiratory tract continue to be a major cause of morbidity and mortality despite the development of broad-spectrum antibiotics (11). Infections originating in the lung more frequently lead to sepsis than those originating in the abdomen or the urinary tract, with community-acquired pneumonia (CAP) emerging as one of the leading causes of death worldwide (4). Klebsiella pneumoniae is an increasingly important CAP pathogen, particularly in individuals with impaired pulmonary defenses, and is also a key pathogen in nosocomial pneumonia (42). Pulmonary infections caused by K. pneumoniae are often characterized by a rapid clinical course that includes multilobar involvement, formation of abscesses, and dissemination of bacteria from the pulmonary air space into the bloodstream, leading to widespread systemic effects and death (27, 31, 43). Treatment of K. pneumoniae infections can be complicated by the presence of extended-spectrum β-lactamases (ESBLs), which confer resistance to many broad-spectrum penicillin and cephalosporin antibiotics. K. pneumoniae in nosocomial infections is becoming more prevalent, with the global incidence of ESBLs in Klebsiella clinical isolates ranging from 8% to 44% (15, 33, 36, 43). In the United States, TEM- and SHV-type ESBLs predominate among Enterobacteriaceae, while in Canada and Europe, SHV and CTX ESBLs, respectively, are most frequently reported (8). Carbapenems are resistant to hydrolysis by most serine β-lactamases and are the drugs of choice for suspected ESBL- or AmpC β-lactamase-producing organisms (26).
Doripenem (DOR) is the most recently approved carbapenem, with MIC values up to 8-fold lower than that of imipenem (IPM) and comparable to that of meropenem (MEM) against ESBL-producing K. pneumoniae (25, 55). In the United States, doripenem is approved for complicated intra-abdominal infections (cIAI) and complicated urinary tract infections (cUTI). In Europe, and in various countries in Latin America and Asia, doripenem is approved for cIAI and cUTI and nosocomial pneumonia, including ventilator-associated pneumonia. Carbapenems are associated with low levels of lipopolysaccharide (LPS; also referred to as endotoxin) release relative to those associated with cephalosporins in the treatment of Gram-negative infections (21, 23, 41). Since sepsis and its accompanying sequelae are risk factors for mortality and antibiotic-induced release of LPS can aggravate septic shock (29, 32, 46), it was of interest to examine the effects of the different carbapenems on the pathogenesis of an ESBL-producing K. pneumoniae isolate in an established murine pneumonia model of infection.
MATERIALS AND METHODS
Bacterial strains.
Klebsiella pneumoniae OC4105, a TEM-26-producing isolate, was obtained from the Johnson & Johnson Pharmaceutical Research & Development collection (35).
MIC.
MICs were determined by the broth microdilution method, according to CLSI guidelines (10). The MIC was defined as the lowest concentration of antibiotic that prevented visible growth after an incubation period of 16 to 20 h.
Compound preparation.
Doripenem (DOR) was obtained from Shionogi & Co., Ltd. (Osaka, Japan). Ampicillin, imipenem, cilastatin, and meropenem were obtained from the United States Pharmacopeia (Rockville, MD). Imipenem (IPM) and meropenem (MEM) were prepared with cilastatin in a 1:1 dilution ratio for rodent dosing because of their instability to murine renal dehydropeptidase I (20, 40). Compounds were prepared fresh daily and refrigerated between doses.
Preparation of inoculum.
K. pneumoniae was inoculated into brain heart infusion (BHI) broth (Becton-Dickinson, Franklin Lakes, NJ) containing 100 μg/ml ampicillin and incubated at 37°C and 200 rpm for 18 h. Following centrifugation, the cell pellet was washed twice by resuspension in 0.85% NaCl. After the second wash, the pellet was resuspended to 1/10 the original culture volume in 0.85% NaCl to yield the infecting inoculum. The number of CFU in the inoculum was confirmed on tryptic soy agar (TSA) using spiral plate methodology (Autoplate 4000 and QCount; Spiral Biotech, Norwood, MA).
In vivo models.
All animal studies were approved by the Johnson & Johnson Institutional Animal Care and Usage Committee. The studies were conducted in an Association for Accreditation and Assessment Laboratory Animal Care (AAALAC)-accredited facility in compliance with U.S. regulations governing the housing and use of animals.
Klebsiella respiratory infection.
Four- to six-week-old female C3H/HeJ mice (Jackson Laboratory, Bar Harbor, ME) were briefly anesthetized with 3% isoflurane (IsoFlo; Abbott, Chicago, IL) maintained with oxygen at 3 liters/min and then infected intranasally with approximately 9 × 108 CFU of K. pneumoniae OC4105, an inoculum that was experimentally determined to yield a lethal infection within 3 to 4 days. At 1.5 h postinfection, a group of mice was euthanized, and the lungs were removed aseptically. Additional groups of animals were dosed subcutaneously with doripenem, meropenem-cilastatin, or imipenem-cilastatin at 1.5, 3, 6, 9, 12, 24, 27, 30, 33, 48, 51, 54, and 57 h postinfection. This dosing schedule was designed to achieve levels of plasma exposure (area under the concentration-time curve [AUC]) similar to those reported for doripenem, imipenem, and meropenem at the approved 500-mg clinical dose (3, 9, 24). Lung tissue samples were aseptically taken from groups of mice prior to dosing at 6, 12, 24, and 48 h postinfection and at 72 and 168 h postinfection. All lungs were weighed, diluted in 1 ml 0.85% saline, and homogenized (Omni Prep multisample homogenizer; Omni International, Marietta, GA). Serial 100-fold dilutions were performed and plated on TSA as described above. The numbers of CFU for each drug and dose were determined, and efficacy was assessed by the reduction in bacterial load (log10 CFU/g lung tissue) compared to those of the infected, untreated controls. At least three independent studies were conducted, with 3 to 5 mice per dose group in each study.
Telemetry.
Four- to six-week-old female C3H/HeJ mice were anesthetized, and TA-F20 transmitters (PhysioTel; DSI, St. Paul, MN) were implanted intraperitoneally. Animals were monitored for 5 days prior to being placed in the study. Mice were infected and dosed as described above (3 to 4 mice per group). Temperatures were monitored hourly, and the average value for each dose group was used for analysis.
Single-dose pharmacokinetics.
Four- to six-week-old female C3H/HeJ mice were infected as described above. At 1.5 hours postinfection, mice were given a single 50-mg/kg body weight subcutaneous dose of doripenem or meropenem-cilastatin or a 100-mg/kg dose of imipenem-cilastatin. At time points ranging from 0.13 to 3 h after dosing, mice (5 mice per time point) were euthanized, and blood samples were collected into lithium heparin tubes by cardiac puncture. Plasma was obtained from whole-blood samples following centrifugation at 10,000 × g for 5 min. Lungs were removed following blood collection and homogenized with 1 ml saline. The concentrations of doripenem and imipenem in plasma and lung extracts were quantitated by agar diffusion bioassay using Escherichia coli ATCC 25922 as the indicator organism. The limits of detection for doripenem and imipenem were 0.156 μg/ml and 1.25 μg/ml, respectively. Meropenem concentrations in plasma specimens and lung tissue were determined by reverse-phase high-pressure liquid chromatography (HPLC) using a Zorbax SB-C8 column (Agilent). Samples were analyzed in an increasing linear gradient of acetonitrile-methanol (4:1) in 5 mM ammonium acetate, pH 4.5, against a decreasing aqueous mobile phase consisting of 5 mM ammonium acetate, pH 4.5. Elution of meropenem was monitored by diode array detection at 295 nm. The limit of detection for meropenem was 0.5 μg/ml. Plasma protein and lung tissue binding were determined by ultrafiltration (13).
In vivo endotoxin release.
Mice were infected intranasally with K. pneumoniae as described above. At 1.5, 3, 6, and 9 h postinfection, groups of mice were dosed subcutaneously with doripenem, meropenem-cilastatin, or imipenem-cilastatin. At 3, 6, and 12 h after dosing, mice (5 per time point) were euthanized, whole-blood samples were obtained via cardiac puncture, and the blood samples were allowed to clot and then were centrifuged for 10 min at 10,000 × g. Lungs were removed following blood collection, homogenized in saline, and centrifuged. Serum samples and lung homogenate supernatants were frozen at −80°C pending analysis. Endotoxin release in sera and lung homogenates was quantitated enzymatically using a Limulus amebocyte lysate (LAL) assay kit (Lonza, Walkersville, MD) (23, 49). Untreated infected and naive animals served as controls.
Cytokine expression.
Mice were infected and dosed as described above. At 1.5, 3, 4.5, 6, 7.5, 12, and 24 h postinfection, groups of 5 animals were euthanized. Whole-blood samples were obtained via cardiac puncture, and the blood was allowed to clot. Sera were obtained as described above and were frozen at −80°C until assayed. Nonperfused lungs were collected into FastPrep lysing matrix D tubes (MP Biomedicals, Solon, OH) containing 1 ml of phosphate-buffered saline (Invitrogen, Carlsbad, CA). The tubes were then processed in the FastPrep machine (MP Biomedicals) at 4.0 m/s for 40 s. After homogenization, the tubes were centrifuged at 16 × g for 5 min to ensure that cellular debris was limited in the assay. Thawed serum and lung homogenate samples were tested for levels of specific cytokines and chemokines using a mouse 22-multiplex bead kit (Millipore, Billerica, MA) by following the manufacturer's protocol.
Statistical analyses of serum and lung cytokine concentrations were performed using analysis of variance (ANOVA), followed by the Tukey posttest.
Histology.
At 12, 24, 48, 72, and 168 h postinfection, groups of 3 mice were euthanized, and lungs were removed, inflated, and fixed in 10% neutral buffered formalin (NBF; Poly Scientific R&D Corp., Bay Shore, NY). The lungs were trimmed in a frontal plane and processed for light microscopy, and sections were cut at 5 to 7 μm on a rotary microtome. All sections were dried overnight and placed in a 60°C oven for 15 min prior to staining with basic hematoxylin (Gill 2; Thermo Scientific, Pittsburgh, PA) and eosin (alcoholic eosin Y; Thermo Scientific) on 1 set of slides. A second set of slides from the same tissue sections as the hematoxylin and eosin (H&E) slides were stained using the Brown-Hopps method (Poly Scientific R&D Corp.) for Gram-positive and Gram-negative bacteria (6). Photographs of stained lung sections were taken using a SPOT Idea camera, model number 27.2-3.1 MP (Diagnostic Instruments, Inc., Sterling Heights, MI). Images were adjusted (color balance, brightness, contrast, and unsharp mask) using Adobe Photoshop CS3 (Adobe Systems, Incorporated, Ottawa, ON, Canada). The histological sections of the lungs were reviewed in a blinded fashion by the pathologist, without knowledge of the treatment groups.
Statistical analyses.
The significances of mean response treatment differences for both lung weights and bacterial counts at the 6-, 12-, 24-, 48-, 72-, and 168-h sampling times were determined using a one-sided Wilcoxon test (14). The differences in treatment values were considered significant if the P values were less than or equal to 0.05. For cytokine analyses (see Fig. 3), a one-way ANOVA with Tukey's posttest was performed. Significance relative to that of untreated controls around P values less than 0.001, 0.01, or 0.05 are described.
RESULTS
Klebsiella lower respiratory infection.
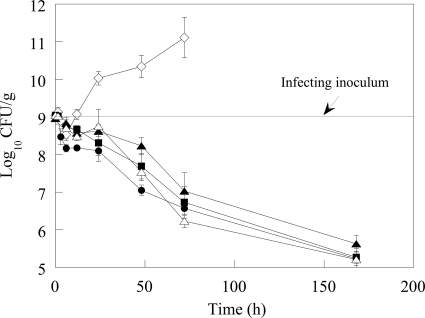
MIC values of doripenem and meropenem against the TEM-26-producing K. pneumoniae strain used in the lower respiratory infection model were 0.06 μg/ml. Imipenem was 16-fold less potent (Table 1). The time course of the infection is shown in Fig. 1 (a tabulated form of Fig. 1 can be found in Table S1 in the supplemental material). Following intranasal instillation of approximately 9 log10 CFU of K. pneumoniae, over the first 1.5 h of the infection, approximately 9 log10 CFU/g were recovered from the lung. At 6 h after intranasal infection, the number of CFU in the lungs of untreated animals decreased by 0.5 log10 CFU/g; this slight decrease in the number of CFU is consistent with an initial lag in growth of the K. pneumoniae strain in vivo, in conjunction with an observed influx in neutrophils into the lungs by 3 h postinfection (data not shown). This initial lag in K. pneumoniae growth in control animals is similar to that shown in results obtained by Ye et al. in a K. pneumoniae mouse lung infection model (54). By 12 h postinfection, the number of CFU in untreated animals rebounded to 9 log10 CFU/g. Thereafter, the lung burden of the untreated animals continued to increase over time, with most animals expiring by 48 h. Animals that survived for 72 h had Klebsiella counts of approximately 11 log10 CFU/g. In contrast, there was a reduction in the number of CFU throughout the dosing and recovery phases in all carbapenem-treated animals. At 6 h postinfection, doripenem-treated animals had 8.2 log10 CFU/g lung, a 0.5-log10 CFU/g greater reduction in Klebsiella than meropenem and imipenem-treated animals and untreated controls (Fig. 1; see also Table S1 in the supplemental material), although this difference did not achieve statistical significance. At 12 h postinfection, doripenem-treated mice had 0.6-log10 CFU/g fewer bacteria than meropenem-treated animals (statistically significant; P < 0.01) and approximately 0.3-log10 CFU/g fewer bacteria than imipenem-treated animals (statistically significant for the 50-mg/kg imipenem dose [P < 0.01] but not for the 100-mg/kg imipenem dose). By 24 h, the number of CFU of doripenem-treated mice was 0.2 log10 lower than that of meropenem and approximately 0.6 log10 lower than those at both imipenem doses (Fig. 1; see also Table S1 in the supplemental material); these differences between doripenem and the comparators were statistically significant (P < 0.01). By 48 h, the lung burden in doripenem-treated animals was 7 log10 CFU/g, approximately 0.5 log10 CFU/g lower than that in meropenem (statistically significant; P < 0.04) and imipenem (statistically significant at either imipenem dose; P < 0.01) treatment groups and 3 log10 CFU/g lower than that in untreated control animals. Seven days postinfection, doripenem-treated animals had a lung burden of 5.1 log10 CFU/g, nearly a 4 log10 decrease in the number of CFU relative to that in the infecting inoculum, and decreases in the numbers of meropenem and imipenem CFU were similar to that of doripenem CFU.
TABLE 1.
Plasma and lung PK/PD parameters following a single 50- or 100-mg/kg subcutaneous dose of drug in mice infected with K. pneumoniae OC4105
| Drug | Dose (mg/kg) | PK/PD parametersd |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma |
Lung |
|||||||||||
| MIC (μg/ml) | fCmax (μg/ml)a | Tmax (h) | t1/2 (h) | fAUC∞ (μg·h/ml) | fT>MIC (h) | fCmax (μg/g)b | Tmax (h) | t1/2 (h) | fAUC∞ (μg·h/g) | fT>MIC (h) | ||
| DOR | 50 | 0.06 | 75 | 0.5 | 0.24 | 54 | 2 | 12.3 | 0.5 | 0.25 | 12 | 1.5 |
| MEM | 50 | 0.06 | 87 | 0.13 | 0.42 | 41 | 3 | 21.7 | 0.13 | 0.17 | 11.4 | 1.5 |
| IPM | 50 | 1 | 43 | 0.13 | 0.26 | 16 | 1.5 | 11 | 0.25 | 0.32 | 7.2 | NDc |
| IPM | 100 | 1 | 108 | 0.25 | 0.52 | 68 | 3 | 25 | 0.25 | 0.86 | 25 | 2 |
Protein binding values of 19% and 2.5% for meropenem and imipenem were used, respectively (20). A protein binding value of 5% for doripenem was used (1, 50).
Free drug in lung tissue was determined experimentally using protein binding values of 6.6%, 1%, and 0% for doripenem, meropenem, and imipenem, respectively.
ND, not detectable in the lung after 0.75 h.
t1/2, half-life.
FIG. 1.
Decrease in viable counts of K. pneumoniae in lung homogenates following treatment with DOR, IPM, or MEM. Error bars represent the standard errors of the means. Symbols: ⋄, untreated; •, DOR; ▪, MEM; ▴, IPM (50 mg/kg); Δ, IPM (100 mg/kg).
Telemetry.
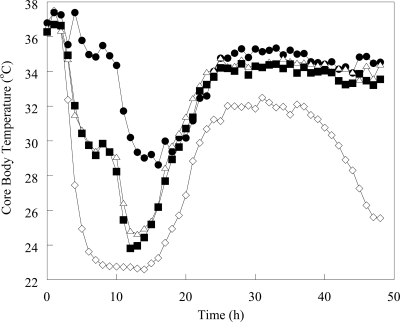
The average core body temperature of uninfected mice was approximately 36°C. The core body temperatures of meropenem- and imipenem-treated animals decreased by 1.8 and 1.9°C, respectively, at 3 h postinfection. In doripenem-treated animals, the temperature at 3 h postinfection decreased by 1.2°C, followed by a 1.8°C increase in temperature at 4 h postinfection (Fig. 2). Meropenem- and imipenem-treated animals experienced a decrease in temperature of 11 to 12°C until 13 to 14 h postinfection, at which time core body temperatures began to increase. Mice treated with doripenem experienced a biphasic temperature decline, stabilizing at 35°C (a 2°C decrease) at between 5 and 12 h and then decreasing to approximately 29°C by 14 h postinfection The maximum core body temperature decrease in doripenem-treated mice was 8.5°C. By 24 h postinfection, core body temperatures for all treated animals had increased to 34°C. The core body temperature of doripenem-treated mice reached 35°C by 49 h postinfection, whereas the temperatures of meropenem- and imipenem-treated mice did not reach 35°C until 84 and 81 h postinfection, respectively. The average core body temperature reached 36°C at 64 h in doripenem-treated animals; however, it took 142 h to reach 36°C in imipenem-treated animals. Core body temperatures in untreated mice decreased until 15 h postinfection, but in contrast with treated animals, at 24 h, the average temperature was only 28°C. Temperatures averaged 30°C until 52 h postinfection, when core body temperatures decreased until the animals expired. No untreated animals survived beyond 99 h postinfection.
FIG. 2.
Core body temperature changes in mice infected with K. pneumoniae and treated with DOR, IPM, or MEM. Individual data points were no more than 3 standard error units from the mean. Symbols: ⋄, untreated; •, DOR; ▪, MEM; Δ, IPM.
Pharmacokinetic and pharmacodynamic (PK/PD) analyses.
Plasma and lung pharmacokinetic parameters are shown in Table 1. Using plasma protein binding values of 5%, 19%, and 2.5% and lung protein binding values of 6.6%, 1%, and 0% for doripenem, meropenem, and imipenem, respectively, maximal free-drug concentrations (fCmax) and exposures for unbound drug in plasma samples and lung homogenates (1, 20, 50) were calculated. At a dose of 50 mg/kg, doripenem reached an fCmax of 75 μg/ml and 12 μg/g in the plasma and lung homogenates, respectively. Meropenem (50 mg/kg) achieved 14 and 43% higher fCmax in the plasma and lungs than doripenem, respectively. In contrast, the plasma free-drug infinite AUC (fAUC∞) value for an equivalent dose of imipenem was approximately 3-fold lower, and the plasma fCmax was 1.8- to 2.4-fold lower than those of doripenem and meropenem, respectively (Table 1). Increasing the dose of imipenem to 100 mg/kg resulted in plasma fAUC∞, fCmax, and free-drug time above the MIC (fT>MIC) values that more closely matched those of doripenem and meropenem, and as such, the 100-mg/kg dose was used for studies described in further detail in this study. The time required to achieve the maximal concentration (Tmax) of doripenem was 0.5 h in plasma and lung homogenates, 2- and 4-fold longer than those of imipenem and meropenem, respectively.
The fAUC∞s for equivalent doses of doripenem and meropenem were 54 and 41 μg·h/ml, respectively. The 100-mg/kg dose of imipenem yielded an fAUC∞ value of 68 μg·h/ml. Notably, representative human clinical AUC values reported for doripenem, meropenem, and imipenem (using a 500-mg dose with a 0.5-h infusion) are 36 μg·h/ml, 30 μg·h/ml, and 64 μg·h/ml, respectively (3, 9, 24), indicating that the murine exposure levels observed in our studies were similar to those obtained clinically. In whole-lung extracts, fAUC∞ values of doripenem and meropenem were similar, 12 and 11 μg·h/g, respectively, and one-half the value of imipenem.
The calculated pharmacodynamic parameters are also shown in Table 1. At the 50-mg/kg dose, doripenem exceeded the fT>MIC in plasma and lung homogenates for 2 and 1.5 h, respectively. The fT>MIC for meropenem was 3 h in plasma and 1.5 h in the lung. The fT>MIC for imipenem was 3 h in plasma and 2 h in the lung.
In vivo endotoxin release.
Liberation of LPS from Klebsiella in the lungs and sera of carbapenem-treated mice was determined using the Limulus amebocyte assay. In the sera, doripenem-treated animals had 3.8-fold-greater levels of LPS than untreated control mice at 3 h postinfection; however, endotoxin concentrations of both groups of animals were similar at 6 and 12 h (Table 2). Meropenem- and imipenem-treated animals had serum LPS levels averaging 1,100 endotoxin units (EU)/ml and 1,300 EU/ml, respectively, at all time points, which were 10-fold higher than that of doripenem-treated animals.
TABLE 2.
Endotoxin LPS release in the sera and lungs of mice infected with K. pneumoniae OC4105
| Time (h)b | Endotoxin LPS concn (EU/ml [mean ±SEM])a |
|||||||
|---|---|---|---|---|---|---|---|---|
| Serum |
Lung |
|||||||
| None | DOR | MEM | IPM | None | DOR | MEM | IPM | |
| 0 | 6 ± 2 | NT | NT | NT | ND | ND | ND | ND |
| 1.5 | 6 ± 1 | NT | NT | NT | NT | NT | NT | NT |
| 3 | 35 ± 27 | 132 ± 26 | 1,269 ± 246 | 1,436 ± 262 | 8,220 ± 1,927 | 39,501 ± 13,356 | 111,700 ± 9,116 | 110,820 ± 5,165 |
| 6 | 80 ± 20 | 117 ± 41 | 1,082 ± 147 | 1,467 ± 277 | 8,553 ± 972 | 53,206 ± 3,238 | 317,750 ± 37,282 | 145,890 ± 2,616 |
| 12 | 118 ± 34 | 109 ± 27 | 1,049 ± 118 | 995 ± 78 | 25,499 ± 4,774 | 49,119 ± 13,947 | 273,900 ± 24,665 | 162,160 ± 11,714 |
NT, not tested; ND, not determined, below the limit of detection (<5 EU/ml).
Times are postinfection.
The amount of LPS in the lungs of untreated mice increased by 44-fold to 8,220 EU/ml at 3 h postinfection (Table 2). At 3 h postinfection, the amounts of endotoxin released into the lungs of treated mice were 39,501, 111,700, and 110,820 EU/ml for doripenem-, meropenem-, and imipenem-treated mice, respectively. In untreated animals, the concentration of LPS in the lungs tripled after 12 h to 25,499 EU/ml. Endotoxin concentrations in the lungs of doripenem- and meropenem-treated animals peaked after 6 h (53,206 EU/ml and 317,750 EU/ml, respectively) before decreasing slightly after 12 h. The concentration of LPS in the lungs of imipenem-treated mice increased at all time points, reaching 162,160 EU/ml after 12 h.
Cytokine/chemokine expression.
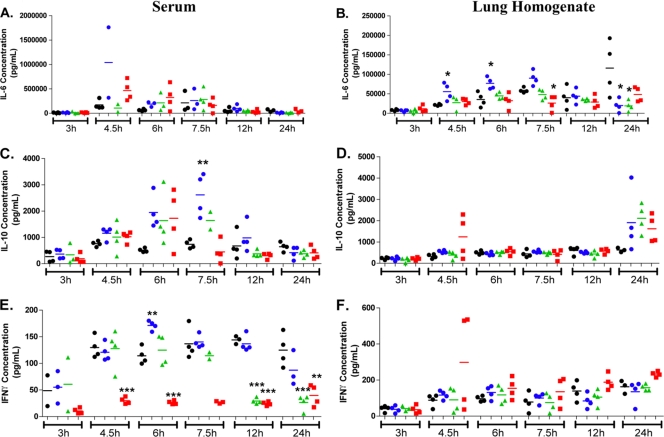
Cytokine and chemokine levels were determined in lung homogenates and sera following intranasal K. pneumoniae inoculation (Fig. 3 and data not shown). In lung homogenates, there was a common trend toward increased levels of the cytokines gamma interferon (IFN-γ) (Fig. 3F), interleukin 1β (IL-1β), granulocyte colony-stimulating factor (G-CSF) IL-9, IL-12p70, IL-13, IL-15, IP-10, and granulocyte-macrophage colony-stimulating factor (GM-CSF) following K. pneumoniae inoculation that was not significantly modulated by any of the three drug treatments (data not shown). However, the level of IL-6 was significantly higher in lung homogenates of doripenem-treated animals relative to those in meropenem-treated or imipenem-treated animals or untreated controls at 4.5 and 6 h postinoculation, respectively (Fig. 3B). This same trend was also observed in lung homogenates with G-CSF and KC but was not statistically significant (data not shown). A trend toward increased levels of IL-10 (Fig. 3D), MCP-1 (data not shown), and MIP-1α (data not shown) in doripenem-, meropenem-, or imipenem-treated animals relative to those in untreated animals at 24 h post-K. pneumoniae inoculation was observed but was not statistically significant.
FIG. 3.
Serum and lung homogenate cytokine levels following intranasal Klebsiella pneumoniae infection in mice. Animals (2 to 4 mice/group) were either dosed subcutaneously with doripenem (blue circles), meropenem-cilastatin (green triangles), or imipenem-cilastatin (red squares) at 1.5, 3, 6, 9, and 12 h postinfection with Klebsiella pneumoniae on day 1 or remained untreated (black circles). Each data point represents the cytokine level of a single mouse, and the bar represents the group mean. Statistical analysis consisted of a one-way ANOVA with Tukey's posttest (*, P < 0.05; **, P < 0.01; ***, P < 0.001, relative to the untreated control at the same time point).
In sera, a trend toward an increased level of IL-6 in doripenem-treated animals at 4.5 h postinoculation was observed but was not statistically significant (Fig. 3A). An increased level of the anti-inflammatory cytokine IL-10 (Fig. 3C) was also observed in the sera of doripenem-treated animals relative to that of meropenem-treated, imipenem-treated, or untreated animals at 7.5 h postinoculation. It is especially interesting to note that (i) animals treated with imipenem had consistently lower serum levels of the important macrophage-activating cytokine IFN-γ relative to those of doripenem-treated, meropenem-treated, or untreated animals at 4.5, 6, 12, and 24 h postinoculation and that (ii) meropenem-treated animals also had reduced serum IFN-γ levels at 12 and 24 h postinoculation (Fig. 3E).
Histopathology.
The lung sections of animals infected with K. pneumoniae were evaluated microscopically using H&E and Brown-Hopps staining (Fig. 4 and 5). During the early phase of the infection (6 h), there was an increase in the number of neutrophils, and free bacteria were visible in the alveolar spaces as well as within macrophages (data not shown).
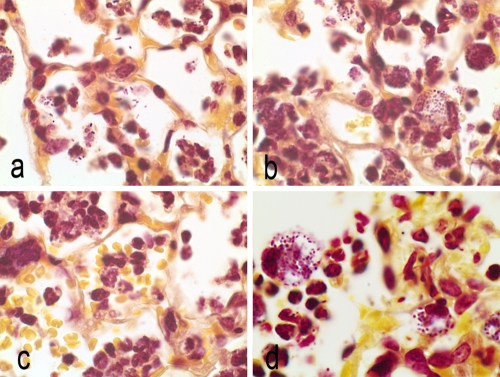
FIG. 4.
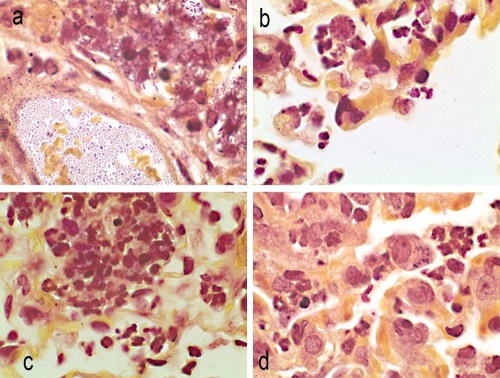
Brown-Hopps stain of lung sections at 12 h postinfection. Magnification, ×100. (a) Untreated control animals, free organisms, and organisms in phagocytic cells. (b) Doripenem treatment. Alveolar macrophages contain bacteria. Mixed infiltrates of macrophages and neutrophils. (c) Meropenem treatment. Alveolar hemorrhage and macrophages in alveoli. (d) Imipenem treatment. Alveoli filled with neutrophils and macrophages containing organisms.
FIG. 5.
Brown-Hopps stain of lung sections at 48 h postinfection. Magnification, 100×. (a) Untreated control animals. Numerous rod-shaped organisms in blood vessels and in adjacent alveoli. (b) Doripenem treatment. Organisms in alveolar macrophages, surrounded by a few neutrophils. Note the rounded appearance of cells. (c) Meropenem treatment. Aggregating neutrophils present in alveoli, with a mixture of mature and immature cells. (d) Imipenem treatment. Alveolar macrophages containing organisms/neutrophils in the alveoli.
At 12 hours postinfection, an influx of neutrophils and numerous free K. pneumoniae cells were visible in the alveoli of all animals (Fig. 4a to d). Bacteria were also present within the alveolar macrophages of untreated and imipenem-treated animals (Fig. 4a and d). In contrast, few free bacteria were seen in lungs of doripenem- and meropenem-treated animals (Fig. 4b and c). K. pneumoniae cells in the macrophages of carbapenem-treated animals had a more rounded appearance compared to the rod-shaped organisms seen in alveoli or in macrophages of untreated animals. In contrast to those of doripenem-treated animals, lungs of meropenem- and imipenem-treated animals (Fig. 4d) had increased numbers of neutrophils and alveolar macrophages containing bacteria, with congested capillaries and hemorrhage into the alveoli.
Numerous free bacteria were visible in the lung parenchyma and in the alveolar macrophages of untreated animals at 24 h postinfection (data not shown). Meropenem-treated animals continued to show congestion in the pulmonary capillary vasculature, whereas the lungs of imipenem-treated animals showed interstitial and alveolar infiltrates composed of acute inflammatory cells, primarily neutrophils and alveolar macrophages filled with organisms. Multiple areas of emphysema were also present in the lungs from both of these treatment groups. In doripenem-treated animals, most organisms were contained within the alveolar macrophages; however, multifocal infiltrates of neutrophils were also evident.
At 48 hours postinfection, numerous free organisms were seen within the pulmonary vasculature and in the alveolar macrophages of untreated animals (Fig. 5a to d). Increased numbers of acute inflammatory cells (neutrophils and macrophages) were present, and exudates were also visible in the alveoli of the lungs (Fig. 5a). Animals treated with either meropenem or doripenem had fewer free organisms in the alveoli or lung parenchyma, and most of the bacteria were located in alveolar macrophages; however, focal areas of neutrophil infiltrates were present (Fig. 5b and c). Animals treated with imipenem similarly had focal neutrophilic infiltrates, in addition to edema in the bronchioles and alveoli (Fig. 5d).
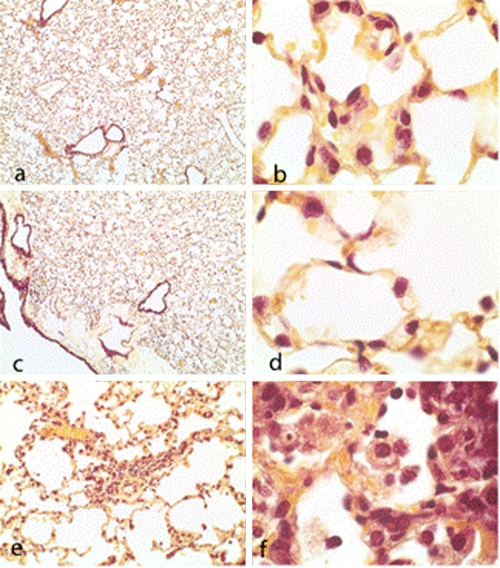
At 7 days (168 h) postinfection, bronchioles and alveoli from both doripenem- and meropenem-treated animals contained a limited number of type II pneumocytes but appeared to be clear of exudates and bacteria (Fig. 6.). Imipenem-treated mice had focal areas of fibroblast infiltrates, resulting in the thickening of the interstitia. Alveoli from these mice contained numerous foamy macrophages and neutrophils, and the capillaries in the alveolar septa remained congested.
FIG. 6.
Brown-Hopps stain of bronchioles and alveoli from lung sections at 168 h postinfection. (a and c) Doripenem and meropenem treatment, respectively (magnification, ×4). (b, d, and f) Doripenem, meropenem, and imipenem treatment, respectively (magnification, ×100). (e) Imipenem treatment (magnification, ×20). Note focal areas of interstitial fibroblast infiltrates, macrophages, neutrophils, and septal thickening.
DISCUSSION
In these studies of doripenem, meropenem, and imipenem in a K. pneumoniae mouse lung infection model, although the in vitro activities of doripenem and meropenem were comparable, there were marked differences in their in vivo efficacy and lung pathology.
In the murine lower respiratory infection model, doripenem significantly reduced the lung burden of K. pneumoniae more rapidly and to a greater extent than meropenem or imipenem over the course of the infection. The bacterial lung burdens in meropenem- and imipenem-treated mice were similar, though a higher imipenem dose was required to achieve a similar reduction in bacteria (Fig. 1).
The pharmacodynamic parameter associated with in vivo efficacy of β-lactam agents is fT>MIC, with a target of 40 to 60% for Gram-negative pathogens (12, 28). In our studies, at the selected doses, the fT>MIC for meropenem and imipenem was 60% of the dosing interval, while that for doripenem was 52%. In the lung, the fT>MIC value for doripenem, meropenem, and imipenem was approximately 50% of the dosing interval. Thus, the fT>MIC values achieved in our studies are within the target range for efficacy. Although doripenem and meropenem had equivalent lung percent T>MICs, doripenem-treated animals had lower Klebsiella lung burdens at most time points examined. In spite of greater exposure (fAUC∞) and equivalent or greater fT>MICs in both the plasma and lung, imipenem was the least effective agent in vivo against this isolate of K. pneumoniae.
It has been well documented that decreased core body temperature is a sign of deteriorating health in rodents challenged with an infectious agent (2, 30). In our studies, mice treated with doripenem were better able to maintain normal core body temperature than those treated with meropenem and imipenem. Decreases in temperature are also associated with greater pathological changes in the lung (2). Consistent with these findings, doripenem-treated mice had less alveolar consolidation, fewer neutrophil infiltrates, and less edema than imipenem or meropenem-treated animals.
Increased levels of LPS release in Gram-negative bacteria have been associated with antibiotics such as cephalosporins that bind predominantly to PBP 3 and cause cell filamentation, whereas carbapenems demonstrate higher affinity to PBP 2 and typically induce spheroplasting and overall reduced amounts of endotoxin release (7, 21-23, 51). Consistent with past studies, in our in vivo studies, treatment of K. pneumoniae with doripenem, meropenem, or imipenem yielded spherical or ovoid cells (Fig. 5). Notably, in spite of similar cell morphologies, doripenem treatment was associated with significantly lower levels of LPS release than meropenem or imipenem treatment. Among the carbapenems, there are differences in binding to PBPs. In E. coli, all three carbapenems bind with equal affinity to PBP 2; however, meropenem also has a high affinity for PBP 3 (16). The affinity of imipenem for PBP 1 is reportedly greater than that of doripenem and meropenem (16, 53), which may contribute to enhanced bacterial lysis. Thus, in our studies, the smaller amounts of LPS released from doripenem-treated mice may result from the impact of differing carbapenem PBP-binding profiles on the cell physiology of this K. pneumoniae isolate. LPS release from bacteria has been associated with a decrease in body temperature (19). Furthermore, Rudaya et al. have demonstrated that mice injected with 104 mg/kg LPS have a greater decrease in body temperature than mice injected with 103 mg/kg LPS or those injected with saline (45). The diminished hypothermic response observed in the doripenem-treated animals relative to those receiving meropenem or imipenem is consistent with the reduced amount of endotoxin released by Klebsiella exposed to doripenem.
Innate immunity is the principal pathway for elimination of virulent extracellular Gram-positive and Gram-negative pathogens, including K. pneumoniae, from the lung (27). Resident alveolar macrophages and recruited neutrophils (polymorphonuclear leukocytes [PMNs]) are essential in host defense against bacterial pneumonia, and selective depletion of either cell population results in defects in the clearance of bacteria from the alveolar space (27). In response to larger inocula or more-virulent organisms, alveolar macrophages synthesize and secrete a wide array of proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α), IFN-γ, IL-1β, and IL-6, several chemokines, and arachidonic acid metabolites, which initiate the inflammatory response, activate macrophages, and recruit activated neutrophils into the alveolar spaces (5, 18, 44, 47). In our studies, after 12 h, all infected animals exhibited an influx of neutrophils into the alveolar space (Fig. 4). After 24 h, doripenem-treated animals exhibited fewer multifocal infiltrates (primarily neutrophils) than meropenem- and imipenem-treated animals, who exhibited higher levels of neutrophils and macrophages. Forty-eight hours postinfection, imipenem-treated animals had multifocal neutrophilic infiltrates and macrophages, whereas animals treated with meropenem and doripenem had fewer focal areas of neutrophilic infiltrates (Fig. 5). Taken together, through 24 h, the lesser degree of infiltration of acute inflammatory cells in doripenem-treated animals is consistent with doripenem's improved efficacy relative to those of imipenem and meropenem. Seven days postinfection, all treated animals had similar bacterial burdens; however, imipenem-treated animals still exhibited pathological changes not seen in doripenem- and meropenem-treated mice, including septal thickening, neutrophil infiltrates, and congestion (Fig. 6).
In addition to its role in infection, the alveolar macrophage also plays a role in resolving inflammation within the air space (34, 44). In our studies, with doripenem-treated animals, there is an increase in macrophages at 48 h postinfection (Fig. 5) that is not observed in meropenem- or imipenem-treated animals until 72 h and 168 h postinfection, respectively (data not shown). Seven days postinfection, lungs of imipenem-treated animals continued to show numerous focal areas of interstitial thickening, indicative of a chronic inflammatory response (Fig. 6). The earlier accumulation of macrophages in doripenem- and meropenem-treated animals may contribute to the more rapid resolution of the infection and inflammation.
We observed that doripenem-treated animals had an increased level of IL-6 in the lungs and a trend toward increased IL-6 in the sera at 4.5 h, relative to those of imipenem- and meropenem-treated mice. IL-6 is considered to be an important mediator of acute inflammatory responses and has been reported to play a protective role in systemic Klebsiella infection by enhancing the ability of neutrophils to kill bacteria (48). We also found that meropenem- and imipenem-treated animals, but not doripenem-treated animals, had reduced serum levels of the important macrophage-activating cytokine IFN-γ relative to those of the untreated controls at various time points postinfection (meropenem treatment reduced IFN-γ levels at 12 and 24 h postinfection, whereas imipenem treatment reduced IFN-γ levels at 4.5, 6, 12, and 24 h postinfection). IFN-γ has been demonstrated to play a protective role in pulmonary Klebsiella pneumoniae infection (38). Although IFN-γ in the lungs is likely more important in the protective immune response to pulmonary K. pneumoniae infection, systemic levels of IFN-γ may be important in controlling the systemic manifestations following dissemination of the bacteria from the lung to the blood (38). Therefore, it is tempting to speculate that the improved in vivo efficacy of doripenem treatment relative to those of meropenem and imipenem treatment may be a result of enhanced IL-6 production and sustained systemic IFN-γ production that lead to enhanced macrophage- and neutrophil-mediated killing of Klebsiella pneumoniae in the lungs of mice. However, additional studies will be needed to support this hypothesis.
Anti-inflammatory cytokines such as IL-10 are thought to control and downregulate the inflammatory response by limiting further recruitment of activated neutrophils, macrophages, and proinflammatory mediators (IL-Iβ, IL-6, and TNF-α) (17, 18, 52). The elevated levels of IL-10 observed selectively with doripenem-treated animals at 7.5 h postinfection suggest that doripenem-treated animals may be more effective at mounting a compensatory anti-inflammatory response, reducing the lung damage observed in doripenem-treated animals. Consistent with these results, mice treated with doripenem had a more rapid resolution of infection, less lung pathology, and exhibited less lethargy than mice treated with either imipenem or meropenem. These findings are likely the result of enhanced antibacterial activity, a reduction in endotoxin release, and a more favorable immunological response.
Supplementary Material
Acknowledgments
We thank Colleen Santoro for technical assistance in conducting PK/PD studies, Jean McCarty for histology slide preparations, and Anne Marie Queenan for in vitro susceptibility assays.
Footnotes
Published ahead of print on 6 December 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Andes, D., and W. A. Craig. 2003. In-vivo pharmacodynamic activity of doripenem against multiple bacteria in a murine thigh infection model, abstr. A-308. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)/46th Infect. Dis. Soc. Am. (IDSA), American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 2.Bast, D. J., et al. 2004. Novel murine model of pneumococcal pneumonia: use of temperature as a measure of disease severity to compare the efficacies of moxifloxacin and levofloxacin. Antimicrob. Agents Chemother. 48:3343-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bax, R. P., et al. 1989. The pharmacokinetics of meropenem in volunteers. J. Antimicrob. Chemother. 24:311-320. [DOI] [PubMed] [Google Scholar]
- 4.Bocchino, M., A. Marruchella, and C. Saltini. 2005. Immunogenetics of severe respiratory infections: models for the development of new therapeutic strategies. Respiration 72:449-457. [DOI] [PubMed] [Google Scholar]
- 5.Broug-Holub, E., et al. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65:1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, R. C., and H. C. Hopps. 1973. Staining of bacteria in tissue sections: a reliable Gram stain method. Am. J. Clin. Pathol. 60:234-240. [DOI] [PubMed] [Google Scholar]
- 7.Buijs, J., A. S. M. Dofferhoff, J. W. Mouton, and J. W. M. van der Meer. 2007. Continuous administration of PBP-2- and PBP-3-specific beta-lactams causes higher cytokine responses in murine Pseudomonas aeruginosa and Escherichia coli sepsis. J. Antimicrob. Chemother. 59:926-933. [DOI] [PubMed] [Google Scholar]
- 8.Bush, K. 2008. Extended-spectrum β-lactamases in North America, 1987-2006. Clin. Microbiol. Infect. 14:134-143. [DOI] [PubMed] [Google Scholar]
- 9.Cirillo, I., V. N. K. Turner, B. Solanki, J. Natarajan, and R. Redman. 2009. Pharmacokinetics, safety, and tolerability of doripenem after 0.5-, 1-, and 4-hour infusions in healthy volunteers. J. Clin. Pharmacol. 49:798-806. [DOI] [PubMed] [Google Scholar]
- 10.CLSI. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 8th ed. Approved standard M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA.
- 11.Craig, A., J. Mai, S. Cai, and S. Jeyaseelan. 2009. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 77:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 13.Craig, W. A., and B. Suh. 1991. Protein binding and the antimicrobial effects: methods for the determination of protein binding, p. 367-374. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. Williams & Wilkins, Baltimore, MD.
- 14.Cytel, Inc. 2007. StatXact 8 with Cytel software; statistical software for exact nonparametric inference. User manual, Cytel statistical software & services. Cytel Inc., Cambridge, MA.
- 15.da Silva Dias, R. C., et al. 2008. Prevalence of AmpC and other beta-lactamases in enterobacteria at a large urban university hospital in Brazil. Diagn. Microbiol. Infect. Dis. 60:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies, T. A., W. Shang, K. Bush, and R. K. Flamm. 2008. Affinity of doripenem and comparators to penicillin-binding proteins in Escherichia coli and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:1510-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty, T. M., R. Kastelein, S. Menon, S. Andrade, and R. L. Coffman. 1993. Modulation of murine macrophage function by IL-13. J. Immunol. 151:7151-7160. [PubMed] [Google Scholar]
- 18.Gogos, C. A., E. Drosou, H. P. Bassaris, and A. Skoutelis. 2000. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J. Infect. Dis. 181:176-180. [DOI] [PubMed] [Google Scholar]
- 19.Habicht, G. S. 1981. Body temperature in normal and endotoxin-treated mice of different ages. Mech. Ageing Dev. 16:97-104. [DOI] [PubMed] [Google Scholar]
- 20.Hori, T., M. Nakano, Y. Kimura, and K. Murakami. 2006. Pharmacokinetics and tissue penetration of a new carbapenem, doripenem, intravenously administered to laboratory animals. In Vivo 20:91-96. [PubMed] [Google Scholar]
- 21.Horii, T., M. Kobayashi, K. Sato, S. Ichiyama, and M. Ohta. 1998. An in-vitro study of carbapenem-induced morphological changes and endotoxin release in clinical isolates of gram-negative bacilli. J. Antimicrob. Chemother. 41:435-442. [DOI] [PubMed] [Google Scholar]
- 22.Horii, T., H. Muramatsu, A. Monji, and D. Miyagishima. 2005. Release of exotoxin A, peptidoglycan and endotoxin after exposure of clinical Pseudomonas aeruginosa isolates to carbapenems in vitro. Chemotherapy 51:324-331. [DOI] [PubMed] [Google Scholar]
- 23.Jackson, J. J., and H. Kropp. 1996. Differences in mode of action of β-lactam antibiotics influence morphology, LPS release and in vivo antibiotic efficacy. J. Endotoxin Res. 3:201-218. [Google Scholar]
- 24.Jaruratanasirikul, S., N. Raungsri, J. Punyo, and S. Sriwiriyajan. 2005. Pharmacokinetics of imipenem in healthy volunteers following administration by 2 h or 0.5 h infusion. J. Antimicrob. Chemother. 56:1163-1165. [DOI] [PubMed] [Google Scholar]
- 25.Jones, R. N., H. S. Sader, and T. R. Fritsche. 2005. Comparative activity of doripenem and three other carbapenems tested against Gram-negative bacilli with various β-lactamase resistance mechanisms. Diagn. Microbiol. Infect. Dis. 52:71-74. [DOI] [PubMed] [Google Scholar]
- 26.Joseph, J., and K. A. Rodvold. 2008. The role of carbapenems in the treatment of severe nosocomial respiratory tract infections. Expert Opin. Pharmacother. 9:561-575. [DOI] [PubMed] [Google Scholar]
- 27.Karaolis, D. K. R., et al. 2007. Cyclic di-GMP stimulates protective innate immunity in bacterial pneumonia. Infect. Immun. 75:4942-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiffer, C. R. V., J. L. Kuti, K. J. Eagye, C. Mendes, and D. P. Nicolau. 2006. Pharmacodynamic profiling of imipenem, meropenem and ertapenem against clinical isolates of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. from Brazil. Int. J. Antimicrob. Agents 28:340-344. [DOI] [PubMed] [Google Scholar]
- 29.Kirikae, T., et al. 1998. Biological characterization of endotoxins released from antibiotic-treated Pseudomonas aeruginosa and Escherichia coli. Antimicrob. Agents Chemother. 42:1015-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kort, W. J., J. M. Hekking-Weijma, M. T. TenKate, V. Sorm, and R. VanStrik. 1998. A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Lab. Anim. 32:260-269. [DOI] [PubMed] [Google Scholar]
- 31.Lau, H. Y., S. Clegg, and T. A. Moore. 2007. Identification of Klebsiella pneumoniae genes uniquely expressed in a strain virulent using a murine model of bacterial pneumonia. Microb. Pathog. 42:148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepper, P. M., et al. 2002. Clinical implications of antibiotic-induced endotoxin release in septic shock. Intensive Care Med. 28:824-833. [DOI] [PubMed] [Google Scholar]
- 33.Marra, A. R., et al. 2006. Nosocomial bloodstream infections caused by Klebsiella pneumoniae: impact of extended-spectrum beta-lactamase (ESBL) production on clinical outcome in a hospital with high ESBL prevalence. BMC Infect. Dis. 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marriott, H. M., et al. 2006. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia. J. Immunol. 177:6480-6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medeiros, A. A., and J. Crellin. 1997. Comparative susceptibility of clinical isolates producing extended spectrum beta-lactamases to ceftibuten: effect of large inocula. Pediatr. Infect. Dis. J. 16:S49-S55. [DOI] [PubMed] [Google Scholar]
- 36.Mendes, R. E., P. R. Rhomberg, J. M. Bell, J. D. Turnidge, and H. S. Sader. 2009. Doripenem activity tested against a global collection of Enterobacteriaceae, including isolates resistant to other extended-spectrum agents. Diagn. Microbiol. Infect. Dis. 63:415-425. [DOI] [PubMed] [Google Scholar]
- 37.Reference deleted.
- 38.Moore, T. A., M. L. Perry, A. G. Getsoian, M. W. Newstead, and T. J. Standiford. 2002. Divergent role of gamma interferon in a murine model of pulmonary versus systemic Klebsiella pneumoniae infection. Infect. Immun. 70:6310-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reference deleted.
- 40.Petersen, P. J., N. V. Jacobus, W. J. Weiss, and R. T. Testa. 1991. In vitro and in vivo activities of LJC10,627, a new carbapenem with stability to dehydropeptidase I. Antimicrob. Agents Chemother. 35:203-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prins, J. M., S. J. H. Van Deventer, E. J. Kuijper, and P. Speelman. 1994. Clinical relevance of antibiotic-induced endotoxin release. Antimicrob. Agents Chemother. 38:1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regueiro, V., M. A. Campos, J. Pons, S. Alberti, and J. A. Bengoechea. 2006. The uptake of a Klebsiella pneumoniae capsule polysaccharide mutant triggers an inflammatory response by human airway epithelial cells. Microbiology 152:555-566. [DOI] [PubMed] [Google Scholar]
- 43.Romero, E. D. V., et al. 2007. Prevalence of clinical isolates of Escherichia coli and Klebsiella spp. producing multiple extended-spectrum beta-lactamases. Diagn. Microbiol. Infect. Dis. 59:433-437. [DOI] [PubMed] [Google Scholar]
- 44.Rubins, J. B. 2003. Alveolar macrophages: wielding the double-edged sword of inflammation. Am. J. Respir. Crit. Care Med. 167:103-104. [DOI] [PubMed] [Google Scholar]
- 45.Rudaya, A. Y., A. A. Steiner, J. R. Robbins, A. S. Dragic, and A. A. Romanovsky. 2005. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am. J. Physiol. 289:R1244-R1252. [DOI] [PubMed] [Google Scholar]
- 46.Sawada, H., et al. 1997. Low levels of free endotoxin result from carbapenem treatment of gram-negative bacteria. J. Infect. Chemother. 3:27-32. [Google Scholar]
- 47.Standiford, T. J., S. L. Kunkel, M. J. Greenberger, L. L. Laichalk, and R. M. Strieter. 1996. Expression and regulation of chemokines in bacterial pneumonia. J. Leukoc. Biol. 59:24-28. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland, R. E., J. S. Olsen, A. McKinstry, S. A. Villalta, and P. J. Wolters. 2008. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J. Immunol. 181:5598-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuji, M., H. Matsuda, H. Miwa, and S. Miyazaki. 2003. Antimicrobial-induced release of endotoxin from Pseudomonas aeruginosa: comparison of in vitro and animal models. J. Antimicrob. Chemother. 51:353-359. [DOI] [PubMed] [Google Scholar]
- 50.Van Wart, S. A., D. R. Andes, P. G. Ambrose, and S. M. Bhavnani. 2009. Pharmacokinetic-pharmacodynamic modeling to support doripenem dose regimen optimization for critically ill patients. Diagn. Microbiol. Infect. Dis. 63:409-414. [DOI] [PubMed] [Google Scholar]
- 51.Vianna, R. C. S., et al. 2004. Antibiotic treatment in a murine model of sepsis: impact on cytokines and endotoxin release. Shock 21:115-120. [DOI] [PubMed] [Google Scholar]
- 52.Wynn, T. A. 2003. IL-13 effector functions. Annu. Rev. Immunol. 21:425-456. [DOI] [PubMed] [Google Scholar]
- 53.Yang, Y., N. Bhachech, and K. Bush. 1995. Biochemical comparison of imipenem, meropenem and biapenem: permeability, binding to penicillin-binding proteins, and stability to hydrolysis by beta-lactamases. J. Antimicrob. Chemother. 35:75-84. [DOI] [PubMed] [Google Scholar]
- 54.Ye, P., et al. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhanel, G. G., et al. 2007. Comparative review of the carbapenems. Drugs 67:1027-1052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.