Abstract
Subtilase cytotoxin (SubAB), which is produced by certain strains of Shiga-toxigenic Escherichia coli (STEC), causes the 78-kDa glucose-regulated protein (GRP78/BiP) cleavage, followed by induction of endoplasmic reticulum (ER) stress, leading to caspase-dependent apoptosis via mitochondrial membrane damage by Bax/Bak activation. The purpose of the present study was to identify SubAB receptors responsible for HeLa cell death. Four proteins, NG2, α2β1 integrin (ITG), L1 cell adhesion molecule (L1CAM), and hepatocyte growth factor receptor (Met), were identified to be SubAB-binding proteins by immunoprecipitation and purification, followed by liquid chromatography-tandem mass spectrometry analysis. SubAB-induced Bax conformational change, Bax/Bak complex formation, caspase activation, and cell death were decreased in β1 ITG, NG2, and L1CAM small interfering RNA-transfected cells, but unexpectedly, BiP cleavage was still observed. Pretreatment of cells with a function-blocking β1 ITG antibody (monoclonal antibody [MAb] P5D2) enhanced SubAB-induced caspase activation; MAb P5D2 alone had no effect on caspase activation. Furthermore, we found that SubAB induced focal adhesion kinase fragmentation, which was mediated by a proteasome-dependent pathway, and caspase activation was suppressed in the presence of proteasome inhibitor. Thus, β1 ITG serves as a SubAB-binding protein and may interact with SubAB-signaling pathways, leading to cell death. Our results raise the possibility that although BiP cleavage is necessary for SubAB-induced apoptotic cell death, signaling pathways associated with functional SubAB receptors may be required for activation of SubAB-dependent apoptotic pathways.
Subtilase cytotoxin (SubAB) was first identified as a product of Shiga-toxigenic Escherichia coli (STEC) O113:H21, which caused an outbreak of hemolytic-uremic syndrome (HUS) (58). Subsequently, SubAB was found only in STEC strains. Recently, however, SubAB was identified in Shiga toxin (Stx)-negative E. coli strains isolated from unrelated cases of childhood diarrhea (70). SubAB cleaved the molecular chaperone BiP, which triggered an endoplasmic reticulum (ER) stress response (57, 73). It also caused other effects, including transient inhibition of protein synthesis (51), G0/G1 cell cycle arrest (50, 51), caspase-dependent apoptosis via mitochondrial membrane damage (45), activation of the Akt-NF-κB signaling (78), and downregulation of gap junction expression (32). In addition, high concentrations of SubAB induced vacuole formation in Vero cells (51, 76). Although several studies have examined the molecular mechanisms responsible for ER stress-induced cell death (61, 67, 74), the relationship between perturbation in protein folding in the ER following SubAB-induced BiP cleavage and activation of death pathways remains poorly understood. We found, however, that SubAB-induced apoptosis in Vero cells was caused by cytochrome c release via mitochondrial permeabilization, followed by caspase activation (45).
It is well-known that cell surface receptors are responsible for bacterial toxin binding and entry into cells, effects on various signal transduction pathways, and morphological changes of the target cell. SubB has a strong preference for binding to cell surface glycans terminating in the sialic acid N-glycolylneuraminic acid (Neu5Gc), a monosaccharide that is not synthesized in humans (7). An earlier report (10) showed that lipid rafts were not required for SubAB cytotoxicity. Moreover, Kondo et al. also reported that glycolipids were not important receptors for SubAB (37). These data suggested that glycolipids are not involved in SubAB-induced cytotoxicity. On the basis of these reports and other data, we hypothesized that glycoproteins on HeLa cells served as functional SubAB receptors. We reported that, in Vero cells, SubAB-induced vacuole formation required binding of toxin to N-linked carbohydrate-modified α2β1 integrin (ITG) (76).
However, significant inhibition of SubAB-induced apoptosis in Vero cells was not observed following β1 ITG knockdown (unpublished data). The ITG family is composed of 18 α subunits and 8 β subunits located in cell membranes and known to assemble into 24 distinct heterodimers (30, 34). β1 ITG is transported through the Golgi apparatus in association with α ITGs, and nonheterodimerized β1 ITG is either degraded prior to transport to the Golgi apparatus or retained in the ER, ready to associate with newly synthesized α ITGs (28, 40). Interaction between ligands and ITGs affects a large variety of signal transduction events that serve to modulate many aspects of cell behavior, including proliferation, survival/apoptosis, shape, polarity, motility, gene expression, and differentiation (34). ITG-mediated cell attachment promotes survival signaling in many types of normal cells, even in the presence of various apoptotic stimuli (2-4, 16, 19, 39, 56, 79), while ITGs have been shown to enhance signal transduction pathways leading to cell death (9, 29, 38, 44, 52, 66). These reports indicate that ITG-mediated signaling regulates both cell survival and cell death, but the detailed mechanisms of integrin-mediated apoptosis are poorly understood.
Here, we demonstrate that in HeLa cells SubAB bound to the terminal sialic acids of NG2, L1 cell adhesion molecule (L1CAM), α2β1 ITG, and hepatocyte growth factor receptor (Met). On the basis of data from small interfering RNA (siRNA) knockdown of these proteins, we conclude that β1 ITG, NG2, and L1CAM play a pivotal role in SubAB-promoted cell death induced by Bax/Bak oligomerization, cytochrome c release, and caspase activation.
MATERIALS AND METHODS
Subtilase cytotoxin preparation.
Escherichia coli producing recombinant His-tagged wild-type SubAB and catalytic inactivated mutant SubA(S272A)B (mSubAB) were used as the source of toxin for purification, according to a published procedure (51).
Antibodies and other reagents.
Anti-NG2 chondroitin sulfate proteoglycan antibody (AB5320), which recognizes both intact proteoglycan and core protein, was purchased from Millipore; anti-cleaved caspase-7, anti-cleaved procyclic acidic repetitive protein (PARP), anti-Bax, anti-Bak, anti-focal adhesion kinase (anti-FAK), and anti-Met antibodies were from Cell Signaling; mouse monoclonal antibodies (MAbs) reactive with NG2 (LHM2), β1 integrin (P5D2), α2 integrin (C-9), and cytochrome c (7H8) were from Santa Cruz Biotechnologies; rabbit polyclonal antibodies reactive with GAPDH (FL335), normal mouse IgG, and normal rabbit IgG were from Santa Cruz Biotechnologies; mouse monoclonal antibodies reactive with BiP/GRP78 and conformation-specific anti-active Bax (clone 3) were from BD Biosciences. Conformation-specific anti-active Bak (Ab-2) antibody was purchased from Calbiochem; anti-L1CAM monoclonal antibody was from eBioscience. Caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp (methoxy) fluoromethylketone (Z-VAD-FMK, or ZVAD) was purchased from BD Biosciences. Calpain inhibitor 1 (N-acetyl-leucyl-leucyl-norleucinal [ALLN]) was purchased from Calbiochem. Calpeptin was purchased from Enzo Life Sciences, and lactacystin was from Cayman Chemical. MG132 and thapsigargin (Tg) were purchased from Sigma Aldrich.
Immunoprecipitation.
Immunoprecipitation of SubAB receptors on a HeLa cell surface was performed as described previously, with some modification (76). In brief, biotinylated HeLa cell lysates (100 μg/200 μl) were incubated at 4°C for 1 h with 1 μg of native SubAB or heat-inactivated SubAB (100°C, 10 min), followed by incubation at 4°C overnight with 1 μl of an anti-SubAB antibody. Antibody-bound proteins were collected after addition of 20 μl of rProtein G-agarose (Invitrogen), 50% (vol/vol) in Sol buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10% glycerol, 1% Triton X-100, with protease inhibitor cocktail [Roche Diagnostics, Mannheim, Germany]), and incubated at 4°C for 1.5 h. After the beads were washed three times with Sol buffer, proteins were solubilized in SDS-PAGE sample buffer. After SDS-PAGE, proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P membranes; Millipore), which were incubated with streptavidin-horseradish peroxidase (HRP; Amersham Pharmacia Biotech). Biotinylated proteins were detected using an enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech). Coimmunoprecipitation of the Bax/Bak complex was performed as described previously (47). Briefly, HeLa cells (3 × 105/6-cm plate) were treated with SubAB or control (phosphate-buffered saline [PBS]) for 6 h. After the cells were washed with ice-cold PBS, they were solubilized with lysis buffer {10 mM HEPES, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), pH 7.4} containing protease inhibitor cocktail (Roche Diagnostics) and incubated for 30 min on ice. After centrifugation, solubilized extracts (200 μg/200 μl) were collected and incubated with 0.25 μg of anti-Bax antibody (BD Bioscience) at 4°C for 2 h. Immunoprecipitates were collected following incubation with rProtein G-agarose (Invitrogen) for 1 h, followed by centrifugation for 1 min at 4°C. After immunocomplexes were washed with lysis buffer three times, proteins were dissolved in SDS sample buffer, analyzed by SDS-PAGE, transferred to PVDF membranes, and then quantified by Western blotting using anti-Bax or anti-Bak antibodies (Cell Signaling).
Purification of SubAB-binding proteins p250, p175, and p135.
To purify p250 by using affinity columns, biotinylated HeLa cells (5 × 107 cells) were washed twice with PBS and suspended in 10 ml of Sol buffer for 15 min on ice. After centrifugation (20 min, 17, 400 × g), the supernatant was filtered, and the filtrate (10 ml) applied to a Datura stramonium agglutinin-agarose column (bed volume, 2 ml; Seikagaku Corporation). The column was washed with 10 ml of Sol buffer, and then Sol buffer containing 1% chitooligosaccharide was used to elute the carbohydrate-containing proteins in 1-ml fractions. To confirm the presence of p250 in eluted fractions, proteins in the effluents were immunoprecipitated with SubAB as described previously (76). After SDS-PAGE, proteins were transferred to PVDF membranes, which were incubated with streptavidin-HRP. Biotinylated p250 was detected using enhanced ECL. To identify p250, proteins in effluents were precipitated with chloroform-methanol (72). The precipitated samples were heated in SDS-PAGE sample buffer, separated in gels, and transferred to PVDF membranes, which were stained with Coomassie brilliant blue (CBB).
To purify p175 from biotinylated HeLa cell lysates, samples were applied to a Maackia amurensis agglutinin-agarose column (bed volume, 2 ml; Seikagaku Corporation). After the column was washed, 0.5× Sol buffer containing 50 mM ethylenediamine was used to elute the carbohydrate-containing proteins in 1-ml fractions. To confirm the presence of p175 in eluted fractions, proteins in effluents were immunoprecipitated with SubAB as described above. To identify p175 and p135, proteins in effluents were precipitated with chloroform-methanol. Precipitated samples were heated in 1× SDS-PAGE sample buffer, separated in 6% gels, and transferred to PVDF membranes, which were stained with CBB. The stained bands were used for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
LC-MS/MS analysis.
Proteins bands were separated by 6% SDS-PAGE, transferred to PVDF membranes, and then fragmented into peptides using an on-membrane digestion method (1). Tryptic digestion of p250, p175, and p135 bands was performed individually for 24 h at 37°C in a digesting solution that contained 2 ng/ml proteome-grade trypsin (Roche Diagnostics) and 25 mM NH4HCO3. Digested peptides were injected into a trap column (0.3- by 5-mm L-trap column) and an analytical column (0.1- by 150-mm L-column2) (Chemicals Evaluation and Research Institute, Saitama, Japan), which were attached to a NanoSpace high-pressure liquid chromatography (HPLC) system (Shiseido Fine Chemicals, Tokyo, Japan). Purified peptides were introduced from the HPLC column to LTQ XL (Thermo Scientific, San Jose, CA), an ion-trap mass spectrometer, via an attached PicoTip (New Objective, Woburn, MA). The Mascot search engine (Matrixscience, London, United Kingdom) was used to identify proteins from the mass and tandem mass spectra of peptides. Peptide mass data were matched by searching the International Protein Index database using the Mascot engine (Matrix Science). The minimum criterion of the probability-based Mascot/Mowse score was set, with 5% being the significant threshold level.
Cell culture and gene silencing in HeLa cells.
HeLa cells were cultured in Eagle's minimum essential medium (EMEM; Sigma) containing 10% fetal bovine serum (FBS). HeLa cells were plated into 24-well dishes (5 × 104 cells/well) or 12-well dishes (1 × 105 cells/well) in EMEM containing 10% fetal calf serum. When cells reached 50 to 60% confluence, they were treated with 20 nM NG2 siRNA (Dharmacon), β1 ITG siRNA (HSS105561; Invitrogen), Met siRNA (Qiagen), L1CAM siRNA (Qiagen), or negative-control medium GC duplex RNAi (Invitrogen) in Lipofectamine RNAiMax transfection reagent (Invitrogen), according to the manufacturer's instructions.
Immunofluorescence staining.
HeLa cells were plated on glass coverslips in six-well dishes (1 × 105 cells/well) in EMEM containing 10% FBS and then treated with SubAB for the indicated times. Cells were washed with PBS, fixed for 10 min with 3% paraformaldehyde in PBS, washed with PBS, permeabilized for 5 min with 0.1% Triton X-100, washed with PBS, and blocked with 4% BlockAce buffer (Snow Bland) for 30 min. Primary antibodies were diluted in 0.4% BlockAce buffer and incubated overnight at 4°C. Cells were washed three times with PBS and incubated for 1 h with the indicated concentration of secondary Alexa 488 antibodies (Invitrogen) or Cy3-conjugated antibodies (Sigma) diluted in 0.4% BlockAce buffer. The washed coverslips were mounted in Prolong Gold and SlowFade Gold antifade reagents (Invitrogen) and observed by immunofluorescence microscopy or confocal microscopy (Fluoview FV10i; Olympus). For analysis of localization of NG2 or SubAB on the living cell surface, immunofluorescence labeling was performed as described previously (14). In brief, HeLa cells were incubated with the indicated primary antibodies for 30 min at room temperature, washed with EMEM containing 2% FBS, and incubated with secondary Alexa 488- or Cy3-conjugated antibodies in EMEM containing 2% FBS for 30 min. After the cells were washed with PBS and fixation with 95% ethanol, the coverslips were mounted as described above.
Detection of apoptotic marker proteins in cytosolic fraction following SubAB treatment.
To detect cytochrome c release from mitochondria into the cytosol and cleaved caspase-7, the cytosolic fraction was collected by following the method described previously (45).
Cell cytotoxicity assay.
A cytotoxicity detection kit for detection of lactate dehydrogenase (LDH) (Cytotoxicity Detection KitPLUS [LDH]; Roche Diagnostics) was used to evaluate cell cytotoxicity, according to the manufacturer's instructions. Briefly, the indicated siRNA-transfected cells were treated with or without SubAB (0.2 μg/ml) for 30 h and then assayed for LDH release in culture medium. To determine the percent cytotoxicity, the average absorbance values of the triplicate samples and controls were calculated. Maximal LDH release from cells was determined by addition of lysis solution provided in the kit, followed by incubation for 5 min at room temperature and quantification of LDH. Cell cytotoxicity (percent growth compared with that for the control) was calculated using the following formula (absorbance of SubAB-treated or untreated indicated siRNA-transfected cells minus absorbance of untreated cells)/(maximal absorbance for the control by cell lysis solution treatment minus absorbance of untreated cells) × 100. Following SubAB treatment, apoptotic cells were assessed by use of an enzyme-linked immunosorbent assay (ELISA) kit (Cell Death Detection ELISAPLUS; Roche Diagnostics) as described in the manufacturer's instructions. Briefly, the indicated siRNA-transfected cells were treated with or without SubAB (0.2 μg/ml) for 30 h and were lysed with 200 μl of cell lysis buffer. Lysates were added to a streptavidin-coated microplate and incubated with a mixture of anti-histone-biotin and anti-DNA peroxidase. After the plate was washed with incubation buffer, peroxidase substrates [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] were added to each well and the absorbance was measured at 405 nm. The enrichment factor quantified the specific enrichment of mono- and oligonucleosomes released into the cytoplasm and was calculated using the following formula: (absorbance of SubAB-treated siRNA-transfected cells)/(absorbance of untreated siRNA-transfected cells).
RESULTS
Interaction of SubAB with cell surface proteins on HeLa cells.
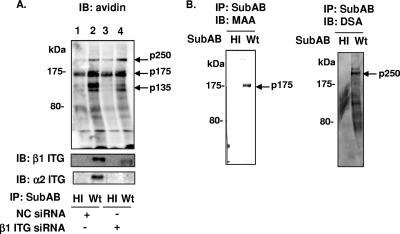
We previously reported that SubAB bound to α2β1 ITG, leading to vacuole formation in Vero cells (76). However, we could not demonstrate that in Vero cells α2β1 ITG-dependent signaling induces cell death. To identify the SubAB receptor, which is responsible for cell death, we looked for SubAB-binding proteins on HeLa cells. After SDS-PAGE of the immunoprecipitates, we found that three strongly reactive bands, those for p135, p175, and p250, were visualized by streptavidin-HRP (Fig. 1A, lane 2). Two bands, those for p175 and p135, were recognized with anti-α2 ITG and anti-β1 ITG antibodies, respectively (Fig. 1A, bottom). SubAB binding to α2β1 ITG was confirmed using β1 ITG siRNA-treated cells. β1 ITG siRNA treatment reduced the expression of α2 ITG. When β1 ITG expression was suppressed, SubAB binding to a p135 protein was significantly decreased, but the p175 band intensity did not decrease (Fig. 1A, lane 4), suggesting that there was another SubAB-binding protein at p175 other than α2 ITG.
FIG. 1.
Detection of SubAB-binding proteins in HeLa cells. (A) HeLa cells were treated for 48 h with 20 nM β1 ITG siRNA (β1 ITG) or negative-control (NC) siRNA. Biotinylated HeLa cell lysates were immunoprecipitated (IP) with heat-inactivated SubAB (HI) or wild-type SubAB (Wt), as described in Materials and Methods. SubAB-binding proteins were detected with streptavidin-HRP or anti- β1 ITG or anti-α2 ITG antibodies. (B) Proteins immunoprecipitated with SubAB were separated by SDS-PAGE and transferred to PVDF membranes, which were visualized with M. amurensis agglutinin (MAA) lectin (left) and involved in apoptosis lectin (right) as described previously (76). Arrows in panels A and B show the locations of p135, p175, and p250. Data are representative of at least three separate experiments. IB, immunoblot.
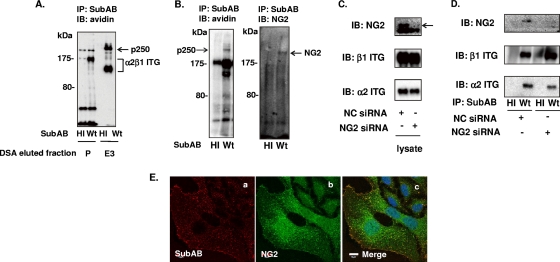
We characterized the sugar modification of p175 and p250 by lectin blot analysis using SubAB-immunoprecipitated complexes. Two binding proteins, p175 and p250, reacted with the M. amurensis lectin agglutinin and D. stramonium agglutinin lectin, respectively (Fig. 1B). M. amurensis lectin is known to recognize terminal SAα(2-3)-Gal(18, 54), and D. stramonium agglutinin lectin reacted with Galβ(1-4)GlcNAc (26). To purify p250, biotin-labeled HeLa cell lysates were incubated with D. stramonium agglutinin-agarose and eluted with 1% chitooligosaccharide. Proteins in the effluents were immunoprecipitated with SubAB. The effluents contained α2β1 ITG and p250 but not p175 (Fig. 2A). The effluents were concentrated, separated by 6% SDS-PAGE, and transferred to PVDF membranes, which were stained with CBB. After trypsin hydrolysis, LC-MS/MS analysis of the p250 protein was performed. The protein had sequences identical to the sequence of a membrane protein, chondroitin sulfate proteoglycan 4 (CSPG4/NG2). Consistent with this finding, anti-NG2 antibody reacted with p250 (Fig. 2B). Furthermore, HeLa cells transfected with NG2 siRNA showed reduced NG2 expression (Fig. 2C, arrow), while α2β1 ITG expression was not affected (Fig. 2C). Binding of SubAB to NG2 was less in cells treated with NG2 siRNA than in negative-control siRNA-treated cells (Fig. 2D, lane 4), whereas α2β1 ITG binding was not affected (Fig. 2D). Next, Cy3-labeled SubAB was used to monitor the colocalization with NG2 in HeLa cells. In U251/NG2 cells, NG2 was associated by immunostaining of living cells at 4°C with a meshwork of plasma membrane protrusions widely distributed over the apical surface (43). In HeLa cells, NG2 was also distributed on the cell surface and Cy3-labeled SubAB colocalized with NG2 (Fig. 2E).
FIG. 2.
Identification of SubAB-binding protein p250. (A) After biotinylated HeLa cell lysates were applied to a DSA-agarose column, the pass-thorough fraction was used as the pass (P) fraction. Bound proteins were eluted with six 1-ml volumes of Sol buffer containing 1% chitooligosaccharide and collected as fractions E3. Samples of fractions P and E3 were incubated with SubAB and immunoprecipitated as described in the legend to in Fig. 1A, before reaction with avidin-HRP. (B) Proteins immunoprecipitated with heat-inactivated SubAB (HI) or wild-type SubAB (Wt) using anti-SubAB antibody were separated by SDS-PAGE and transferred to PVDF membranes, which were reacted with HRP-conjugated streptavidin (left) or anti-NG2 antibody (right). (C) Proteins from cells incubated with the indicated siRNA for 48 h were separated and transferred to PVDF membranes, as described in the legend to Fig. 1A, which were incubated with antibodies against NG2 (top panel, arrow), β1 ITG (middle panel), or α2 ITG (bottom panel), followed by ECL detection. (D) The indicated siRNA-treated cell lysates were incubated with heat-inactivated SubAB or wild-type SubAB and immunoprecipitated using anti-SubAB antibody, separated, and transferred to PVDF membranes, which were reacted with antibodies against anti-NG2 (top panel), anti-β1 ITG (middle panel), or anti-α2 ITG (bottom panel), followed by ECL detection. (E) HeLa cells were incubated with Cy3-labeled SubAB (a) for 30 min at 4°C and then stained for NG2 (b) as described in Materials and Methods. A merged picture (c) shows intense colocalization of Cy3-SubAB and Alexa 488-NG2 at the membrane. Bar, 10 μm. Data are representative of those from at least three separate experiments.
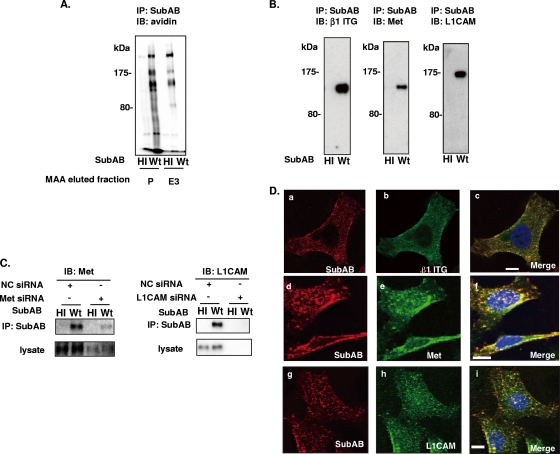
As p175 was shown to bind to M. amurensis agglutinin lectin (Fig. 1B), we purified p175 from HeLa cell lysates using M. amurensis agglutinin lectin-agarose columns. Biotinylated HeLa cell lysates were applied to M. amurensis agglutinin lectin-agarose, and the absorbed proteins were eluted with 50 mM ethylenediamine. Proteins in the pass-through fraction or in effluents were immunoprecipitated with SubAB and detected with streptavidin-HRP (Fig. 3A). Unexpectedly, all three bands (p250, p175, and p135) were detected in the eluted fractions, although M. amurensis lectin specifically bound to p175 (Fig. 1B). Both p175 and p135 were analyzed by LC-MS/MS analysis. p175 had sequences identical to those of L1CAM and α2 ITG, and p135 contained sequences identical to those of β1 ITG and Met. Thus, these results suggest that there are at least four SubAB-binding proteins in the p175 and p135 range. In agreement, p175 reacted with anti-L1CAM antibody, whereas p135 reacted with both anti-Met and anti-β1 ITG antibodies (Fig. 3B). Further experiments with L1CAM or Met siRNA showed that reduction of L1CAM and Met expression, respectively, resulted in inhibition of SubAB binding to L1CAM or Met (Fig. 3C). Confocal microscopy analysis indicated that Cy3-labeled SubAB colocalized with β1 ITG, L1CAM, or Met on the cell surface (Fig. 3D). These data are consistent with interaction of SubAB with NG2 (p250), α2β1 ITG (p175/p135), L1CAM (p175), and Met (p135) in HeLa cells.
FIG. 3.
Identification of SubAB-binding proteins p175 and p135. (A) Biotinylated HeLa cell lysates were incubated overnight with an M. amurensis-agarose column. The pass-through fraction was used as the pass (P) fraction. Bound proteins were eluted with six 1-ml volumes of Sol buffer containing 50 mM ethylenediamine and collected as fraction E3. Samples of fractions P and E3 were incubated with SubAB and immunoprecipitated as described in the legend to Fig. 2A before reaction with avidin-HRP. (B) Proteins immunoprecipitated with heat-inactivated SubAB (HI) or wild-type SubAB (Wt) using anti-SubAB antibody were separated and transferred to PVDF membranes, which were reacted with anti-β1 ITG (left panel), anti-Met (middle panel), and anti-L1CAM (right panel) antibodies. (C) Proteins from cells treated with 20 nM the indicated siRNA for 48 h were incubated with heat-inactivated or wild-type SubAB. Immunoprecipitated complexes and the lysate from siRNA-treated cells were separated and transferred to PVDF membranes, which were reacted with antibodies against anti-Met (right panel) or anti-L1CAM (left panel) antibodies, followed by ECL detection. (D) HeLa cells were incubated with Cy3-labeled SubAB (a, d, and g) for 30 min at 4°C, fixed with 3% paraformaldehyde, and reacted with anti-β1 ITG (b), anti-Met1 (e), or anti-L1CAM (h) antibodies as described in Materials and Methods. Merged pictures (c, f, and i) show colocalization in HeLa cells. Panel a to c, d to f, and g to i are at the same magnification. Bars, 10 μm. Data are representative of those from at least three separate experiments.
Effect of receptor knockdown on cell death by SubAB.
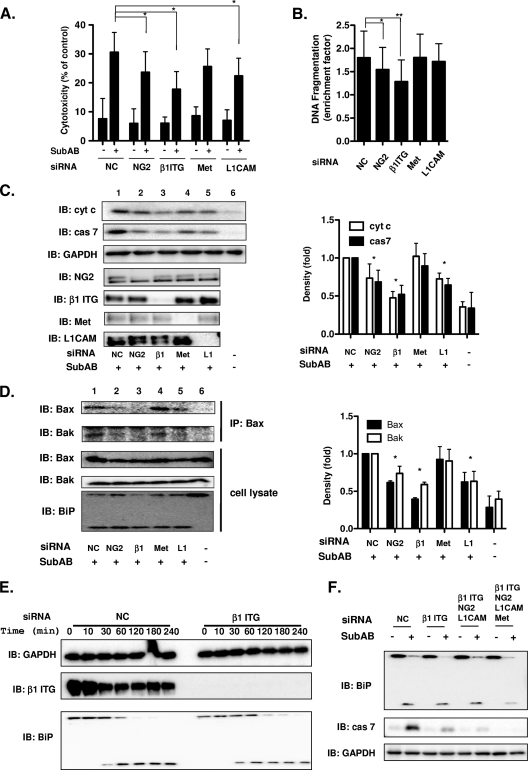
To clarify whether these SubAB-binding proteins contribute to cell death by SubAB, we examined the cytotoxicity using LDH activity (32) and DNA fragmentation assays. Cells were treated with SubAB for 30 h after transfection with the indicated siRNA. SubAB-induced cytotoxicity and DNA fragmentation were significantly suppressed in β1 ITG siRNA-treated cells and slightly suppressed in NG2 or L1CAM siRNA-treated cells, but no inhibition was observed in Met siRNA-treated cells (Fig. 4A and B). Reduction of β1 ITG expression by siRNA also dramatically inhibited cytochrome c release and activation of caspase-7 (Fig. 4C, lane 3), whereas NG2 and L1CAM siRNA treatment slightly inhibited these activities and Met siRNA treatment did not inhibit these activities, although expression levels of NG2, β1 ITG, Met, and L1CAM were significantly suppressed (Fig. 4C). In addition to caspase-7 activation, SubAB-induced caspase-3 and -9 activation with similar kinetics, while caspase-8 activation was noted 6 to 18 h after intoxication (data not shown). Since we found that SubAB-induced cytochrome c release was dependent on Bax/Bak conformational change in HeLa cells (75), we next examined the effect of SubAB on Bax conformational change and formation of the Bax/Bak complex in the indicated siRNA-transfected cells. The anti-Bax clone 3 monoclonal antibody, which reacted to amino acids 55 to 178 (spanning Bcl-2 homology domains 1 to 3) of Bax and which appeared to be conformation specific (17), was used to immunoprecipitate Bax from siRNA-transfected cells solubilized in CHAPS. This nonionic detergent was chosen because it maintains the quaternary structure of monomeric Bax in its native conformation (31, 47). The amount of conformationally changed Bax following SubAB treatment was decreased in cells treated with NG2, β1 ITG, or L1CAM siRNA (Fig. 4D, lanes 2, 3, and 5) but not in the cells treated with negative-control or Met siRNA (Fig. 4D, lanes 1 and 4). In addition, SubAB induced Bax/Bak complexes in negative-control or Met siRNA-transfected cells (Fig. 4D, lanes 1 and 4) and, to a lesser extent, in NG2 and L1CAM siRNA-transfected cells (Fig. 4D, lanes 2 and 5) but not in β1 ITG siRNA-transfected cells (Fig. 4D, lane 3). Interestingly, BiP cleavage was not inhibited in these siRNA-treated cells (Fig. 4D, bottom). Even at early time points, BiP cleavage occurred, although it was slightly delayed (but not significantly so) in β1 ITG-knockdown cells (Fig. 4E). We next examined SubAB-induced caspase activation in siRNA-treated cells in which four proteins were suppressed. Caspase activation was dramatically decreased when these receptors were suppressed; however, BiP cleavage was still observed (Fig. 4F).
FIG. 4.
Effect of gene silencing of SubAB-binding proteins on SubAB-induced cell death. (A) The indicated siRNA-transfected HeLa cells were incubated with or without SubAB (0.2 μg/ml) for 30 h. Cytotoxicity was assessed by measurement of LDH activity as described in Materials and Methods. NC, negative-control siRNA. Data are means ± standard deviations of values from three independent assays performed in triplicate experiments. *, P < 0.01. (B) The indicated siRNA-treated cells were incubated with or without SubAB for 30 h, and then DNA fragmentation was detected by a cell death detection ELISA as described in Materials and Methods. Data are means ± standard deviations of values from three independent assays performed in triplicate experiments. *, P < 0.04; **, P < 0.01. (C) The indicated siRNA-transfected cells were incubated with SubAB (0.2 μg/ml; lanes 1 to 5) or not incubated (control; lane 6) for 6 h. Cytochrome c (cyt c), cleaved caspase-7 (cas 7), and GAPDH were detected by Western blotting. The knockdown level of protein expression was also confirmed by Western blotting with antibodies against NG2, β1 ITG, Met, or L1CAM. The levels of cytochrome c and cleaved caspase-7 observed in untreated siRNA-transfected cells were similar to those observed in control cells. A representative blot of three separate experiments is shown. Quantification of cytochrome c and cleaved caspase 7 was performed by analysis with a densitometer (right panel). Data are the means ± standard deviations from three separate experiments. *, P < 0.05; **, P < 0.01. (D) Coimmunoprecipitation of Bax/Bak complex. CHAPS (2%) extracts of the indicated siRNA-transfected cells were treated with or without SubAB or were not treated, and proteins were then immunoprecipitated with conformation-specific anti-active Bax monoclonal antibody as described in Materials and Methods. The immunocomplexes or cell lysates were analyzed by SDS-PAGE, followed by immunoblotting with anti-Bax, anti-Bak, or anti-BiP antibodies. A representative blot of two separate experiments is shown. Quantification of conformationally changed Bax and coprecipitated Bak was performed by densitometer analysis (right panel). Data are the means ± standard deviations of the values from two separate experiments. *, P < 0.05. (E) SubAB-induced BiP cleavage at early time points in β1 ITG-knockdown cells. The indicated siRNA-transfected cells (1 × 105 cell/well) were incubated with SubAB (0.2 μg/ml) for the indicated times at 37°C. GAPDH, β1 ITG, and BiP were detected by Western blotting. A representative blot of three separate experiments is shown. (F) The indicated siRNA-transfected cells were incubated or without SubAB (0.2 μg/ml) for 3 h at 37°C. GAPDH, cleaved caspase-7 (cas 7), and BiP were detected by Western blotting. A representative blot of two separate experiments is shown. The knockdown levels of the proteins were also confirmed by Western blotting with antibodies against NG2, β1 ITG, Met, or L1CAM (data not shown).
Effect of β1 integrin knockdown on thapsigargin-induced caspase activation in HeLa cells.
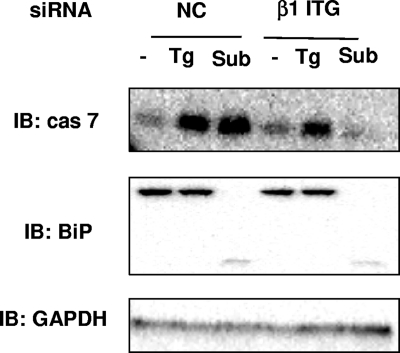
To show that SubAB-induced caspase activation is regulated by specific receptors, we investigated the effect of the ER stress-inducing agent Tg, as a positive control for ER stress, on caspase activation in β1 ITG-knockdown cells. Although SubAB-induced caspase activation was inhibited in β1 ITG siRNA-transfected cells, Tg-induced caspase activation was not suppressed in β1 ITG-knockdown cells (Fig. 5). Thus, SubAB-induced caspase activation participates in a pathway different from that regulated by Tg, and these results suggest that SubAB-induced apoptosis may be dependent on β1 ITG, NG2, and L1CAM-related signaling pathways, in addition to SubAB-induced BiP cleavage.
FIG. 5.
Effect of gene silencing of β1 integrin on thapsigargin-induced cell death. The indicated siRNA-transfected cells were incubated with or without 1 μM Tg or 0.2 μg/ml SubAB (Sub) or were not treated for 3 h at 37°C. GAPDH, cleaved caspase-7 (cas 7), and BiP were detected by Western blotting. A representative blot of two separate experiments is shown. The knockdown levels of the proteins were also confirmed by Western blotting with antibodies against β1 ITG (data not shown). A representative blot of three separate experiments is shown.
SubAB-induced caspase activation was enhanced by function-blocking β1 ITG antibody treatment.
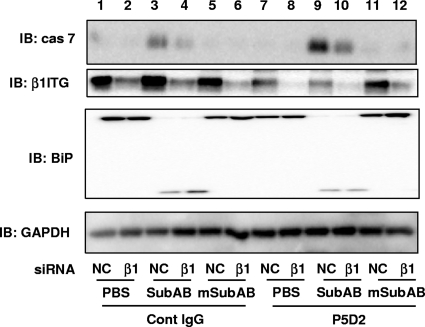
As shown in Fig. 4, β1 ITG signaling seems to be important in SubAB-induced apoptosis in HeLa cells. To investigate whether β1 ITG signaling affects SubAB-induced cell death, HeLa cells were pretreated with control IgG or anti-β1 ITG antibody (MAb P5D2), which was shown to suppress the functional activity of α2β1 ITG and to induce inhibitory signaling for cell adhesion (6), and then incubated with SubAB or mSubAB for 3 h. Although the extent of BiP cleavage in control mouse IgG- and MAb P5D2-treated cells was not different (Fig. 6, lanes 3, 4, 9, and 10), SubAB-induced caspase-7 activation was enhanced in MAb P5D2-treated cells (Fig. 6, lane 9). In addition, control mouse IgG or MAb P5D2 treatment did not affect caspase activation in mSubAB-treated cells (Fig. 6, lanes 5, 6, 11, and 12). In contrast, in β1 ITG-knockdown cells, caspase-7 activation by SubAB was clearly suppressed in the presence or absence of MAb P5D2 (Fig. 6, lanes 4 and 10), suggesting that β1 ITG signaling may be involved in SubAB-induced apoptotic signaling.
FIG. 6.
Effect of β1 ITG inhibitory signaling and BiP cleavage on SubAB-induced caspase activation. Control or β1 ITG siRNA-transfected HeLa cells were pretreated for 30 min with control mouse IgG or anti-β1 ITG antibodies (MAb P5D2) (1 μg/ml) that inhibit function and were then treated with 0.2 μg/ml of SubAB, mSubAB, or PBS (control) for 3 h. Cell lysates were separated by SDS-PAGE, followed by immunoblotting with anti-cleaved caspase-7 (cas 7), anti-β1 ITG, anti-BiP, or anti-GAPDH antibodies. A representative blot of two separate experiments is shown.
SubAB-induced FAK fragmentation.
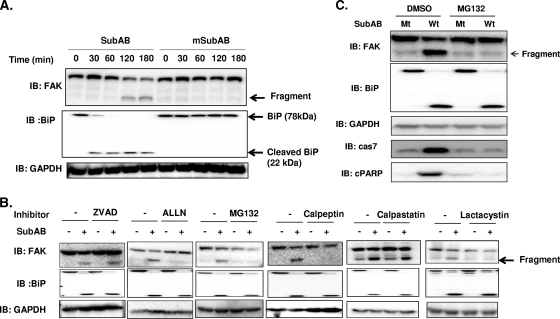
β1 ITG is known to form protein complexes at focal adhesion plaques, consisting of several classes of proteins, including FAK and src family members, which initiate tyrosine phosphorylation events that regulate growth and differentiation of many cell types (48, 49, 77). We hypothesized that β1 ITG-associated molecules might contribute to SubAB-induced cell death signaling after ER stress resulting from SubAB-induced BiP cleavage. We observed that BiP was cleaved after 30 min of incubation with SubAB, followed by FAK cleavage; mutant SubAB was inactive (Fig. 7A). Thus, ER stress resulting from SubAB-induced BiP cleavage precedes FAK degradation. Proteolytic cleavage of FAK has been reported in various cell types during apoptosis (13, 25, 41, 46). FAK cleavage was shown to be induced by activation of calpain-like intracellular proteases (8) or caspases (23, 41, 42, 62, 71) in fibroblasts transformed by the v-src or myc oncogene (13, 20) or by a proteasome-dependent pathway (36, 53). To clarify how FAK cleavage was induced in SubAB-treated cells, HeLa cells were incubated with SubAB in the presence of caspase inhibitor (ZVAD), calpain, and proteasome inhibitors (ALLN, calpeptin, and MG132), calpain inhibitor (acetyl-calpastatin [Ac-calpastatin]) or proteasome inhibitor (lactacystin) for 3 h at 37°C. Cell lysates were analyzed by Western blotting using an anti-FAK antibody. Although SubAB-induced BiP cleavage was not inhibited, FAK cleavage was significantly suppressed in the presence of ALLN, calpeptin, MG132, or lactacystin but not ZVAD and Ac-calpastatin compared with the level of cleavage for the control (Fig. 7B). These results suggest that SubAB-induced ER stress promotes FAK proteolysis through a proteasome-dependent event. To determine whether proteasomal degradation, in addition to SubAB-induced ER stress, is involved in regulating apoptosis, we examined SubAB-induced caspase activation in the presence of MG132. MG132 pretreatment significantly inhibited SubAB-induced caspase-7 activation and PARP cleavage (Fig. 7C).
FIG. 7.
SubAB-induced ER stress caused FAK proteolysis. (A) HeLa cells (1 × 105 cell/well) were incubated with wild-type SubAB or mSubAB (0.2 μg/ml) for the indicated times at 37°C. Cell lysate proteins were analyzed by Western blotting using antibodies against BiP, FAK, or GAPDH, as a control. Data are representative of those from three separate experiments. (B) HeLa cells were preincubated in the presence of either dimethyl sulfoxide or PBS (control), ZVAD (50 μM), ALLN (100 μM), MG132 (20 μM), calpeptin (60 μM), calpastatin (20 μM), or lactacystin (10 μM) for 30 min and were then incubated with or without SubAB for 3 h prior to preparation of cell lysates, which were analyzed with the indicated antibodies. Data are representative of those from at least three separate experiments. (C) HeLa cells were preincubated with dimethyl sulfoxide (DMSO) or MG132 (20 μM) for 30 min and then incubated with 0.2 μg/ml of wild-type SubAB (Wt) or mSubAB (Mt) for 3 h. Cell lysate proteins were analyzed by Western blotting using antibodies against BiP, FAK, cleaved caspase-7 (cas 7), cleaved PARP (cPARP), or GAPDH. Data are representative of those from three separate experiments.
DISCUSSION
Here we show some of the specific SubAB receptors in HeLa cells which directly contribute to cell death. Analysis of SubAB-binding proteins in HeLa cells using immunoprecipitation, purification using lectin, and identification by LC-MS/MS led us to conclude that the membrane proteins responsible for binding to SubAB were α2β1 ITG, NG2, Met, and L1CAM. We previously reported that SubAB bound to α2β1 ITG, leading to vacuole formation in Vero cells (76). However, we could not demonstrate that α2β1 ITG-dependent signaling induces cell death. It is possible that Neu5Gc-modified proteins differ between Vero cells and HeLa cells. Recently, it was reported that SubAB had a strong preference for binding to glycans terminating in Neu5Gc (7). It was postulated that a monosaccharide, Neu5Gc, was the SubAB receptor, even though Neu5Gc is not synthesized in humans and protein-bound Neu5Gc is generated by metabolic incorporation of the Neu5Gc contained in food products. In fact, Neu5Gc-modified proteins are abundant in Vero cells compared to their prevalence in HeLa cells (data not shown). Thus, we hypothesize that following β1 ITG knockdown, Vero cells still have low-affinity SubAB-binding proteins containing Neu5Gc which are responsible for cell death. Therefore, we are unable to conclude that α2β1 ITG-dependent signaling induces cell death since β1 ITG-knockdown Vero cells may still have an alternative SubAB-dependent signaling pathway(s). Regarding structural features of receptor proteins required for SubAB-binding activity, neuraminidase treatment of HeLa cell lysate resulted in inhibition of SubAB binding (data not shown). Lectin blot analysis indicated that L1CAM contained O-glycan and terminal SAα(2-3)-Gal, while NG2, α2β1 ITG, and Met were modified by N-glycan-linked Gal-β(1-4)-GlcNAc and terminal sialic acids, and the terminal sialic acids of these binding proteins are essential for interaction with SubAB.
At the purification step using M. amurensis lectin-agarose, the effluents contained not only L1CAM but also NG2, α2β1 ITG, and Met, suggesting the possibility that NG2, α2β1 ITG, or Met bound to M. amurensis lectin under these undenatured conditions or that these receptors might form a complex in HeLa cells. In fact, these membrane proteins are known to interact or cross talk with each other and regulate various cellular functions. NG2 interacts with the galectin/α3β1 ITG complex on the cell surface, resulting in enhanced β1 ITG signaling, with effects on cell motility, endothelial tube formation in vitro, and angiogenesis (21, 59, 65). L1CAM functionally interacts with β1 ITG to potentiate neuronal migration toward extracellular matrix proteins through endocytosis and mitogen-activated protein kinase signaling (69), and the RGD site in L1CAM involved in the interaction with ITG in tumors is known to play an important role in cell-cell binding, cell motility, invasiveness, and tumor growth (22). Further, phosphorylated Met formed a complex with β1 ITG and was colocalized with vinculin and FAK at focal adhesions in epithelial cells expressing activated Src (33). Since β1 ITG is a key protein in these cellular pathways, binding of SubAB to β1 ITG may induce interaction or cross talk with other proteins and affect cell movement, focal adhesion, or signal transduction.
To define which binding protein is associated with SubAB-induced apoptosis, we examined in HeLa cells the effect of gene silencing of SubAB-binding proteins on Bax/Bak activation, cytochrome c release, and caspase-7 activation. We found that SubAB-induced Bax/Bak activation, cytochrome c release, and apoptosis were significantly and most effectively decreased in β1 ITG-knockdown cells; SubAB-induced BiP cleavage, however, was observed at a level similar to that in control siRNA-transfected cells at the early and late time points, suggesting that SubAB-induced BiP cleavage might be mediated by other SubAB-binding proteins in Triton X-100-insoluble fractions or by terminal sialic acid-modified minor proteins. Similarly, NG2 and L1CAM but not Met siRNA treatment also slightly but significantly inhibited SubAB-induced cell death, cytochrome c release, and Bax activation but not BiP cleavage. In addition, even siRNA knockdown of four proteins (NG2, β1 ITG, L1CAM, and Met) also significantly inhibited SubAB-induced caspase activation but not BiP cleavage (Fig. 4F). There are several reports that these SubAB-binding proteins regulate cell death. Stimulation of β1 ITG signal- ing markedly upregulated anchorage-dependent apoptosis (anoikis) of adenocarcinoma cells (52). NG2, a novel proapoptotic receptor (35), regulated anoikis of fibroblasts via changes in FAK phosphorylation through a protein kinase Cα-dependent pathway. Further, treatment with L1CAM monoclonal antibody inhibited tumor growth (5). Thus, these findings suggest that these proteins regulate cell proliferation and death. Our results indicate that SubAB recognizes these proteins as functional receptors and thereby regulates cell death.
Although SubAB-induced BiP cleavage was observed in NG2-, β1 ITG-, L1CAM-. or all four protein-knockdown cells, SubAB-induced apoptosis was significantly decreased to a different extent dependent on each receptor. These results raise the possibility that SubAB-induced cell death requires, in addition to BiP cleavage-induced ER stress, additional signaling pathways resulting from SubAB binding to surface receptors. We demonstrated that Tg-induced caspase activation was not inhibited in β1 ITG-knockdown cells (Fig. 5). Thus, our results suggest that SubAB receptors may be regulated by a SubAB-induced apoptotic pathway, which is different from that regulated by Tg. The importance of β1 ITG signaling was demonstrated using MAb P5D2, which is known to inhibit β1 ITG signaling. SubAB-induced caspase-7 activation was significantly enhanced in cells pretreated with MAb P5D2 and clearly decreased in β1 ITG siRNA-treated cells, while BiP cleavage was at a level similar to that in control IgG-treated cells. Caspase-7 activation by mSubAB was not observed in cells pretreated with MAb P5D2, suggesting that β1 ITG-related signaling alone is not sufficient to induce caspase activation. β1 ITG-induced signaling needs to be coupled with BiP cleavage to enhance apoptosis. SubAB binding to NG2 and L1CAM also might alter signal transduction, leading to effects on apoptosis. Differences in SubAB-induced cytotoxicity in each receptor-knockdown cell might depend on the signaling pathway associated with the particular SubAB receptor.
There were several reports of apoptosis in response to ER stress (63, 64). Bax and Bak operate in both ER and mitochondria as part of an essential gateway for selected apoptotic signals. Interferon gene regulatory element 1 (IRE1) forms a heterotrimeric complex with tumor necrosis factor-associated factor 2 and apoptosis signal-regulating kinase 1 (ASK1), resulting in activation of c-Jun N-terminal protein kinase (JNK), leading to cell death (55, 68). Our previous work demonstrated that SubAB-induced cell death appears to be induced through Bax/Bak-dependent cytochrome c release, which was not mediated by the IRE1 or JNK pathway (75). Here we show that SubAB induced Bax conformational changes and that Bax/Bak complex formation was dramatically suppressed in β1 ITG-knockdown cells. However, it is unclear how, in SubAB-treated HeLa cells, β1 ITG regulates Bax/Bak conformational changes and their oligomerization, cytochrome c release, and cell death. It was reported that (i) ITG regulates the apoptotic function of Bax through FAK activity, (ii) ITG-mediated positional control is required for maintaining homeostasis, and (iii) defective ITG signaling resulted in apoptosis (24). We found that FAK was cleaved following BiP cleavage after 30 min of incubation with SubAB but not mutant SubAB (Fig. 7A). FAK regulates proliferation and migration of normal and tumor cells (12). It has been proposed that FAK cleavage attenuates its autokinase activity, participates in disassembly of the focal adhesion complex, interrupts survival signals, and then finally promotes cell death (11, 15, 41). ER-associated degradation (ERAD) has been known to mediate the ER stress-induced decrease in several membrane proteins (27, 32, 60). Recently, it was reported that ER stress by cis-hydroxyproline leads to activation of an intracellular proteolytic process, including caspase-independent FAK degradation, resulting in cell damage (53). We found here that SubAB-induced FAK fragmentation was caused by a proteasome-dependent pathway. Interestingly, SubAB-induced apoptotic activation was inhibited by MG132 treatment at the early time points. Our data indicate that the inhibition of proteasomal degradation of SubAB receptor-related signal molecules including FAK may block SubAB-induced cell death. We proposed that ER stress by SubAB-induced BiP cleavage caused FAK proteolysis and thereby might alter FAK activity in an ITG-regulated signaling pathway, resulting in defective signaling, followed by Bax conformational changes and cell death.
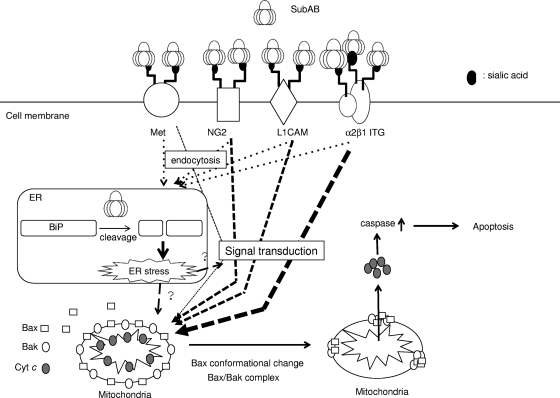
Finally, a proposed model is shown in Fig. 8. First, SubAB interacts with terminal sialic acids of NG2, L1CAM, α2β1 ITG, or Met. After SubAB is delivered to the ER, SubAB directly cleaves BiP, leading to induction of ER stress signals, which induces activation of the proteasome pathway. At the same time, SubAB binding to NG2, L1CAM, α2β1 ITG, or Met induces signals from each receptor. It remains unclear how SubAB-induced ER stress signals and signals from the receptors affect mitochondria or modify receptor-mediated signaling pathways. However, β1 ITG in HeLa cells is the major receptor for SubAB-induced cell death, and NG2 and L1CAM are also involved, at least in part, in cell death.
FIG. 8.
Proposed model of SubAB-induced apoptosis signaling pathway in HeLa cells. See text for additional details.
Acknowledgments
This work was supported by grants in aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan and Improvement of Research Environment for Young Researchers from the Japan Science and Technology Agency. Joel Moss was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health.
We thank T. Hirayama (Institute of Tropical Medicine, Nagasaki University) for helpful discussions and review of the manuscript. We acknowledge the expert technical assistance of C. Noritake and A. Kiuchi.
Editor: S. M. Payne
Footnotes
Published ahead of print on 22 November 2010.
REFERENCES
- 1.Aebersold, R. H., J. Leavitt, R. A. Saavedra, L. E. Hood, and S. B. Kent. 1987. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc. Natl. Acad. Sci. U. S. A. 84:6970-6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoudjit, F., and K. Vuori. 2000. Engagement of the alpha2beta1 integrin inhibits Fas ligand expression and activation-induced cell death in T cells in a focal adhesion kinase-dependent manner. Blood 95:2044-2051. [PubMed] [Google Scholar]
- 3.Aoudjit, F., and K. Vuori. 2001. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene 20:4995-5004. [DOI] [PubMed] [Google Scholar]
- 4.Aoudjit, F., and K. Vuori. 2001. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J. Cell Biol. 152:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arlt, M. J., et al. 2006. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 66:936-943. [DOI] [PubMed] [Google Scholar]
- 6.Beauvais, D. M., and A. C. Rapraeger. 2003. Syndecan-1-mediated cell spreading requires signaling by alphavbeta3 integrins in human breast carcinoma cells. Exp. Cell Res. 286:219-232. [DOI] [PubMed] [Google Scholar]
- 7.Byres, E., et al. 2008. Incorporation of a non-human glycan mediates human susceptibility to a bacterial toxin. Nature 456:648-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carragher, N. O., B. Levkau, R. Ross, and E. W. Raines. 1999. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J. Cell Biol. 147:619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheresh, D. A., and D. G. Stupack. 2002. Integrin-mediated death: an explanation of the integrin-knockout phenotype? Nat. Med. 8:193-194. [DOI] [PubMed] [Google Scholar]
- 10.Chong, D. C., J. C. Paton, C. M. Thorpe, and A. W. Paton. 2008. Clathrin-dependent trafficking of subtilase cytotoxin, a novel AB5 toxin that targets the endoplasmic reticulum chaperone BiP. Cell. Microbiol. 10:795-806. [DOI] [PubMed] [Google Scholar]
- 11.Cooray, P., et al. 1996. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem. J. 318(Pt 1):41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox, B. D., M. Natarajan, M. R. Stettner, and C. L. Gladson. 2006. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J. Cell. Biochem. 99:35-52. [DOI] [PubMed] [Google Scholar]
- 13.Crouch, D. H., V. J. Fincham, and M. C. Frame. 1996. Targeted proteolysis of the focal adhesion kinase pp125 FAK during c-MYC-induced apoptosis is suppressed by integrin signalling. Oncogene 12:2689-2696. [PubMed] [Google Scholar]
- 14.Dahlin-Huppe, K., E. O. Berglund, B. Ranscht, and W. B. Stallcup. 1997. Mutational analysis of the L1 neuronal cell adhesion molecule identifies membrane-proximal amino acids of the cytoplasmic domain that are required for cytoskeletal anchorage. Mol. Cell. Neurosci. 9:144-156. [DOI] [PubMed] [Google Scholar]
- 15.Dedieu, S., et al. 2004. Myoblast migration is regulated by calpain through its involvement in cell attachment and cytoskeletal organization. Exp. Cell Res. 292:187-200. [DOI] [PubMed] [Google Scholar]
- 16.de la Fuente, M. T., B. Casanova, M. Garcia-Gila, A. Silva, and A. Garcia-Pardo. 1999. Fibronectin interaction with alpha4beta1 integrin prevents apoptosis in B cell chronic lymphocytic leukemia: correlation with Bcl-2 and Bax. Leukemia 13:266-274. [DOI] [PubMed] [Google Scholar]
- 17.Dewson, G., R. T. Snowden, J. B. Almond, M. J. Dyer, and G. M. Cohen. 2003. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene 22:2643-2654. [DOI] [PubMed] [Google Scholar]
- 18.Edge, A. S., and R. G. Spiro. 1987. Presence of an O-glycosidically linked hexasaccharide in fetuin. J. Biol. Chem. 262:16135-16141. [PubMed] [Google Scholar]
- 19.Estrugo, D., et al. 2007. Ligand bound beta1 integrins inhibit procaspase-8 for mediating cell adhesion-mediated drug and radiation resistance in human leukemia cells. PLoS One 2:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fincham, V. J., J. A. Wyke, and M. C. Frame. 1995. v-Src-induced degradation of focal adhesion kinase during morphological transformation of chicken embryo fibroblasts. Oncogene 10:2247-2252. [PubMed] [Google Scholar]
- 21.Fukushi, J., I. T. Makagiansar, and W. B. Stallcup. 2004. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol. Biol. Cell 15:3580-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gast, D., et al. 2008. The RGD integrin binding site in human L1-CAM is important for nuclear signaling. Exp. Cell Res. 314:2411-2418. [DOI] [PubMed] [Google Scholar]
- 23.Gervais, F. G., N. A. Thornberry, S. C. Ruffolo, D. W. Nicholson, and S. Roy. 1998. Caspases cleave focal adhesion kinase during apoptosis to generate a FRNK-like polypeptide. J. Biol. Chem. 273:17102-17108. [DOI] [PubMed] [Google Scholar]
- 24.Gilmore, A. P., A. D. Metcalfe, L. H. Romer, and C. H. Streuli. 2000. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J. Cell Biol. 149:431-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossmann, J., et al. 2001. Hierarchical cleavage of focal adhesion kinase by caspases alters signal transduction during apoptosis of intestinal epithelial cells. Gastroenterology 120:79-88. [DOI] [PubMed] [Google Scholar]
- 26.Hart, G. W., R. S. Haltiwanger, G. D. Holt, and W. G. Kelly. 1989. Glycosylation in the nucleus and cytoplasm. Annu. Rev. Biochem. 58:841-874. [DOI] [PubMed] [Google Scholar]
- 27.Hegde, N. R., et al. 2006. The role of BiP in endoplasmic reticulum-associated degradation of major histocompatibility complex class I heavy chain induced by cytomegalovirus proteins. J. Biol. Chem. 281:20910-20919. [DOI] [PubMed] [Google Scholar]
- 28.Heino, J., R. A. Ignotz, M. E. Hemler, C. Crouse, and J. Massague. 1989. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J. Biol. Chem. 264:380-388. [PubMed] [Google Scholar]
- 29.Hess, F., D. Estrugo, A. Fischer, C. Belka, and N. Cordes. 2007. Integrin-linked kinase interacts with caspase-9 and -8 in an adhesion-dependent manner for promoting radiation-induced apoptosis in human leukemia cells. Oncogene 26:1372-1384. [DOI] [PubMed] [Google Scholar]
- 30.Hood, J. D., and D. A. Cheresh. 2002. Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2:91-100. [DOI] [PubMed] [Google Scholar]
- 31.Hsu, Y. T., and R. J. Youle. 1997. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272:13829-13834. [DOI] [PubMed] [Google Scholar]
- 32.Huang, T., et al. 2009. Downregulation of gap junction expression and function by endoplasmic reticulum stress. J. Cell. Biochem. 107:973-983. [DOI] [PubMed] [Google Scholar]
- 33.Hui, A. Y., et al. 2009. Src and FAK mediate cell-matrix adhesion-dependent activation of Met during transformation of breast epithelial cells. J. Cell. Biochem. 107:1168-1181. [DOI] [PubMed] [Google Scholar]
- 34.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 35.Joo, N. E., et al. 2008. NG2, a novel proapoptotic receptor, opposes integrin alpha4 to mediate anoikis through PKCalpha-dependent suppression of FAK phosphorylation. Cell Death Differ. 15:899-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim, B., C. M. van Golen, and E. L. Feldman. 2003. Degradation and dephosphorylation of focal adhesion kinase during okadaic acid-induced apoptosis in human neuroblastoma cells. Neoplasia 5:405-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo, Y., et al. 2009. Glycosphingolipids are not pivotal receptors for subtilase cytotoxin in vivo: sensitivity analysis with glycosylation-defective mutant mice. Biochem. Biophys. Res. Commun. 378:179-181. [DOI] [PubMed] [Google Scholar]
- 38.Kozlova, N. I., G. E. Morozevich, A. N. Chubukina, and A. E. Berman. 2001. Integrin alphavbeta3 promotes anchorage-dependent apoptosis in human intestinal carcinoma cells. Oncogene 20:4710-4717. [DOI] [PubMed] [Google Scholar]
- 39.Lee, J. W., and R. L. Juliano. 2000. alpha5beta1 integrin protects intestinal epithelial cells from apoptosis through a phosphatidylinositol 3-kinase and protein kinase B-dependent pathway. Mol. Biol. Cell 11:1973-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenter, M., and D. Vestweber. 1994. The integrin chains beta 1 and alpha 6 associate with the chaperone calnexin prior to integrin assembly. J. Biol. Chem. 269:12263-12268. [PubMed] [Google Scholar]
- 41.Levkau, B., B. Herren, H. Koyama, R. Ross, and E. W. Raines. 1998. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J. Exp. Med. 187:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, J. J., and D. Xie. 2007. Cleavage of focal adhesion kinase (FAK) is essential in adipocyte differentiation. Biochem. Biophys. Res. Commun. 357:648-654. [DOI] [PubMed] [Google Scholar]
- 43.Makagiansar, I. T., et al. 2004. Phosphorylation of NG2 proteoglycan by protein kinase C-alpha regulates polarized membrane distribution and cell motility. J. Biol. Chem. 279:55262-55270. [DOI] [PubMed] [Google Scholar]
- 44.Marco, R. A., C. M. Diaz-Montero, J. N. Wygant, E. S. Kleinerman, and B. W. McIntyre. 2003. Alpha 4 integrin increases anoikis of human osteosarcoma cells. J. Cell. Biochem. 88:1038-1047. [DOI] [PubMed] [Google Scholar]
- 45.Matsuura, G., et al. 2009. Novel subtilase cytotoxin produced by Shiga-toxigenic Escherichia coli induces apoptosis in Vero cells via mitochondrial membrane damage. Infect. Immun. 77:2919-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mian, M. F., et al. 2008. Cleavage of focal adhesion kinase is an early marker and modulator of oxidative stress-induced apoptosis. Chem. Biol. Interact. 171:57-66. [DOI] [PubMed] [Google Scholar]
- 47.Mikhailov, V., et al. 2003. Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochrome c release. J. Biol. Chem. 278:5367-5376. [DOI] [PubMed] [Google Scholar]
- 48.Miyamoto, S., S. K. Akiyama, and K. M. Yamada. 1995. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 267:883-885. [DOI] [PubMed] [Google Scholar]
- 49.Miyamoto, S., et al. 1995. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 131:791-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morinaga, N., K. Yahiro, G. Matsuura, J. Moss, and M. Noda. 2008. Subtilase cytotoxin, produced by Shiga-toxigenic Escherichia coli, transiently inhibits protein synthesis of Vero cells via degradation of BiP and induces cell cycle arrest at G1 by downregulation of cyclin D1. Cell. Microbiol. 10:921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morinaga, N., et al. 2007. Two distinct cytotoxic activities of subtilase cytotoxin produced by Shiga-toxigenic Escherichia coli. Infect. Immun. 75:488-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morozevich, G. E., et al. 2006. The role of beta1 integrin subfamily in anchorage-dependent apoptosis of breast carcinoma cells differing in multidrug resistance. Biochemistry 71:489-495. [DOI] [PubMed] [Google Scholar]
- 53.Mueller, C., et al. 2006. Cis-hydroxyproline-induced inhibition of pancreatic cancer cell growth is mediated by endoplasmic reticulum stress. World J. Gastroenterol. 12:1569-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nilsson, B., N. E. Norden, and S. Svensson. 1979. Structural studies on the carbohydrate portion of fetuin. J. Biol. Chem. 254:4545-4553. [PubMed] [Google Scholar]
- 55.Nishitoh, H., et al. 2002. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16:1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Brien, V., S. M. Frisch, and R. L. Juliano. 1996. Expression of the integrin alpha 5 subunit in HT29 colon carcinoma cells suppresses apoptosis triggered by serum deprivation. Exp. Cell Res. 224:208-213. [DOI] [PubMed] [Google Scholar]
- 57.Paton, A. W., et al. 2006. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443:548-552. [DOI] [PubMed] [Google Scholar]
- 58.Paton, A. W., P. Srimanote, U. M. Talbot, H. Wang, and J. C. Paton. 2004. A new family of potent AB(5) cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 200:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pluschke, G., et al. 1996. Molecular cloning of a human melanoma-associated chondroitin sulfate proteoglycan. Proc. Natl. Acad. Sci. U. S. A. 93:9710-9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rab, A., et al. 2007. Endoplasmic reticulum stress and the unfolded protein response regulate genomic cystic fibrosis transmembrane conductance regulator expression. Am. J. Physiol. Cell Physiol. 292:C756-C766. [DOI] [PubMed] [Google Scholar]
- 61.Ron, D., and P. Walter. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8:519-529. [DOI] [PubMed] [Google Scholar]
- 62.Sawhney, R. S., M. M. Cookson, Y. Omar, J. Hauser, and M. G. Brattain. 2006. Integrin alpha2-mediated ERK and calpain activation play a critical role in cell adhesion and motility via focal adhesion kinase signaling: identification of a novel signaling pathway. J. Biol. Chem. 281:8497-8510. [DOI] [PubMed] [Google Scholar]
- 63.Schroder, M., and R. J. Kaufman. 2005. ER stress and the unfolded protein response. Mutat. Res. 569:29-63. [DOI] [PubMed] [Google Scholar]
- 64.Scorrano, L., et al. 2003. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300:135-139. [DOI] [PubMed] [Google Scholar]
- 65.Stallcup, W. B., and F. J. Huang. 2008. A role for the NG2 proteoglycan in glioma progression. Cell Adh. Migr. 2:192-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stupack, D. G., X. S. Puente, S. Boutsaboualoy, C. M. Storgard, and D. A. Cheresh. 2001. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 155:459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szegezdi, E., S. E. Logue, A. M. Gorman, and A. Samali. 2006. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7:880-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan, Y., et al. 2006. Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 281:16016-16024. [DOI] [PubMed] [Google Scholar]
- 69.Thelen, K., et al. 2002. The neural cell adhesion molecule L1 potentiates integrin-dependent cell migration to extracellular matrix proteins. J. Neurosci. 22:4918-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tozzoli, R., et al. Production of the subtilase AB5 cytotoxin by Shiga toxin-negative Escherichia coli. J. Clin. Microbiol. 48:178-183. [DOI] [PMC free article] [PubMed]
- 71.Wen, L. P., et al. 1997. Cleavage of focal adhesion kinase by caspases during apoptosis. J. Biol. Chem. 272:26056-26061. [DOI] [PubMed] [Google Scholar]
- 72.Wessel, D., and U. I. Flugge. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138:141-143. [DOI] [PubMed] [Google Scholar]
- 73.Wolfson, J. J., et al. 2008. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell. Microbiol. 10:1775-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu, J., and R. J. Kaufman. 2006. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 13:374-384. [DOI] [PubMed] [Google Scholar]
- 75.Yahiro, K., N. Morinaga, J. Moss, and M. Noda. 2010. Subtilase cytotoxin induces apoptosis in HeLa cells by mitochondrial permeabilization via activation of Bax/Bak, independent of C/EBF-homologue protein (CHOP), Ire1alpha or JNK signaling. Microb. Pathog. 49:153-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yahiro, K., et al. 2006. Identification and characterization of receptors for vacuolating activity of subtilase cytotoxin. Mol. Microbiol. 62:480-490. [DOI] [PubMed] [Google Scholar]
- 77.Yamada, K. M., and S. Miyamoto. 1995. Integrin transmembrane signaling and cytoskeletal control. Curr. Opin. Cell Biol. 7:681-689. [DOI] [PubMed] [Google Scholar]
- 78.Yamazaki, H., et al. 2009. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 183:1480-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, Z., K. Vuori, J. C. Reed, and E. Ruoslahti. 1995. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc. Natl. Acad. Sci. U. S. A. 92:6161-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]