Abstract
The success of Mycobacterium tuberculosis as a pathogen relies on its ability to regulate the host immune response. M. tuberculosis can manipulate adaptive T cell responses indirectly by modulating antigen-presenting cell (APC) function or by directly interacting with T cells. Little is known about the role of M. tuberculosis molecules in direct regulation of T cell function. Using a biochemical approach, we identified lipoproteins LprG and LpqH as major molecules in M. tuberculosis lysate responsible for costimulation of primary human CD4+ T cells. In the absence of APCs, activation of memory CD4+ T cells with LprG or LpqH in combination with anti-CD3 antibody induces Th1 cytokine secretion and cellular proliferation. Lipoprotein-induced T cell costimulation was inhibited by blocking antibodies to Toll-like receptor 2 (TLR2) and TLR1, indicating that human CD4+ T cells can use TLR2/TLR1 heterodimers to directly respond to M. tuberculosis products. M. tuberculosis lipoproteins induced NF-κB activation in CD4+ T cells in the absence of TCR co-engagement. Thus, TLR2/TLR1 engagement alone by M. tuberculosis lipoprotein triggered intracellular signaling, but upregulation of cytokine production and proliferation required co-engagement of the TCR. In conclusion, our results demonstrate that M. tuberculosis lipoproteins LprG and LpqH participate in the regulation of adaptive immunity not only by inducing cytokine secretion and costimulatory molecules in innate immune cells but also through directly regulating the activation of memory T lymphocytes.
Mycobacterium tuberculosis is an ancient pathogen that continues to cause substantial human disease. The success of M. tuberculosis as a chronic and persistent pathogen depends to great extent on its ability to manipulate the host immune response in diverse and paradoxical ways. M. tuberculosis triggers potent proinflammatory and antigen-specific T cell responses while, at the same time, it displays a wide arrange of immune evasion strategies, such as inhibition of intracellular killing and antigen processing (8, 18, 21, 23, 37, 40). Understanding the complex mechanisms by which M. tuberculosis regulates the host immune response is essential to developing more effective drug treatments and tuberculosis (TB) vaccines.
T cells are critical for an effective immune response against M. tuberculosis. Regulation of T cell function in M. tuberculosis infection is traditionally considered an indirect result of M. tuberculosis-induced functional changes in antigen-presenting cells (APCs). Inhibition of antigen processing and presentation, induction of proinflammatory or inhibitory cytokines, and control of costimulatory molecule expression in APCs are among the indirect mechanisms used by M. tuberculosis to regulate T cell function (21). However, direct interactions between M. tuberculosis molecules and T cells may occur when vesicles containing mycobacterial components are released by infected macrophages (5-7). Different M. tuberculosis molecules have been implicated in direct regulation of T cell function. PIM binds α5β1 integrin on CD4+ T cells and triggers T cell adhesion to fibronectin (45) and lipoarabinomannan inserts into the T cell membrane, resulting in the inhibition of type I cytokine production (32, 48). ESAT-6 may directly inhibit gamma interferon (IFN-γ) secretion and T cell proliferation by interfering with T cell receptor (TCR) signaling (54). By directly controlling T cell function, M. tuberculosis molecules may influence development of protective responses. Therefore, identification of mycobacterial molecules that can bind to non-TCRs on T lymphocytes and influence activation, adhesion, or migration may not only provide new insights into the pathogenesis of M. tuberculosis infection and disease but also open new avenues for TB treatment and/or prevention.
Toll-like receptors (TLRs) are pattern recognition receptors that trigger rapid antimicrobial responses (1). They are prototypic receptors of the innate immune system and are mainly expressed on APCs (macrophages and dendritic cells) and NK cells. Recent studies demonstrate that TLRs are also expressed on T lymphocytes, including CD4+, CD8+, and Foxp3+ regulatory T cells. TLR2, TLR5, and TLR7-TLR8 (TLR7/8) can provide a second signal for activation of T cells upon TCR engagement (3, 10, 25, 27, 30, 33). M. tuberculosis expresses several TLR ligands, including the cell membrane-associated lipoproteins LpqH (Rv3763), LprG (Rv1411c), and LprA (Rv1270c); cell wall glycolipids (TLR2); and mycobacterial DNA containing CpG motifs (TLR9) (18, 19, 28, 37, 41). Mycobacterial TLR2 ligands have diverse effects on cells of the innate immune system, such as the induction of cytokine production, costimulatory molecule expression, and major histocompatibility complex class II (MHC-II) expression (18, 19, 21, 37, 40). Recently, mycobacterial TLR2 ligands, including lipoprotein LpqH, were identified in exosomes secreted by M. tuberculosis-infected macrophages (7). This suggests that TLR2 ligands can gain access to the extracellular environment and interact with uninfected cells such as T cells (5-7). Thus, TLR2 ligands could directly regulate T cell function and impact the outcome of M. tuberculosis infection.
Here we identified liproproteins LprG and LpqH as major components of the M. tuberculosis lysate with direct costimulatory effects for CD4+ T cells. These effects were independent of APCs and mediated by engagement of TLR2/1 on the T cells. The M. tuberculosis lipoproteins LpqH and LprG upregulated Th1 cytokine secretion and cellular proliferation in response to immobilized anti-CD3. Although both memory (CD45RO+) and naive (CD45RA+) CD4+ T cells expressed TLR2, only memory cells responded to TLR2 ligands when stimulated with anti-CD3 simultaneously. Consequently, M. tuberculosis lipoproteins serve as primary costimulators of memory T cells. Lipoproteins induced NF-κB activation in resting CD4+ T cells in the absence of TCR coengagement. Thus, TLR2 engagement alone triggered intracellular signaling, but upregulation of cytokine production and proliferation required coengagement of TCR. Our data extend previous reports of TLR2 expression by human CD4+ T cells and suggest that TLR2/TLR1 may have a particularly important role in maintenance of memory Th1 responses in M. tuberculosis infection. In addition, these findings support the use of TLR2 agonists as adjuvants in TB vaccines, since these molecules may directly enhance CD4+ T cell memory responses and thus protective immunity against M. tuberculosis (29, 53).
MATERIALS AND METHODS
Cell isolation and culture.
Human peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation over sodium diatrizoate/Hypaque (GE Healthcare, Uppsala, Sweden) from 14 healthy, tuberculin skin test (TST)-negative donors (18 to 45 years old) recruited among laboratory staff. All protocols were approved by Case Western Reserve University/University Hospital's Case Medical Center institutional review board. Informed written consent was obtained from all participants.
Highly purified CD3+ CD4+ T cells were isolated from peripheral blood mononuclear cells using a magnetic CD4+ selection kit according to the manufacturer's instructions (Miltenyi Biotec, Inc., Auburn, CA), followed by fluorescence-activated cell sorting (FACS) using APC-conjugated anti-CD4 plus a cocktail of phycoerythrin (PE)-labeled monoclonal antibodies (MAbs; anti-CD19, anti-CD123, anti-CD1c, anti-CD14, and anti-CD56). Forward-scatter/side-scatter (FSC/SSC) gates were drawn on intact lymphocytes and CD4+ (CD19, CD123, CD1c, CD14, CD56)− cells were sorted. Anti-CD4 APC, anti-CD14 PE, and anti-CD56 were purchased from BD Biosciences (San Jose, CA); anti-CD19 PE and anti-CD123 PE were from eBioscience (San Diego, CA); and anti-CD1c PE was from Miltenyi Biotec, Inc. FACS sorting was performed using a BD FACSAria Cell-Sorting System (BD Biosciences). The T cell purity of the resulting cell population was assessed by FACS, and samples with >99.4% CD3+ CD4+ were used in all experiments (range, 99.4 to 100%; mean, 99.7% ± 0.2%) (30). As previously reported by our group (30), the absence of functionally relevant APC contamination was demonstrated by the failure of purified CD4+ T cells to respond to phytohemagglutinin (PHA) stimulation (data not shown). In other experiments, peripheral blood CD4+ T cells were separated into CD45RA+ and CD45RO+ cells by FACS using CD3 APC (SK-7 clone; BD Biosciences), γδ2 PE (BD Biosciences), CD45RA fluorescein isothiocyanate (FITC; Biolegend, San Diego, CA), and CD45RO PEcy5 (eBioscience). FSC/SSC gates were drawn on intact lymphocytes, and CD4+ γδ2− cells were selected, followed by selection for CD45RA+ or CD45RO+ cells. Nonviable cells were detected after each isolation protocol by propidium iodide incorporation using a dead cell discriminator kit (Caltag; Invitrogen, Carlsbad, CA) and by the trypan blue exclusion method.
All experiments were performed with FACS-purified CD3+ CD4+ T cells cultured in serum free medium (X-VIVO 15; Lonza, Walkersville, MD) supplemented with 20 mM HEPES, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids (BioWhittaker, Walkersville, MD), 100 U of penicillin/ml, and 100 μg of streptomycin (Lonza)/ml in 96-well flat-bottom plates (Becton Dickinson) at 37°C in 5% CO2.
Preparation of mycobacterial fractions.
M. tuberculosis strain H37Ra lysate was prepared and fractionated by biphasic extraction with Triton X-114 and phenol, followed by preparative electroelution as described previously (18). Thirty fractions were obtained and analyzed in 13% sodium dodecyl sulfate (SDS) polyacrylamide gels.
LprG cloning and purification.
Lipoprotein cloning was done as previously described (40) in E. coli DH5α (Invitrogen). Full-length LprG (Rv1411c) was amplified from M. tuberculosis H37Rv genomic DNA by PCR using the following oligodeoxynucleotide primers (sequences are written 5′ to 3′; the underlined portions are the NdeI and HindIII restriction enzyme recognition sites): the 5′ primer GCATATCCATATGCGGACCCCCAGACGCCACTG and the 3′ primer GTACAAGCTTGCTCACCGGGGGCTTCG. A nonacylated (NA) variant of LprG was cloned by using a 5′ primer that excluded the signal sequence and changed the acylated cysteine to a methionine. NA-LprG was cloned with the 5′ primer GCAATTCCATATGTCGTCGGGCTC and the 3′ primer GTACAAGCTTGCTACCGGGGGCTTCG. Products were ligated into the shuttle vector pVV16 (J. Belisle, Colorado State University, Fort Collins, CO) behind the mycobacterial hsp60 promoter and in-frame with a C-terminal His6 tag. LprG was expressed in M. smegmatis MC2 1-2C (from R. Wilkinson, Imperial College, London, United Kingdom) cultivated in Middlebrook 7H9 broth (Difco, Lawrence, KS) supplemented with 1% Casamino acids, 0.2% glycerol, 0.2% glucose, and 0.05% Tween 80. Kanamycin was used for selection at 30 μg/ml. All constructs were verified by sequencing. M. smegmatis was transformed by electroporation with a Gene Pulser (Bio-Rad, Hercules, CA).
To purify LprG or NA-LprG, a 4-liter culture of M. smegmatis expressing the His6-tagged protein was grown to late log phase and harvested by centrifugation. The pellet was resuspended in 40 ml of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, and 20 mM imidazole), 2.5% protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO), and 75 U/ml of benzonase (Sigma-Aldrich). The suspension was passed through a French press four times and centrifuged for 60 min at 100,000 × g. The supernatant was incubated with nickel-nitrilotriacetic acid (Ni-NTA) agarose beads (Qiagen, Valencia, CA) for 2 h at 4°C. After a washing step with wash buffer (50 mM NaH2PO4, 1 M NaCl, 20 mM imidazole, 10% [vol/vol] glycerol), the His6-tagged LprG or NA-LprG was disassociated from the resin in elution buffer (50 mM NaH2PO4, 300 mM NaCl, 450 mM imidazole). PD-10 columns (Amersham Biosciences, Uppsala, Sweden) were used to exchange the buffer to 20 mM Tris. Both LprG and NA-LprG were further purified by ion-exchange chromatography with a 1-ml QFF anion exchange column (Amersham Biosciences) and elution in 20 mM Tris (pH 8.0) with 50 mM NaCl. Eluted proteins were concentrated in Amicon Ultra-4 spin columns, and the protein concentrations were determined with a BCA assay (Pierce, Rockford, IL). rLprG acylation was inferred from the physicochemical behavior of the full-length product, which clearly differed it from NA-LprG. As reported previously, indirect evidence of lipoprotein acylation included the presence of a highly conserved cysteine located at the N terminus, which is part of the lipobox [LVI][ASTVI][GAS][C] (44); a double-band appearance on SDS-PAGE; a tendency to form aggregates; a partition to the hydrophobic phase in detergent-phase separations; and incomplete solubility in water-based solvents (56).
Flow cytometric analysis of cell surface markers.
The cell surface expression of CD25, CD69, TLR2, and TLR1 was assessed immediately after T cell isolation and after overnight rest or stimulation at 37°C in 5% CO2 with plate-bound anti-CD3 MAb (10 μg/ml) with or without soluble anti-CD28 MAb (1 μg/ml) and with either anti-TLR2.5-PE (eBioscience), anti-TLR1-PE (eBioscience), anti-CD25-FITC (BD Bioscience), or anti-CD69-PE (BD Bioscience). Cells were acquired by using a FACSCalibur flow cytometer (BD Bioscience) using CellQuest software (BD Bioscience). Ten thousand events were recorded for each cell surface marker. The cutoff lines for positive and negative fluorescence were set manually based on the distribution of cells stained with APC-, FITC-, and PE-conjugated isotype controls and was kept constant within each experiment. Further analysis was performed using FlowJo software (Tree Star, Stanford, CA).
T cell stimulation and proliferation assay.
CD4+ T cells (105/well) were cultured in the presence of medium alone or 10 μg of plate-bound anti-CD3 MAb (clone HIT3a; BD Bioscience)/ml with or without 1 μg of soluble anti-CD28 MAb (clone CD28.2; BD Bioscience)/ml and the following: M. tuberculosis fractions (0.5 μg/ml), nonacylated recombinant (NA-rLprG) or acylated recombinant LprG (rLprG) (1 μg/ml), native H37Rv LpqH (nLpqH, 1 μg/ml) provided by the Tuberculosis Vaccine Testing and Research Materials Contract at Colorado State University (NIAID HHSN266200400091C), or N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-[R]-cysteinyl-[S]-seryl-[S]-lysyl-[S]-lysyl-[S]-lysyl-[S]-lysine × 3HCl (Pam3CSK4; EMC Microcollections GmbH, Tubingen, Germany) at 0.5 μg/ml. All conditions were performed in triplicate. CD4+ T cells were incubated for 72 h in 96-well flat-bottom plates (Becton Dickinson). Cell-free culture supernatants (100 μl) were collected at 18 h (interleukin-2 [IL-2]) and 72 h (IFN-γ) for cytokine quantification by enzyme-linked immunosorbent assay (ELISA). Cells were pulsed during final 16 h of culture with 1 μCi of [3H]thymidine (Amersham Pharmacia Biotech, Piscataway, NJ)/well. [3H]thymidine incorporation was measured by liquid scintillation counting, and the results are expressed as mean counts per minute (cpm) of triplicate values.
For TLR1 and TLR2 blocking assays, FACS-purified CD4+ T cells (105/well) were stimulated overnight with anti-CD3 and anti-CD28 MAbs as described previously and plated in triplicate onto 96-well flat-bottom plates that had been blocked with 0.2% bovine serum albumin for 2 h and washed in cold phosphate-buffered saline. Polyclonal antibodies against TLR1 (Invivogen) and/or TLR2 (Invivogen) or against control immunoglobulin (rat IgG) were added at 10 μg/ml, and the cells were incubated for 1 h at 37°C in 5% CO2. rLprG (2 μg/ml), Pam3CSK4 (0.5 μg/ml), or medium alone were then added, and the cells were incubated for an additional 72 h at 37°C in 5% CO2. Cell-free culture supernatants were collected at 18 h and 72 h, and the cells were pulsed for the final 16 h of culture with 1 μCi of [3H]thymidine/well as previously described.
Cytokine quantification.
IL-2 was measured by using a commercial kit according to the manufacturer's instructions (Ready-Set-Go human IL-2 detection kit; eBioscience). IFN-γ was determined in culture supernatants by sandwich ELISA with the anti-IFN-γ antibody pair M-700A and biotinylated M-701B (Thermo Scientific, Waltham, MA).
Immunoblotting of M. tuberculosis fractions.
Portions (3 μg) of each fraction were resolved on a 4 to 20% gradient SDS-PAGE gel and transferred to Trans-Blot nitrocellulose membrane. Blots were incubated with mouse anti-Rv1411 (LprG) or mouse anti-LpqH (IT-19) MAbs (Tuberculosis Vaccine Testing and Research Materials Contract, Colorado State University, NIAID contract HHSN266200400091C), followed by goat anti-mouse (IRDye 800 CW; LI-COR Biosciences, Lincoln, NE). Membranes were subsequently incubated with rabbit anti-BCG (Dako) overnight, followed by goat anti-rabbit (IRDye 700 DX; LI-COR Biosciences) for 1 h. Western blots were imaged with an Odyssey infrared imaging system (LI-COR Biosciences), and the images were analyzed with ImageJ software (National Institutes of Health). Band density was expressed as a relative optical value.
Immunoblotting of T cell lysates.
FACS-purified CD4+ T cells (2 × 106) were left unstimulated or were stimulated for 15 min with CD3/CD28-coated beads (Invitrogen), Pam3CSK4 (0.5 μM), or rLprG (0.5 μM). In selected experiments, FACS-purified CD4+ T cells (2 × 106) were stimulated overnight with anti-CD3 and anti-CD28 MAbs as described previously and subsequently exposed for 15 min to Pam3CSK4 (0.5 μM) or rLprG (0.5 μM). The cells were resuspended in cell lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton), 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg of leupeptin/ml) and lysed on ice. Cell lysate proteins (7 μg/lane) were resolved on a 4 to 20% gradient SDS-PAGE gel and transferred to Trans-Blot nitrocellulose membrane (Bio-Rad). Blots were sequentially incubated with anti-phospho-NF-κBp65 MAb (Cell Signaling, Beverly, MA) and anti-NF-κBp65 MAb (Cell Signaling). Bands were visualized with secondary horseradish peroxidase-conjugated mouse anti-rabbit IgG (Jackson, West Grove, PA) and the SuperSignal West Pico chemiluminescent substrate (Thermo Scientific).
RT-PCR.
TLR1 and TLR2 expression was analyzed by reverse transcription-PCR (RT-PCR) on FACS-sorted CD4+ T cells (2 × 106). Total cytoplasmic RNA was extracted by using an RNeasy minikit and treated with RNase-free DNase (Qiagen). RT was performed from 0.5 μg of total RNA using Superscript III/RNaseOUT (Invitrogen) according to the manufacturer's instructions. The resulting cDNA was subjected to 40-cycle PCR amplification using the TaqMan Universal 2× gene expression master mix and the ready-made primer and probe sets for TLR1 and TLR2 (catalog no. Hs00413978_m1 [TLR1] and Hs01014511_m1 [TLR2]; Applied Biosystems, Carlsbad, CA) in a Step One-Plus system (Applied Biosystems). The amount of TLR1 and TLR2 mRNA expression was normalized to β-actin mRNA and calculated by using the comparative cycle threshold method as described by Applied Biosystems.
Statistical analysis.
SAS Proc Mixed was used to test for differences in means between specific treatment groups. The null hypothesis of no difference in means between specific treatment groups was tested using a t-statistic, with a P value of <0.05 taken as evidence of a significant difference between groups.
RESULTS
Lipoproteins LprG and LpqH are two major CD4+ T cell costimulatory ligands expressed by M. tuberculosis.
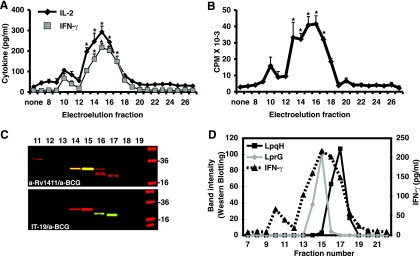
To determine whether M. tuberculosis molecules could directly affect human CD4+ T cell activation in an APC-independent manner, we used highly purified (>99% CD3+ CD4+) resting CD4+ T cells and serum-free medium. Preliminary experiments demonstrated that hydrophobic material extracted from M. tuberculosis lysate increased CD4+ T cell proliferation and IL-2 production triggered by anti-CD3/anti-CD28 (data not shown). To identify the molecules responsible for this effect, the hydrophobic extract was separated by SDS-PAGE, and constituents of different molecular weight obtained by electroelution. As shown in Fig. 1 A and B, fractions 13 to 17 (16 to 36 kDa) significantly enhanced IL-2 and IFN-γ secretion and cell proliferation by CD4+ T cells when combined with anti-CD3 and anti-CD28 stimulation (P < 0.005, n = 5). None of the fractions induced CD4+ T cell proliferation or cytokine secretion in the absence of TCR triggering (data not shown). Lipoproteins LprG (24 kDa) and LpqH (19 kDa) were identified in fractions 13 to 17 by Western blotting (Fig. 1C). These lipoproteins were the major immunoreactive constituents in active fractions, as demonstrated by subsequent incubation of immunoblots with anti-BCG serum and anti-lipoprotein-specific MAbs (Fig. 1C). Furthermore, relative concentration of these lipoproteins in electroelution fractions assessed by densitometry correlated with magnitude of costimulatory activity in T cell stimulation assay (Fig. 1D). The presence of LprG and LpqH in active fractions was confirmed by mass spectrometry analysis (data not shown). These results demonstrate that lipoproteins LprG and LpqH isolated from M. tuberculosis directly enhance CD4+ T cell activation triggered by anti-CD3 and anti-CD28. These results in an APC-free system suggest that M. tuberculosis lipoproteins increase CD4+ T cell activation by directly interacting with receptors on CD4+ T cells. In addition, since we used cells derived from TST-negative donors, the effects are not a result of antigen recognition by memory CD4+ T cells.
FIG. 1.
Lipoproteins LprG and LpqH are major CD4+ T cell costimulatory ligands in M. tuberculosis lysate. CD4+ T cells (105 cells/well) were stimulated with plate-bound anti-CD3 (10 μg/ml) and soluble anti-CD28 (1 μg/ml) in presence or absence of fractions obtained by electroelution from M. tuberculosis lysate. (A) IL-2 and IFN-γ were measured in 18- and 72-h culture supernatants, respectively, by ELISA. (B) Proliferation was measured by [3H]thymidine incorporation assay. Shown are means ± the standard errors of the mean (SEM) of five independent experiments with different donors. (C) M. tuberculosis fractions were analyzed by Western blotting with anti-LprG (a-Rv1411c; top panel, yellow bands) or anti-LpqH (IT-19; bottom panel, green bands) MAbs, followed by polyclonal anti-BCG and correspondent secondary antibodies. Western blots were imaged with an Odyssey infrared imaging system (LI-COR Biosciences). (D) Western blot images were analyzed with ImageJ software (NIH), and the band intensity was expressed as a relative optical value. Band intensities from anti-LprG and anti-LpqH MAbs blots were plotted separately for fractions 7 to 21, and plots were laid over the IFN-γ secretion chart obtained with same fractions. *, P < 0.005 compared to no treatment control (“none”).
Acylation of lipoproteins is required for upregulation of CD4+ T cell activation.
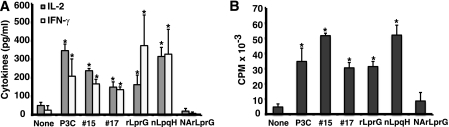
Mature bacterial lipoproteins are characterized by an N-terminal, triacylated cysteine, and this acyl modification is important for signaling through TLR2 (2, 9, 22, 34, 37, 46). To assess the role of acylation in LprG-induced CD4+ T cell costimulation, acylated rLprG and NA-rLprG were amplified from H37Rv genomic DNA and expressed in M. smegmatis (14, 40). Acylated rLprG, but not NA-rLprG, significantly upregulated CD4+ T cell cytokine production (IL-2 and IFN-γ, Fig. 2A) and proliferation (Fig. 2B) in the context of anti-CD3 and anti-CD28 stimulation (P < 0.05, n = 3). This demonstrates that acylation of lipoproteins is required for direct upregulation of T cell activation. In addition, rLprG's effect on CD4+ T cells was comparable to that of fractions 15 and 17 obtained from H37Ra, of native LpqH purified from H37Rv and of synthetic TLR2 ligand Pam3CSK4. These results indirectly suggest that acylated lipoproteins from both virulent and avirulent M. tuberculosis strains upregulate CD4+ T cell activation initiated by TCR triggering through interaction with T cell TLR2.
FIG. 2.
Acylation is required for lipoprotein-induced costimulation of human CD4+ T cells. CD4+ T cells (105 cells/well) were stimulated with anti-CD3 and anti-CD28 plus medium (None), synthetic lipopeptide Pam3CSK4 (P3C), M. tuberculosis fractions 15 and 17 (#15 and #17), full-length acylated recombinant LprG (rLprG), native LpqH from H37Rv (nLpqH), or nonacylated recombinant LprG (NArLprG). (A) IL-2 and IFN-γ were measured in culture supernatants by ELISA. (B) Proliferation was determined by [3H]thymidine incorporation. Shown are the means ± the standard deviation (SD) of three independent experiments with different donors. *, P < 0.05 compared to a no-treatment control (None).
M. tuberculosis lipoproteins can deliver signal 2 for activation of resting CD4+ T cells and synergize with other costimulatory ligands for cytokine secretion.
Previous results indicate that M. tuberculosis lipoproteins deliver costimulatory signals for CD4+ T cell activation. Full activation of resting T cells requires two signals. Signal 1 is generated by TCR stimulation, while signal 2 can be generated by CD28, TNF-α family receptors, or cytokines (31). To determine whether lipoproteins can provide signal 2, resting CD4+ T cells were stimulated with anti-CD3 and either rLprG, nLpqH, anti-CD28 or a combination of one lipoprotein and anti-CD28. CD4+ T cells stimulated with anti-CD3 alone did not secrete detectable levels of cytokines or proliferate. Low levels of IL-2 and IFN-γ (Fig. 3 A and B) were detected in supernatants of CD4+ T cells stimulated with anti-CD3 and either rLprG, nLpqH, or anti-CD28. IFN-γ production from T cells stimulated with anti-CD3 and rLprG was significantly increased compared to those treated with anti-CD3 alone (none) (P = 0.0005, n = 4, Fig, 3D). CD4+ T cell proliferation was readily induced in the presence of lipoproteins, especially rLprG (Fig. 3C), and this was consistent and statistically significant across different donors (P < 0.0001, n = 4 [data not shown]). Costimulation with two different ligands, i.e., lipoprotein plus anti-CD28, had a synergistic effect on cytokine secretion and an additive effect on proliferation compared to costimulation with a single ligand (Fig. 3A to C). Therefore, both LprG and LpqH can deliver costimulatory signals to resting human CD4+ T cells that, when combined with TCR engagement, induce cytokine secretion and proliferation.
FIG. 3.
M. tuberculosis lipoproteins trigger a second signal for CD4+ T cell activation that results in cytokine secretion and proliferation. CD4+ T cells (105 cells/well) were stimulated with anti-CD3 and either medium alone (none), anti-CD28MAb, recombinant LprG (rLprG), native LpqH (nLpqH) or a combination of anti-CD28 and rLprG or nLpqH. (A and B) IL-2 and IFN-γ were measured in culture supernatants by ELISA. (C) Proliferation was determined by [3H]thymidine incorporation. Shown are means ± the SD of triplicates from one representative donor (A to C, n = 4). (D) Cumulative data for IFN-γ secretion from four donors. Boxes indicate the interquartile ranges, horizontal lines transecting the boxes indicate the medians, plus signs indicate the means, and whiskers indicate the highest and lowest values. *, P = 0.0005 compared to a no-treatment control (None); **, P < 0.0001 compared to a no-treatment control (None).
LprG upregulates expression of T cell early activation markers CD25 and CD69.
To determine early events associated with the increased cytokine secretion and proliferation triggered by lipoproteins, we measured CD25 and CD69 expression on CD4+ T cells before and after activation with anti-CD3 with or without rLprG. Surface expression of CD25 and CD69 were low on resting CD4+ T cells after 18 h of culture (Table 1). Stimulation of CD4+ T cells with anti-CD3 MAb alone for 18 h increased the percentage of CD25+ and CD69+ cells, whereas stimulation with rLprG alone did not. Costimulation of CD4+ T cells with rLprG significantly enhanced CD25 and CD69 expression compared to stimulation with anti-CD3 alone, demonstrating that LprG-induced upregulation of cytokine secretion and proliferation in CD4+ T cells is preceded by increased expression of activation markers (P < 0.05, n = 4, Table 1).
TABLE 1.
LprG upregulates CD25 and CD69 expression induced by anti-CD3
| CD | Expression (%) ± SEMa |
|||
|---|---|---|---|---|
| None | Anti-CD3 | LprG | Anti-CD3 + LprG | |
| CD25 | 8.6 ± 2.0 | 20.7 ± 6.7 | 9.9 ± 3.3 | 51.5 ± 9.3b |
| CD69 | 1.1 ± 0.9 | 56.5 ± 11 | 2.4 ± 0.3 | 80.7 ± 7.9 |
n = 4.
Significantly different (P < 0.05) from the value for T cells treated with anti-CD3 alone, as calculated by the Student t test.
Memory but not naive CD4+ T cells are costimulated by M. tuberculosis lipoproteins.
Naive and memory CD4+ T cells have different costimulatory requirements. Therefore, we investigated whether these subsets differ in their responses to LprG costimulation. CD4+ T cells were separated into naive (CD45RA+) and memory (CD45RO+) subsets by flow sorting and stimulated with anti-CD3 MAb and either rLprG, anti-CD28 MAb, or a combination of rLprG and anti-CD28. Memory CD4+ T cells had higher cytokine responses compared to naive T cells in all of the conditions tested. LprG and the control TLR2 ligand Pam3CSK4, provided substantial costimulation to memory but not naive CD4+ T cells. Although this costimulation was only statistically significant for Pam3CSK4 (P < 0.05, n = 3, Fig. 4), when rLprG and a second costimulator (i.e., anti-CD28) were provided, the upregulation of IL-2 production by memory CD4+ T cells was highly significant (P < 0.0001, n = 3, Fig. 4). Thus, the costimulatory signal triggered by LprG is strong enough to activate memory CD4+ T cells but not naive CD4+ T cells. This suggests that lipoprotein-induced costimulation of CD4+ cells can play a role in the rapid induction of secondary responses or maintenance of immunologic memory.
FIG. 4.
M. tuberculosis lipoproteins costimulate memory but not naive CD4+ T cells. Memory (CD45 RO+) or naive (CD45 RA+) CD4+ T cells (105 cells/well) were stimulated with medium (none), synthetic lipopeptide Pam3CSK4 (P3C), or recombinant LprG (rLprG), along with anti-CD3 with or without anti-CD28, as indicated. IL-2 was measured in culture supernatants by ELISA. Shown are means ± the SD of three independent experiments with different donors. *, P < 0.05; **, P < 0.0001 (compared to a no-treatment control [none]).
CD4+ T cells upregulate TLR2 expression upon activation.
Since mycobacterial lipoproteins bind and signal through TLR2/TLR1 heterodimers on APCs (9, 18, 37, 50), we examined the role of TLR2 and TLR1 in recognition of M. tuberculosis lipoproteins by human CD4+ T cells. First, we measured surface TLR2 and TLR1 expression on resting and activated CD4+ T cells by using flow cytometry. Resting human CD4+ T cells expressed low levels of surface TLR2 (Fig. 5 A, D, and G). After overnight stimulation with anti-CD3 plus anti-CD28, TLR2 expression increased in T cells undergoing activation, i.e., lymphoblasts (Fig. 5B, LB, and Fig. 5H) compared to small lymphocytes (Fig. 5B, LC, and Fig. 5E) or resting CD4+ T cells (Fig. 5A, D, and G). Addition of the TLR2 ligand Pam3CSK4 during stimulation (Fig. 5C, F, and I) significantly increased CD4+ T cell activation and upregulation of surface TLR2 expression compared to resting cells (Fig. 5A, D, and G) (also, data not shown; P < 0.05, n = 5). This suggests that TLR2 ligands enhance expression of their own receptor in activated CD4+ T cells. TLR1 expression could not be detected under any conditions, and this may be a related to low sensitivity of flow cytometry for this receptor (data not shown).
FIG. 5.
Activation of human CD4+ T cells increases the expression of TLR2. TLR2 surface expression was measured on CD4+ T cells by FACS either at rest (A, D, and G) or after overnight stimulation with anti-CD3 and anti-CD28 (B, E, and H), or anti-CD3 and anti-CD28 plus Pam3CSK4 (C, F, and I). Cells were gated on the basis of forward and side-scatter parameters (A to C) and classified as small lymphocytes (LC) or activated lymphoblasts (LB). A surface TLR2 expression (shaded histogram) was determined in lymphoblasts (G, H, and I) and compared to small lymphocytes (D, E, and F). Matched isotype immunoglobulin was used as control (clear histogram). Shown are the results of a representative experiment of five performed.
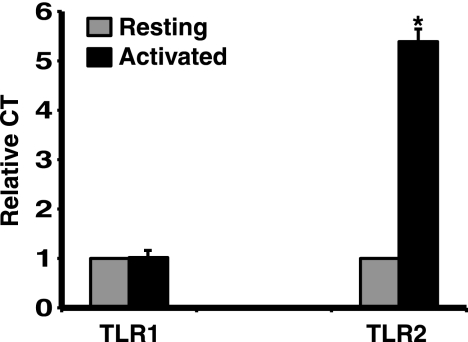
Since TLR1 expression could not be assessed by flow cytometry, and in order to extend flow cytometry findings, we next measured TLR2 and TLR1 on CD4+ T cells by quantitative PCR. Resting CD4+ T cells expressed low but detectable levels of TLR1 and TLR2 mRNA. After overnight activation with anti-CD3 plus anti-CD28 MAb, TLR2 mRNA expression in CD4+ T cells was significantly increased, while the expression of TLR1 remained unchanged (P < 0.01, n = 3, Fig. 6). There was no significant difference in upregulation of TLR1 or TLR2 expression between memory and naive CD4+ T cells (data not shown). Taken together, our data demonstrate that TLR2 and TLR1 expression is low in resting human CD4+ T cells and that TLR2 expression is upregulated after T cell activation, while TLR1 expression remains unchanged. This suggests that regulation of TLR2 but not TLR1 expression determines the CD4+ T cell ability to respond to TLR2 ligands.
FIG. 6.
TLR2 but not TLR1 mRNA expression is upregulated in activated CD4+ T cells. CD4+ T cells (2 × 106) were either left unstimulated or stimulated overnight with anti-CD3 and anti-CD28 MAbs. Total cellular RNA was isolated, and TLR1 and TLR2 mRNA expression was measured by RT-PCR and normalized to β-actin mRNA levels. The TLR1 and TLR2 mRNA level in unstimulated CD4+ T cells was set at 1.0, and all other conditions were plotted as a relative measure to that value (relative comparative threshold value [CT]). Each bar represents the mean ± the SD of values from three experiments with different donors. *, P < 0.01 compared to no treatment control (unstimulated cells).
M. tuberculosis lipoprotein LprG costimulates CD4+ T cells via TLR2 and TLR1.
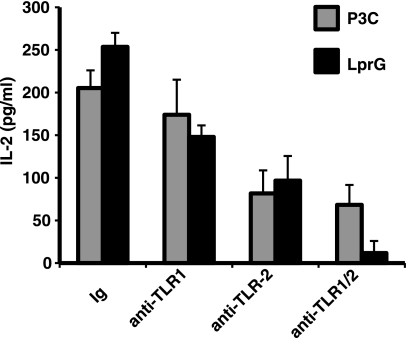
Recognition of M. tuberculosis lipoproteins LprG and LpqH by TLR-transfected HEK 293 cells is dependent on TLR2 and TLR1 expression and independent of TLR6 (14). To confirm the role of TLR2 and TLR1 in LprG-induced costimulation of CD4+ T cells, prestimulated CD4+ T cells were treated with blocking polyclonal antibodies (pAbs) directed to TLR2 or TLR1 or with control Ig. These particular antibodies were selected from a panel of anti-TLR1/anti-TLR2 antibodies due to their capacity to effectively block Pam3CSK4 induced activation of TLR2- and TLR1-transfected HEK293 cells (data not shown). CD4+ T cells were then treated with recombinant LprG (rLprG) or Pam3CSK4 for 18 h, and IL-2 was measured in culture supernatants (Fig. 7). Anti-TLR2 pAb resulted in a substantial reduction in rLprG- and Pam3CSK4-induced IL-2 secretion, and maximum reduction in IL-2 secretion was achieved by using a combination of anti-TLR2 and anti-TLR1 antibodies (P = 0.04 compared to cells treated with control immunoglobulin; n = 4). LprG-induced IL-2 production was minimally reduced in the presence of anti-TLR1 antibodies alone (Fig. 7). These results indicate that human, primary CD4+ T cells use TLR2/TLR1 heterodimers to interact with M. tuberculosis lipoproteins, and this interaction results in direct T cell costimulation.
FIG. 7.
LprG induces CD4+ T cell costimulation via TLR1 and TLR2. CD4+ T cells (105 cells/well) were stimulated overnight with anti-CD3 and anti-CD28 MAbs. Cells were then treated with control immunoglobulin (Ig), anti-TLR1, anti-TLR2 or a combination of both antibodies for 1 h at 37°C, followed by either Pam3CSK4 (P3C) or rLprG for 18 h, and IL-2 was measured in culture supernatant by ELISA. Shown are means ± the SD of triplicates from one representative experiment (n = 4).
M. tuberculosis LprG induces NF-κB activation in resting and preactivated CD4+ T cells.
Our previous data demonstrated that LprG costimulates CD4+ T cell via TLR2 and TLR1. Activation of CD4+ T cells results in intracellular signaling, nuclear translocation of transcription factors, and upregulation of genes involved in cell cycle and cytokine secretion. NF-κB is one of these transcription factors, which is also involved in the transduction of TLR initiated signals (26). Thus, we hypothesize that M. tuberculosis LprG triggers intracellular signaling via TLR2 and leads to NF-κB phosphorylation in CD4+ T cells. As shown in Fig. 8, short treatment (15 min) of resting CD4+ T cells with either rLprG or Pam3CSK4 induced NF-κB p65 phosphorylation in the absence of TCR engagement (Fig. 8A). rLprG- and Pam3CSK4-induced NF-κB phosphorylation was higher in CD4+ T cells that had been preactivated with anti-CD3 and anti-CD28 overnight compared to resting cells (Fig. 8B). This correlated with increased expression of TLR2 in activated compared to resting CD4+ T cells (Fig. 5). Although TLR2 ligands triggered NF-κB activation in resting cells, they did not induce expression of activation markers, cytokine secretion, or proliferation in the absence of TCR engagement (data not shown). This demonstrates that mycobacterial lipoproteins induce intracellular signaling in resting CD4+ T cells that, when combined with TCR signaling, leads to activation and expression of effector functions.
FIG. 8.
M. tuberculosis lipoprotein LprG induces NF-κB phosphorylation in the absence of TCR signaling. CD4+ T cells (106 cells/well) were left unstimulated (Resting [A]) or stimulated overnight with anti-CD3 and anti-CD28 (Prestimulated [B]). (A) Resting cells were either left untreated, stimulated with anti-CD3/CD28 beads, or treated with Pam3CSK4 or rLprG for 15 min. (B) Prestimulated cells were either left untreated or treated with Pam3CSK4 or recombinant LprG for 15 min. The levels of total (NF-κB p65) and phosphorylated (pNF-κB p65) NF-κB p65 were determined by Western blotting (top and middle panels). Western blots were analyzed with ImageJ software (NIH), and the band density of pNF-κB p65/pNF-κB expressed as a relative optical value (bottom panel). Shown are the results from one representative experiment of five performed.
DISCUSSION
Direct binding of M. tuberculosis molecules to receptors expressed on T cells can occur when mycobacterial components are released by infected macrophages (5-7). Lipoprotein LpqH and mycobacterial glycolipids have been identified in exosomes isolated from mycobacterium-infected macrophages (7). Furthermore, M. tuberculosis-infected macrophage-derived microvesicles directly present M. tuberculosis peptide-MHC-II complexes to T cells (42). Exosomes isolated from M. tuberculosis-infected macrophages (7) directly costimulate primary CD4+ T cells (R. E. Rojas, unpublished data). This release of vesicles from the infected APC provides a mechanism by which M. tuberculosis molecules could directly regulate T cell function, and this may influence infection or disease outcomes. Considering the central role of CD4+ T cells in protection against TB, we undertook a thorough screening of M. tuberculosis lysate in search of molecules that directly modulate the activation and effector functions of this T cell subset. We identified lipoproteins LprG and LpqH as major components of the mycobacterial lysate with direct costimulatory activity on CD4+ T cells and characterized their mechanism of action. Previous studies have demonstrated that LpqH and LprG regulate human dendritic cell and macrophage functions (9, 18, 19, 37, 40). Our current findings extend the immunomodulatory role of mycobacterial lipoproteins to human T cells, demonstrating LpqH and LprG trigger signals that, when combined with TCR triggering, lead to T cell activation and induction of effector functions.
The genome of M. tuberculosis is estimated to encode 48 to 100 lipoproteins among which, LprG, LpqH, LprA, and PhoS1 are known TLR2 ligands. Although numerous studies have reported the role of TLR2 ligands on the regulation of innate immune cells, their contribution to direct regulation of T cells is only starting to be deciphered. A few reports have shown that TLR2, TLR5, and TLR7/8 ligands upregulate human CD4+ T cell cytokine production and proliferation in the context of TCR stimulation (3, 10, 27, 30). We demonstrated for the first time that two natural M. tuberculosis TLR2 ligands, i.e., LpqH and LprG, have effects on CD4+ T cell costimulation comparable to those of synthetic Pam3CSK4. Unlike previous reports (12), our study used T cell purification and stimulation methods that effectively exclude the participation of APCs in the activation of T cells (30). In addition, results with primary cells were confirmed with cells from two lymphoblastic T cell lines, CD4+ HuT78 and Jurkat E6-1, that secreted increased amounts of IL-2 in response to anti-CD3 plus rLprG compared to anti-CD3 alone (data not shown). These approaches establish the role of direct TLR2 signaling in T cells in the responses to M. tuberculosis lipoproteins. Importantly, cells used in our studies were isolated from TST-negative, healthy donors with no immunological memory for M. tuberculosis antigens. Therefore, we can conclude that the lipoprotein-induced T cell costimulation in our system was not due to TLR2 triggering in APCs or antigen recognition by memory T cells.
CD4+ T cell activation requires two signals and is associated with early activation markers and late events (cytokines, proliferation). Signal 1 is delivered after TCR engagement, while signal 2 requires engagement of costimulatory receptors such as CD28, TNF-α family receptors, or cytokine receptors (31). Our data demonstrate that cells activated through the TCR alone upregulated activation markers, but expression of effector functions such as cytokine secretion or proliferation required a second signal. M. tuberculosis lipoproteins contributed to the induction of both early and late T cell activation events. When used alone as costimuli, either lipoproteins or anti-CD28 MAbs, induced low levels of cytokines. This is likely due to the use of these ligands in soluble form, which do not induce enough cross-linking of receptors. However, stimulation with both lipoprotein and anti-CD28 MAbs induced high cytokine levels and cell proliferation. Importantly, lipoproteins and anti-CD28 MAbs had a synergistic effect on cytokine secretion and an additive effect on cell proliferation. This suggests that TLR2 and CD28 use different signaling pathways or transcription factors to regulate cytokine production, while they share a pathway for regulation of cell proliferation. The existence of additional costimulatory pathways evolved to recognize mycobacterial molecules could be of significance if classical costimulatory molecules become limited or downregulated by the pathogen.
T cell activation is controlled by different transcription factors such as NF-κB, NFAT, and AP-1. NF-κB is a central player in TLR2 signaling. Our data showed that engagement of TLR2 by mycobacterial lipoproteins directly induced NF-κB activation in resting CD4+ T cells in the absence of TCR triggering or preactivation. Considering the low level of TLR2 expression in resting T cells, these results indicate that engagement of a very low number of receptors can induce signaling. This suggests a very efficient costimulation mechanism that may have in vivo implications in M. tuberculosis infection. Studies on the M. tuberculosis granuloma structure suggest that infected macrophages may be spatially separated from recruited T cells. Therefore, the accessibility of T cells to the APC costimulatory receptors in the granuloma may be limited. Under these circumstances, an efficient costimulatory mechanism that is physically separated from the distant infected macrophage and that is coupled to peptide recognition, i.e., exosomes, may be important in the generation or maintenance of T cell responses.
Although ligation of TLR2/TLR1 alone triggered NF-κB phosphorylation, additional signaling (TCR engagement) was required to induce cytokines or cell proliferation. As shown with other costimulatory receptors, full and productive activation of T cells depends on the simultaneous delivery of TCR- and coreceptor-derived signals. The way TCR and costimulatory signals are integrated to induce functional responses is still unclear. CD28 is the best-known costimulatory receptor, and most of its effects are mediated through NF-κB activation (47). CD28-triggered NF-κB phosphorylation regulates prosurvival genes such as Bcl-xL, cell cycle through cdk6/4-cyclin, and IL-2 transcription. Additional studies will be required to determine whether TLR2 and CD28 signaling pathways differ and if they target different genes. Differential gene regulation between CD28 and TLR2 may lead to divergent T cell differentiation, and this may impact infection control.
The lack of functional responses in CD4+ T cells after TLR2-induced NF-κB activation (in the absence of TCR signal) differs from the profound functional response of APCs when stimulated through TLR2 alone. This likely reflects the importance of preventing TLR2 signals from inducing nonspecific T cell activation. More comprehensive studies are in progress to determine the signaling pathways and genes targeted by lipoprotein mediated costimulation in human CD4+ T cells.
Here we confirmed that human primary CD4+ T cells express TLR1 and TLR2. Although both memory and naive T cells expressed TLR2, only memory T cells responded to lipoprotein stimulation. This is in agreement with well-established differences between memory and naive T cells in activation signal requirements (10, 27).
Prevention of reactivation in chronically infected individuals depends on sustained memory CD4+ T cells, as demonstrated by a high occurrence of reactivation TB in AIDS patients. If this depends on long lasting steady memory responses or on constant stimulation of antigen specific responses by persistent bacteria is unknown. The failure of BCG as a vaccine is often attributed to its lack of persistence in the host and the subsequent waning of the memory immune response. This suggests that continuous stimulation of antigen-specific T cells is important for protection. Persistent, slowly proliferating, antigen-specific CD4+ T cell responses have been described in mice chronically infected with M. tuberculosis (55). This indicates a continuous turnover in the memory population, presumably antigen driven. A recent report indicates that, in contrast to the traditional view of nonreplicating persistence in chronic TB, there is a substantial ongoing bacterial replication during chronic infection in the mouse (20). In humans, detection of persistent organisms in latently infected individuals has been difficult, and therefore the bacterial load is presumed to be low. In this context, i.e., low but continuous antigen presentation, efficient costimulatory mechanisms to amplify local responses in the granuloma are of paramount importance for the control of latent infection. The type and distribution of costimulatory molecules in the TB granuloma during latency is unknown. We speculate that the continuous release of TLR2 ligands from infected macrophages, along with antigen presentation, may contribute to sustained activation of memory CD4+ T cells within the granuloma.
TLR2 forms heterodimers with either TLR1 or TLR6, and this confers binding specificity for different lipopeptides (17, 34, 39, 50). Triacylated lipoproteins are known to bind TLR2/TLR1 heterodimers. Our data indicate that resting CD4+ T cells express both TLR1 and TLR2. However, TLR2 but not TLR1 expression is upregulated following T cell activation with anti-CD3 and anti-CD28 MAbs and is further enhanced by TLR2 ligands. To our knowledge, this is the first report on the regulation of the two components of this heterodimer in primary human T cells.
The structural requirements for the TLR2-ligand interaction are beginning to be deciphered. Crystal structures show that the thioether-linked diacylglycerol binds a hydrophobic pocket in TLR2, and the amide-linked third acyl chain binds TLR1A (24). In agreement with this model, our data indicate that acylation of the lipoprotein is required for TLR2 triggering in T cells. Interestingly, a recent report from our group indicates that NA-LprG retains TLR2 agonistic activity in APCs and TLR2/CD14-transfected HEK293 cells. This was due to the presence of another type of mycobacterial TLR2 ligands, i.e., glycolipids, carried in a binding pocket of NA-LprG (15). We postulate that the unresponsiveness of CD4+ T cells to NA-LprG, and its carried glycolipids, is due to their lack of CD14, a required coreceptor for TLR2 engagement by glycolipids (36).
The absence of TLR2 in mice results in a greater susceptibility to chronic or high-dose aerosol M. tuberculosis infection, suggesting a defect in acquired immunity (4, 16, 43, 49). Polymorphisms in TLR2 have been associated with an increased risk of TB in different populations (13, 38, 52), as well as with severe, disseminated forms of TB (11, 35, 51). Although these genetic association studies cannot provide a direct causal relationship between TLR2/1 polymorphisms and risk of TB, they do suggest that TLR2 signaling may have a role in development of protective immune responses to M. tuberculosis. Our data support a role for TLR2 in directly enhancing memory CD4+ T cell responses. These direct mechanisms likely happen in concert with indirect control of T cell responses through the innate immune system and may contribute to protection in M. tuberculosis infection. The biological relevance of TLR2-mediated direct and indirect regulation of adaptive immune response will require further testing in an M. tuberculosis infection animal model.
In conclusion, we demonstrated for the first time that M. tuberculosis lipoproteins LpqH and LprG, in addition to their multiple effects on APCs, directly regulate human memory CD4+ T cell function, trigger costimulatory signals and upregulate T cell activation. To our knowledge, this is the first report of a systematic screening of molecules expressed by M. tuberculosis for direct regulatory effects on human CD4+ T cells. Little is known on how human memory CD4+ T cell responses are regulated during latency and why they fail to prevent reactivation disease. Recognition of lipoproteins by TLR2 on memory CD4+ T cells may have evolved as a mechanism to compensate for lack of expression by or poor accessibility to costimulatory molecules expressed by APCs infected with M. tuberculosis. Thus, secretion of pathogen-derived costimulatory molecules such as LprG and LpqH from infected macrophages, and their subsequent recognition by T cell TLR2, may have a role in the development and/or maintenance of cell-mediated immunity to M. tuberculosis.
Acknowledgments
This study was supported by National Institutes of Health (NIH) grants AI083739 to C.L.L.; NIH grants AI027243 and HL055967 and contract HHSN266200700022C/NO1-AI-70022 to W.H.B.; and NIH grants AI069085, AI034343, and AI035726 to C.V.H. Additional support was provided by a Rainbow Babies & Children's Hospital Fellowship Research Award in Pediatrics to C.L.L. and an American Lung Association grant RG48786N to R.E.R.
We thank the blood donors who volunteered to participate in this study. We also thank the Case Comprehensive Cancer Center Cytometry Core Facility, the Center for AIDS Research at CWRU, and the Veterans Hospital of Cleveland for their assistance in purifying T cells by flow cytometry. We thank Derek W. Abbott, Department of Pathology, for helpful advice on NF-κB studies; Jon C. D. Houtman for kindly providing HuT 78 and Jurkat T cell lines; and Daniel Linfesty for his assistance in experiments with the T cell lines.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 15 November 2010.
REFERENCES
- 1.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., et al. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Babu, S., C. P. Blauvelt, V. Kumaraswami, and T. B. Nutman. 2006. Cutting edge: diminished T cell TLR expression and function modulates the immune response in human filarial infection. J. Immunol. 176:3885-3889. [DOI] [PubMed] [Google Scholar]
- 4.Bafica, A., et al. 2005. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 202:1715-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatty, W. L., et al. 2000. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1:235-247. [DOI] [PubMed] [Google Scholar]
- 6.Beatty, W. L., H. J. Ullrich, and D. G. Russell. 2001. Mycobacterial surface moieties are released from infected macrophages by a constitutive exocytic event. Eur. J. Cell Biol. 80:31-40. [DOI] [PubMed] [Google Scholar]
- 7.Bhatnagar, S., K. Shinagawa, F. J. Castellino, and J. S. Schorey. 2007. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110:3234-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt, K., and P. Salgame. 2007. Host innate immune response to Mycobacterium tuberculosis. J. Clin. Immunol. 27:347-362. [DOI] [PubMed] [Google Scholar]
- 9.Brightbill, H. D., et al. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 10.Caron, G., et al. 2005. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J. Immunol. 175:1551-1557. [DOI] [PubMed] [Google Scholar]
- 11.Caws, M., et al. 2008. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 4:e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, X., et al. 2009. Engagement of Toll-like receptor 2 on CD4+ T cells facilitates local immune responses in patients with tuberculous pleurisy. J. Infect. Dis. 200:399-408. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Y. C., et al. 2010. Toll-like receptor 2 gene polymorphisms, pulmonary tuberculosis, and natural killer cell counts. BMC Med. Genet. 11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drage, M. G., et al. 2009. TLR2 and its coreceptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell. Immunol. 258:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drage, M. G., et al. 2010. Mycobacterium tuberculosis lipoprotein LprG (Rv1411c) binds triacylated glycolipid agonists of Toll-like receptor 2. Nat. Struct. Mol. Biol. 17:1088-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drennan, M. B., et al. 2004. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am. J. Pathol. 164:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farhat, K., et al. 2008. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J. Leukoc. Biol. 83:692-701. [DOI] [PubMed] [Google Scholar]
- 18.Gehring, A. J., K. M. Dobos, J. T. Belisle, C. V. Harding, and W. H. Boom. 2004. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J. Immunol. 173:2660-2668. [DOI] [PubMed] [Google Scholar]
- 19.Gehring, A. J., et al. 2003. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect. Immun. 71:4487-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill, W. P., et al. 2009. A replication clock for Mycobacterium tuberculosis. Nat. Med. 15:211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding, C. V., and W. H. Boom. 2010. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat. Rev. Microbiol. 8:296-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertz, C. J., et al. 2001. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol. 166:2444-2450. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs, M., et al. 2007. Tumor necrosis factor is critical to control tuberculosis infection. Microbes Infect. 9:623-628. [DOI] [PubMed] [Google Scholar]
- 24.Jin, M. S., et al. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130:1071-1082. [DOI] [PubMed] [Google Scholar]
- 25.Kabelitz, D. 2007. Expression and function of Toll-like receptors in T lymphocytes. Curr. Opin. Immunol. 19:39-45. [DOI] [PubMed] [Google Scholar]
- 26.Kawai, T., and S. Akira. 2007. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 13:460-469. [DOI] [PubMed] [Google Scholar]
- 27.Komai-Koma, M., L. Jones, G. S. Ogg, D. Xu, and F. Y. Liew. 2004. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. U. S. A. 101:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krutzik, S. R., and R. L. Modlin. 2004. The role of Toll-like receptors in combating mycobacteria. Semin. Immunol. 16:35-41. [DOI] [PubMed] [Google Scholar]
- 29.Lahiri, A., P. Das, and D. Chakravortty. 2008. Engagement of TLR signaling as adjuvant: toward smarter vaccine and beyond. Vaccine 26:6777-6783. [DOI] [PubMed] [Google Scholar]
- 30.Lancioni, C. L., J. J. Thomas, and R. E. Rojas. 2009. Activation requirements and responses to TLR ligands in human CD4+ T cells: comparison of two T cell isolation techniques. J. Immunol. Methods 344:15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leitner, J., K. Grabmeier-Pfistershammer, and P. Steinberger. 2010. Receptors and ligands implicated in human T cell costimulatory processes. Immunol. Lett. 128:89-97. [DOI] [PubMed] [Google Scholar]
- 32.Mahon, R. N., et al. 2009. Mycobacterium tuberculosis cell wall glycolipids directly inhibit CD4+ T-cell activation by interfering with proximal T-cell-receptor signaling. Infect. Immun. 77:4574-4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarron, M., and D. J. Reen. 2009. Activated human neonatal CD8+ T cells are subject to immunomodulation by direct TLR2 or TLR5 stimulation. J. Immunol. 182:55-62. [DOI] [PubMed] [Google Scholar]
- 34.Morr, M., O. Takeuchi, S. Akira, M. M. Simon, and P. F. Muhlradt. 2002. Differential recognition of structural details of bacterial lipopeptides by Toll-like receptors. Eur. J. Immunol. 32:3337-3347. [DOI] [PubMed] [Google Scholar]
- 35.Motsinger-Reif, A. A., et al. 2010. Polymorphisms in IL-1beta, vitamin D receptor Fok1, and Toll-like receptor 2 are associated with extrapulmonary tuberculosis. BMC Med. Genet. 11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nigou, J., et al. 2008. Mannan chain length controls lipoglycans signaling via and binding to TLR2. J. Immunol. 180:6696-6702. [DOI] [PubMed] [Google Scholar]
- 37.Noss, E. H., et al. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 38.Ogus, A. C., et al. 2004. The Arg753GLn polymorphism of the human Toll-like receptor 2 gene in tuberculosis disease. Eur. Respir. J. 23:219-223. [DOI] [PubMed] [Google Scholar]
- 39.Ozinsky, A., et al. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. U. S. A. 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pecora, N. D., A. J. Gehring, D. H. Canaday, W. H. Boom, and C. V. Harding. 2006. Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J. Immunol. 177:422-429. [DOI] [PubMed] [Google Scholar]
- 41.Quesniaux, V. J., et al. 2004. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J. Immunol. 172:4425-4434. [DOI] [PubMed] [Google Scholar]
- 42.Ramachandra, L., et al. 2010. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing MHC-II molecules capable of antigen presentation. Infect. Immun. [DOI] [PMC free article] [PubMed]
- 43.Reiling, N., et al. 2002. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480-3484. [DOI] [PubMed] [Google Scholar]
- 44.Rezwan, M., T. Grau, A. Tschumi, and P. Sander. 2007. Lipoprotein synthesis in mycobacteria. Microbiology 153:652-658. [DOI] [PubMed] [Google Scholar]
- 45.Rojas, R. E., et al. 2006. Phosphatidylinositol mannoside from Mycobacterium tuberculosis binds α5β1 integrin (VLA-5) on CD4+ T cells and induces adhesion to fibronectin. J. Immunol. 177:2959-2968. [DOI] [PubMed] [Google Scholar]
- 46.Sander, P., et al. 2004. Lipoprotein processing is required for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 52:1543-1552. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz, M. L., and D. Krappmann. 2006. Controlling NF-κB activation in T cells by costimulatory receptors. Cell Death Differ. 13:834-842. [DOI] [PubMed] [Google Scholar]
- 48.Shabaana, A. K., et al. 2005. Mycobacterial lipoarabinomannans modulate cytokine production in human T helper cells by interfering with raft/microdomain signaling. Cell Mol. Life Sci. 62:179-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sugawara, I., et al. 2003. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol. Immunol. 47:327-336. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi, O., et al. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 51.Thuong, N. T., et al. 2007. A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 8:422-428. [DOI] [PubMed] [Google Scholar]
- 52.Velez, D. R., et al. 2010. Variants in Toll-like receptors 2 and 9 influence susceptibility to pulmonary tuberculosis in Caucasians, African-Americans, and West Africans. Hum. Genet. 127:65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, B., et al. 2007. A Toll-like receptor-2-directed fusion protein vaccine against tuberculosis. Clin. Vaccine Immunol. 14:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, X., et al. 2009. ESAT-6 inhibits production of IFN-gamma by Mycobacterium tuberculosis-responsive human T cells. J. Immunol. 182:3668-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winslow, G. M., A. D. Roberts, M. A. Blackman, and D. L. Woodland. 2003. Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J. Immunol. 170:2046-2052. [DOI] [PubMed] [Google Scholar]
- 56.Young, D. B., and T. R. Garbe. 1991. Lipoprotein antigens of Mycobacterium tuberculosis. Res. Microbiol. 142:55-65. [DOI] [PubMed] [Google Scholar]