Abstract
During early urinary tract infection (UTI) the interplay between invading bacteria and the urothelium elicits a mucosal response aimed at clearing infection. Unfortunately, the resultant inflammation and associated local tissue injury are responsible for patient symptoms. Interleukin-6 (IL-6), a cytokine released during acute UTI, has both pro- and anti-inflammatory effects on other body systems. Within the urothelium, the IL-6 native-tissue origin, the target cell type(s), and ultimate effect of the cytokine on target cells are largely unknown. In the present study we modeled the UTI IL-6 response ex vivo using canine bladder mucosa mounted in Ussing chambers to determine the inflammatory and reparative role of IL-6. We demonstrated that uropathogenic Escherichia coli infection stimulates the synthesis of IL-6 by all urothelial cell layers, with the urothelial cells alone representing the only site of unequivocal IL-6 receptor expression. Autocrine effects of IL-6 were supported by the activation of urothelial STAT3 signaling and SOCS3 expression. Using exogenous IL-6, a microarray approach, and quantitative reverse transcriptase PCR (q-RT-PCR), 5 target genes (tumor necrosis factor alpha, interleukin-1β, matrix metallopeptidase 2, heparan sulfate d-glucosaminyl 3-O-sulfotransferase 3A1, and hyaluronan synthase 2) that have direct or indirect roles in promoting a proinflammatory state were identified. Two of these genes, heparan sulfate d-glucosaminyl 3-O-sulfotransferase 3A1 and hyaluronan synthase 2, are also potentially important mediators of wound repair via the production of glycosaminoglycan components. These findings suggest that IL-6 secretion during acute UTI may serve a dual biological role by initiating the inflammatory response while also repairing urothelial defenses.
Urinary tract infections (UTIs) are the second most common infection of the human body. Nearly 11% of women 18 years of age or older experience at least one UTI per year, with combined annual medical costs exceeding 1 billion dollars (6). Even patients with uncomplicated UTI experience on average 6.1 days with symptoms and 2.4 days of restricted activity; therefore, each episode can be debilitating (7). Given the prevalence, costs, and morbidity associated with these infections, there has been ongoing emphasis placed on an understanding of the mucosal response to UTI and how the response can be modified to improve clinical outcomes.
Paramount to UTI prevention is the maintenance of the complex multicellular urothelium that lines the bladder lumen. There are numerous urothelial elements that contribute to the prevention of the bacterial colonization of the bladder. Three of the most integral components include uroplakin plaques within the umbrella cell apical membranes (21, 30), tight junctions adjoining umbrella cells (18, 27), and a hydrophilic glycosaminoglycan (GAG)-rich mucus layer covering the luminal surface of the umbrella cells (26, 36). Collectively, these three components restrict the translocation of ions, solutes, and bacteria into the bladder interstitium and protect the urothelial microenvironment from injury. During urinary tract infections these defenses are compromised, and the urothelium initiates a local response that includes the release of cytokines, growth factors, and other inflammatory mediators (12, 41). This inflammatory response is both an asset and a detriment. The initial innate response is essential for the recruitment of phagocytic cells and the clearance of infection; however, inflammation is also associated with tissue injury resulting in clinical signs such as painful, frequent, and difficult urination (41). The urothelial balance of pro- and anti-inflammatory responses is therefore essential to bacterial eradication while minimizing tissue pathology.
The synthesis of interleukin-6 (IL-6) is an important component of the urinary bladder response to bacterial infection (2, 8, 14, 15); however, the target cell type(s) and functional effects of IL-6 within the urinary bladder are incompletely understood. Throughout the body, IL-6 is an important pleiotropic cytokine that promotes tissue homeostasis after acute injury and infection (9). Three well-described roles of IL-6 include cytoprotection, maintenance of the cellular microenvironment, and modulation of inflammation (1, 43, 45). All three of these actions could prove important to the maintenance and repair of damaged urothelial defenses during the acute phase of UTI. An enhanced understanding of the role of IL-6 during UTI may be vital to defining the role of therapeutic agents that enhance urothelial repair.
In the present study, we first characterized the IL-6 secretory response of canine bladder mucosa to uropathogenic E. coli infection ex vivo in Ussing chambers. We then recapitulated the concentration and location of IL-6 secretion in the absence of Escherichia coli using exogenous canine IL-6. This approach facilitated sustained viability, the physical separation of suburothelial and lumen influences, and the isolation of the IL-6 response. As this model maintains viable intact tissue in the absence of a blood supply, the system is able to specifically isolate the action of IL-6 on the urothelium without the confounding effects of recruited inflammatory cells. Given these unique features, this approach allowed us to isolate changes in gene expression associated with IL-6 induction and tissue remodeling.
The central hypothesis of the described work contends that locally secreted IL-6 binds to urothelial membrane-associated IL-6 receptors and alters gene expression within the mucosa. Upregulated genes encode proteins that modify the inflammatory response, thereby enhancing the repair of the damaged urothelial microenvironment.
MATERIALS AND METHODS
Animals.
Urinary tract infections in both dogs and humans mirror each other, with similar clinical signs caused primarily by uropathogenic strains of E. coli (3, 20, 40); hence, canine urinary bladders were chosen as the experimental tissue in our ex vivo model. Intact urinary bladders were obtained from beagle dogs (aged 6 months to 1 year; Covance Laboratories) immediately after intravenous sodium pentobarbital euthanasia. The urine sterility of each animal was confirmed by aerobic culture (10% blood agar for 14 days at 37°C) of urine samples aspirated from the bladder. All studies were approved by the North Carolina State University Institutional Animal Care and Use Committee.
Ussing chamber parameters.
Urinary bladders were bathed in an oxygenated Ringer's solution (154.1 mM Na+, 6.3 mM K+, 1.2 mM Ca2+, 0.7 mM Mg2+, 137.3 mM Cl−, 24 mM HCO3−, 1.65 mM HPO42−). Sterile Ringer's solutions were filtered (0.22 μm) and treated with antibiotics (streptomycin, 50 μg/ml; penicillin, 50 IU/ml). E. coli-infected Ringer's solutions were prepared similarly; however, antibiotics were omitted. The urinary bladder was bisected longitudinally, and the seromuscular and submucosal layers were removed by sharp dissection. The resulting mucosal sheets (urothelium and lamina propria) were mounted in 3.14-cm2-aperture Ussing chambers, and both surfaces were bathed with Ringer's solution containing glucose (10 mM submucosal) and mannitol (10 mM lumen). Solutions were oxygenated (95% O2, 5% CO2), circulated by gas lift, and maintained at 37°C by water-jacketed reservoirs.
Bacteria.
Uropathogenic E. coli J96, originally isolated from a human patient with pyelonephritis (22) (kindly supplied by Paul Orndorff, North Carolina State University), was grown to log phase at 37°C in Luria-Bertani broth and washed three times in Ringer's solution prior to addition to the lumen reservoir of Ussing-chambered bladder mucosae to achieve a final concentration of 1 × 108 CFU/ml. This concentration of E. coli was chosen based on previously reported work demonstrating that E. coli concentrations of 1 × 108 CFU/ml and 1 × 109 CFU/ml were able to stimulate IL-6 secretion in vivo and in vitro, respectively (15, 17).
Exogenous interleukin-6.
Recombinant canine IL-6 (R&D Systems, Minneapolis, MN) was added to the submucosal reservoir of Ussing-chambered bladder mucosae at a concentration of 20 ng/ml. This recapitulated the 5-h submucosal reservoir concentration of IL-6 induced by the ex vivo infection of the bladder mucosa with uropathogenic E. coli and falls within the range of IL-6 levels measured in humans with naturally occurring UTI (23).
Immunofluorescence microscopy.
After removal from the Ussing chamber, mucosae were embedded in optimal-temperature cutting medium and frozen-sectioned at a 4-μm thickness. Sections were fixed in 100% ethanol and blocked with 1% (vol/wt) bovine serum albumin (BSA) and 2% (vol/vol) goat serum in PBS+ (1× phosphate-buffered saline [PBS], 0.12% 1 M CaCl2 [pH 7.4]) for 1 h at 4°C prior to incubation with primary antibodies. Primary antibodies (1:50 in blocking buffer) were applied for 1 h at room temperature and included biotinylated polyclonal goat anti-canine IL-6 and biotinylated polyclonal goat anti-human soluble IL-6 receptor (R&D Systems, Minneapolis, MN). Fluorescence labeling was performed by using streptavidin-conjugated Alexa Fluor 488 (1:100; Invitrogen, Eugene, OR) and FITC-labeled control mouse IgG1 (1:500; BD Pharmingen, Franklin Lakes, NJ) for 30 min at room temperature. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and visualized by using an epifluorescence microscope. For the demonstration of cytokine synthesis, bladder mucosae were treated with the Golgi protein transport inhibitor monensin (1 μl/ml of 1.5× solution; BioLegend, San Diego, CA) applied to both the lumen and submucosal reservoir of the Ussing chamber.
IL-6 ELISA.
A canine-specific IL-6 chemiluminescence enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) was used to assay IL-6 in duplicate samples obtained from the lumen and submucosal reservoirs bathing the bladder mucosa. Components of the ELISA included capture (800 ng/ml) and detection (25 ng/ml) goat anti-canine polyclonal IL-6 antibodies, streptavidin-horseradish peroxidase (HRP) (1:200 in Femto Luminol/Enhancer), and Femto stable peroxide substrate (Pierce, Woburn, MA). Recombinant canine IL-6 (R&D Systems, Minneapolis, MN) was used to create the standard curve, and results were reported as pg of IL-6 per ml Ringer's solution.
Western blot analysis.
Soluble protein was extracted from liquid nitrogen-frozen samples of bladder mucosa by homogenization (Mini-Beadbeater; BioSpec Products, Bartlesville, OK) with stainless steel beads (3.2 mm) in radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 1% EDTA [pH 7.6]) containing antiproteases (1% each of Halt antiprotease cocktail [Pierce, Woburn, MA] and anti-phosphatase inhibitor cocktails 1 and 2 [Sigma-Aldrich, St. Louis, MO]). Equal protein concentrations, measured by a BCA assay, were electrophoretically separated in 4 to 12% Bis-Tris gradient gels (Invitrogen, Carlsbad, CA), transferred onto nitrocellulose, and sequentially immunoblotted using anti-phosphorylated STAT3 (pSTAT3) (Ser727) (0.5 μg/ml; Assay Designs, Ann Arbor, MI) and anti-total STAT3 (1:2,500; BD Biosciences, San Jose, CA) monoclonal antibodies or goat anti-human polyclonal anti-SOCS3 (1 μg/ml; Life Span Biosciences, Seattle, WA). Secondary reagents included anti-mouse IgG-HRP (1:10,000; Santa Cruz, Santa Cruz, CA) or donkey anti-goat IgG-HRP (1:10,000; Santa Cruz, Santa Cruz, CA), respectively, and a chemiluminescence substrate (Supersignal West Pico; Pierce, Woburn, MA). A pervanadate-treated HepG2 cell lysate (Axxora, San Diego, CA) was included as a positive control for pSTAT3, and a MOLT-4 cell lysate (Santa Cruz, Santa Cruz, CA) was used as a positive control for SOCS3. For the STAT blots, protein loading was normalized by calculating the pSTAT3-to-STAT3 ratio. For the SOCS3 blots, equal protein loading was confirmed by the immunoblotting of cell lysates with monoclonal anti-mouse actin (1:2,000; Chevicon, Billerica, MA), followed by secondary anti-mouse IgG-HRP antibodies (1:5,000; Santa Cruz, Santa Cruz, CA). Densitometry was performed by using SigmaScan software (Systat, San Jose, CA) and is reported as pSTAT3 normalized to total STAT3 or simply SOCS3.
RNA extraction.
Approximately 100 mg of chambered bladder mucosa was placed into 600-μl aliquots of RLT lysis buffer (Qiagen, Valencia, CA) and flash-frozen in liquid nitrogen for storage at −20°C prior to extraction. Tissues were homogenized using two 3.2-mm stainless steel beads (BioSpec Products, Bartlesville, OK) agitated with a Mini-Beadbeater (BioSpec Products, Bartlesville, OK). RNA was extracted from the resultant suspension by using the RNeasy Plus minikit (Qiagen, Valencia, CA) according to the manufacturer's recommendations and stored at −80°C. RNA purity and quality were assessed by using an Agilent Bioanalyzer 2100 (Hewlett Packard, Corvallis, OR). RNA integrity numbers (RIN) for all samples met or exceeded 8.80.
Microarray.
The RNA samples were amplified by using an Affymetrix GeneChip one-cycle cDNA synthesis kit and labeled by using an Affymetrix GeneChip IVT labeling kit (Affymetrix, Santa Clara, CA). The cRNA was hybridized to GeneChip Canine Genome 2.0 arrays (Affymetrix, Santa Clara, CA) containing probes for >20,000 genes. The images were captured by using an Affymetrix GeneChip Scanner 3000 7G (Affymetrix, Santa Clara, CA).
The data were analyzed via principal-components analysis (PCA) to first identify outlier samples. A repeated-measures permutation analysis of differential expression (RM-PADE) was then utilized in combination with an overlap analysis to identify target genes. Genes were required to have a minimum of a 2-fold increase in levels of transcripts over control RNA, a P value of less than 0.05, and concordance in gene expression for all three dogs to be considered for further analysis.
q-RT-PCR.
Seven target genes were validated via quantitative reverse transcriptase PCR (q-RT-PCR) using the above-mentioned extracted RNA. Total RNA was reverse transcribed with random hexamers (Applied Biosystems, Carlsbad, CA) using the Superscript II RT enzyme kit (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Using a Bio-Rad ICycler, 100 ng of converted cDNA was amplified by using 100 nM both forward and reverse primers and SYBR green PCR master mix (Applied Biosciences, Framingham, MA). Primer pairs (Table 1) were designed by using the canine predicted base sequences found in the NCBI database. To ensure that cDNA and not genomic DNA was amplified, all primers were amplified across at least one intron-exon junction. Products of the q-RT-PCR were validated by gel electrophoresis to confirm product sizes. The product melting temperature and sequence were also verified (Davis Sequencing, Davis, CA). The q-RT-PCR negative control was a q-RT-PCR that included no template. Relative fold changes in gene transcription (2−ΔΔCT) were determined by using the hypoxanthine phosphoribosyltransferase (HPRT) canine housekeeping gene and comparative threshold cycle (CT) analysis as described by ABI Prism 7700 sequence detection system user bulletin 2.
TABLE 1.
Primer sequences, product sizes, and melting temperatures used for q-RT-PCR of designated target genes
| Genea | GenBank accession no. | Primer sequence (5′→3′) | Product size (bp) | Product Tmb |
|---|---|---|---|---|
| HPRT | NM_001003357 | CGC TGA GGA TTT GGA AAA AG | 150 | 82.5 |
| AAT CCA GCA GGT CAG CAA AG | ||||
| TNF-α | NM_001003244 | TGC CTG CTG CAC TTT GG | 125 | 84.0 |
| GCT ACT GGC TTG TCA CTT GG | ||||
| MMP-1 | XM_546546 | CGC GTA AAT CCC TTC TAT CC | 157 | 80.5 |
| CAT CCT GAC CCT GAA CAA CC | ||||
| MMP-2 | XM_535300 | TGG AGC AAG AAC AAG AAG ACC | 182 | 82.0 + 85.5 |
| CCC TTG AAG AAG TAG CTA TGA CC | ||||
| MMP-3 | NM_001002967 | ACA CCA GCT GCA TGT GAC C | 123 | 82.0 |
| GAA CCC AGG TTC AAG TGT CC | ||||
| HS3ST3A1 | XM_546631 | TCA TCG GCG TGA AGA AGG | 200 | 91.0 |
| GCG GGT GAC AAA GTA ACT GG | ||||
| IL-1β | NM_001037971 | CAG GAC ATA AGC CAC AAA TAC C | 109 | 80.0 |
| CAA AGC TCA TGT GGA ACA CC | ||||
| HS2 | XM_539153.2 | TTC AGA CAC CAT GCT TGA CC | 142 | 59.5 |
| TCT CAC ACT GCT GAG GAA GG |
HPRT, hypoxanthine phophoribosyltransferase, TNF-α, tumor necrosis factor alpha; MMP-1, matrix metalloproteinase 1; MMP-2, matrix metallopeptidase 2; MMP-3, matrix metallopeptidase 3; HS3ST3A1, heparan sulfate d-glucosaminyl 3-O-sulfotransferase 3A1; IL-1β, interleukin-1β; HS2, hyaluronan synthase 2.
Tm, melting temperature.
Statistical analysis.
Statistical analyses were performed by using commercially available software (SigmaStat software; Systat, San Jose, CA). Values are reported as means ± standard errors. Data were tested for normality and analyzed by using parametric or nonparametric tests where appropriate. Parametric data were analyzed by using the Student t test. Nonparametric data were analyzed by using a Mann-Whitney rank sum test. Data were paired when appropriate. In all cases, n equals the number of dogs.
RESULTS
Uropathogenic E. coli stimulates basolateral secretion of interleukin-6 by the urothelium.
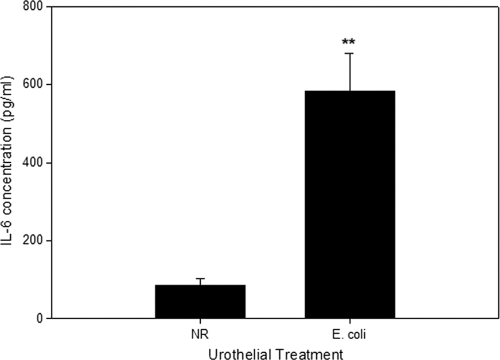
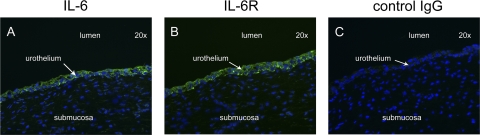
Urothelial cells in culture will secrete IL-6 in response to infection with E. coli (2, 8, 14), and bacterial urinary tract infection in vivo results in increases in urinary IL-6 concentrations (15). Using intact canine bladder mucosae, we sought to first establish if the E. coli-induced synthesis of IL-6 is confined to the urothelium and whether the primary secretion is selectively directed to either the lumen or lamina propria of the bladder. Five hours after the inoculation of E. coli J96 (1 × 108 CFU/ml) into the luminal reservoir of the Ussing chamber containing bladder mucosa, significant increases in concentrations of IL-6 were measured in the submucosal reservoir but not in the luminal reservoir (Fig. 1). The identification of IL-6-secreting cells by means of immunofluorescence demonstrated that IL-6 synthesis was restricted to the urothelial cells, with no apparent contribution by cells residing in the underlying lamina propria. Moreover, all layers of the urothelium were identified as participating in the synthesis of IL-6 (Fig. 2A).
FIG. 1.
Secretion of IL-6 by urothelium in response to normal Ringer's solution (NR) alone or luminal infection with uropathogenic E. coli J96 (1 × 108 CFU/ml). IL-6 concentrations were measured in the submucosal reservoir of the Ussing chamber after 5 h of infection. IL-6 was below the limit of detection in the lumen reservoir (data not shown). **, P < 0.01 (n = 6 dogs for each treatment condition).
FIG. 2.
Immunofluorescence localization of IL-6 and IL-6 receptors in E. coli-treated canine urothelium. Urothelia mounted in Ussing chambers were exposed to 1 × 108 CFU/ml of E. coli J96. The tissue was simultaneously exposed to monensin. (A and B) Both IL-6 (A) and IL-6 receptors (IL-6R) (B) were restricted in location to the urothelial cells. (C) Fluorescence was not observed in sections incubated with mouse IgG1 control antibodies.
The urothelium is an autocrine recipient of IL-6 signals in response to uropathogenic E. coli infection.
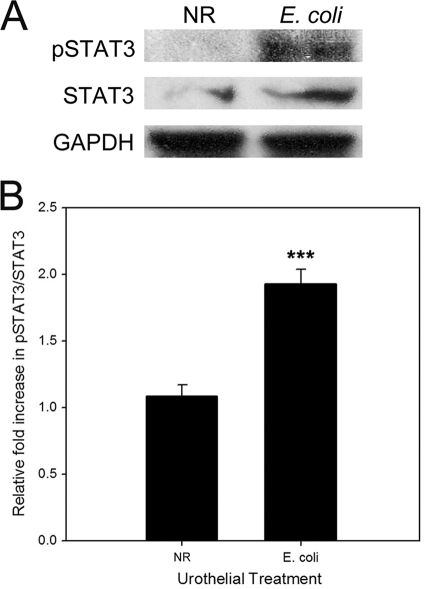
While the synthesis of IL-6 is a salient response of the urinary bladder to bacterial infection, the target cell type(s) and functional effects of IL-6 remain unknown. To identify the cellular target(s) of IL-6, we located IL-6 receptor-expressing cells by means of immunofluorescence. In both uninfected and E. coli-infected bladder mucosa, the constitutive expression of IL-6 receptors was confined to the urothelial cell layer. There was little evidence to support IL-6 receptor expression by cells residing within the underlying lamina propria (Fig. 2B). A nonspecific binding of antibody was not observed for tissues treated with nonimmune mouse IgG1 (Fig. 2C). We next examined if IL-6-mediated signaling pathways were activated within the urothelium in response to E. coli exposure. Accordingly, uninfected and E. coli-infected urothelia were removed from the Ussing chamber after 5 h, and the cellular lysates were immunoblotted for total and phosphorylated STAT3. A significant phosphorylation of STAT3 was observed for urothelia infected with E. coli compared to uninfected urothelia, indicating that an IL-6-dependent signaling pathway was activated in the E. coli-treated tissues (Fig. 3).
FIG. 3.
Infection of canine urothelium with E. coli promotes phosphorylation of STAT3. After a 5-h treatment in Ussing chambers with normal Ringer's solution (NR) or uropathogenic E. coli J96 (1 × 108 CFU/ml), canine bladder mucosae were immunoblotted for phosphorylated STAT3 (pSTAT3), total STAT3, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (protein loading control). E. coli significantly increased the phosphorylation of STAT3 compared to uninfected tissue (normal Ringer's solution). Densitometric data represent relative fold increases in pSTAT3 compared to total STAT3. ***, P < 0.001 (n = 4 dogs each).
Exogenous IL-6 is sufficient to activate urothelial gene transcription.
As a prelude to examining the isolated effect of IL-6 on urothelial gene expression, we first examined whether exogenous IL-6 alone was sufficient to activate urothelial STAT3 signaling in a manner similar to that observed with E. coli. Accordingly, tissue was exposed to normal Ringer's solution (NR) or exogenous canine recombinant IL-6 at a polarity and a concentration that recapitulated E. coli-induced IL-6 secretion. After 5 h of treatment, protein lysates were prepared from the harvested urothelium and immunoblotted for total and phospho-STAT3. Unexpectedly, there was no difference in STAT3 phosphorylation between the IL-6- and NR-treated tissues (P = 0.12).
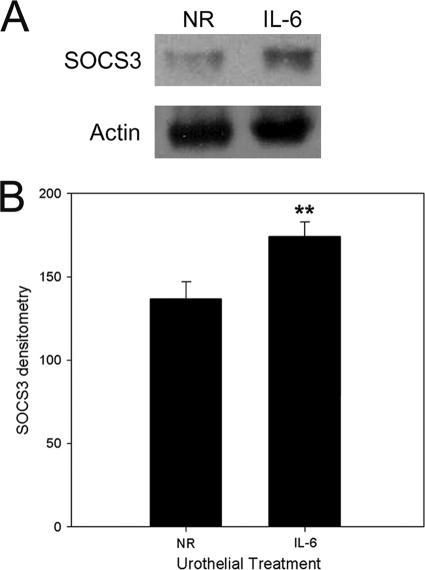
In contrast to E. coli, which must first stimulate IL-6 secretion before STAT signaling is activated, exogenous IL-6 activates STAT signaling immediately. Therefore, we were suspicious that negative regulatory factors such as SOCS3 may have reduced pSTAT3 concentrations in the urothelial cells after 5 h of direct exposure to IL-6. Since pSTAT3 induces the transcription of SOCS3, the demonstration of an increased expression of SOCS3 would support this observation as well as urothelial gene expression in response to the exogenously added IL-6.
Predictably, urothelia mounted within Ussing chambers and exposed to exogenous IL-6 had significantly more SOCS3 protein detected by immunoblotting than the NR control (P < 0.05) (Fig. 4). These results support the hypothesis that exogenously added IL-6 activates STAT3 signaling and SOCS3 expression and confirmed that exogenous IL-6 is sufficient to mediate urothelial cell transcription during the 5-h experimental timeframe.
FIG. 4.
Exogenous IL-6 activates urothelial cells and initiates transcription. After a 5-h treatment in Ussing chambers with normal Ringer's solution (NR), exogenous IL-6 canine bladder mucosae were immunoblotted for SOCS3 and actin (protein loading control). Exogenous IL-6 significantly increased SOCS3 production compared to untreated tissue (NR). **, P < 0.05 (n = 4 dogs each).
Differential urothelial gene expression induced by IL-6.
To determine the identity of urothelial genes whose transcription is induced by IL-6, sheets of canine urothelium were treated with exogenous canine recombinant IL-6 or the NR control for a period of 5 h, after which the tissue was harvested from the Ussing chambers and the RNA was extracted. Comparing gene lists generated from PCA, overlap, and random-measure statistical analyses, a total of 49 common genes had ≥2-fold increases (Table 2) or decreases (Table 3) in gene expression, with concordant results for all dogs, and a P value of <0.05. Seven target genes important for acute inflammation and tissue remodeling were selected for verification by q-RT-PCR, including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), matrix metalloproteinase 1 (MMP-1), matrix metallopeptidase 2 (MMP-2), matrix metallopeptidase 3 (MMP-3), heparan sulfate d-glucosaminyl 3-O-sulfotransferase 3A1 (HS3ST3A1), and hyaluronan synthase 2 (HS2). Increases in levels of gene transcription (fold change ± SE) were confirmed by q-RT-PCR as statistically significant for TNF-α (3.98 ± 1.40), IL-1β (3.20 ± 0.85), MMP-2 (2.79 ± 0.41), HS3ST3A1 (4.40 ± 1.15), and hyaluronan synthase 2 (2.81 ± 0.66) compared to tissue treated with NR alone (P < 0.05).
TABLE 2.
IL-6-dependent urothelial transcriptome upregulation
| Functional category and Affymetrix ID | Fold change | Max P value | Gene description |
|---|---|---|---|
| Cytokine/chemokine response | |||
| Cfa.54.1.S1_s_at | 4.73 | 0.000060 | Tumor necrosis factor |
| CfaAffx.11741.1.S1_s_at | 2.92 | 0.000040 | Interleukin 1 beta |
| Cfa.3554.1.S1_at | 2.78 | 0.000040 | Interleukin 1 beta |
| CfaAffx.21295.1.S1_at | 2.84 | 0.000040 | Chemokine (C-C motif) receptor-like 2 |
| Cfa.14352.1.A1_at | 2.68 | 0.000040 | Chemokine (C-C motif) ligand 3 |
| Cfa.15815.1.S1_s_at | 2.05 | 0.000080 | Chemokine (C-C motif) ligand 3 |
| Cfa.16590.1.S1_s_at | 2.36 | 0.000040 | Chemokine (C-X-C motif) ligand 10 |
| Cfa.16590.1.S2_at | 2.22 | 0.000040 | Chemokine (C-X-C motif) ligand 10 |
| CfaAffx.1705.1.S1_at | 2.20 | 0.000046 | Gamma interferon-inducible protein 47 |
| Cfa.3528.1.S1_s_at | 2.14 | 0.000156 | Interleukin-6 |
| Matrix metallopeptidases | |||
| Cfa.3447.1.S1_s_at | 3.28 | 0.000040 | Matrix metallopeptidase 3 |
| CfaAffx.23166.1.S1_s_at | 3.10 | 0.000040 | Matrix metalloproteinase 1 |
| CfaAffx.23166.1.S1_at | 2.62 | 0.000040 | Matrix metalloproteinase 1 |
| Cfa.3597.1.S1_at | 2.13 | 0.000260 | Matrix metallopeptidase 2 |
| CfaAffx.14851.1.S1_s_at | 2.03 | 0.000046 | Matrix metallopeptidase 2 |
| Mediators of cell adhesion | |||
| Cfa.3868.1.S1_at | 3.20 | 0.000040 | Selectin E |
| CfaAffx.23326.1.S1_s_at | 2.51 | 0.000040 | Selectin E |
| CfaAffx.23464.1.S1_s_at | 3.00 | 0.001104 | Dermatopontin precursor |
| Cfa.6133.1.S1_at | 2.02 | 0.000040 | Dermatopontin precursor |
| CfaAffx.13511.1.S1_at | 2.93 | 0.000040 | Immunoglobulin superfamily, member 10 |
| Cfa.3983.1.A1_s_at | 2.45 | 0.000260 | Immunoglobulin superfamily, member 10 |
| Glycosaminoglycan production | |||
| CfaAffx.27384.1.S1_s_at | 2.46 | 0.000054 | Heparan sulfate d-glucosaminyl 3-O-sulfotransferase 3A1 |
| CfaAffx.2351.1.S1_at | 2.14 | 0.000334 | Hyaluronan synthase 2 |
| CfaAffx.24924.1.S1_at | 2.38 | 0.000060 | Colony-stimulating factor 3 isoform b precursor |
| CfaAffx.4373.1.S1_s_at | 2.21 | 0.000040 | Bone morphogenetic protein 5 precursor (BMP-5) |
| Cfa.9659.1.A1_at | 2.16 | 0.000104 | Vascular smooth muscle cell growth-promoting factor |
| Growth and differentiation factors | |||
| Cfa.12671.1.A1_at | 2.14 | 0.000546 | Signaling lymphocyte activation molecule family member 7 |
| CfaAffx.5498.1.S1_at | 2.12 | 0.000040 | CXCL7; leukocyte-derived growth factor; macrophage-derived growth factor |
| CfaAffx.18191.1.S1_s_at | 2.11 | 0.002402 | Myeloid cell nuclear differentiation antigen |
| CfaAffx.5548.1.S1_at | 2.10 | 0.000546 | Epiregulin |
| Cfa.3484.1.S1_at | 2.05 | 0.002972 | Progesterone receptor |
| Negative regulators | |||
| CfaAffx.1046.1.S1_at | 2.34 | 0.002972 | Urokinase inhibitor |
| CfaAffx.2230.1.S1_s_at | 2.31 | 0.000040 | Thrombospondin 2 precursor |
| CfaAffx.1632.1.S1_s_at | 2.26 | 0.000040 | Radiation-inducible immediate-early gene IEX-1 |
| CfaAffx.13597.1.S1_s_at | 2.09 | 0.000614 | Chondroitin sulfate proteoglycan 2 |
| CfaAffx.25712.1.S1_at | 2.02 | 0.000040 | Jumonji domain-containing 3 |
| Miscellaneous | |||
| CfaAffx.13553.1.S1_at | 2.83 | 0.001932 | Succinate receptor 1 |
| Cfa.13912.1.A1_at | 2.35 | 0.000104 | Ribonucleotide reductase small chain |
| Cfa.3973.1.A1_at | 2.35 | 0.000984 | Proteasome alpha 3 subunit isoform 2 |
| Cfa.1370.1.A1_at | 2.33 | 0.000040 | Solute carrier family 2 |
| Cfa.825.1.S2_at | 2.10 | 0.000040 | Solute carrier family 2 |
| CfaAffx.13491.1.S1_at | 2.22 | 0.000482 | G-protein-coupled receptor 171 |
| CfaAffx.4930.1.S1_s_at | 2.21 | 0.000070 | Alpha 1 type XII collagen short-isoform precursor |
| CfaAffx.1247.1.S1_s_at | 2.18 | 0.000040 | Vanin 1 |
| Cfa.6170.1.A1_at | 2.09 | 0.001384 | GMP-PDE gammaa |
| CfaAffx.14532.1.S1_at | 2.08 | 0.000040 | Nuclear receptor subfamily 4, group A, member 2 |
| Cfa.1416.1.A1_at | 2.03 | 0.000876 | Proenkephalin A precursor |
| Cfa.3589.1.S1_s_at | 2.03 | 0.000070 | Caspase 4, apoptosis-related cysteine peptidase |
PDE, phosphodiestrase.
TABLE 3.
IL-6-dependent urothelial transcriptome downregulation
| Functional category and Affymetrix ID | Fold change | Max P value | Gene description |
|---|---|---|---|
| Cytoskeleton | |||
| CfaAffx.23771.1.S1_s_at | 4.03 | 0.000040 | Desmin |
| Cfa.45.1.S2_at | 3.67 | 0.000614 | Desmin |
| Cfa.12195.5.S1_at | 3.15 | 0.000080 | Actin |
| CfaAffx.11658.1.S1_s_at | 2.97 | 0.002972 | Myosin binding protein C |
| CfaAffx.835.1.S1_s_at | 2.87 | 0.001236 | Myosin regulatory light chain 2 |
| Cfa.12198.1.A1_at | 2.40 | 0.000080 | Myosin regulatory light chain 2 |
| Cfa.12841.1.S1_at | 2.11 | 0.000334 | Similar to alpha-1-syntrophin |
| Transport proteins | |||
| CfaAffx.24259.1.S1_at | 2.28 | 0.000692 | Mitochondrial 2-oxoglutarate/malate carrier protein |
| Cfa.305.1.A1_at | 2.04 | 0.000294 | Heat shock 22-kDa protein 8 |
| Cfa.305.1.A1_at | 2.04 | 0.000294 | Heat shock 22-kDa protein 8 |
| Miscellaneous | |||
| Cfa.9045.1.A1_s_at | 2.72 | 0.000228 | EF hand calcium binding domain 1 |
| CfaAffx.24807.1.S1_at | 2.54 | 0.000692 | Glutathione peroxidase-related protein 2 |
| Cfa.12202.1.A1_at | 2.33 | 0.000040 | Glutathione peroxidase-related protein 2 |
| Cfa.17108.1.S1_at | 2.41 | 0.000544 | Mesothelin isoform 1 preproprotein |
DISCUSSION
A key biological response of the bladder to urinary tract infection is the secretion of IL-6 (15). While not normally present in the urine (16), IL-6 is commonly found in the urine of patients with cystitis or pyelonephritis (16, 32) and appears in human urine within 4 h of experimental infection with E. coli (15). Using cell culture models, previous studies have shown that urothelial cells secrete IL-6 in response to infection with E. coli (2, 8, 14). Whether IL-6 synthesis is confined to the urothelium, is secreted directly into the urine, or translocates into the urine secondary to an increase in bladder permeability is unknown. By clarifying the source and polarity of IL-6 secretion in the context of the entire bladder mucosa, the present studies have provided insights into the cellular targets and function of this cytokine.
Using an ex vivo preparation of canine bladder mucosa, these studies demonstrate that IL-6 is synthesized by all layers of the urothelium and is secreted exclusively into the underlying lamina propria but was not secreted into the luminal chamber after exposing the urothelium to E. coli. Whereas elevations in urine IL-6 concentrations were measured within hours of experimental urinary tract infection in humans (15), similar elevations were not detected during the same time frame in our ex vivo studies. A key difference between the two models is the absence of recruited inflammatory cells ex vivo compared to the immunologically intact host. These observations suggest that IL-6 may secondarily leak into the urine in association with the influx and transmigration of neutrophils (4). This may further explain why urine IL-6 concentrations appear to correlate with clinical disease severity, as the inflammatory process may be responsible for disrupting barrier function, resulting in the leakage of IL-6 from the urothelium (32).
While IL-6 is a predominant cytokine secreted by the urothelium in response to urinary tract infection, the cellular target and biological responses mediated by IL-6 in this microenvironment have remained poorly understood. In this Ussing chamber model, the urothelium was the only site of unequivocal IL-6 receptor expression within the bladder mucosa. As such, urothelial cells appear to be key autocrine recipients of IL-6 signals, an assertion which is supported by our demonstration of increased urothelial STAT3 signaling and SOCS3 expression. However, a direct effect of E. coli on STAT3 activation cannot be ruled out.
A local autocrine response of the urothelium to IL-6 is a potentially important observation. Despite being a frequent topic of research investigations, a consensus regarding the precise role of IL-6 in the various epithelia of different body systems remains ill defined. Generally, during acute injury, IL-6 has been associated with an attempt to return the tissue to a state of homeostasis. For both the epidermis and respiratory epithelium, IL-6 has been identified as being important for promoting tissue barrier repair after injury (44, 45). In models of endotoxic lung injury and endotoxemia, the presence of IL-6 is associated with decreased TNF-α concentrations and neutrophil infiltration (28, 46). Increased concentrations of IL-6 have also been found to promote the production of anti-TNF-α and anti-IL-1β molecules to modulate the inflammatory response (1, 39, 42). In contrast, IL-6 has been associated with proinflammatory actions such as increased neutrophil recruitment during E. coli pneumonia and when lung tissue is exposed to certain Gram-positive cell wall components such as peptidoglycan (PepG) (24, 25, 37). Given these seemingly opposing effects, the cellular role of IL-6 within different tissues should be investigated further. If applicable to other organ systems, our findings are important for the ongoing development and use of pharmaceuticals that alter IL-6 responses (31, 38).
Because patient morbidity during UTI is associated with the inflammatory response to bacteria (41), an understanding of the extent to which IL-6 augments or blunts this response is essential. By specifically targeting the intact urothelium using the Ussing chamber model, the role of IL-6 in gene transcription and protein expression can be studied. Using microarrays and q-RT-PCR, 5 genes that appear to play a role in IL-6-induced urothelial inflammation were identified.
In contrast to the effects observed for other tissues, IL-6 upregulated the transcription of genes in the urothelium that are responsible for both TNF-α and IL-1β synthesis. These two cytokines are known proinflammatory molecules with important functions during the acute-phase immune response, including promoting the migration of inflammatory cells and the production of cyclooxygenase-2, type 2 phospholipase A, and inducible nitric oxide synthase (5). These roles argue against the proposition that IL-6 is an anti-inflammatory cytokine within the urothelium. While it is generally accepted that these two early-response cytokines are responsible for inducing local IL-6 production (29), a direct effect of IL-6 on TNF-α and IL-1β expression would be a novel observation and suggests a urothelial positive-feedback circuit that promotes tissue inflammation.
The upregulation of the MMP-2, HS3ST3A1, and HS2 genes provides additional evidence supporting positive-feedback effects of IL-6 within the urothelium. MMP-2 converts the IL-1β precursor to its active form (5, 10). Increasing concentrations of IL-1β would augment the inflammatory response within the epithelium. Heparan sulfate d-glucosaminyl 3-O-sulfotransferase 3A1 is an enzyme responsible for the synthesis of heparan sulfate. Heparan sulfate is a glycosaminoglycan carbohydrate that during inflammatory processes interacts with heparan binding proteins to play integral roles in leukocyte extravasation and chemotaxis as well as promote the release of the above-mentioned inflammatory cytokines TNF-α and IL-1 from macrophages (11). The HS2 gene also synthesizes a glycosaminoglycan carbohydrate, hyaluronan. As such, HS2 may play a role similar to that of HS3ST3A1, as hyaluronan has been associated with the increased macrophage production of IL-1 (19).
The importance of the IL-6-mediated upregulation of the HS3ST3A1 and HS2 genes is not limited to the production of proinflammatory cytokines. Both of these genes have potentially important effects on wound repair. As stated above, these enzymes are responsible for the production of glycosaminoglycan components. In the urinary bladder GAG serves a particularly important role in tissue defense as a mucus-rich GAG layer that lines the luminal surface of the bladder (35). This anionic layer is hydrophilic and traps water, creating a wall of water between the urothelial cells and the urine, which contains cationic solutes and potentially bacteria (33). Prior research demonstrated that a disruption of the GAGs will increase epithelial cell permeability (34). IL-6-induced heparan sulfate and hyaluronan production may represent a preemptive effort to repair a damaged GAG layer. If the increased production of these components can be linked to GAG layer repair, it would seem probable that they may also increase barrier function. Treatment with GAG replacers such as pentosan polysulfate sodium and chondroitin sulfate has shown promise in relieving pain and discomfort associated with interstitial cystitis as well as preventing bacterial adherence and barrier repair (13, 33). Therefore, a potential role for IL-6 in increasing GAG production during infection is a potentially important observation that may provide an alternate pathway for targeting therapy.
In conclusion, by isolating the urothelial microenvironment from recruited inflammatory cells, the present studies demonstrate that the inoculation of uropathogenic E. coli results in the synthesis of IL-6 by all urothelial layers, with selective secretion into the lamina propria. Furthermore, the urothelium was the only site of unequivocal IL-6 receptor expression identified within the resident bladder mucosa. In addition, autocrine effects of IL-6 were supported by the activation of urothelial STAT3 signaling and SOCS3 production. IL-6 also mediated the expression of genes responsible for the production of TNF-α and IL-β through both direct and indirect mechanisms. Besides promoting the synthesis of proinflammatory cytokines, the upregulation of the HS3ST3A1 and HS2 genes by IL-6 suggests a possible role for IL-6 in urothelial tissue repair and as a defense against invading bacteria. At this time, the latter evidence remains circumstantial.
Further application of the Ussing chamber model is likely to provide an important and relevant tool for identifying mechanisms of E. coli urothelial pathogenesis and for establishing the role of IL-6 in promoting or ameliorating the urothelial immune response. The model facilitates the isolation of the urothelial response to IL-6 following bacterial infection without the confounding effects of inflammatory cells. Future assessments of the bacterial/cytokine interplay and how manipulating the IL-6 response affects urothelial defense against bacterial colonization are crucial to our understanding of the mucosal response to UTI.
Acknowledgments
Michael W. Wood was supported by the Ruth L. Kirschstein National Research Service award (T32 RR024394) as part of North Carolina State University's Comparative Medicine and Translational Research Training Program.
We thank Maria Stone, Stephen Stauffer, Mitsu Suyemoto, and the Laboratory for Advanced Electron and Light Optical Methods for their valuable technical assistance.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 29 November 2010.
REFERENCES
- 1.Aderka, D., J. M. Le, and J. Vilcek. 1989. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human-monocytes, U937 cells, and in mice. J. Immunol. 143:3517-3523. [PubMed] [Google Scholar]
- 2.Agace, W., et al. 1993. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect. Immun. 61:602-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartges, J. W. 2004. Diagnosis of urinary tract infections. Vet. Clin. North Am. Small Anim. Pract. 34:923-933. [DOI] [PubMed] [Google Scholar]
- 4.Chin, A. C., W. Y. Lee, A. Nusrat, N. Vergnolle, and C. A. Parkos. 2008. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J. Immunol. 181:5702-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinarello, C. A. 2003. Interleukin=1 family [IL=1∓1, F2], p. 643-668. In A. W. Thompson and M. T. Lotze (ed.), The cytokine handbook, 4th ed., vol. 2. Academic Press, Boston, MA. [Google Scholar]
- 6.Foxman, B., R. Barlow, H. D'Arcy, B. Gillespie, and J. D. Sobel. 2000. Urinary tract infection: self-reported incidence and associated costs. Ann. Epidemiol. 10:509-515. [DOI] [PubMed] [Google Scholar]
- 7.Foxman, B., and R. R. Frerichs. 1985. Epidemiology of urinary-tract infection. 2. Diet, clothing, and urination habits. Am. J. Public Health 75:1314-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funfstuck, R., et al. 2001. Secretion of cytokines by uroepithelial cells stimulated by Escherichia coli and Citrobacter spp. Int. J. Antimicrob. Agents 17:253-258. [DOI] [PubMed] [Google Scholar]
- 9.Gabay, C. 2006. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 8(Suppl. 2):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill, S. E., and W. C. Parks. 2008. Metalloproteinases and their inhibitors: regulators of wound healing. Int. J. Biochem. Cell Biol. 40:1334-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotte, M. 2003. Syndecans in inflammation. FASEB J. 17:575-591. [DOI] [PubMed] [Google Scholar]
- 12.Hang, L., B. Wullt, Z. X. Shen, D. Karpman, and C. Svanborg. 1998. Cytokine repertoire of epithelial cells lining the human urinary tract. J. Urol. 159:2185-2192. [DOI] [PubMed] [Google Scholar]
- 13.Hauser, P. J., et al. 2009. Restoring barrier function to acid damaged bladder by intravesical chondroitin sulfate. J. Urol. 182:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedges, S., et al. 1994. Uroepithelial cells are part of a mucosal cytokine network. Infect. Immun. 62:2315-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedges, S., P. Anderson, G. Lidin-Janson, P. de Man, and C. Svanborg. 1991. Interleukin-6 response to deliberate colonization of the human urinary tract with Gram-negative bacteria. Infect. Immun. 59:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedges, S., et al. 1992. Comparison of urine and serum concentrations of interleukin-6 in women with acute pyelonephritis or asymptomatic bacteriuria. J. Infect. Dis. 166:653-656. [DOI] [PubMed] [Google Scholar]
- 17.Hedges, S., M. Svensson, and C. Svanborg. 1992. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect. Immun. 60:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicks, R. M., B. Ketterer, and R. C. Warren. 1974. Ultrastructure and chemistry of the luminal plasma membrane of mammalian urinary bladder: a structure with low permeability to water and ions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 268:23-38. [DOI] [PubMed] [Google Scholar]
- 19.Hiro, D., A. Ito, K. Matsuta, and Y. Mori. 1986. Hyaluronic acid is an endogenous inducer of interleukin-1 production by human monocytes and rabbit macrophages. Biochem. Biophys. Res. Commun. 140:715-722. [DOI] [PubMed] [Google Scholar]
- 20.Hooton, T. M., and W. E. Stamm. 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. North Am. 11:551-581. [DOI] [PubMed] [Google Scholar]
- 21.Hu, P., et al. 2002. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am. J. Physiol. Renal Physiol. 283:F1200-F1207. [DOI] [PubMed] [Google Scholar]
- 22.Hull, R. A., R. E. Gill, P. Hsu, B. H. Minshew, and S. Falkow. 1981. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect. Immun. 33:933-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jantausch, B. A., R. O'Donnell, and B. L. Wiedermann. 2000. Urinary interleukin-6 and interleukin-8 in children with urinary tract infection. Pediatr. Nephrol. 15:236-240. [DOI] [PubMed] [Google Scholar]
- 24.Jones, M. R., et al. 2006. Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia. J. Infect. Dis. 193:360-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leemans, J. C., M. Vervoordeldonk, S. Florquin, K. P. van Kessel, and T. van der Poll. 2002. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am. J. Respir. Crit. Care Med. 165:1445-1450. [DOI] [PubMed] [Google Scholar]
- 26.Lewis, S. A. 2000. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am. J. Physiol. Renal Physiol. 278:F867-F874. [DOI] [PubMed] [Google Scholar]
- 27.Lewis, S. A., D. C. Eaton, and J. M. Diamond. 1976. The mechanism of Na+ transport by rabbit urinary bladder. J. Membr. Biol. 28:41-70. [DOI] [PubMed] [Google Scholar]
- 28.Mehrad, B., and T. J. Standiford. 1999. Role of cytokines in pulmonary antimicrobial host defense. Immunol. Res. 20:15-27. [DOI] [PubMed] [Google Scholar]
- 29.Naugler, W. E., and M. Karin. 2008. The wolf in sheep's clothing: the role of interieukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 14:109-119. [DOI] [PubMed] [Google Scholar]
- 30.Negrete, H. O., J. P. Lavelle, J. Berg, S. A. Lewis, and M. L. Zeidel. 1996. Permeability properties of the intact mammalian bladder epithelium. Am. J. Physiol. 271:F886-F894. [DOI] [PubMed] [Google Scholar]
- 31.Ohsugi, Y. 2007. Recent advances in immunopathophysiology of interleukin-6: an innovative therapeutic drug, tocilizumab (recombinant humanized anti-human interieukin-6 receptor antibody), unveils the mysterious etiology of immune-mediated inflammatory diseases. Biol. Pharm. Bull. 30:2001-2006. [DOI] [PubMed] [Google Scholar]
- 32.Otto, G., J. Braconier, A. Andreasson, and C. Svanborg. 1999. Interleukin-6 and disease severity in patients with bacteremic and nonbacteremic febrile urinary tract infection. J. Infect. Dis. 179:172-179. [DOI] [PubMed] [Google Scholar]
- 33.Parsons, C. L. 2007. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 69:9-16. [DOI] [PubMed] [Google Scholar]
- 34.Parsons, C. L., D. Boychuk, S. Jones, R. Hurst, and H. Callahan. 1990. Bladder surface glycosaminoglycans: an epithelial permeability barrier. J. Urol. 143:139-142. [DOI] [PubMed] [Google Scholar]
- 35.Parsons, C. L., and S. G. Mulholland. 1978. Bladder surface mucin—its antibacterial effect against various bacterial species. Am. J. Pathol. 93:423-432. [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons, C. L., C. W. Stauffer, and J. D. Schmidt. 1988. Reversible inactivation of bladder surface glycosaminoglycan antibacterial activity by protamine sulfate. Infect. Immun. 56:1341-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinton, L. J., et al. 2008. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am. J. Respir. Cell Mol. Biol. 38:699-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose-John, S., G. H. Waetzig, J. Scheller, J. Grotzinger, and D. Seegert. 2007. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin. Ther. Targets 11:613-624. [DOI] [PubMed] [Google Scholar]
- 39.Schindler, R., et al. 1990. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 75:40-47. [PubMed] [Google Scholar]
- 40.Senior, D. F. 2007. Management of urinary tract infections, p. 282-289. In J. Elliott and G. Grauer (ed.), BSAVA manual of canine and feline nephrology and urology, 2nd ed. British Small Animal Veterinary Association, Gloucester, United Kingdom.
- 41.Svanborg, C., G. Godaly, and M. Hedlund. 1999. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Curr. Opin. Microbiol. 2:99-105. [DOI] [PubMed] [Google Scholar]
- 42.Tilg, H., E. Trehu, M. B. Atkins, C. A. Dinarello, and J. W. Mier. 1994. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor P55. Blood 83:113-118. [PubMed] [Google Scholar]
- 43.Umegaki, H., et al. 1996. Protective effect of interleukin-6 against the death of PC12 cells caused by serum deprivation or by the addition of a calcium ionophore. Biochem. Pharmacol. 52:911-916. [DOI] [PubMed] [Google Scholar]
- 44.Wang, X. P., et al. 2004. The interleukin-6 cytokine system regulates epidermal permeability barrier homeostasis. J. Invest. Dermatol. 123:124-131. [DOI] [PubMed] [Google Scholar]
- 45.Wolters, P. J., et al. 2009. Neutrophil-derived IL-6 limits alveolar barrier disruption in experimental ventilator-induced lung injury. J. Immunol. 182:8056-8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xing, Z., et al. 1998. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest. 101:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]