Abstract
Zinc metalloprotease B (ZmpB) is present in all isolated pneumococcal strains and contributes to the pathogenesis of pneumococcal infection. In this study, recombinant ZmpB was cloned and expressed in Escherichia coli. The expression of ZmpB by different pneumococcal strains was detectable by Western blotting with antisera raised to recombinant ZmpB. Flow cytometry analysis demonstrated that anti-ZmpB polyclonal antibodies could bind to the cell surface of the pneumococcal strains analyzed. Both recombinant ZmpB protein and anti-ZmpB polyclonal antibodies significantly inhibited the adhesion of Streptococcus pneumoniae D39 to A549 cells. In mouse models, mucosal immunization with recombinant ZmpB could significantly reduce pneumococcal lung colonization caused by S. pneumoniae serotypes 19F and 14 and significantly increase mice survival times following invasive pneumococcal challenge with different pneumococcal strains, including serotypes 2, 3, 6B, and 14. Furthermore, intraperitoneal immunization with recombinant ZmpB in combination with the recombinant pneumolysin mutant (DeltaA146 Ply) and heat shock protein 40 (DnaJ) could enhance the protection against pneumococcal infection compared to protection provided by single-protein antigens. Passive immunization with hyperimmune antisera against these three antigens also demonstrated that the combination of three hyperimmune antisera could provide better protection than single antisera. Taken together, our results suggest that ZmpB is a good candidate pneumococcal vaccine antigen.
Streptococcus pneumoniae is able to cause a variety of mucosal and invasive infectious diseases, including otitis media, sinusitis, pneumonia, bacteremia, and meningitis. S. pneumoniae is responsible for approximately 1.6 million deaths and at least 1.2 million infant deaths worldwide each year (5, 25, 41). Using antibiotics to control pneumococcal infections has become increasingly difficult because of widespread and progressive antimicrobial resistance (32, 42). Therefore, vaccination is a promising way to prevent the occurrence of pneumococcal diseases.
At present, there are two pneumococcal vaccines based on capsular polysaccharides available to prevent pneumococcal infections. The 23-valent polysaccharide vaccine is T-cell independent and is poorly immunogenic in infants younger than 2 years of age. Also, it is not effective in elderly people and immunocompromised patients (6, 19, 36). The conjugation of pneumococcal capsular polysaccharides to carrier proteins could overcome the deficiency of immunogenicity to polysaccharide antigens in the first 2 years of life and induce T-cell-dependent immune response and memory cells (33). However, the effectiveness of conjugate vaccine is limited to several serotypes of S. pneumoniae. Moreover, the induction of pneumococcal conjugate vaccine has the propensity to result in serotype replacement by nonvaccine serotypes (6, 8, 39).
Over the past years, it has been recognized that immunization with some surface-exposed or secreted pneumococcal proteins could elicit protection against pneumococcal infection in different animal models (22). These candidate vaccine antigens include pneumococcal surface protein A (PspA), pneumococcal surface adhesion A (PsaA), putative protease maturation protein A (PpmA), choline-binding protein A (CbpA), pneumolysin (Ply), sortase A (SrtA), glutamyl tRNA synthetase (GtS), protein required for the cell wall separation of group B streptococcus (PcsB), serine/threonine protein kinase (StkP), neuraminidase A (NanA), pneumococcal pilus subunits (RrgA, RrgB, and RrgC), iron uptake ABC transporters PiuA and PiaA, pneumococcal histidine triad (Pht) proteins PhtB and PhtE, and heat shock proteins (ClpP and DnaJ) (11, 15, 16, 17, 18, 24, 28, 31). Although protein-based vaccines are considered the next generation of pneumococcal vaccines, it is possible that no single-protein antigen could effectively protect against all pneumococcal strains. Continuing the identification of new protein antigens would accelerate the development of protein-based pneumococcal vaccines.
Zinc metalloprotease B (ZmpB) is present in all isolated pneumococcal strains (10, 29). The first 300 to 400 amino acids, at the N-terminal region of ZmpB, show 99% similarity between all pneumococcal serotypes examined, while the remainder of this protein displays high sequence variation (4). In mouse models of pneumococcal septicemia, there were increased survival times and decreased blood bacterial counts in the mice infected with ZmpB-mutant pneumococcus compared to those of wild-type D39 (4). Also, ZmpB mutant TIGR4 pneumococcus had a 36-fold reduction of the mouse 50% lethal dose (LD50) with respect to the parent strain (13). A recent study further confirmed a pivotal role of ZmpB in a model of complicated pneumonia in young mice infected with clinical strains of S. pneumoniae from children with complicated pneumonia, and this model proved that the allelic variation of ZmpB affects the virulence of S. pneumoniae (20). In addition, pneumococcal ZmpB also is involved in modulating the production of tumor necrosis factor alpha (TNF-α) in the lower respiratory tract (4). All of these findings indicate that ZmpB is a novel target for treating pneumococcal infection. In fact, Beghetto et al. found that there were human B-cell epitopes in ZmpB by using a pneumococcal genome display library, and antigenic regions of ZmpB reacted with 77% of human adult sera, which is higher than the reactivity of PspA (60%), suggesting a broad recognition of ZmpB antigen (3). Therefore, ZmpB has the potential to be a candidate pneumococcal vaccine.
In this study, we investigated the ability of immunization with recombinant ZmpB (rZmpB), alone or in combination with recombinant nontoxic Ply (DeltaA146 Ply), and rDnaJ to elicit protection against pneumococcal colonization and invasive infection in mice. We found that ZmpB is a good candidate vaccine antigen for the development of protein-based pneumococcal vaccines.
MATERIALS AND METHODS
Bacterial strains.
Escherichia coli DH5α (Invitrogen, CA) was used as the host for routine plasmid cloning. Recombinant proteins were expressed in E. coli BL21(DE3) (Novagen). Pneumococcal strain D39 (NCTC 7466, serotype 2) was purchased from the National Collection of Type Cultures (London, United Kingdom), while pneumococcal strains CMCC 31436 (serotype 3), CMCC 31207 (serotype 6B), CMCC 31614 (serotype 14), and CMCC 31693 (serotype 19F) were obtained from the China Medical Culture Collection (CMCC; Beijing, China) center. The ZmpB-negative D39 mutant was constructed as described previously (4). All pneumococcal strains have been confirmed by Gram staining, optochin sensitivity, the presence of α-hemolysis on blood agar base plates, and multilocus sequence typing. The Quellung reaction was used to confirm the capsular serotype.
Mice.
Female 5- to 6-week-old BALB/c mice were obtained from and raised at Chongqing Medical University. The genotype of BALB/c mice has been confirmed recently. All animal experiments were done in accordance with the Institutional Animal Care and Use Committee's guidelines at the Chongqing Medical University.
Cloning, expression, and purification of rZmpB in E. coli.
The fragment of ZmpB was amplified with forward and reverse primers (5′-CCGGAATTCATGGTTGTAGGCTCTGTATTTC-3′ and 5′-CCGCTCGAGTTATAGGGTGTAACCTTGATA-3′, which incorporate EcoRI and XhoI sites, respectively) from genomic DNA isolated from S. pneumoniae strain D39 by PCR. The approximately 1.7-kb amplified coding sequence corresponds to the region encoding amino acids 15 to 590 of the mature N-terminal of ZmpB. The PCR fragment was digested with the same enzymes and cloned into the corresponding restriction sites in pSUMO (LifeSensors, Malvern, PA) to generate plasmid pSUMO-ZmpB, forming a sequence that encodes a fusion protein of SUMO-ZmpB and an N-terminal His6-tag. The construct then was transformed into competent E. coli. BL21(DE3) and grown at 37°C in 1 liter of Luria broth supplemented with 30 μg/ml kanamycin, and the production of recombinant ZmpB (rZmpB) was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) exposure for 4 h. The soluble protein in the supernatant was collected by refrigerate centrifugation after ultrasound sonication, and rZmpB was purified by the use of metal affinity chromatography columns and buffers (Novagen) according to the manufacturer's instructions and dialyzed against 10 mM sodium phosphate buffer (pH 7.0). The expression of His6-tagged SUMO-ZmpB was analyzed by Western blotting with an anti-His6-tag mouse monoclonal antibody (Invitrogen). Recombinant SUMO protein from the BL21(DE3) strain transformed with empty pSUMO plasmid was prepared and used as the control protein.
The expression and purification of recombinant nontoxic Ply and DnaJ were performed as described previously (6, 45). All recombinant proteins were filter sterilized (Millipore) and stored at −20°C.
Immunization of mice.
In mucosal immunization studies, groups of BALB/c mice (12 per group) were immunized three times at 14-day intervals with 10 μg of recombinant antigens in cholera toxin (CT). The dose of antigen solution corresponded to a mixture of 25 μl of antigen and 5 μl (1 mg/ml) of CT. The 23-valent pneumococcal polysaccharide vaccine PPV23 (Chengdu Institute of Biological Products, Chengdu, China) was used as the positive control. The intranasal immunization was carried out with the BALB/c mouse held in a supine position with the head down while 30 μl of the antigen solution was delivered slowly with a micropipette onto the nares. The sera and saliva were collected 7 days after the last immunization and stored at −70°C for further studies.
In intraperitoneal immunization studies, groups of BALB/c mice (12 per group) were primed with either rZmpB alone, rPly alone, rDnaJ alone, rZmpB plus rPly, rZmpB plus rDnaJ, rPly plus rDnaJ, rZmpB plus rPly plus rDnaJ, or control protein, each in complete Freund's adjuvant (1:1 ratio, vol/vol), and each mouse received 10 μg of each protein antigen on day 0 and was boosted with the same concentration of each protein antigen in incomplete Freund's adjuvant (1:1 ratio, vol/vol) on days 14 and 28. Mice injected with PPV23 served as positive controls. The sera were collected 7 days after the last immunization and stored at −70°C for further studies.
ELISA and Western blot analysis.
The levels of specific antibodies in immunized mice were determined by enzyme-linked immunosorbent assay (ELISA) (11). Total immunoglobulin G (IgG), IgG subclass (IgG1, IgG2a, and IgG2b), and IgA antibodies produced against the vaccine antigens were detected by using horseradish peroxidase-conjugated anti-mouse IgA or anti-mouse IgG (heavy-plus-light chains) and subclass antibodies (Invitrogen), with tetramethylbenzidine as the substrate. Whole-cell lysates or concentrated culture supernatants prepared from S. pneumoniae also were analyzed for reactivity with antisera against recombinant antigens by Western blotting.
Surface staining by flow cytometry.
S. pneumoniae in the early-logarithmic growth phase were collected and washed twice in phosphate-buffered saline (PBS) supplemented with 2% (vol/vol) fetal calf serum and 0.05% sodium azide. Pneumococcal suspensions were aliquoted in 300-μl samples, and anti-ZmpB or preimmune mouse sera were added for 1 h of incubation at 4°C, followed by the addition of fluorescein isothiocyanate-conjugated goat anti-mouse IgG antibodies (R&D systems) for a further 30 min of incubation at 4°C in the dark. After the samples were washed, the surface binding of anti-ZmpB antibodies to pneumococci was analyzed by flow cytometry (FACSCalibur; BD Biosciences).
Inhibition assays.
A549 cells (type II epithelial lung carcinoma cells; ATCC) were grown to 100% confluence as described previously (28). rZmpB at concentrations ranging from 0 to 50 μg/ml was added to the cells and incubated for 1 h. After the samples were washed extensively, S. pneumoniae D39 (1 × 106 CFU) was added to A549 cells and then incubated for 1 h. A549 cells finally were liberated and plated onto blood agar plates.
To analyze the inhibitory activity of anti-ZmpB sera, S. pneumoniae D39 (1 × 106 CFU) was added to A549 cells before or after 30 min of incubation with antisera. The experiment was continued as described above. All adhesion experiments were performed three times, and the total numbers of adherent pneumococci (CFU/well) were recorded.
Cytokine assays.
Spleens were removed from immunized and control BALB/c mice 7 days after the last immunization. The splenocytes were collected aseptically, and 200 μl of cells was cultured (1 × 105 cells/ml) in a 96-well plate. The cells then were stimulated with rZmpB in vitro for 72 h at 37°C in a 5% CO2 incubator. The levels of IL-4, IFN-γ, IL-10, and IL-17A in the culture supernatants were detected by ELISA kits (Biolegend) according to the manufacturer's recommendations.
Challenge studies.
In pneumococcal lung colonization models, BALB/c mice were lightly anesthetized with 3% (vol/vol) halothane over oxygen by using a methacrylate box connected to a Fluovac 240 (Anesthetizing Systems) to suppress the ciliary clearance of inhaled pneumococci. Following anesthetization, BALB/c mice were intranasally inoculated with pneumococcal strain CMCC 31614 (1.5 × 106 CFU) or CMCC 31693 (1.0 ×107 CFU). To determine lung colonization, the lungs were removed and homogenized in PBS at day 3 after challenge. Samples were serially diluted and plated on blood agar plates, and viable counts were determined after overnight incubation.
In the sepsis models, both intranasal and intraperitoneal challenge experiments were performed as described previously (31, 46). In intranasal challenge studies, at 14 days after the last immunization, BALB/c mice were lightly anesthetized as described above and then intranasally challenged with either CMCC 31436 (6.0 × 108 CFU), CMCC 31207 (3.0 × 108 CFU), CMCC 31614 (2.0 × 108CFU), or D39 (7.0 × 107 or 2 × 108 CFU). The challenge dose for each strain was more than 50 times the LD50 for BALB/c mice. In intraperitoneal challenge studies, BALB/c mice were challenged with 1 ×107 CFU of D39. The challenged BALB/c mice were observed twice daily by an experienced person, and signs of sickened and dead BALB/c mice were recorded. BALB/c mice still alive after 21 days were considered to have survived the infection.
Passive protection against intraperitoneal infection.
To further investigate the ability of antisera from BALB/c mice immunized with protein antigens to elicit protection against invasive pneumococcal infection, groups of BALB/c mice were injected in the tail vein with 150 μl of mouse antisera. Control mice were injected with 150 μl of pooled sera from nonimmunized normal mice. Combinations of two antisera (anti-ZmpB plus anti-Ply, anti-ZmpB plus anti-DnaJ, and anti-Ply plus anti-DnaJ sera at 150 μl each) or three antisera (anti-ZmpB plus anti-Ply plus anti-DnaJ sera at 150 μl each) were administered to mice under the same conditions as those for single antisera. An hour later, mice were infected intraperitoneally with 1 × 107 CFU D39. Mice received a second dose of antisera 36 h after infection under the same conditions as those for the first dose. Mice then were observed for 21 days, and the survival time of each mouse was recorded.
Statistics.
A nonparametric test (Mann-Whitney) was used to compare numbers of pneumococci (CFU) or median survival times for groups of mice. Statistical analysis in adhesion assays was performed using one-way analysis of variance (ANOVA) with Bonferroni's multiple comparison post-hoc test. The limit of statistical significance was a P value of 0.05.
RESULTS
Purification and characterization of rZmpB.
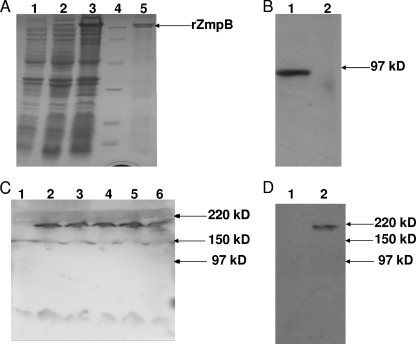
Purified rZmpB was >95% pure, as judged by SDS-PAGE, after it was stained with Coomassie brilliant blue R250. rZmpB migrated with the expected molecular size of approximately 90 kDa in SDS-PAGE (Fig. 1A). The ZmpB band appeared to be a double band; the cause of this may be that there was some protein degradation during purification. It is also plausible that additional processing during protein translation causes this size difference of rZmpB. On the other hand, because ZmpB is an acidic protein, SDS may not have uniformly coated rZmpB, causing the aberrant migration of some recombinant proteins in SDS-PAGE. The purified rZmpB reacted with anti-His monoclonal antibodies in a Western blot assay, and it was further confirmed by N-terminal amino acid sequencing (data not shown). Western blot analysis using anti-ZmpB sera generated from mice immunized with rZmpB demonstrated a specific antibody response to the purified rZmpB (Fig. 1B), which suggests that rZmpB was a single antigen and it was not contaminated by other proteins.
FIG. 1.
Purification of rZmpB protein from E. coli. (A) SDS-PAGE analysis (10% gel) of protein samples. Lanes: 1, lysate of untransformed E. coli expression strain; 2, lysate of recombinant E. coli expression construct before induction; 3,supernatant of E. coli lysate centrifuged at 100,000 × g after a 4-h induction with IPTG before being loaded onto Ni-NTA resin; 4, protein markers; 5, purified His6-tagged ZmpB protein (approximate mass, 90 kDa) after elution from Ni-NTA. (B) Western blot analysis of purified rZmpB. SDS-PAGE (10%) was loaded with recombinant SUMO protein (lane 1) and rZmpB (lane 2) purified from E. coli. (C) Western blot analysis of pneumococcal lysates. SDS-PAGE (10%) was loaded with whole-cell lysates obtained from ZmpB mutant D39 (lane 1), wild-type D39 (lane 2), CMCC 31436 (lane 3), CMCC 31207 (lane 4), CMCC 31614 (lane 5), and CMCC 31693 (lane 6). (D) Western blot analysis of pneumococcal supernatants. SDS-PAGE (10%) was loaded with concentrated supernatants of late-exponential-phase broth cultures of ZmpB mutant (lane 1) and wild-type D39 (lane 2). PVDF membrane strips then were reacted with anti-ZmpB sera. Apparent molecular mass in kilodaltons is indicated by arrows.
Characterization of ZmpB expression in pneumococcal strains.
Anti-ZmpB polyclonal antibodies reacted with a band of approximately 210 kDa in fractions of all pneumococcal strains tested, and it did not react with lysates of ZmpB-negative D39 mutant pneumococci (Fig. 1C). These results are consistent with previous reports that the 5.7-kb ZmpB gene in D39 encodes a full-length ZmpB protein of 1,908 amino acids with a molecular mass of approximately 210 kDa (3, 10). In addition to ZmpB, anti-ZmpB sera showed a very low cross-reactivity with a band of approximately 150 kDa in the lysates of pneumococcal strains tested. It is unlikely that this protein was a degradation product of ZmpB, as it still was present when anti-ZmpB sera were reacted with lysates of the ZmpB-negative D39 mutant. However, this cross-reactivity of 150 kDa could not be seen in the concentrated supernatants of late-exponential-phase broth cultures of both the wild type and ZmpB mutant D39, while a band for ZmpB was identified only in the supernatants of wild-type D39 (Fig. 1D).
Surface expression of ZmpB in S. pneumoniae.
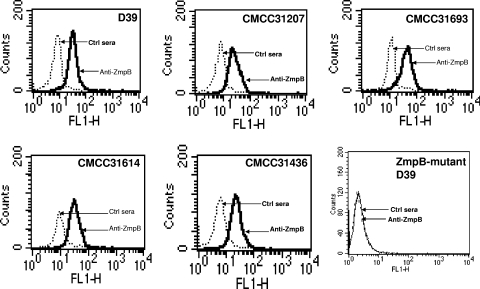
Binding studies were performed to investigate whether anti-ZmpB sera could bind to the surface of S. pneumoniae by flow cytometry. The analysis revealed that ZmpB was accessible to antibodies on the surface of D39 and four clinical isolates of S. pneumoniae, including CMCC 31436, CMCC 31207, CMCC 31614, and CMCC 31693, while there was no detectable level of ZmpB on the surface of the ZmpB mutant D39 (Fig. 2). Therefore, pneumococcal ZmpB is in fact surface exposed.
FIG. 2.
Flow-cytometric analysis of different S. pneumoniae isolates. Flow cytometry analysis was performed using anti-ZmpB sera obtained from immunized mice. Shown are overlays of flow-cytometric histograms demonstrating the staining of D39, CMCC 31436, CMCC 31207, CMCC 31614, CMCC 31693, and ZmpB mutant D39 with anti-ZmpB or preimmune control sera.
Inhibition of S. pneumoniae adhesion to cultured A549 cells.
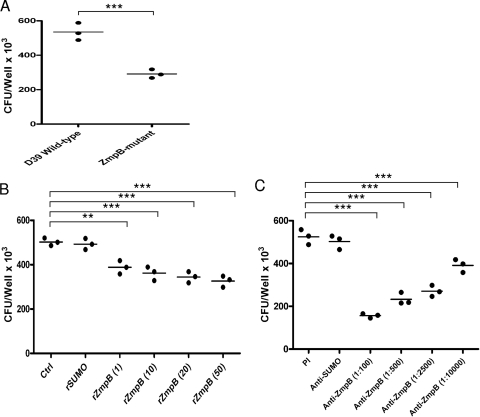
Wild-type and mutant D39 were characterized for their ability to adhere to A459 cells, and the number of ZmpB mutant D39 cells adhered to A549 cells was significantly decreased compared to that for wild-type D39 (P < 0.001) (Fig. 3A). To further determine whether rZmpB or anti-ZmpB sera have a functional significance, we tested their ability to interfere with the adhesion of D39 to A549 cells. Both rZmpB and anti-ZmpB sera could significantly inhibit D39 adhesion to A549 cells in a dose-dependent manner (P < 0.01 in all cases) (Fig. 3B and C), while SUMO control protein or preimmune sera lacked the ability to interfere with the adhesion of D39.
FIG. 3.
Analysis of S. pneumoniae adhesion to A549 cells. (A) Adhesion of ZmpB mutant and wild-type D39 cells to A549 cells. The ZmpB mutant and wild-type D39 were added to A549 cells and incubated for 1 h. (B) The effect of rZmpB on S. pneumoniae adhesion. rZmpB at serial concentrations ranging from 0 to 50 μg/ml was added to the cells and incubated for 1 h. S. pneumoniae D39 (1 × 106 CFU) and then were added to A549 cells. Recombinant SUMO protein was used as the control protein. (C) The effect of antibodies against ZmpB on S. pneumoniae adhesion. S. pneumoniae D39 (1 × 106 CFU) was added to A549 cells before or after 30 min of incubation with antisera obtained from mice immunized with recombinant ZmpB. The sera from preimmune mice were used as the control. All adhesion experiments were performed in triplicate, and the total numbers of adherent pneumococci (CFU/well) were recorded. Statistical analysis was performed using 1-way ANOVA. ** and ***, P < 0.01 and < 0.001, respectively, compared between groups denoted by horizontal lines.
Immune responses elicited by mucosal immunization with rZmpB.
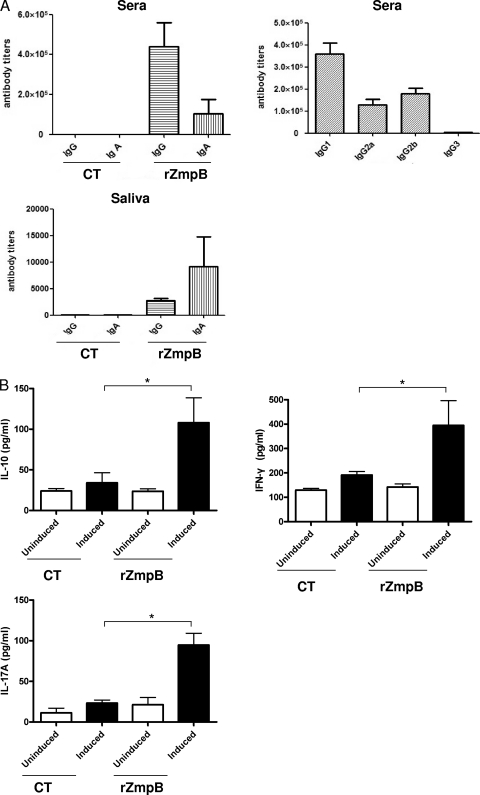
To study antigen-specific responses generated by intranasal immunization with rZmpB, both humoral and cell-mediated responses were investigated. As shown in Fig. 4 A, intranasal immunization with rZmpB could induce high-level production of ZmpB-specific IgG and IgA antibodies in sera. IgG subclass analysis revealed that the IgG1 response was predominant, followed by those of IgG2b and IgG2a, and the IgG3 response was little. Besides, it could induce ZmpB-specific IgA and IgG antibodies in saliva. In contrast, no such antibody response was elicited when CT was given alone.
FIG. 4.
Immune responses elicited by mucosal immunization with rZmpB. The mice were intranasally immunized with rZmpB, with CT as the mucosal adjuvant. (A) The levels of specific total IgG, IgG1, IgG2a, IgG2b, IgG3, or IgA against rZmpB in sera or saliva samples were tested by ELISA. The antibody titers were defined as the reciprocal of the dilution of sera giving a 2.1-fold value of the highest absorbance value versus the background level at 450 nm. The antibody titers were measured 7 days after the third immunization. (B) Effect of mucosal immunization with rZmpB on cytokine production in splenocytes. Seven days after the last immunization, the splenocytes (1 × 105 cells/well) were cultured in the absence (uninduced) or in the presence (induced) of 5 μg of rZmpB for 72 h at 37°C. After 72 h, the culture supernatants were assayed for the levels of IFN-γ, IL-10, and IL-17A by ELISA. *, P < 0.05 compared between groups denoted by horizontal lines.
Furthermore, splenocytes isolated from control and immunized mice were stimulated with rZmpB in vitro. As shown in Fig. 4B, the levels of cytokines IFN-γ, IL-10, and IL-17A in culture supernatants of splenocytes isolated from rZmpB-immunized mice were significantly higher than those from control mice (P < 0.05). However, there was no significant difference for the production of IL-4 (data not shown).
Protection against pneumococcal pneumonia by mucosal immunization with rZmpB.
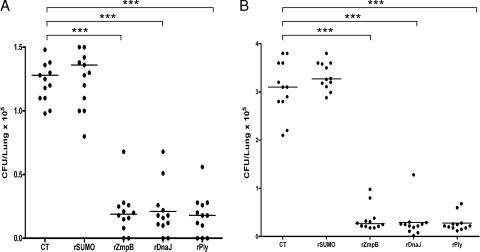
Two pneumococcal pneumonia models have been successfully established using serotypes 19F and 14 (43, 44, 45). In the CMCC 31693 (serotype 19F) challenge model, intranasal immunization with rZmpB could reduce a bacterial load in the lungs of approximately six times greater relative to levels for mice vaccinated with CT alone, similarly to rDnaJ and rPly immunization (Fig. 5A). This way also was effective in reducing lung infection caused by CMCC 31614 (serotype 14), and rZmpB resulted in an approximately 10 times-reduced median bacterial load in the lungs, whereas rZmpB was as effective as rDnaJ and rPly (Fig. 5B). As a control protein, intranasal immunization with rSUMO could not protect pneumococcal lung colonization.
FIG. 5.
Protection against pneumococcal pneumonia by mucosal immunization with rZmpB. Mice immunized with rZmpB by the nasal route were intranasally challenged with pneumococcal strain CMCC 31693 (1.0 × 107 CFU) (A) or CMCC 31614 (1.5 × 106 CFU) (B). The lung colonization of individual mice was determined at day 3 after challenge. Each dot represents one mouse. The horizontal lines indicate the median CFU per lung. ***, P < 0.001 compared between groups denoted by horizontal lines.
Protection against invasive pneumococcal infection by mucosal immunization with rZmpB.
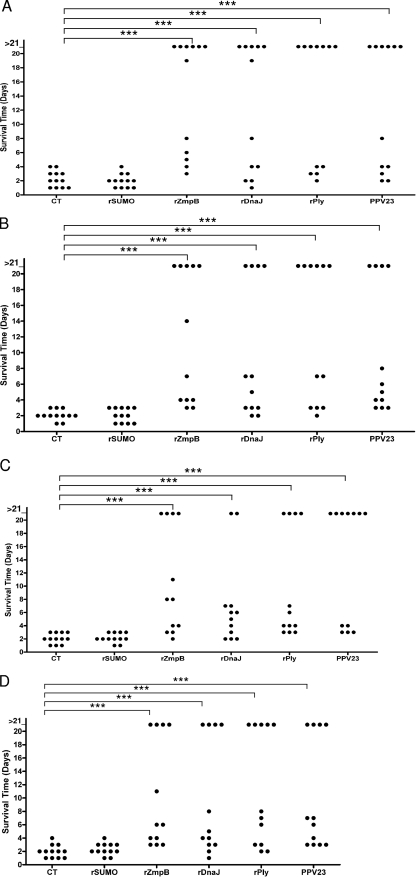
To evaluate protection against death afforded by mucosal immunization with rZmpB, invasive pneumococcal infection models by intranasal inoculation of different serotypes of S. pneumoniae were established in mice that mimic the natural route of pneumococcal infection. Figure 6 shows that the median survival times (>6 days) for mice treated with rZmpB were significantly longer than those for mice that received CT alone upon challenge with D39, CMCC 31436, CMCC 31207, or CMCC 31614 (P < 0.001 in all cases). When the median survival times for mice that received rZmpB were compared to those that obtained with either rDnaJ (>4 days) or rPly (>6 days), there were no significant differences in all groups. Besides, PPV23 was as protective as rZmpB, rPly, or rDnaJ against challenge by these pneumococcal strains.
FIG. 6.
Survival times for mice after intranasal challenge. Groups of 12 BALB/c mice were immunized intranasally with the indicated antigens and challenged 14 days after the third immunization with D39 (7.0 × 107 CFU) (A), CMCC 31436 (6.0 × 108 CFU) (B), CMCC 31207 (3.0 × 108 CFU) (C), or CMCC 31614 (2.0 × 108 CFU) (D). Each dot represents one mouse. The horizontal lines denote the median survival time for each group. ***, P < 0.001 compared between groups denoted by horizontal lines.
Enhanced protection against invasive pneumococcal infection by immunization with rZmpB in combination with rPly and rDnaJ.
We further evaluated the possibility that immunization with rZmpB in combination with rPly and rDnaJ would confer an additive protection against invasive pneumococcal infection. Groups of mice were immunized intraperitoneally with rZmpB, rPly, and rDnaJ either singly or in combination, and ELISA analysis of pooled sera from immunized mice shows strong and antigen-specific antibody responses (Table 1). There was no detectable antagonistic effect of combining these recombinant antigens, since antigen-specific antibody titers were not suppressed when they were treated in combination. Besides, Western blot analysis also demonstrated the specific reactivity of the antisera with whole-cell lysates of D39 (data not shown).
TABLE 1.
Antibody titers (total IgG) obtained from mice immunized with rZmpB, rPly, and rDnaJc
| Immunization group | Antibody titera response to: |
||
|---|---|---|---|
| rZmpB | rPly | rDnaJ | |
| Adjuvant | -b | - | - |
| rZmpB | 680,000 ± 250,000 | ND | ND |
| rPly | ND | 400,000 ± 110,000 | ND |
| rDnaJ | ND | ND | 320,000 ± 120,000 |
| rZmpB+rPly | 385,000 ± 80,000 | 385,000 ± 90,000 | ND |
| rZmpB+rDnaJ | 420,000 ± 90,000 | ND | 225,000 ± 50,000 |
| rPly+rDnaJ | ND | 328,000 ± 70,000 | 280,000 ± 85,000 |
| rZmpB+rPly+rDnaJ | 400,000 ± 60,000 | 360,000 ± 85,000 | 310,000 ± 900,000 |
ELISA titers were defined as the reciprocal of the dilution of sera giving a value 2.1-fold above the highest absorbance value versus the background level at 450 nm.
-, no reactivity (value of <100).
Antibody titers obtained from mice prior to challenge with S. pneumoniae. Groups of 12 mice were immunized with the indicated antigens by intraperitoneal injection, and sera were collected from all mice by retroorbital bleeding 1 week after the third immunization.
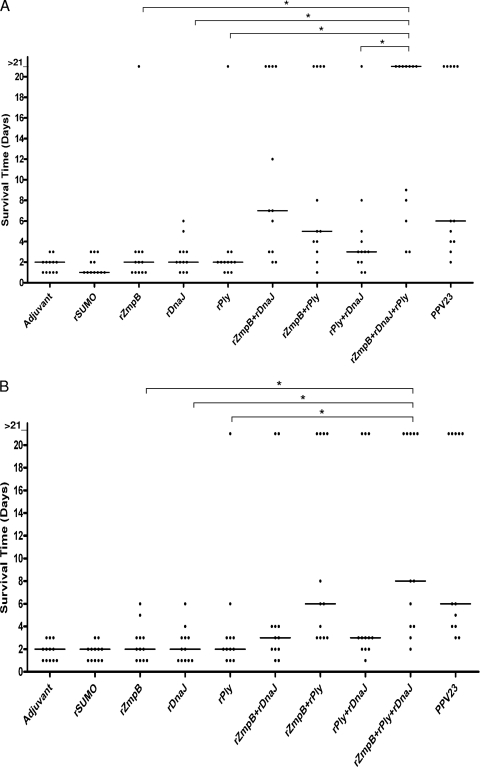
Both intranasal and intraperitoneal challenge experiments were performed, and high challenge doses were employed to assess additive protection achieved with the combinations of rZmpB, rDnaJ, and rPly. In the first experiment, immunized mice were challenged intranasally with 2 × 108 CFU of D39. As shown in Fig. 7 A, the median survival times for mice that received rZmpB, rDnaJ, or rPly singly were not significantly different from those for mice that received adjuvant alone or SUMO control protein. However, mice that received rZmpB plus rDnaJ survived significantly longer than those that received either rZmpB alone (P < 0.05) or rDnaJ alone (P < 0.05), and mice immunized with rZmpB plus rPly survived significantly longer than those immunized with either rZmpB alone (P < 0.05) or rPly alone (P < 0.05). Besides, the median survival time for mice that received rZmpB plus rDnaJ plus rPly was significantly longer than those for mice that received rZmpB alone (P < 0.05), rDnaJ alone (P < 0.05), rPly alone (P < 0.05), or rPly plus rDnaJ (P < 0.05).
FIG. 7.
Survival times for mice after intranasal or intraperitoneal challenge. Groups of 12 BALB/c mice were immunized intraperitoneally with the indicated antigens. Vaccinated mice then were challenged with 2.0 × 108 CFU of D39 intranasally (A) or 1 ×107 CFU of D39 intraperitoneally (B) after the third immunization. Each dot represents one mouse. The horizontal lines denote the median survival times for each group. *, P < 0.05 compared between groups denoted by horizontal lines.
In the intraperitoneal challenge experiment, mice that received single antigens could not live significantly longer than those that received either adjuvant alone or SUMO control protein (Fig. 7B). However, mice immunized with rZmpB plus rPly survived significantly longer than those that received either rZmpB alone (P < 0.05) or rPly alone (P < 0.05). Moreover, the median survival time for mice that received rZmpB plus rDnaJ plus rPly was significantly longer than those for mice that received rZmpB alone (P < 0.05), rDnaJ alone (P < 0.05), or rPly alone (P < 0.05). As the positive control, immunization with PPV23 could elicit effective protection against D39 in these experiments.
Passive protection against invasive pneumococcal infection by antigen-specific antibodies.
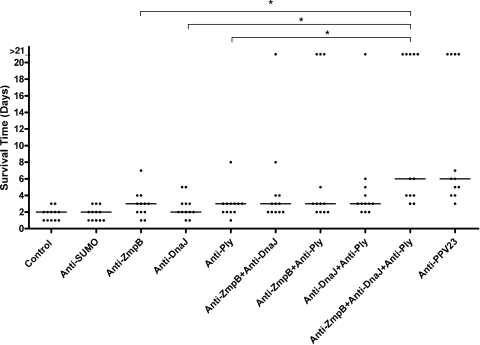
To confirm that the protection elicited by ZmpB-based protein vaccine is antibody mediated, anti-ZmpB, anti-DnaJ, and anti-Ply sera were administered singly or in combinations, and mice then were intraperitoneally challenged by D39. As shown in Fig. 8, mice that received single-antigen-specific sera could not live significantly longer than those that received control sera. Moreover, the combinations of two antigen-specific sera (anti-ZmpB plus anti-DnaJ, anti-ZmpB plus anti-Ply, or anti-DnaJ plus anti-Ply) could not confer significant protection against pneumococcal infection. However, a significant increase in the median survival time was obtained for mice treated with the triple combination of anti-ZmpB, anti-DnaJ, and anti-Ply sera compared to that for mice that received control sera (P < 0.05). This treatment also provided better protection than anti-ZmpB alone (P < 0.05), anti-DnaJ alone (P < 0.05), or anti-Ply alone (P < 0.05), and it was as effective as anti-PPV23 sera.
FIG. 8.
Survival times for mice after intraperitoneal challenge. Groups of 12 BALB/c mice were immunized with the indicated antisera containing specific polyclonal antibodies against the indicated antigens and challenged with 1 ×107 CFU of D39 intraperitoneally. Each dot represents one mouse. The horizontal lines denote the median survival time for each group. *, P < 0.05 compared between groups denoted by horizontal lines.
DISCUSSION
There are a number of pneumococcal surface proteins that play important roles in the pathogenesis of pneumococcal infection. Current studies suggest that they are promising nonpolysaccharide vaccine candidates that could provide effective and non-serotype-dependent protection against pneumococcal infection (40). In the present study, we described a new vaccine candidate, ZmpB, which was found to elicit a protective immune response against pneumococcal infection caused by different serotypes of S. pneumoniae. Moreover, we found that a combination of ZmpB with nontoxic Ply and DnaJ could afford enhanced protection against pneumococcal infection. Our findings therefore suggest that ZmpB is a new pneumococcal protein antigen for inclusion in the development of an effective pneumococcal multiprotein vaccine.
Extracellular zinc metalloproteinases have been recognized to contribute essentially to the virulence of several human pathogens, such as Bacillus anthracis, Clostridium botulinum, Clostridium tetani, Pseudomonas aeruginosa, Staphylococcus aureus, Vibrio cholerae, and Listeria monocytogenes (13). S. pneumoniae has two to four zinc metalloproteinases depending on the strains. The best characterized of them is IgA1 protease, which is involved in pneumococcal adherence and colonization, and it could elicit significant protection against fatal pneumococcal pneumonia in mice (2, 37). ZmpC has the capacity to cleave human matrix metalloproteinase-9 (MMP-9) and to stimulate syndecan-1 shedding (12, 30), while the role of ZmpD in pneumococcal infection still is unknown. Unlike ZmpC and ZmpD in some pneumococcal strains, ZmpB is ubiquitous in all strains of S. pneumoniae and contributes to the virulence of this pathogen (13). Here, we expressed the N terminus of ZmpB by exploiting the natural chaperoning properties of the SUMO tag, which could enhance the expression, folding, solubility, and purification of target proteins (9). We have conducted a comparison of the effectiveness of other fusion tags, including glutathione-S-transferase (GST) and thioredoxin (Trx), and found that SUMO was superior to other tags for the expression of ZmpB. Romanello et al. have successfully produced a nearly complete pneumococcal IgA1 protease using PET-19b expression vector in E. coli to characterize its enzymatic activity and immunogenicity (34). In this study, we have tried to express complete pneumococcal ZmpB using different expression vectors. However, we could not get a large quantity of recombinant soluble ZmpB because of its large size. Since N termini of pneumococcal surface proteins generally are thought to exert their biological functions and to be the candidate vaccine components as described for PspA and CbpA (18, 31), we expressed the first 15 to 590 amino acids of the N terminus of ZmpB, which contained two transmembrane domains and an LPXTG processing motif. We hypothesized that this fragment of ZmpB can be exploited as a vaccine component.
Although ZmpB exhibits variability in different pneumococcal strains (4), Western blot and flow-cytometric analyses demonstrated that polyclonal antibodies against ZmpB antigen were cross-reactive and could bind to the cell surface of different serotypes of S. pneumoniae. These results are reasonable for the hypothesis that surface antigens that exhibit variability caused by immunological selection (such as PspA and CbpA) are more readily accessible to antibodies on the surface of intact pneumococci than highly conserved antigens (such as PsaA and PpmA) (18). Unexpectedly, a protein of approximately 150 kDa present in different serotypes of pneumococcus was found to have a very low cross-reaction with anti-ZmpB sera. The cross-reaction with anti-ZmpB sera may be due to some homology of the N terminus of ZmpB to other pneumococcal choline-binding proteins (4). However, we could not detect the expression of this 150-kDa protein on the surface of the ZmpB mutant D39 using anti-ZmpB sera, and this cross-reactivity of 150-kDa protein could not be seen in the concentrated culture supernatants of both the wild type and ZmpB mutant D39, which suggests that this protein is not a surface-exposed or secreted antigen and thus does not satisfy the criteria for pneumococcal candidate protein vaccines (17). Therefore, the low cross-reaction of this protein with anti-ZmpB sera may not complicate the protective effects of ZmpB antigen against S. pneumoniae. At this stage, we still do not know the characteristics of this 150-kDa protein, and it is difficult to assess the level of protection elicited specifically by ZmpB.
In human cell models, we found that both rZmpB and anti-ZmpB sera could inhibit the adhesion of S. pneumoniae to A549 cells, which is consistent with previous reports that ZmpB contributed to pneumococcal pneumonia in mouse models (4, 20). However, since ZmpB is a zinc metalloprotease, we cannot exclude the possibility that the addition of external recombinant ZmpB may decrease the adherence of pneumococci through the proteolytic degradation of pneumococcal adhesions. To the best of our knowledge, the substrates for ZmpB have not been reported. Therefore, identifying specific substrates or host cell receptors for pneumococcal ZmpB would further delineate the role of ZmpB in the pathogenesis of pneumococcal pneumonia.
In mucosal immunization studies, we found that the intranasal application of rZmpB could induce mucosal and systemic immunity to S. pneumoniae, and there was a significant increase of Th1 cytokine IFN-γ, T-reg cytokine IL-10, or Th17 cytokine IL-17A in rZmpB-immunized mice, while the increase of Th2 cytokine IL-4 was marginal and did not reach statistical significance. Previous studies have suggested that the Th1 immune response was critical in overcoming pneumococcal infection (1, 21, 23), while IL-17A regulates acquired immunity to pneumococcal colonization, and it could enhance pneumococcal killing by human neutrophils. In addition, IL-17A plays an important role in suppressing pneumococcal nasal carriage in mice vaccinated mucosally (26, 27). IL-10 is an anti-inflammatory cytokine involved in regulating immune responses. It also could promote B-cell survival, proliferation, and antibody production (35). Although the increase of IL-4 stimulated by ZmpB antigen was not significant, we cannot exclude the active role of IL-4 in inducing the Th2 immune response, since the IgG subclass revealed a higher IgG1 response than that of IgG2a in mice sera. We suggest that an effective vaccination strategy against S. pneumoniae is associated with mixed Th1, Th2, Th17, and T-reg responses.
In pneumococcal pneumonia models established with serotypes 19F and 14, we found that intranasal immunization with rZmpB could reduce pneumococcal lung colonization in mice. ZmpB is as effective as StkP and PcsB in reducing pneumococcal lung colonization, because subcutaneous immunization with StkP or PcsB also was found to reduce WU2 (serotype 3) and EF3030 (serotype 19) colonization in lungs by about 10 times relative to levels for mock-immunized mice and C3H/HeNHsd mice (17). However, in CBA/N mice, subcutaneous immunization with PspA was found to be more effective in protecting pneumococcal pneumonia, which could result in more than 100 times-reduced bacterial loads in the lungs (7). The formulations of antigens, vaccine delivery, and mouse models may account for the difference of degree in reducing lung colonization described above. We also found that mucosal immunization with rZmpB could protect against death caused by pneumococcal serotypes 2, 3, 6B, and 14. This is the first study showing that mucosal immunization with rZmpB could protect against pneumococcal infection.
There are a growing number of studies demonstrating that immunization with a combination of multiple-protein antigens could elicit additive or synergistic protection, such as ClpP plus PspA plus PspA or Ply plus PspA plus PspC (11, 31). Here, we found that a triple combination of recombinant ZmpB, Ply, and DnaJ provided better protection against invasive pneumococcal infection than single antigens. Furthermore, passive immunization studies showed that a triple combination of anti-ZmpB, anti-DnaJ, and anti-Ply sera also could confer increased protection. The enhanced protection elicited by the combination of these antigens indicates that they contribute to different stages of pneumococcal infection and have different, complementary or synergistic effects on the virulence of S. pneumoniae. Further studies are required to investigate whether the simultaneous mutagenesis of all three genes of ZmpB, Ply, and DnaJ would result in the additive or synergistic attenuation of pneumococcal virulence compared to that of the mutagenesis of single genes.
In conclusion, this study indicates that immunization with ZmpB protects against pneumococcal diseases. A recent study reported that the proline-rich region of PspA and CbpA contains surface-accessible epitopes common to all pneumococci and was able to elicit antibody-mediated protection against sepsis (14), suggesting that epitope vaccine is a promising option for vaccination against S. pneumoniae (38). Sine ZmpB is a large protein present in all pneumococcal strains, we would identify conserved epitopes of ZmpB and develop multiple-epitope vaccines in future work.
Acknowledgments
This study is supported by National Natural Science Foundation grants of China (no. 30700914, 81000711, and 30970110), Natural Science Foundation grants of Chongqing key projects (NO.CSTC, 2008BA7027), and the Chongqing Municipal Education Commission (KJ100310).
We declare that no competing interests exist.
Editor: A. Camilli
Footnotes
Published ahead of print on 22 November 2010.
REFERENCES
- 1.Arvå, E., and B. Andersson. 1999. Induction of phagocyte-stimulating and Th1-promoting cytokines by in vitro stimulation of human peripheral blood mononuclear cells with Streptococcus pneumoniae. Scand. J. Immunol. 49:417-423. [DOI] [PubMed] [Google Scholar]
- 2.Audouy, S. A., et al. 2007. Development of lactococcal GEM-based pneumococcal vaccines. Vaccine 25:2497-2506. [DOI] [PubMed] [Google Scholar]
- 3.Beghetto, E., et al. 2006. Discovery of novel Streptococcus pneumoniae antigens by screening a whole-genome lambda λ-display library. FEMS. Microbiol. Lett. 262:14-21. [DOI] [PubMed] [Google Scholar]
- 4.Blue, C. E., et al. 2003. ZmpB, a novel virulence factor of Streptococcus pneumoniae that induces tumor necrosis factor alpha production in the respiratory tract. Infect. Immun. 71:4925-4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogaert, D., et al. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871-1872. [DOI] [PubMed] [Google Scholar]
- 6.Bogaert, D., R. De Groot, and P. W. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. [DOI] [PubMed] [Google Scholar]
- 7.Briles, D. E., et al. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339-348. [DOI] [PubMed] [Google Scholar]
- 8.Brueggemann, A. B., R. Pai, D. W. Crook, and B. Beall. 2007. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS. Pathog. 3:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butt, T. R., S. C. Edavettal, J. P. Hall, and M. R. Mattern. 2005. SUMO fusion technology for difficult-to-express proteins. Protein Expr. Purif. 43:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilli, R., et al. 2006. Zinc metalloproteinase genes in clinical isolates of Streptococcus pneumoniae: association of the full array with a clonal cluster comprising serotypes 8 and 11A. Microbiology 152:313-321. [DOI] [PubMed] [Google Scholar]
- 11.Cao, J., et al. 2007. Enhanced protection against pneumococcal infection elicited by immunization with the combination of PspA, PspC, and ClpP. Vaccine 25:4996-5005. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Y., A. Hayashida, A. E. Bennett, S. K. Hollingshead, and P. W. Park. 2007. Streptococcus pneumoniae sheds syndecan-1 ectodomains through ZmpC, a metalloproteinase virulence factor. J. Biol. Chem. 282:159-167. [DOI] [PubMed] [Google Scholar]
- 13.Chiavolini, D., et al. 2003. The three extra-cellular zinc metalloproteinases of Streptococcus pneumoniae have a different impact on virulence in mice. BMC. Microbiol. 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels, C. C., et al. 2010. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect. Immun. 78:2163-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianfaldoni, C., et al. 2007. Streptococcus pneumoniae pilus subunits protect mice against lethal challenge. Infect. Immun. 75:1059-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gianfaldoni, C., et al. 2009. Sortase A confers protection against Streptococcus pneumoniae in mice. Infect. Immun. 77:2957-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giefing, C., et al. 2008. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J. Exp. Med. 205:117-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gor, D. O., X. Ding, D. E. Briles, M. R. Jacobs, and N. S. Greenspan. 2005. Relationship between surface accessibility for PpmA, PsaA, and PspA and antibody-mediated immunity to systemic infection by Streptococcus pneumoniae. Infect. Immun. 73:1304-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene, C. M., et al. 2006. Preventability of invasive pneumococcal disease and assessment of current polysaccharide vaccine recommendations for adults: United States, 2001-2003. Clin. Infect. Dis. 43:141-150. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh, Y. C., et al. 2008. Establishment of a young mouse model and identification of an allelic variation of zmpB in complicated pneumonia caused by Streptococcus pneumoniae. Crit. Care. Med. 36:1248-1255. [DOI] [PubMed] [Google Scholar]
- 21.Jahn-Schmid, B., et al. 1996. Toward selective elicitation of TH1-controlled vaccination responses: vaccine applications of bacterial surface layer proteins. J. Biotechnol. 44:225-231. [DOI] [PubMed] [Google Scholar]
- 22.Kadioglu, A., J. N. Weiser, J. C. Paton, and P. W. Andrew. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288-301. [DOI] [PubMed] [Google Scholar]
- 23.Khan, A. Q., Q. Chen, Z. Q. Wu, J. C. Paton, and C. M. Snapper. 2005. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect. Immun. 73:298-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan, M. N., et al. 2006. Immunogenicity and protective efficacy of DnaJ (hsp40) of Streptococcus pneumoniae against lethal infection in mice. Vaccine 24:6225-6231. [DOI] [PubMed] [Google Scholar]
- 25.Levine, O. S., et al. 2006. Pneumococcal vaccination in developing countries. Lancet 367:1880-1882. [DOI] [PubMed] [Google Scholar]
- 26.Lu, Y. J., et al. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS. Pathog. 4:e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu, Y. J., S. Forte, C. M. Thompson, P. W. Anderson, and R. Malley. 2009. Protection against pneumococcal colonization and fatal pneumonia by a trivalent conjugate of a fusion protein with the cell wall polysaccharide. Infect. Immun. 77:2076-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizrachi Nebenzahl, Y., et al. 2007. Streptococcus pneumoniae surface-exposed glutamyl tRNA synthetase, a putative adhesin, is able to induce a partially protective immune response in mice. J. Infect. Dis. 196:945-953. [DOI] [PubMed] [Google Scholar]
- 29.Novak, R., et al. 2000. Extracellular targeting of choline-binding proteins in Streptococcus pneumoniae by a zinc metalloprotease. Mol. Microbiol. 36:366-376. [DOI] [PubMed] [Google Scholar]
- 30.Oggioni, M. R., et al. 2003. Pneumococcal zinc metalloproteinase ZmpC cleaves human matrix metalloproteinase 9 and is a virulence factor in experimental pneumonia. Mol. Microbiol. 49:795-805. [DOI] [PubMed] [Google Scholar]
- 31.Ogunniyi, A. D., M. Grabowicz, D. E. Briles, J. Cook, and J. C. Paton. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinert, R. R. 2009. The antimicrobial resistance profile of Streptococcus pneumoniae. Clin. Microbiol. Infect. 15(Suppl. 3):7-11. [DOI] [PubMed] [Google Scholar]
- 33.Reinert, R. R., P. Paradiso, and B. Fritzell. 2010. Advances in pneumococcal vaccines: the 13-valent pneumococcal conjugate vaccine received market authorization in Europe. Expert Rev. Vaccines 9:229-236. [DOI] [PubMed] [Google Scholar]
- 34.Romanello, V., et al. 2006. Cloning, expression, purification, and characterization of Streptococcus pneumoniae IgA1 protease. Protein Expr. Purif. 45:142-149. [DOI] [PubMed] [Google Scholar]
- 35.Saraiva, M., and A. O'Garra. 2010. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10:170-181. [DOI] [PubMed] [Google Scholar]
- 36.Scott, J. A. 2007. The preventable burden of pneumococcal disease in the developing world. Vaccine 25:2398-2405. [DOI] [PubMed] [Google Scholar]
- 37.Senior, B. W., and J. M. Woof. 2006. Sites in the CH3 domain of human IgA1 that influence sensitivity to bacterial IgA1 proteases. J. Immunol. 177:3913-3919. [DOI] [PubMed] [Google Scholar]
- 38.Sette, A., et al. 2009. Definition of epitopes and antigens recognized by vaccinia specific immune responses: their conservation in variola virus sequences, and use as a model system to study complex pathogens. Vaccine 27(Suppl. 6):G21-G26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spratt, B. G., and B. M. Greenwood. 2000. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet 356:1210-1211. [DOI] [PubMed] [Google Scholar]
- 40.Swiatlo, E., and D. Ware. 2003. Novel vaccine strategies with protein antigens of Streptococcus pneumoniae. FEMS. Immunol. Med. Microbiol. 38:1-7. [DOI] [PubMed] [Google Scholar]
- 41.van der Poll, T., and S. M. Opal. 2009. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 374:1543-1556. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein, M. P., K. P. Klugman, and R. N. Jones. 2009. Rationale for revised penicillin susceptibility breakpoints versus Streptococcus pneumoniae: coping with antimicrobial susceptibility in an era of resistance. Clin. Infect. Dis. 48:1596-1600. [DOI] [PubMed] [Google Scholar]
- 43.Wu, H. Y., et al. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb. Pathog. 23:127-137. [DOI] [PubMed] [Google Scholar]
- 44.Wu, H. Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]
- 45.Wu, K., et al. 2010. Immunization with a combination of three pneumococcal proteins confers additive and broad protection against Streptococcus pneumoniae infections in mice. Infect. Immun. 78:1276-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xin W., Y. Li, H. Mo, K. L. Roland, and R. Curtiss III. 2009. PspA family fusion proteins delivered by attenuated Salmonella enterica serovar Typhimurium extend and enhance protection against Streptococcus pneumoniae. Infect. Immun. 77:4518-4528. [DOI] [PMC free article] [PubMed] [Google Scholar]