Abstract
Thrombopoietin (Tpo), which primarily regulates megakaryopoiesis, and its receptor (c-Mpl) are expressed in the brain, where Tpo exhibits proapototic effects on neurons. In the present study, we investigated the implication of Tpo in experimental pneumococcal meningitis. Following intrathecal infection with the encapsulated Streptococcus pneumoniae strain D39, we observed upregulation of Tpo mRNA expression at 12 h and 24 h in brain homogenates of wild-type C57BL/6 mice. c-Mpl mRNA expression was upregulated at 12 h and returned to baseline at 24 h. Compared to wild-type mice, mutants with homozygous Tpo receptor ablation (c-Mpl−/−) displayed reduced microglial activation and neuronal apoptosis in the dentate gyrus. Concentrations of bacteria in blood or cerebrospinal fluid (CSF), as well as CSF pleocytosis, were not significantly different between wild-type and c-Mpl−/− mice. In human postmortem brain, Tpo protein was colocalized to macrophages during encephalitis. In murine primary microglia and RAW264.7 macrophages, upregulation of Tpo mRNA was induced by D39-conditioned medium but not by bacterial lipopeptide or by medium conditioned by pneumococcal mutants defective in hydrogen peroxide formation (ΔspxB) or pneumolysin (Δpln). We conclude that Tpo acts as a mediator of neuronal damage in bacterial meningitis.
Acute bacterial meningitis is a life-threatening disease. Even with modern therapies, mortality of pneumococcal meningitis, the most prevalent subgroup, reaches 30% (35). There is a high frequency of neurological sequelae in surviving patients. Neuropsychological deficits have been linked to neuronal loss and resulting atrophy of hippocampal structures (13, 16, 28). Both bacterial toxins and host-derived mediators contribute to neuronal damage (36). In the case of Streptococcus pneumoniae, hydrogen peroxide and pneumolysin have been identified as two major bacterial exotoxins with neurotoxic properties (4, 5, 18). The initial host immune responses to bacterial invasion of the cerebrospinal fluid (CSF) are driven by the influx of granulocytes and monocytes from the blood and by the activation of local microglia (23). Activated immune cells release cytotoxic products, including reactive nitrogen and oxygen species as well as proapoptotic cytokines such as tumor necrosis factor alpha (TNF-α).
Thrombopoietin (Tpo), a 70-kDa glycoprotein, is the primary regulator of megakaryopoiesis (22). It is mainly synthesized in the liver, with additional production in the kidneys (38). Prior to the isolation of Tpo, its receptor was identified as the cellular proto-oncogene targeted by murine myeloproliferative leukemia virus and was therefore named c-Mpl. c-Mpl is predominantly expressed on megakaryocytes, mature platelets, and a subset of CD34+ hematopoietic stem cells (27). Tpo plasma concentrations are not primarily related to changes in hepatic synthesis but rather depend on the removal of circulating Tpo via c-Mpl binding by megakaryocytic progenitors and platelets, thus providing an end-cell-mediated regulation (10). However, synthesis of Tpo in the liver can be increased by interleukin-6 (IL-6) in the context of systemic inflammation and infection (21). Importantly, both Tpo expression and c-Mpl expression have also been detected in nonhematopoietic tissues, including the brain (9, 11, 20, 26). Proaptotic effects of Tpo on neurons were reported in the context of hypoxia and ischemia (12), suggesting a neurotoxic effect of Tpo in brain diseases.
The effects of Tpo have not been studied in acute brain inflammation. We have previously reported increased concentrations of Tpo protein in the CSF of neonates suffering from meningitis or sepsis (30). Our present study was therefore designed to investigate a potential role of Tpo as a modulator of neuronal damage in acute central nervous system (CNS) infection.
MATERIALS AND METHODS
Bacteria and culture.
Meningitis was induced by inoculation with encapsulated serotype 2 Streptococcus pneumoniae strain D39. Bacteria were grown to log phase in casein plus yeast liquid medium (C+Y medium) at 37°C in the presence of 5% CO2 (24). Using a standard curve, the number of CFU per ml was determined photometrically to obtain defined inocula of 5 × 105 CFU per animal, diluted in phosphate-buffered saline (PBS). To produce bacterial conditioned media for cell culture experiments, D39 and its pneumolysin A-defective Δpln (3) and H2O2-defective ΔspxB (32) isogenic mutants were grown to log phase in C+Y medium and then transferred to the appropriate cell culture medium at a final concentration of 107 CFU/ml. After 3 h of incubation at 37°C with 5% CO2, the supernatant was purified by passing it through a 0.45-nm-pore-size filter (Becton Dickinson) and stored at −80°C until use.
Murine model of meningitis.
All animal experiments fully complied with federal and institutional guidelines and were approved by state authorities. Experiments were conducted in 8- to 12-week-old C57BL/6 wild-type mice and transgenic mice with homozygous c-Mpl ablation (c-Mpl−/−) (1), using a well-established model of experimental meningitis (15-18). During anesthesia with intraperitoneal ketamine (100 mg/kg of body weight; DeltaSelect) and xylazine (20 mg/kg; Bayer), a skin incision was made to expose the lumbar spine. Using a 30-gauge needle, 40 μl of bacterial suspension containing 5 × 105 CFU was slowly injected into the spinal canal below vertebra L2 or L3. This relatively large volume was applied, considering a potential leakage of the inoculum at the site of injection. Noninfected controls received an equal volume of pyrogen-free PBS. In additional mice, sterile meningeal inflammation was induced by intrathecal injection of the synthetic bacterial lipopeptide Pam3CysSK4 (10 μg per animal; EMC Microcollections) (15). Following wound closure with dermal clips, animals were allowed to wake up and were kept under standard conditions. After 24 h, mice were again deeply anesthetized using sodium thiopental (100 mg/kg). Under a preparation microscope, skin and suboccipital muscles were dissected. The cisterna magna was punctured with a 27-gauge butterfly cannula to obtain a CSF specimen. White blood cell concentrations in the CSF were determined microscopically using a Fuchs-Rosenthal chamber. Blood samples were obtained by puncture of the left atrium, followed by transcardial perfusion with cold PBS. Brains were removed and snap-frozen in methylbutane on dry ice. Bacterial concentrations in the CSF and blood were determined by plating of serial dilutions on blood agar plates.
Histology.
Neuronal damage in mice was assessed by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling (TUNEL) staining. Cryosections (20 μm) were thaw mounted on coated glass slides, air dried, and postfixed in methanol at −20°C. We performed TdT-TUNEL using a commercially available kit (Qbiogene). Labeled sites were visualized using a fluorescein isothiocyanate (FITC)-conjugated antibody. Sections were counterstained with Hoechst 33258 (1/10,000; Invitrogen Life Technologies). For quantification, the number of TUNEL+ nuclei in the dentate gyrus on all sections was divided by the area of the dentate gyrus as determined using Stereo Investigator software (version 4.0; MicroBrightfield). For immunostaining of murine microglia, sections were prepared as described above, followed by blocking in donkey serum (3% in PBS with 0.3% Triton X-100) for 30 min and incubation with a rabbit polyclonal anti-F4/80 antibody (Chemicon, 1:200 in blocking solution) at 4°C overnight. A Texas Red-conjugated donkey anti-rabbit secondary antibody (Jackson, 1:100) was used for visualization. In addition, nuclei were counterstained with Hoechst 33258 (1:10,000).
To determine Tpo expression in infected human CNS tissue, an autopsy specimen from a cytomegalovirus (CMV)-infected fetus (22nd week of gestation) was studied by double immunofluorescence. The use of archival human tissue was in strict observance of institutional ethical guidelines. Serial sections (4 μm) were deparaffinized and incubated with primary antibodies against major histocompatibility complex (MHC) class II 1:10 (mouse monoclonal; Dako) at 4°C overnight and human Tpo 1:500 (rabbit polyclonal; Abcam) at 20°C for 2 h. Species-specific, biotinylated rat immunoglobulin and goat immunoglobulin (Dianova) were used as secondary antibodies, followed by Cy3 (Sigma) or Alexa 488 (Dianova) (both 1:100 for 1 h at 20°C) labeling, respectively.
Flow cytometry of brain-derived microglia ex vivo.
To assess microglial activation, PBS-perfused brains were harvested 24 h after infection. Cerebral leukocytes were isolated from brains after perfusion with 0.9% NaCl. Brain tissue was minced through a 70-μm mesh (BD), followed by Percoll gradient centrifugation (Amersham-Pharmacia) as described previously (33). Brain-derived leukocytes and microglia from wild-type (n = 6) and c-Mpl−/− (n = 4) mice were subjected to triple immunofluorescence staining followed by flow cytometry. In brief, after being pelleted and resuspended, the cells were blocked for unspecific binding of the Fc receptor (CD16/32; BD Pharmingen) for 10 min at 4°C. They were then incubated for 20 min with a phycoerythrin (PE)-labeled CD11b antibody (BD Pharmingen; 1:400), a Cy7/PE-labeled GR-1 antibody (BD Pharmingen; 1:200) and an allophycocyanin (APC)/Cy7-labeled CD45 antibody (BD Pharmingen; 1:200). After being washed, cells were analyzed on a FACSCanto device. CD11b+CD45dim resting microglial cells, CD11b+CD45high activated microglial cells/macrophages, and GR1+CD45+CD11bdim granulocytes were quantified using the FlowJo software. To quantify activation, the number of CD11b+CD45high amoeboid microglia/macrophages was analyzed relative to that of all CD11b+ cells.
Cell culture.
Primary cultures of murine microglia were prepared as described previously (15). Briefly, brains were harvested from neonatal mice (postnatal days 0 to 3 [P0 to P3]). Following digestion with trypsin and mechanical dissociation, cells were seeded in 75-cm2 flasks in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, 2 mM l-glutamine, and 0.1% glucose. After 8 to 10 days, microglia were detached by shaking for 2 h at 200 rpm, retrieved from the supernatant, and reseeded into 24-well plates in neurobasal medium with B27. Experiments on microglia were conducted after 24 h in culture. RAW264.7 cells were seeded into 24-well plates at a density of 72,000/cm2 and cultured in VLE-RPMI medium with 10% FBS and 1 mM sodium pyruvate. For stimulation, cells were incubated with bacterial conditioned cell culture medium (see above) or challenged with Pam3CysSK4 at a final concentration of 0.1 μg/ml and/or recombinant human Tpo (rTpo) (Immunotools) at a final concentration of 0, 1, 10, or 100 pmol/liter. As a readout of microglia activation, TNF-α bioactivity was measured in the supernatant at 6 h and 24 h using a modified L929 cytotoxicity assay (14).
Tpo and c-Mpl mRNA expression.
Total RNA was isolated from >106 cells, and reverse transcription was performed as described before (39). Real-time PCR was performed using FastStartDNA SYBR green I in a light cycler instrument (Roche). PCR conditions were 10 min at 95°C followed by 45 cycles at 95°C for 15 s, 64°C for 10 s, and 72°C for 15 s (amplification product data acquisition at 81°C). All reactions were performed in duplicate, and the mean threshold cycle was used for analysis. Tpo and c-Mpl mRNA expression levels were normalized against the β-actin mRNA content. The following sequence-specific primers were used: mTpo, forward, 5′-CCA TGC TTC TTG CAG TGG CAA G-3′, reverse, 5′-CAG GCA GCA CAA CAG GGA TAG-3′; mc-Mpl, forward, 5′-CAG CAC CCA GTG GGA CAT AC-3′, reverse, 5′-CAG CAC CCG CTG GAT CAA AG-3′; m-actin, forward, 5′-ACC CAC ACT GTG CCC ATC TA-3′, reverse, 5′-GCC ACA GGA TTC CAT ACC CA-3′.
Statistical analysis.
Normally distributed data are presented as mean ± standard deviation (SD). Two-group comparisons were performed with Student's t tests, while multiple-group comparisons were performed with analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc testing. In the absence of normal distribution, data are presented as median and range. Mann-Whitney U tests were applied for two-group comparisons, and nonparametric ANOVA (Kruskal-Wallis) and Dunn's post hoc analysis were used for multiple-group comparisons.
RESULTS
Impact of c-Mpl on the inflammatory response and neuronal damage in experimental meningitis.
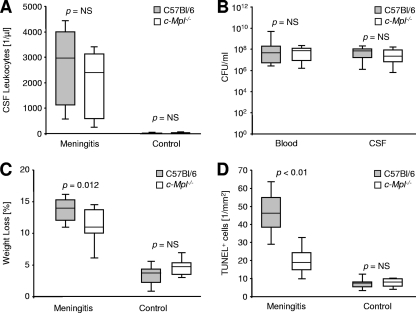
Intrathecal infection with live encapsulated pneumococci caused a pronounced influx of leukocytes into the CSF (Fig. 1A). In wild-type mice (n = 13), the median concentration of leukocytes in the CSF was 2,901/μl (range, 427 to 4,693/μl), compared to 2,389/μl (n = 13; range, 171 to 3,584/μl) in c-Mpl−/− mice (P = not significant [NS], Kruskal-Wallis ANOVA with Dunn's post hoc analysis). In noninfected controls (n = 7), the median concentration of leukocytes in the CSF was 11/μl (range, 0 to 43/μl), compared to 53/μl (n = 7; range, 0 to 171/μl) in c-Mpl−/− mice (P = NS). The median bacterial concentration in blood was 5.0 × 107 CFU per ml (range, 2.2 × 106 to 1.0 × 1010 CFU per ml) in infected wild-type mice, compared to 9.1 × 107 CFU per ml (range, 1.2 × 106 to 2.4 × 108 CFU per ml) in c-Mpl−/− mice (Fig. 1B; P = 0.77, Mann-Whitney U test). In the CSF, plating revealed a median of 7.8 × 107 CFU per ml (range, 1.1 × 105 to 2.3 × 108 CFU per ml) in C57BL/6 mice, compared to 1.8 × 108 CFU per ml (range, 1.0 × 105 to 6.7 × 108 CFU per ml) in c-Mpl−/− mice (P = 0.35, Mann-Whitney U test). As a marker of the severity of systemic inflammation, we compared the individual body weights before and 24 h after surgery (Fig. 1C). In PBS-treated control mice, percentages of weight loss were similar in wild-type and c-Mpl−/− mice (3.4% ± 2.2% versus 4.9% ± 2.1%; NS; one-way ANOVA with Student-Newman-Keuls post hoc analysis). In infected wild-type mice, individual weight loss (13.8% ± 2.3%) was more pronounced than in infected c-Mpl−/− mice (10.9% ± 3.5%; P < 0.05; one-way ANOVA with Student-Newman-Keuls post hoc analysis). Intracranial hemorrhage or other bleeding complications were not observed in c-Mpl−/− or wild-type mice. Neuronal damage in the dentate gyrus was assessed by TUNEL staining (Fig. 1D). The basal levels of apoptosis in noninfected mice were 8.1 ± 5.2 TUNEL+ nuclei/mm2 in C57BL/6 mice and 7.4 ± 3.1/mm2 in c-Mpl−/− mice (NS; one-way ANOVA with Student-Newman-Keuls post hoc analysis). At 24 h after infection, wild-type mice had 45.9 ± 15.1 TUNEL+ nuclei/mm2 (P < 0.05 versus the result for noninfected wild-type mice; see above). By comparison, neuronal damage was reduced in infected c-Mpl−/− mice (20.3 ± 9.7/mm2; P < 0.05 versus the result for infected wild-type mice and P < 0.05 versus the result for noninfected c-Mpl−/− mice; one-way ANOVA with Student-Newman-Keuls post hoc analysis).
FIG. 1.
Impact of c-Mpl in experimental pneumococcal meningitis. (A) Leukocyte counts in the cerebrospinal fluid (CSF) of C57BL/6 wild-type mice (gray bars, n = 13) and transgenic mice with ablation of the Tpo receptor (c-Mpl−/−, open bars, n = 13) 24 h after intrathecal inoculation with 5 × 105 CFU of Streptococcus pneumoniae strain D39. Controls (n = 7 per group) were treated with an equal volume of pyrogen-free PBS. (B) Bacterial concentration (CFU) in the blood and CSF 24 h after inoculation. Statistical significance for CSF leukocyte count and bacterial CFU was determined by Kruskal-Wallis ANOVA and Dunn's post hoc test. (C) Body weight loss within 24 h after infection. (D) Density of apoptotic nuclei in the dentate gyrus in TUNEL staining 24 h after challenge. For the two latter parameters, statistical significance was determined by one-way ANOVA and Student-Newman-Keuls post hoc analysis. Graphs depict the median and 10th, 25th, 75th, and 90th percentiles.
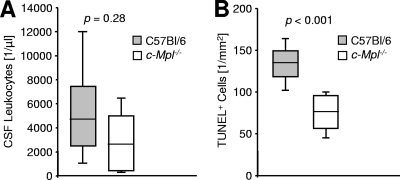
To rule out a potential interaction of bacterial toxins with c-Mpl, we performed additional experiments using Pam3CysSK4 rather than live bacteria to induce meningeal inflammation. At 24 h after intrathecal application, the median concentration of leukocytes in the CSF was 4,697/μl (range, 917 to 19,328/μl; n = 9) in wild-type mice, versus 2,614/μl (range, 192 to 4,715/μl; n = 9) in c-Mpl−/− mice (Fig. 2A; P = 0.28, Mann-Whitney U test). The median density of TUNEL+ nuclei in the dentate gyrus was 67.8/mm2 (range, 46.0 to 83.7/mm2) in wild-type mice, compared to 37.7/mm2 (range, 18.7 to 51.1/mm2) in c-Mpl−/− mice (Fig. 2B; P < 0.001, Mann-Whitney U test).
FIG. 2.
Impact of c-Mpl in sterile TLR2-driven neuroinflammation. (A) Leukocyte counts in the cerebrospinal fluid (CSF) of C57BL/6 wild-type mice (gray bars, n = 9) and transgenic mice with ablation of the Tpo receptor (c-Mpl−/−, open bars, n = 9) 24 h after intrathecal challenge with 10 μg Pam3CysSK4. (B) Density of TUNEL+ apoptotic nuclei in the dentate gyrus 24 h after challenge. Statistical significance was determined by Mann-Whitney U tests. Box plots depict medians and 10th, 25th, 75th, and 90th percentiles.
These findings identify endogenous Tpo as a modulator of neuronal damage and clinical disease severity, while CSF leukocyte or bacterial concentrations in meningitis were not affected.
Effect of recombinant Tpo in experimental meningitis.
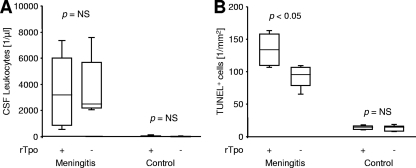
The effect of rTpo was analyzed in a separate experiment. In noninfected wild-type mice (n = 6), intrathecal application of rTpo (1 nmol/kg) did not induce inflammatory processes or apoptosis in the dentate gyrus. At 24 h after intrathecal application of rTpo, noninfected animals displayed a median concentration of 21 leukocytes per μl of CSF (P = NS versus results for PBS-injected controls; see above). The median density of TUNEL-positive neurons in the dentate gyrus was 14.2/mm2 (range, 10.0 to 18.6/mm2) in rTpo-treated animals, compared to 7.7/mm2 (range, 3.0 to 18.8/mm2) in PBS-injected controls (P = NS). At 24 hours after injection of 5 × 105 CFU D39 per animal and rTpo (n = 5), the median concentration of CSF leukocytes was 3,189/μl (range, 341 to 8,277/μl), compared to 2,496/μl (range, 1,963 to 8,874/μl; n = 5) in untreated D39-infected animals (Fig. 3A; P = NS, Kruskal-Wallis ANOVA with Dunn's post hoc analysis). However, the density of TUNEL-positive nuclei in the dentate gyrus was increased by rTpo: compared to 88.6 ± 21.6 in D39-infected mice, 135.2 ± 31.1 apoptotic nuclei per mm2 were detected in the dentate gyrus of infected mice treated with rTpo (Fig. 3B; P < 0.05, one-way ANOVA with Student-Newman-Keuls post hoc analysis). These findings further support a proapoptotic role of Tpo in meningitis, which is apparently independent of the degree of leukocyte recruitment into the CSF.
FIG. 3.
Effect of recombinant Tpo (rTpo) in experimental meningitis. Wild-type mice were challenged by intrathecal application of 5 × 105 CFU S. pneumoniae (meningitis) or PBS (controls). Mice received an additional intrathecal treatment with rTpo (1 nmol/kg) as indicated. (A) Leukocyte count in the CSF after 24 h. Statistical significance was determined by Kruskal-Wallis ANOVA and Dunn's post hoc test. (B) Density of apoptotic nuclei in the dentate gyrus as identified by TUNEL 24 h after challenge. Statistical significance was determined by one-way ANOVA and Student-Newman-Keuls post hoc analysis.
Microglia activation.
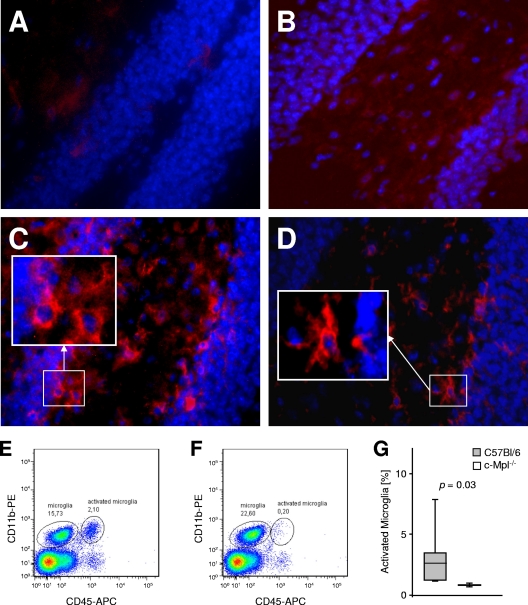
Activation of microglia during experimental meningitis was assessed by immunohistochemistry and fluorescence-activated cell sorter (FACS) analysis. At 24 h after infection, the level of F4/80 antigen expression in brain tissue was below the limit of detection in PBS-injected control mice, irrespective of the c-Mpl genotype (Fig. 4 A and B). By comparison, pronounced immunoreactivity of activated microglia and macrophages in the subarachnoid space was present 24 h after inoculation with D39. In infected wild-type mice, the majority of F4/80-immunopositive cells in the parenchyma displayed amoeboid morphology (Fig. 4C), while in the c-Mpl−/− group, a ramified type signal was predominant (Fig. 4D). FACS analysis of brain-derived cells (Fig. 4E, F, and G) revealed a higher proportion of activated CD45high microglia in wild-type mice than in c-Mpl−/− mice (6.2% ± 6.7% versus 1.2% ± 0.2%; P = 0.04; Mann-Whitney U test). Interestingly, also more granulocytes were retrieved from the brain tissue of wild-type mice than from c-Mpl−/− mice (1.8% ± 2.3% versus 0.1% ± 0.0% of the gated leukocytes; P = NS). These findings demonstrate a lower level of microglia activation and proliferation in c-Mpl−/− mice than in wild-type mice, thus also supporting a proinflammatory role of Tpo and its receptor within the brain parenchyma during experimental meningitis.
FIG. 4.
Impact of c-Mpl on microglia activation. At 24 h after intrathecal application of pyrogen-free PBS, expression of the microglial marker F4/80 (red) was below the detection limit in wild-type C57BL/6 mice (A) and in c-Mpl−/− mice (B). In wild-type mice infected with S. pneumoniae (C), a strong increase in F4/80 immunostaining was observed. Note several F4/80-positive microglial cells with amoeboid shape. Infected c-Mpl−/− mice (D) show weaker immune staining for F4/80, with predominance of ramified morphology. Hoechst 33258 was used as a nuclear counterstain (blue). (E to G) FACS analysis of brain-derived cells 24 h after meningitis induction. Resting microglia was identified as a CD11b+CD45dim population, while activated microglia and macrophages were characterized as CD11b+CD45high cells. Compared to wild-type mice (E) (representative example out of n = 6), c-Mpl−/− mice (F) (representative example out of n = 4) displayed a low abundance of activated microglia and macrophages. (G) Proportion of CD11b+ cells represented by activated microglia and macrophages. Statistical significance was determined by the Mann-Whitney U test.
Regulation of Tpo and c-Mpl mRNA in brain tissue.
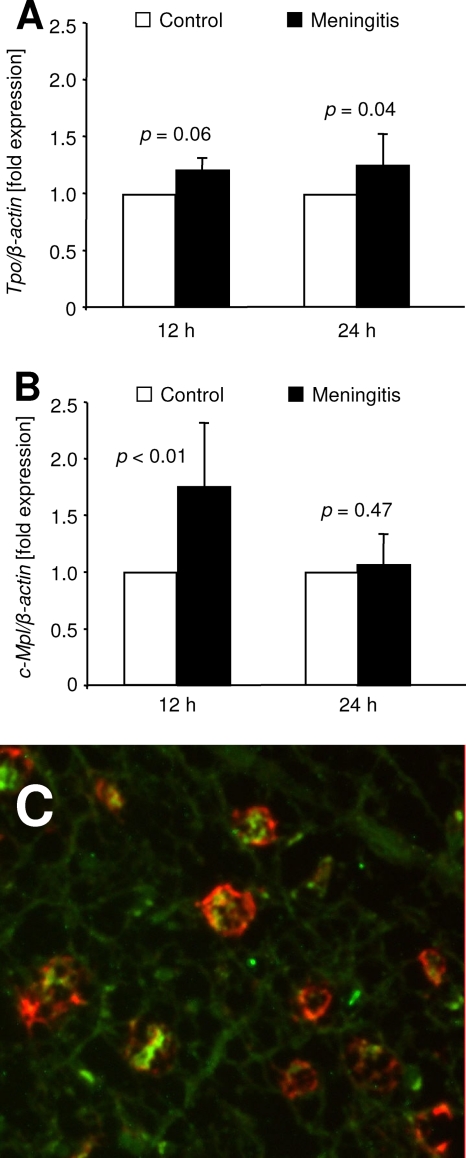
In brain tissue homogenates, real-time PCR revealed an increase of Tpo mRNA during meningitis (Fig. 5A). Compared to results for PBS-treated control mice, infection with D39 resulted in an increase of Tpo transcripts by 21% ± 11% after 12 h (P = 0.06) and by 24% ± 28% after 24 h (P = 0.04). c-Mpl mRNA levels were transiently upregulated at 12 h after infection (175% ± 56% of controls, P < 0.01) and returned to baseline at 24 h (107% ± 25% of controls, P = 0.47; Fig. 5B).
FIG. 5.
Regulation of Tpo and c-Mpl mRNA expression in the brain in response to inflammation. (A) A slight upregulation of Tpo mRNA in brain homogenates was observed at 12 h and 24 h after intrathecal infection of wild-type mice with S. pneumoniae (closed bars), normalized to the mean expression in PBS-challenged controls (open bars). (B) c-Mpl mRNA was upregulated at 12 h and returned to baseline 24 h after meningitis induction. For statistical analysis, Student's t tests were performed. (C) Double immunostaining of a human brain specimen from a fetus with CMV infection. Tpo immune signal (green) is present in cortical macrophages (MHC II antigen, red).
Tpo immunostaining in human encephalitis.
To investigate parenchymal sources of Tpo in acute brain infection, we performed immunostaining of murine brain sections 24 h after induction of meningitis. Using commercially available antibodies, we were not able to establish sufficient sensitivity and specificity (data not shown). Instead, we performed Tpo immunostaining on archival human brain tissue from a CMV-infected fetus. Using a human-specific Tpo antibody, we detected Tpo immunoreactivity within the cytoplasm of MHC class II+ macrophages, which were localized within the cortex of infected CNS tissue (Fig. 5C). This finding supports a role of macrophages as a source of Tpo in acute CNS infection.
Regulation of Tpo mRNA in primary microglia and in macrophages.
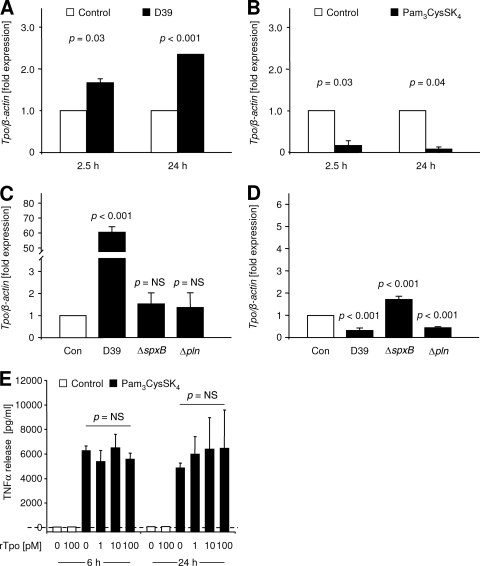
To further elucidate the sources of Tpo during meningitis, we challenged primary murine microglia in vitro with pneumococcal conditioned medium or with the synthetic bacterial lipopeptide Pam3CysSK4. Compared to unstimulated controls, Tpo mRNA was upregulated by 68.0% ± 7.8% (P = 0.03; Student's t test) at 2.5 h and by 134.5% ± 0.7% (P < 0.001; Student's t test) at 24 h after exposure to D39-conditioned medium (Fig. 6A). In contrast, stimulation with Pam3CysSK4 (Fig. 6B) resulted in downregulation of Tpo mRNA by 82.6% ± 11.5% (P = 0.03; Student's t test) at 2.5 h and by 91.7% ± 5.6% (P = 0.04; Student's t test) at 24 h. In the murine macrophage cell line RAW264.7, Tpo mRNA was strongly upregulated by a factor of 60.6 ± 3.5 after 2.5 h of incubation in D39-conditioned medium (Fig. 6C; P < 0.001 versus result for control; one-way ANOVA with Student-Newman-Keuls post hoc analysis). With ΔspxB conditioned medium, Tpo mRNA was increased by 54.5% ± 52% (P < 0.01 versus result for D39), and with Δpln conditioned medium an increase of 37.5% ± 58.0% was observed (P < 0.01 versus result for D39). After 24 h of incubation, Tpo mRNA expression fell below control levels with D39 (−65.8% ± 9.6%, P < 0.001; Fig. 6D) and Δpln (−47.3% ± 3.7%, P < 0.001) conditioned medium while expression remained increased by 73.3% ± 14.9% with ΔspxB conditioned medium (P < 0.001; one-way ANOVA with Student-Newman-Keuls post hoc analysis).
FIG. 6.
Regulation of Tpo and costimulatory effects of Tpo during proinflammatory activation of primary murine microglia in vitro. (A) Expression of Tpo mRNA in primary murine microglia after 2.5 h and 24 h of incubation with D39-conditioned medium (closed bars). (B) Expression of Tpo mRNA in primary murine microglia after 2.5 h and 24 h of exposure to the synthetic bacterial lipopeptide Pam3CysSK4 (0.1 μg/ml, closed bars). For statistical analysis, Student's t tests were performed. (C and D) Expression of Tpo mRNA in RAW264.7 cells after 2.5 h (C) and 24 h (D) of incubation with medium conditioned by wild-type pneumococci (D39), H2O2-deficient pneumococci (ΔspxB), or PlnA-deficient pneumococci (Δpln). Expression was measured with β-actin as the internal standard and normalized to the expression of unstimulated controls at the identical time point (open bars). For statistical analysis, one-way ANOVA and Student-Newman-Keuls tests were used. P values are given for the comparison versus control. (E) Primary murine microglia was incubated with medium (open bars) or with Pam3CysSK4 (0.1 μg/ml, closed bars), and rTpo was added to the medium at a final concentration as indicated. As a readout of activation, TNF-α concentrations were measured in the supernatant after 6 h or 24 h using the L929 cytotoxicity bioassay. Experiments were performed in triplicate, and one-way ANOVA was used for statistical analysis.
In further experiments, we tested whether rTpo modulates activation of microglia during inflammation. Incubation of primary microglia with rTpo up to a concentration of 100 pmol/liter (0, 1, 10, and 100 pmol/liter) did not induce TNF-α release within an interval of 6 h or 24 h (Fig. 6E). Stimulation of microglia with Pam3CysSK4 (0.1 μg/liter) induced a pronounced release of TNF-α into the supernatant, but an additional effect of rTpo up to 100 pmol/liter was not observed (Fig. 6E).
DISCUSSION
Degeneration of hippocampal neurons is a major cause of long-term neuropsychological disabilities in survivors of bacterial meningitis (28). The mechanisms leading to this type of injury have not been elucidated in detail. Here we report the first evidence of an involvement of Tpo and its receptor c-Mpl as modulators of inflammatory neuronal damage. Compared to the result for meningitis in wild-type mice, the density of apoptotic nuclei in the dentate gyrus was decreased in infected Tpo receptor-deficient mice (c-Mpl−/−), and it was increased in infected wild-type mice after intrathecal application of rTpo. Since c-Mpl also modulated neuronal damage in Pam3CysSK4-induced sterile neuroinflammation, a major contribution of bacterial toxins interacting with c-Mpl is unlikely.
Proapoptotic effects of Tpo on neurons in vivo were first reported in a rat model of hypoxic brain ischemia (12). Under the conditions used in that study, newly formed neurons in the dentate gyrus were especially vulnerable to the effects of endogenous and recombinant Tpo. Thus, a physiological role of the Tpo/c-Mpl system was postulated by which excess neurons are removed from the maturing brain (12). In bacterial meningitis, net loss of neurons and atrophy of the hippocampus may in part result from negative effects on neurogenesis (16), which could also involve Tpo signaling. In our mouse model of bacterial meningitis, however, most apoptotic cells in the dentate gyrus are mature, NeuN-positive neurons rather than immature cells (16). In the present study, proapoptotic effects of rTpo were discernible only in infected mice, while no induction of apoptosis was observed in PBS-challenged control mice. In conclusion, it appears that during bacterial meningitis, mature neurons are affected by proapoptotic effects of endogenous and exogenous Tpo. A possible additional impact of the Tpo/c-Mpl system on brain repair after meningitis was not assessed.
An important question relates to the sources of Tpo during meningitis. Tpo and c-Mpl are widely transcribed in the developing human and rodent brain (11, 12, 20, 26). However, the exact cellular sources of Tpo in the brain have not been established in vivo. In vitro, primary cultures of hippocampal neurons and cortical astrocytes express Tpo on mRNA and protein level, and this expression is downregulated after hypoxia (12). In contrast to those results, we found a global increase of Tpo mRNA transcripts in the brains of infected adult mice. Using commercially available antibodies for immunostaining, we were unable to establish sufficient specificity to pinpoint Tpo-producing cells in the murine brain. In human autopsy tissue, however, we detected Tpo signal in a substantial number of macrophages present in the cortex.
During inflammation, a subset of brain macrophages is acutely recruited from the bloodstream, while the majority of brain macrophages appear to derive from highly activated local microglia (31). In a previous study of rat brain-derived primary cultures (39), we had found that microglia and astrocytes consistently downregulate Tpo mRNA expression in response to proinflammatory stimulation with lipopolysaccharide (LPS). In the present study, downregulation was also induced in primary murine microglia by the Toll-like receptor 2 (TLR2) ligand Pam3CysSK4, a synthetic equivalent of the lipopeptides found in Gram-positive bacterial cell walls. Conversely, incubation of primary murine microglia with D39-conditioned medium resulted in an increase of Tpo transcripts at 2.5 and 24 h. In murine macrophages, a transient substantial increase of Tpo mRNA was also observed after 2.5 h of stimulation with D39 supernatant, followed by downregulation at 24 h. This difference in findings points to cell type-specific patterns of regulation in microglia and macrophages as well as differential effects of cell wall components versus other bacterial factors on Tpo transcription in microglia. Furthermore, H2O2-deficient ΔspxB and pneumolysin-deficient Δpln pneumococcal mutants showed a strongly decreased induction of Tpo mRNA in macrophages at 2.5 h compared to the effect of wild-type D39-conditioned medium, and the delayed downregulation at 24 h was absent with the ΔspxB mutant. In conclusion, both bacterial H2O2 production and release of pneumolysin appear to be involved in Tpo regulation in murine microglia and macrophages. In the case of pneumolysin, this effect is probably related to the intrinsic actions of the toxin rather than to its capacity to interact with TLR4, since the prototype TLR4 ligand LPS did not induce Tpo transcription in microglia (39).
A further type of brain cell known to express Tpo is represented by the vascular endothelium. The presence of Tpo and c-Mpl was previously reported in endothelial cells of diverse tissue origins (7, 8, 19). We have found a delayed upregulation of Tpo mRNA in brain microvascular endothelial cells (BMEC) after inflammatory stimulation in vitro (39). In concert with astrocytes, BMEC form the substrate of the blood-brain barrier, and their activation is a crucial first step for the recruitment of leukocytes from the bloodstream into the CSF. We have previously shown the involvement of autocrine positive feedback loops in the activation of BMEC by bacterial cell wall components (14). Other authors have reported an induction of proinflammatory cytokines by Tpo in an endothelial cell line (8) as well as activation of ERK1/2 signaling in astrocytes (6). Taken together, these results suggest that production of Tpo by BMEC could provide proinflammatory stimuli to the endothelium itself as well as to adjacent asctrocytes, thus modulating the local inflammatory response in meningitis.
In the present study, morphological examination as well as FACS analysis revealed a reduced activation of microglia during meningitis in the absence of c-Mpl. Apart from being a source of cytotoxic molecules, microglia has also been ascribed a sentinel function in bacterial meningitis (23). Release of chemoattractants such as CXCL2 and CXCL8 by activated microglia is thought to fuel the inflammatory process by promoting the invasion of leukocytes into the brain (29, 40). This may be reflected by the FACS results in the present study, where reduced activation of microglia in c-Mpl−/− mice was paralleled by a trend toward a lower number of granulocytes present in the brain tissue. Leukocytes invading from the blood mediate early key events of meningitis, such as breakdown of the blood-brain barrier, formation of brain edema, induction of cerebral hyperemia, and intracranial hypertension (37). In particular, we have identified granulocytes as important mediators of neuronal loss in experimental meningitis (17). Thus, activated microglia may directly and indirectly contribute to meningitis-induced neuronal damage.
While these findings may provide a basis for the neuroprotective phenotype of c-Mpl−/− mice, direct effects of Tpo on microglia appear to be limited during meningitis. Resting microglia was not activated in vitro by rTpo itself, and the activation of microglia by Pam3CysSK4 was not enhanced by costimulation with rTpo. Thus, an indirect mode of Tpo action, possibly involving the activation of endothelial cells, has to be hypothesized. Alternatively, reduced activation of microglia could be the result rather than the cause of relative neuronal sparing in c-Mpl−/− mice, since HSP60 released from dying neurons presents an endogenous proinflammatory stimulus to microglia (25).
In interpreting the data obtained in c-Mpl−/− mice, potentially confounding effects of defective Tpo signaling need to be considered. In particular, c-Mpl−/− mice display severe thrombocytopenia, with platelet counts reduced to about 6% of wild-type levels (1). Platelets may participate in antibacterial defense by the release of antibacterial peptides (34) and by the agglutination of pathogens, e.g., via the M protein of streptococci (2). Platelets are, however, not abundant in the CSF during meningitis, and the intrathecal route of infection in our experiments should limit the impact of systemic platelet counts on bacterial growth in this compartment. Indeed, bacterial concentrations in the CSF 24 h after infection were not significantly influenced by the c-Mpl genotype. Systemic spread of the infection occurs as a rule in the given model, but no significant effect of the Tpo receptor on bacterial CFU in the peripheral blood was observed either.
In conclusion, we have identified Tpo as a novel mediator of neuronal damage and microglia activation in bacterial meningitis. Microglia and macrophages may represent intracranial sources of Tpo during acute brain inflammation.
Acknowledgments
The study was supported by a grant from Wilhelm Sander-Stiftung (2007.111.1) to O.H. and C.D.
We thank Warren Alexander (Walter and Eliza Hall Institute of Medical Research, Parkville, Australia) for providing the c-Mpl−/− mouse strain as well as Elaine Tuomanen (St. Jude's Children's Research Hospital, Memphis, TN) for the generous gift of the D39 pneumococcal strain. We also thank Renate Gusinda, Cordula Mahrhofer, and Petra Matylewski for excellent technical assistance.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 13 December 2010.
REFERENCES
- 1.Alexander, W. S., A. W. Roberts, N. A. Nicola, R. Li, and D. Metcalf. 1996. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood 87:2162-2170. [PubMed] [Google Scholar]
- 2.Beachey, E. H., and G. H. Stollerman. 1971. Toxic effects of streptococcal M protein on platelets and polymorphonuclear leukocytes in human blood. J. Exp. Med. 134:351-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton, K. A., M. P. Everson, and D. E. Briles. 1995. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect. Immun. 63:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun, J. S., et al. 2007. Pneumolysin causes neuronal cell death through mitochondrial damage. Infect. Immun. 75:4245-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun, J. S., et al. 2002. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis. J. Clin. Invest. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byts, N., A. Samoylenko, H. Woldt, H. Ehrenreich, and A. L. Sirén. 2006. Cell type specific signalling by hematopoietic growth factors in neural cells. Neurochem. Res. 31:1219-1230. [DOI] [PubMed] [Google Scholar]
- 7.Cardier, J. E., and J. Dempsey. 1998. Thrombopoietin and its receptor, c-Mpl are constituvely expressed by mouse liver endothelial cells: evidence of thrombopoietin as a growth factor for liver endothelial cells. Blood 91:923-929. [PubMed] [Google Scholar]
- 8.Cardier, J. E. 1999. Effects of megakaryocyte growth and development factor (thrombopoietin) on liver endothelial cells in vitro. Microvasc. Res. 58:108-113. [DOI] [PubMed] [Google Scholar]
- 9.Columbyova, L., M. Loda, and D. T. Scadden. 1995. Thrombopoietin receptor expression in human cancer cell lines and primary tissues. Cancer Res. 55:3509-3512. [PubMed] [Google Scholar]
- 10.Dame, C. 2002. Developmental biology of thrombopoietin in the human fetus and neonate. Acta Paediatr. Suppl. 91:54-65. [DOI] [PubMed] [Google Scholar]
- 11.Dame, C., et al. 2003. Thrombopoietin gene expression in the developing human central nervous system. Brain Res. Dev. Brain Res. 143:217-223. [DOI] [PubMed] [Google Scholar]
- 12.Ehrenreich, H., et al. 2005. A hematopoietic growth factor, thrombopoietin, has a proapoptotic role in the brain. Proc. Natl. Acad. Sci. U. S. A. 102:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Free, S. L., L. M. Li, D. R. Fish, S. D. Shorvon, and J. M. Stevens. 1996. Bilateral hippocampal volume loss in patients with a history of encephalitis or meningitis. Epilepsia 37:400-405. [DOI] [PubMed] [Google Scholar]
- 14.Freyer, D., et al. 1999. Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J. Immunol. 163:4308-4314. [PubMed] [Google Scholar]
- 15.Hoffmann, O., et al. 2007. TLR2 mediates neuroinflammation and neuronal damage. J. Immunol. 178:6476-6481. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann, O., et al. 2007. Pneumococcal cell wall-induced meningitis impairs adult hippocampal neurogenesis. Infect. Immun. 75:4289-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann, O., et al. 2007. TRAIL limits excessive host immune responses in bacterial meningitis. J. Clin. Invest. 117:2004-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann, O., et al. 2006. Interplay of pneumococcal hydrogen peroxide and host-derived nitric oxide. Infect. Immun. 74:5058-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishybashi, T., K. Nakazato, and Y. Maruyama. 1997. Expression of thrombopoietin receptor in human endothelial cells and smooth muscle cells of aortic and coronary arteries. (Abstract.) Blood 90(Suppl. 1):76b. [Google Scholar]
- 20.Ivanova, A., et al. 2010. Expression pattern of the thrombopoietin receptor (Mpl) in the murine central nervous system. BMC Dev. Biol. 10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaser, A., et al. 2001. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood 98:2720-2725. [DOI] [PubMed] [Google Scholar]
- 22.Kaushansky, K. 1998. Thrombopoietin and the hematopoietic stem cell. Blood 92:1-3. [PubMed] [Google Scholar]
- 23.Kreutzberg, G. W. 1996. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19:312-318. [DOI] [PubMed] [Google Scholar]
- 24.Lacks, S., and R. D. Hotchkiss. 1960. A study of the genetic material determining an enzyme in Pneumococcus. Biochim. Biophys. Acta 39:508-518. [DOI] [PubMed] [Google Scholar]
- 25.Lehnardt, S., et al. 2008. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J. Neurosci. 28:2320-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, B., H. Pan, J. C. Winkelmann, and W. Dai. 1996. Thrombopoietin and its alternatively spliced form are expressed in human amygdala and hippocampus. Blood 87:5382-5384. [PubMed] [Google Scholar]
- 27.Methia, N., F. Louache, W. Vainchenker, and F. Wendling. 1993. Oligodeoxynucleotides antisense to the proto-oncogene c-Mpl specifically inhibit in vitro megakaryocytopoiesis. Blood 82:1395-1401. [PubMed] [Google Scholar]
- 28.Nau, R., and W. Brück. 2002. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 25:38-45. [DOI] [PubMed] [Google Scholar]
- 29.Ostergaard, C., et al. 2000. Treatment with a monocolonal antibody to IL-8 attenuates the pleocytosis in experimental pneumococcal meningitis in rabbits when given intravenously, but not intracisternally. Clin. Exp. Immunol. 122:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinhold, A., et al. 2007. High thrombopoietin concentrations in the cerebrospinal fluid of neonates with sepsis and intraventricular hemorrhage may contribute to brain damage. J. Interferon Cytokine Res. 27:137-145. [DOI] [PubMed] [Google Scholar]
- 31.Schilling, M., et al. 2005. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 196:290-297. [DOI] [PubMed] [Google Scholar]
- 32.Spellerberg, B., et al. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803-813. [DOI] [PubMed] [Google Scholar]
- 33.Stenzel, W., et al. 2008. Both TLR2 and TLR4 are required for the effective immune response in Staphylococcus aureus-induced experimental murine brain abscess. Am. J. Pathol. 172:132-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang, Y. Q., M. R. Yeaman, and M. E. Selsted. 2002. Antimicrobial peptides from human platelets. Infect. Immun. 70:6524-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Beek, D., et al. 2004. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 351:1849-1859. [DOI] [PubMed] [Google Scholar]
- 36.van der Flier, M., S. P. Geelen, J. L. Kimpen, I. M. Hoepelman, and E. I. Tuomanen. 2003. Reprogramming the host response in bacterial meningitis: how best to improve outcome? Clin. Microbiol. Rev. 16:415-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber, J. R., K. Angstwurm, W. Bürger, K. M. Einhäupl, and U. Dirnagl. 1995. Anti ICAM-1 (CD 54) monoclonal antibody reduces inflammatory changes in experimental bacterial meningitis. J. Neuroimmunol. 63:63-68. [DOI] [PubMed] [Google Scholar]
- 38.Wolber, E. M., et al. 1999. Expression of the thrombopoietin gene in human fetal and neonatal tissues. Blood 94:97-105. [PubMed] [Google Scholar]
- 39.Zhang, J., et al. 2010. Inflammation stimulates thrombopoietin (Tpo) expression in rat brain-derived microvascular endothelial cells, but suppresses Tpo in astrocytes and microglia. J. Interferon Cytokine Res. 30:465-469. [DOI] [PubMed] [Google Scholar]
- 40.Zwijnenburg, P. J., et al. 2001. Experimental pneumococcal meningitis in mice: a model of intranasal infection. J. Infect. Dis. 183:1143-1146. [DOI] [PubMed] [Google Scholar]