Abstract
Activated T and B lymphocytes in periodontal disease lesions express receptor activator of NF-κB ligand (RANKL), which induces osteoclastic bone resorption. The objective of this study was to evaluate the effects of anti-RANKL antibody on periodontal bone resorption in vitro and in vivo. Aggregatibacter actinomycetemcomitans outer membrane protein 29 (Omp29) and A. actinomycetemcomitans lipopolysaccharide (LPS) were injected into 3 palatal gingival sites, and Omp29-specific T clone cells were transferred into the tail veins of rats. Rabbit anti-RANKL IgG antibody or F(ab′)2 antibody fragments thereof were injected into the palatal sites in each rat (days −1, 1, and 3). Anti-RANKL IgG antibody significantly inhibited soluble RANKL (sRANKL)-induced osteoclastogenesis in vitro, in a dose-dependent manner, but also gave rise to a rat antibody response to rabbit IgG in vivo, with no significant inhibition of periodontal bone resorption detected. Lower doses (1.5 and 0.15 μg/3 sites) of F(ab′)2 antibody were not immunogenic in the context of the experimental model. Periodontal bone resorption was inhibited significantly by injection of the anti-RANKL F(ab′)2 antibody into gingivae. The sRANKL concentrations for the antibody-treated groups were decreased significantly compared to those for the untreated group. Osteoclasts on the alveolar bone surface were also diminished significantly after antibody injection. Gingival sRANKL concentration and bone loss showed a significant correlation with one another in animals receiving anti-RANKL F(ab′)2 antibody. These results suggest that antibody to RANKL can inhibit A. actinomycetemcomitans-specific T cell-induced periodontal bone resorption by blockade and reduction of tissue sRANKL, providing an immunological approach to ameliorate immune cell-mediated periodontal bone resorption.
Host immune responses play a key role in periodontal diseases (11, 29). Compelling evidence now indicates that periodontitis is not a conventional infectious disease but is an inflammatory disease triggered by the host immune response to the microorganism(s) associated with periodontal biofilm (22, 26, 28, 30, 34). Recently, a close relationship between the immune and skeletal systems within the interdisciplinary field called “osteoimmunology” has attracted much attention due to accumulating evidence that bone destruction can be caused by an inflammatory activation of the immune system in rheumatoid arthritis and in periodontitis (31). Many studies have demonstrated that host immune responses play a key role in stimulating osteoclastogenesis and in promoting bone resorption in periodontal disease (9, 27). T and B lymphocytes found in periodontal lesions express abundant receptor activator of NF-κB ligand (RANKL). Stimulation of RANKL expression by these lymphocytes is inhibited (in vitro and in vivo) by a decoy receptor for RANKL, osteoprotegerin (OPG), which suggests that bone resorption is RANKL dependent. These findings provide a fundamentally new understanding of the mechanism of periodontal disease and support the hypothesis that tissue destruction in periodontal disease can be ameliorated by inhibition of RANKL activity in immune effector cells. If this hypothesis is correct, then therapeutic strategies based on immune modulation may be effective in preventing bone resorption and tooth loss in people with periodontitis. The hypothesis has been tested in osteoporotic individuals with immunological blockade of RANKL-RANK interaction by use of antibody to RANKL. Such an antibody (denosumab; formerly known as AMG 162) has been shown to effectively increase bone mineral density in postmenopausal women and to decrease bone resorption (20, 21). Preliminary data from clinical trials suggest that denosumab might be an effective treatment for osteoporosis. However, much remains to be determined before such a strategy (capable of modulating the RANKL-RANK interaction) could be administered safely for the management of bone-resorptive diseases other than osteoporosis.
In this study, anti-RANKL antibody treatments were assessed in an in vitro osteoclastogenesis model and in a validated rat model of periodontal disease (12, 31). We evaluated a method of reducing periodontal bone resorption by direct inhibition of RANKL with anti-RANKL neutralizing antibody. This study provides an understanding of the mechanisms by which the host immune response mediates bone resorption and may lead to future clinical translational studies of strategies for preventing tooth loss in periodontitis.
MATERIALS AND METHODS
Animals.
Rowett strain (inbred at the Forsyth Institute for 20 years) 2-month-old female rats (rnu/+; heterozygous normal) with restricted oral flora were bred in plastic isolators and maintained under pathogen-free conditions in laminar flow cabinets. All T-cell clones used in this study were derived from these Rowett strain rats.
Bacteria, bacterial antigens, and LPS.
Aggregatibacter actinomycetemcomitans ATCC 43718 (strain Y4; American Type Culture Collection, Manassas, VA) was grown in pleuropleumonia-like organism broth (Difco Laboratories, Detroit, MI) with glucose (3 g/liter) and sodium bicarbonate (1g/liter) for 72 h at 37°C under increased CO2. Cultured bacteria in the logarithmic growth phase were killed with formalin (5%) and served as a T-cell antigen(s). The A. actinomycetemcomitans 29-kDa outer membrane protein (Omp29) (18, 32) and A. actinomycetemcomitans lipopolysaccharide (LPS) (24) were prepared as previously described.
Preparation of anti-RANKL IgG and F(ab′)2 fragments.
Anti-RANKL antibody was produced in New Zealand White rabbits (Robert Sargeant, Ramona, CA) by immunization with recombinant rat RANKL (19.4 kDa, 174 amino acids; Peprotech, Rocky Hill, NJ). Anti-RANKL IgG antibody and F(ab′)2 fragments were prepared by pepsin digestion and purified with NAb spin kits (Pierce, Rockford, IL), an F(ab′)2 preparation kit (Pierce), and an iCON concentrator (Pierce). Nonantibody rabbit IgG was prepared identically.
Evaluation of osteoclastogenesis in vitro.
The mouse macrophage/monocyte cell line RAW 264.7 (ATCC no. TIB-71) was seeded in 96-well plates at a density of 103 cells/well in 150 μl of alpha minimal essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 100 ng/ml murine soluble RANKL (sRANKL) (R&D Systems, Minneapolis, MN). After 2 days, the medium was replaced with fresh medium without sRANKL, and the RAW 264.7 cells were cocultured with purified A. actinomycetemcomitans Omp29-specific T cells (1.5 × 105 cells/well) or with the culture supernatant (100 μl/well) of purified T cells for another 3 days. Cultures were performed with different doses of rabbit anti-rat RANKL IgG (10 μg/ml, 5 μg/ml, and 1 μg/ml). Cells were stained for tartrate-resistant acid phosphatase (TRAP) by use of a leukocyte acid phosphatase kit (Sigma, St. Louis, MO) according to the manufacturer's instructions. Osteoclasts were identified as multinucleated dark red cells. TRAP-positive cells with three or more nuclei were considered osteoclasts and were counted microscopically.
Determination of rat anti-rabbit IgG antibody or F(ab′)2 antibody fragment.
To verify the immunogenicity of rabbit IgG antibody or F(ab′)2 antibody fragment in recipient animals, the rat serum IgG antibody levels to the rabbit IgG F(ab′)2 fragment were determined by enzyme-linked immunosorbent assay (ELISA). The sera were collected on days 0, 7, and 10 after gingival injection of rabbit IgG antibody or F(ab′)2 fragment on days −1, 1, and 3. Rabbit normal IgG (1:400) was bound to 96-well ELISA plates as previously described (6). Rat serum (1/100 dilution) was applied to the plates and incubated for 2 h. After washing of the plates, alkaline phosphatase (AP)-conjugated rabbit anti-rat IgG (Sigma) was added. After incubation in tetramethylbenzidine (TMB) liquid substrate (Sigma) for 20 min, the reaction was terminated by the addition of 1% sodium dodecyl sulfate (SDS) and the absorbance at 405 nm was determined by use of a spectrophotometer.
Overall experimental protocol for adoptive transfer.
Rowett strain rats were randomly divided into five groups (n = 12 rats/group) that received microinjection of 0.5 μg/site of A. actinomycetemcomitans Omp29 plus 0.5 μg/site of A. actinomycetemcomitans LPS in the right palate at 3 sites, whereas phosphate-buffered saline (PBS) was injected into the symmetric sites of the left palate as a control. Each group of rats (n = 12 rats/group) also received tail vein injection of the T-cell clone (5 × 106/rat) as previously described (12). Th1-type clone cells specific for Omp29 were activated by incubation with sterile formalin-killed A. actinomycetemcomitans and mitomycin-treated rat spleen cells in RPMI complete medium containing 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 mg/ml streptomycin, 2 mM l-glutamine, and 10 mM HEPES buffer. The injection of different doses (10, 1.5, 0.15, and 0.015 μg/3 sites) of anti-RANKL IgG or F(ab′)2 fragment was performed on both sides on days −1, 1, and 3. To determine the formation of osteoclasts in vivo, decalcified maxillary tissues from 3 rats from each group were prepared by cryostat sectioning (20 sections per animal). The sections were subjected to TRAP staining, and osteoclasts were identified as multinucleated TRAP-positive cells on the alveolar bone surface (7 to 9 sections per animal). Also, gingival tissues were removed from each rat in each group (n = 6 rats/group) for the preparation of gingival homogenate. In addition, bone resorption was measured in the defleshed jaws of the residual animals from the original groups of 12 rats.
Flow cytometry analysis.
Cells were cultured for 3 days before collection for analysis. T-cell membrane-bound RANKL expression was detected by double staining for a T-cell marker (phycoerythrin [PE]-conjugated anti-CD3) and OPG-Fc fusion protein as described previously (31), followed by incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG secondary antibody (Sigma). The flow cytometry results are expressed as mean fluorescence intensities of treated cells versus untreated cells. At least 30,000 cells were counted for each sample by flow cytometry.
Detection of gingival homogenate sRANKL by ELISA.
For gingival homogenate collection, gingivae were removed by dissection and were homogenized with a Dounce glass homogenizer in PBS with 0.05% Tween 20, phenylmethane sulfonyl fluoride (Sigma), and proteinase inhibitor cocktail (Sigma). The sRANKL level in gingival homogenate was detected using a murine sRANKL ELISA development kit (Peprotech).
Measurement of bone resorption.
Ten days after T clone transfer and gingival challenge with Omp29 and LPS, animals were sacrificed, the jaws were defleshed, and periodontal bone resorption was measured as previously described (12). Briefly, the distance from the cemento-enamel junction (CEJ) to the alveolar crest (AC) on the palatal side of each root was measured by microscopy with a reticule eyepiece at a magnification of ×25. Recordings were made for the long axis of the root surface for all molar teeth. A total of seven recordings were evaluated for each quadrant, including three roots of the first molar and both roots of the second and the third molars. Measurements were made without prior knowledge of the group designation of the animals, and the recordings were verified by a second examiner. The bone loss was calculated and expressed as the percentage of bone loss, determined by the following equation: [(total CEJ-to-AC distance of right maxilla − total CEJ-to-AC distance of left maxilla)/(total CEJ-to-AC distance of left maxilla)] × 100.
Evaluation of osteoclastogenesis in vivo.
To further investigate the reduction of periodontal bone resorption in this model, the formation and localization of osteoclasts were evaluated by TRAP staining. Rats were killed 10 days after transfer of A. actinomycetemcomitans-specific T cells and gingival injection with antigen and F(ab′)2 fragment antibody. The whole maxilla was dissected and fixed in 5% formaldehyde-saline solution overnight at 4°C. Tissues were decalcified in 10% EDTA-0.1 M Tris (pH 6.9) solution for 3 weeks at 4°C. Decalcified samples were cut in half through the midline of the palate, embedded in OCT compound (Miles), and frozen at −80°C for 1 h. Serial sections (6 μm) were cut by cryostat sectioning. For TRAP staining, the slides were incubated for 10 min in TRAP staining solution at 37°C in the dark. The slides were then counterstained with hematoxylin. Multinucleated dark red cells with three or more nuclei lying along the alveolar bone surface were considered osteoclasts. For quantitative image analysis, the number of TRAP-positive cells per millimeter of the alveolar bone surface was counted using Image-Pro Plus (Media Cybernetics, Inc.).
Statistical analysis.
All assays were conducted at least in triplicate, and each study was repeated 3 times. Quantitative data are expressed as means ± standard deviations (SD). Data for the in vitro and in vivo multiple group experiments, including multinucleated TRAP+ cell formation and sRANKL quantitation by ELISA, were analyzed by the Student-Newman-Keuls (SNK) multiple comparison test following one-way analysis of variance (ANOVA). P values of <0.05 were considered statistically significant.
RESULTS
Influence of rabbit anti-RANKL IgG antibody in vitro and in vivo.
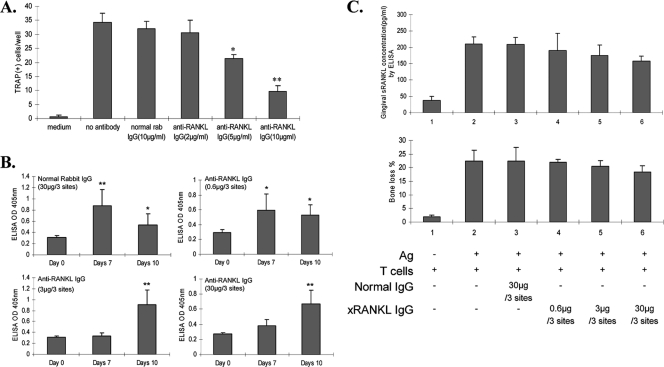
In preliminary experiments, we prepared and tested a rabbit antibody to recombinant sRANKL. The IgG fraction was tested for the ability to inhibit RANKL-mediated osteoclastogenesis in vitro. This antibody significantly inhibited TRAP-positive multinucleated cell formation in vitro, in a dose-dependent fashion (Fig. 1A). This antibody was also tested in the specific T-cell clone adoptive transfer periodontal disease model in vivo. The antibody (4 doses) did not affect levels of sRANKL in the gingivae or periodontal bone loss (Fig. 1B). All intragingivally injected rats demonstrated immune responses to the rabbit IgG antibody, which might have inhibited the effect of antibody to RANKL on periodontal bone resorption (Fig. 1C).
FIG. 1.
(A) Anti-RANKL IgG antibody inhibits osteoclastogenesis by RAW 264.7 cells in vitro. RAW 264.7 cells (2 × 103/well) were cultured with 100 ng/ml recombinant murine soluble RANKL (75 ng/ml) for 2 days. Different concentrations of anti-RANKL IgG antibody were then added to the culture for 3 days. TRAP-positive cells with three or more nuclei were considered osteoclasts. *, P < 0.05; **, P < 0.01 (for cells assayed in triplicate). This experiment was repeated 3 times with similar results. (B) Rabbit anti-rat RANKL IgG does not significantly affect periodontal bone resorption (% increase relative to control side) or gingival sRANKL concentration in the rat T-cell clone transfer model. sRANKL levels in gingival homogenates were detected by ELISA as described in Materials and Methods. Periodontal bone loss was measured as the distance from the CEJ to the AC on each palatal side of the root and is expressed as a percentage relative to the control level (n = 3 rats per treated group). Data are presented as means ± SD for ELISA-determined sRANKL concentrations assayed in triplicate. (C) Immune response of rats to rabbit IgG antibody after injection with various doses (0.6, 3, and 30 μg/3 sites) of anti-RANKL IgG (xRANKL IgG). Rat anti-rabbit IgG antibody levels in sera were measured by ELISA on days 0, 7, and 10 after injection of various doses of rabbit anti-RANKL IgG into the rat palatal gingival papillae. Data are presented as means ± SD for 3 sera, each assayed in triplicate. *, P < 0.05; **, P < 0.01.
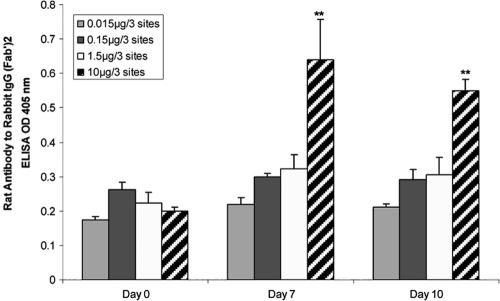
Influence of injected anti-RANKL F(ab′)2 antibody on rat humoral response.
We prepared the F(ab′)2 fragment of rabbit anti-RANKL antibody to determine the status of injected anti-RANKL F(ab′)2 in vivo. Rat serum IgG antibody to rabbit IgG was measured on days 0, 7, and 10 after adoptive transfer and gingival challenge. Only the group injected with 10 μg/rat anti-RANKL showed a significantly higher level of rat serum IgG antibody to the rabbit F(ab′)2 fragment (P < 0.05). Lower anti-RANKL doses (0.15 and 1.5 μg/3 sites) did not result in IgG antibody to rabbit IgG during the indicated period (Fig. 2).
FIG. 2.
Lack of immunogenicity of low doses of rabbit F(ab′)2 antibody to RANKL in rats. Rat anti-rabbit IgG antibody levels in sera were measured by ELISA after injection of T clone cells and various doses (0.015 μg to 10 μg/3 sites) of rabbit anti-RANKL F(ab′)2 antibody into rat gingival papillae. Data are presented as means ± SD for 3 sera, each assayed in triplicate. **, P < 0.01.
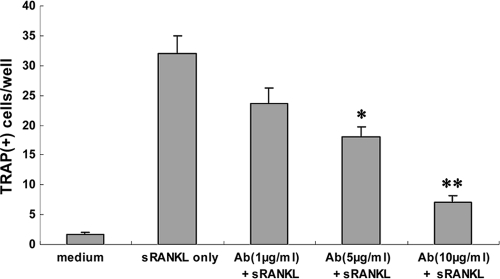
Anti-RANKL F(ab′)2 fragment inhibits osteoclastogenesis by RAW 264.7 cells.
We found that F(ab′)2 antibody to RANKL inhibited osteoclastogenesis by RAW 264.7 cells in vitro, in a dose-dependent manner (Fig. 3). Therefore, we sought to determine if there were effects of this F(ab′)2 antibody on immune-mediated periodontal bone loss in vivo.
FIG. 3.
Anti-RANKL F(ab′)2 antibody fragment inhibits osteoclastogenesis by RAW 264.7 cells in vitro. RAW 264.7 cells were first cultured with 100 ng/ml murine sRANKL for 2 days and then cocultured with different doses of anti-RANKL F(ab′)2 antibody (1 μg/ml, 5 μg/ml, or 10 μg/ml) for another 3 days. For TRAP staining, cells were stained using a leukocyte acid phosphatase kit and counterstained with hematoxylin. Multinucleated TRAP-positive cells were counted. Data are expressed as means ± SD for 4 repeated experiments. *, P < 0.05; **, P < 0.01.
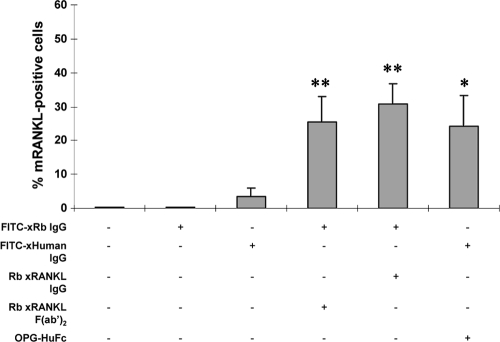
mRANKL expression detection by flow cytometry.
We tested the antibody for reactivity with membrane RANKL (mRANKL) on the surfaces of activated T clone cells by flow cytometry (Fig. 4). F(ab′)2, intact anti-RANKL IgG, and OPG-human Fc bound to the mRANKL of 25 to 30% of the activated T cells, as indicated by reactivity with FITC-conjugated anti-rabbit IgG or FITC-conjugated anti-human IgG. Elimination of either of these reagents resulted in fewer than 5% of the cells being stained. Therefore, these data indicate that the antibody [either intact IgG or F(ab′)2] reacted with both sRANKL and mRANKL (Fig. 4).
FIG. 4.
Anti-RANKL F(ab′)2 antibody binds to RANKL on Omp29-specific T clone cells. After collection of cultured A. actinomycetemcomitans-specific T clone cells, surface RANKL-expressing cells were evaluated by flow cytometry, using rabbit anti-rat RANKL IgG, rabbit anti-rat RANKL F(ab′)2, and OPG-human (Hu) Fc, followed by FITC-anti-rabbit IgG antibody or FITC-anti-human IgG. At least 30,000 cells were counted, and the % RANKL-positive T cells is expressed as the mean ± SD for 3 repeated experiments. *, P < 0.05; **, P < 0.01.
Anti-RANKL F(ab′)2 antibody fragment inhibits osteoclastogenesis by RAW 264.7 cells cocultured with A. actinomycetemcomitans-specific T cells or T-cell supernatant.
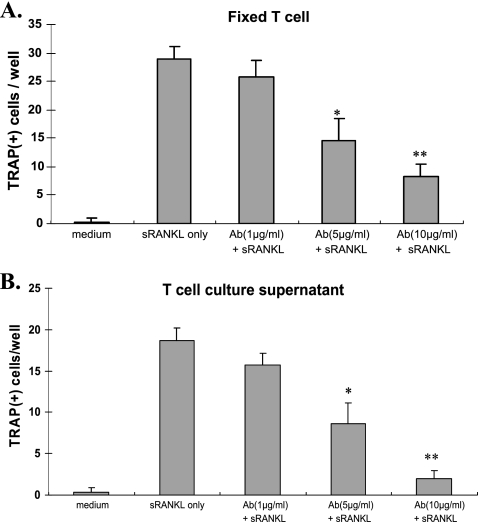
RAW 264.7 cells were cocultured with A. actinomycetemcomitans-specific T cells or T-cell culture supernatant in the presence of various doses of the rabbit anti-RANKL IgG antibody or F(ab′)2 fragments. Anti-RANKL F(ab′)2 fragment significantly reduced TRAP+ cell formation by RAW264.7 cells cocultured with fixed T cells (Fig. 5A) or T-cell culture supernatant (Fig. 5B). TRAP+ cell formation by RAW264.7 cells could be inhibited significantly by 5 and 10 μg/ml F(ab′)2 antibody but not by a concentration of 1 μg/ml (Fig. 5A and B). These findings suggest that the F(ab′)2 antibody can inhibit osteoclastogenesis by RAW264.7 cells induced by antigen-specific T cells.
FIG. 5.
Rabbit F(ab′)2 anti-RANKL antibody significantly inhibits osteoclastogenesis by RAW 264.7 cells. RAW 264.7 cells were cocultured with formalin-fixed A. actinomycetemcomitans-specific T cells (A) or T-cell culture supernatants (B), each with 2, 5, or 10 μg/ml of anti-RANKL antibody. Multinucleated TRAP-positive cells were counted. Data are expressed as means ± SD for 4 repeated experiments. *, P < 0.05; **, P < 0.01 (compared to T cells only or to T-cell supernatant only).
Detection of sRANKL protein in gingival tissue homogenates.
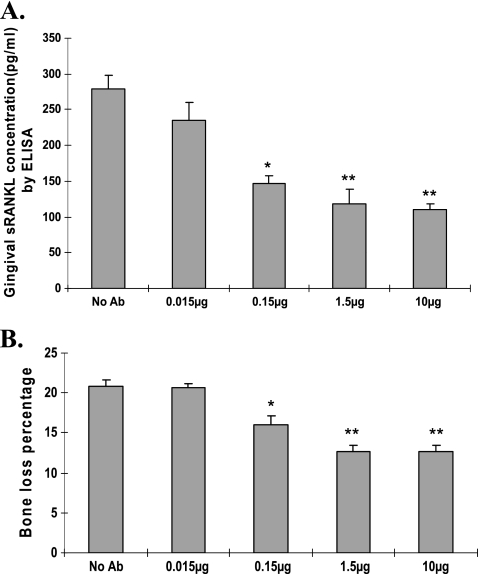
ELISA was performed to measure sRANKL protein in the gingival tissue homogenates of rats that received T-cell transfer and gingival antigen injection at day 10. The concentration of sRANKL protein in gingival tissue was decreased significantly (P < 0.05; t test) in anti-RANKL F(ab′)2 fragment-treated groups compared to the untreated group (Fig. 6A).
FIG. 6.
Effect of administration of rabbit anti-RANKL IgG F(ab′)2 antibody on gingival sRANKL concentration and bone resorption in vivo. (A) Ten days after T-cell transfer, gingival tissue was collected, homogenates were prepared, and the concentrations of sRANKL in the homogenates were measured by ELISA. Data are means ± standard errors (SE) for 6 rats per group. *, P < 0.05; **, P < 0.01. (B) Ten days after transfer, rat heads were processed to remove tissue and measure periodontal bone resorption. Data are means ± SE for 9 rats per group. *, P < 0.05; **, P < 0.01.
Alveolar bone resorption.
Ten days after T-cell transfer, rat heads were processed to measure periodontal bone resorption. Rats injected with anti-RANKL antibody (0.015, 0.15, 1.5, and 10 μg/3 sites) demonstrated significantly decreased alveolar bone resorption (15.4% ± 1.2%, 12.7% ± 2.5%, and 12.6% ± 2.4% for the 0.15-, 1.5-, and 10-μg groups, respectively) compared with the control group (without antibody injection; 20.8% ± 2.3%) (P < 0.05) (Fig. 6B). There was no significant difference in the percentage of bone loss in the 0.015-μg anti-RANKL injection group compared with the control group (Fig. 6B). The correlation between bone loss and gingival sRANKL concentration in rats administered F(ab′)2 antibody was highly significant (r2 = 0.995; P = 0.0046). These results indicate that bone loss in the T-cell transfer model can be inhibited directly by anti-RANKL antibody.
Inhibition of multinucleated TRAP-positive cells on alveolar bone crest in vivo.
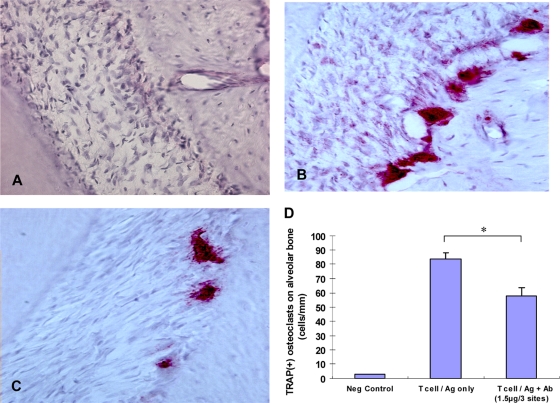
To determine the effect of administration of anti-RANKL antibody in vivo, we used an immune-mediated specific T-cell bone resorption system in vivo where T cells were adoptively transferred and Omp29 and LPS were injected into the gingivae. No TRAP+ cells were observed in the negative-control group receiving no T cells (Fig. 7A). Administration of the anti-RANKL antibody (Fig. 7C) resulted in a reduction of TRAP+ cells on the alveolar crest compared to the group receiving T-cell transfer only (Fig. 7B). The numbers of cells on the alveolar bone crest, presented as numbers of TRAP-positive cells/mm, were decreased more than 31% for animals injected with anti-RANKL antibody compared to the group receiving T-cell transfer only (Fig. 7D).
FIG. 7.
Ten days after adoptive T-cell transfer and gingival microinjection of antigen and anti-RANKL F(ab′)2 fragment, rats were killed and maxillary tissues were decalcified for sectioning. TRAP staining of periodontal tissue sections (8 sections/rat; n = 3 rats/group) was performed for the following groups. (A) Negative control (no T-cell transfer/gingival antigen injection). (B) T-cell transfer/gingival antigen injection only. (C) T-cell transfer/gingival antigen injection plus anti-RANKL F(ab′)2 fragment injection. Slides were counterstained with hematoxylin and analyzed with Image-Pro Plus (Media Cybernetics, Inc.). Multinucleated cells stained red-purple (magnification, ×400) are considered osteoclast-like cells. (D) The numbers of TRAP-positive multinucleated osteoclast-like cells on the alveolar bone crest were quantitated as numbers of cells per mm of bone surface. Data are means ± SD for 3 rats/group. *, P < 0.05.
DISCUSSION
Many studies have demonstrated that host immune responses are associated with the onset and progression of periodontal diseases. We have extensively studied the role of immune responses in rodent experimental periodontal disease (5, 12, 15, 33). In this study, we administered anti-RANKL antibody directly to the gingivae of rats receiving T-cell transfer and gingival antigen injections, resulting in a significant reduction of gingival sRANKL expression and of bone resorption.
The human RANKL gene encodes a 316-amino-acid type II transmembrane protein containing a 47-amino-acid cytoplasmic region, a 21-amino-acid transmembrane region, and a 248-amino-acid extracellular domain (2). The soluble form of RANKL is a 20-kDa polypeptide comprising the tumor necrosis factor (TNF)-homologous region of RANKL and is generated by intracellular proteolytic processing of the full-length RANKL protein (10). Studies have suggested that immunological blockade of RANKL-RANK interaction by use of antibody to RANKL can relieve symptoms of osteoporosis and sustain increased bone density (20). Our hypothesis is that antibody blockade of RANKL interferes with immune cell-mediated periodontal bone resorption by reduction of the sRANKL concentration level (Fig. 6A).
Our previous studies using immunohistochemical analyses and confocal microscopy demonstrated abundant RANKL expression on T and B cells in the gingival lesions of patients with chronic periodontitis. These cells taken from gingival tissues could induce osteoclastic development and associated bone pit formation (14). In other studies, several Th1 antigen-specific clones gave rise to significant bone resorption that could be ablated with CTLA4-Ig or OPG-Fc (5, 12-14), emphasizing the RANKL dependence of bone resorption. In this study, we focused on Th1 cells and anti-RANKL antibody and studied if anti-RANKL can decrease bone resorption, using a T-cell adoptive transfer model. Our data suggest that the effect of RANKL expression by T cells is inhibited directly by anti-RANKL antibody. Our results demonstrated that blockage of antigen-specific RANKL-expressing T cells with anti-RANKL antibody resulted in a markedly decreased osteoclast presence on the alveolar bone surface and reduced periodontal bone resorption.
We previously reported that antigen-specific T cells can be retained in antigen- and LPS-challenged gingivae (33). A mechanism for T-cell migration into inflammatory lesions can involve regulation by chemokines and adhesion molecules (4, 23, 25). In this study, we injected anti-RANKL antibody into gingivae on days 0, 1, and 3. The concentration of sRANKL protein in gingival tissue and the amount of bone resorption were decreased significantly after antibody injection (P < 0.01) compared to those of the control group. In addition, there was a significant positive correlation between the level of sRANKL in tissues and bone resorption. These data suggest that local anti-RANKL antibody injection may directly inhibit RANKL expression on the T-cell surface.
We initially prepared intact anti-RANKL IgG antibody from rabbits immunized with RANKL peptides and tested its effect on inhibition of RANKL-dependent osteoclastogenesis both in vitro and in vivo. The results showed that rabbit anti-RANKL IgG dramatically inhibited osteoclastogenesis in vitro (Fig. 1A) but did not inhibit periodontal bone loss in vivo, probably due to a rat immune response to this rabbit anti-RANKL IgG antibody and to production of anti-rabbit IgG antibody (Fig. 1B and C). In order to circumvent such a consequence, we then generated anti-RANKL F(ab′)2 antibody to demonstrate that low doses of such an antibody (from 0.015 μg/3 sites to 1.5 μg/3 sites) do not sensitize rats and that administration of this antibody significantly inhibits RANKL-dependent osteoclastogenesis both in vitro and in vivo (Fig. 5, 6, and 7). The data indicate that a moderate dose of anti-RANKL F(ab′)2 antibody (1.5 μg/3 sites) would be the optimal intervention regimen to interfere with RANKL-dependent osteoclastogenesis without sensitizing the host. This suggests that removal of the Fc fragment and optimizing the antibody dosage may be among the necessary steps toward clinical usage consideration.
It has been demonstrated that LPS alone can trigger innate as well as adaptive immune responses and induce periodontal bone loss (17). However, in the T-cell transfer model, LPS alone did not generate bone loss, based on the results from our previous studies (12). This was probably due to the fact that our system applies only a single injection of 0.5 μg/site LPS into the gingival tissue, whereas other systems use multiple injections of a much larger amount of LPS (17). An “LPS treatment” control group was not included because LPS had no bone-resorptive effect and therefore such an effect would not be ameliorated by anti-RANKL treatment.
Using the rat adoptive transfer/challenge model of periodontitis, we have demonstrated that bone resorption can be mediated by osteoclasts and is RANKL dependent. Our previous studies (9, 12-15, 27, 31) using rats showed that many antigen-specific T lymphocytes from the gingivae express RANKL, suggesting a crucial role of T cell-derived RANKL in inducing bone resorption. Other studies with similar rodent models have obtained comparable results (5, 33). The purpose of this study was to focus on the effects of anti-RANKL antibody on T cell-mediated RANKL-dependent periodontal bone loss. Based on our previous results, it is conceivable that such an effect may be observed in a B-cell model. Although the current work focuses on T cells, it is warranted that the effect of anti-RANKL antibody on antigen-specific B cell-mediated bone loss should be studied separately with other established model systems (8).
New treatments for periodontal disease must address the major immune cell contribution to periodontal bone resorption. Current treatment for periodontal disease often relies on mechanical procedures and neglects host biological responses, including the immune cells. Therefore, the overall emphasis of this study was to develop and evaluate therapeutic strategies to treat immune cell-mediated periodontal bone resorption. The focus of these treatments was on RANKL. We demonstrated that OPG-Fc is a potent inhibitor of T cell- or B cell-mediated periodontal bone resorption (9). Physiological blockade of RANKL-RANK interaction and subsequent osteoclastogenesis by use of OPG-Fc is very powerful. In a rat model of periodontal disease using adoptive T-cell transfer, systemic administration of osteoprotegerin-Fc significantly reduced periodontal bone loss, by about 90%, compared to that of the control group injected with the control protein L6-Fc (a fusion protein of the irrelevant peptide L6 and the human Fc fragment) (26). However, recent studies with this fusion protein in patients with multiple myeloma were discontinued due to development of antibodies to OPG by the patients (3, 7, 16, 19). In addition, multivariate analyses demonstrated that osteoprotegerin retained a strong association with elevated coronary artery calcification scores after adjustment for age, gender, and other risk factors (1). More recent studies have extended this general strategy to include immunological blockade of RANKL-RANK interaction by use of antibody to RANKL (20, 21). This study suggests that antibody blockage of RANKL interferes with immune cell-mediated periodontal bone resorption by reduction of the sRANKL level.
Acknowledgments
This work was supported by NIH grants DE-03420, DE-07327, DE-018499, and DE-19917 from the National Institute of Dental and Craniofacial Research.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 15 November 2010.
REFERENCES
- 1.Anand, D. V., A. Lahiri, E. Lim, D. Hopkins, and R. Corder. 2006. The relationship between plasma osteoprotegerin levels and coronary artery calcification in uncomplicated type 2 diabetic subjects. J. Am. Coll. Cardiol. 47:1850-1857. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. M., et al. 1997. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175-179. [DOI] [PubMed] [Google Scholar]
- 3.Bone, H. G., et al. 2008. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J. Clin. Endocrinol. Metab. 93:2149-2157. [DOI] [PubMed] [Google Scholar]
- 4.Butcher, E. C., and L. J. Picker. 1996. Lymphocyte homing and homeostasis. Science 272:60-66. [DOI] [PubMed] [Google Scholar]
- 5.Crotti, T., et al. 2003. Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J. Periodontal Res. 38:380-387. [DOI] [PubMed] [Google Scholar]
- 6.Ebersole, J. L., et al. 1984. Serological identification of oral Bacteroides spp. by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 19:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamdy, N. A. 2008. Denosumab: RANKL inhibition in the management of bone loss. Drugs Today (Barcelona) 44:7-21. [DOI] [PubMed] [Google Scholar]
- 8.Han, X., T. Kawai, J. W. Eastcott, and M. A. Taubman. 2006. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J. Immunol. 176:625-631. [DOI] [PubMed] [Google Scholar]
- 9.Han, X., T. Kawai, and M. A. Taubman. 2007. Interference with immune-cell-mediated bone resorption in periodontal disease. Periodontol. 2000 45:76-94. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda, F., et al. 2004. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Invest. 114:475-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz, J., K. P. Black, and S. M. Michalek. 1999. Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infect. Immun. 67:4352-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai, T., et al. 2000. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J. Immunol. 164:2102-2109. [DOI] [PubMed] [Google Scholar]
- 13.Kawai, T., et al. 2006. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 169:987-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai, T., et al. 2007. Cross-reactive adaptive immune response to oral commensal bacteria results in an induction of receptor activator of nuclear factor-kappaB ligand (RANKL)-dependent periodontal bone resorption in a mouse model. Oral Microbiol. Immunol. 22:208-215. [DOI] [PubMed] [Google Scholar]
- 15.Kawai, T., H. Shimauchi, J. W. Eastcott, D. J. Smith, and M. A. Taubman. 1998. Antigen direction of specific T-cell clones into gingival tissues. Immunology 93:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khosla, S. 2005. Magic bullets to kill nasty osteoclasts. Endocrinology 146:3233-3234. [DOI] [PubMed] [Google Scholar]
- 17.Kirkwood, K. L., et al. 2007. A p38alpha selective mitogen-activated protein kinase inhibitor prevents periodontal bone loss. J. Pharmacol. Exp. Ther. 320:56-63. [DOI] [PubMed] [Google Scholar]
- 18.Komatsuzawa, H., et al. 1999. Cloning of the gene encoding the Actinobacillus actinomycetemcomitans serotype b OmpA-like outer membrane protein. Infect. Immun. 67:942-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong, Y. Y., et al. 1999. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315-323. [DOI] [PubMed] [Google Scholar]
- 20.Lewiecki, E. M., et al. 2007. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J. Bone Miner. Res. 22:1832-1841. [DOI] [PubMed] [Google Scholar]
- 21.McClung, M. R., et al. 2006. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 354:821-831. [DOI] [PubMed] [Google Scholar]
- 22.Michalek, S. M., J. Katz, N. K. Childers, M. Martin, and D. F. Balkovetz. 2002. Microbial/host interactions: mechanisms involved in host responses to microbial antigens. Immunol. Res. 26:223-234. [DOI] [PubMed] [Google Scholar]
- 23.Mueller, S. N., et al. 2007. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science 317:670-674. [DOI] [PubMed] [Google Scholar]
- 24.Perry, M. B., L. L. MacLean, R. Gmur, and M. E. Wilson. 1996. Characterization of the O-polysaccharide structure of lipopolysaccharide from Actinobacillus actinomycetemcomitans serotype b. Infect. Immun. 64:1215-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stachowiak, A. N., Y. Wang, Y. C. Huang, and D. J. Irvine. 2006. Homeostatic lymphoid chemokines synergize with adhesion ligands to trigger T and B lymphocyte chemokinesis. J. Immunol. 177:2340-2348. [DOI] [PubMed] [Google Scholar]
- 26.Taubman, M. A., and T. Kawai. 2001. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit. Rev. Oral Biol. Med. 12:125-135. [DOI] [PubMed] [Google Scholar]
- 27.Taubman, M. A., T. Kawai, and X. Han. 2007. The new concept of periodontal disease pathogenesis requires new and novel therapeutic strategies. J. Clin. Periodontol. 34:367-369. [DOI] [PubMed] [Google Scholar]
- 28.Taubman, M. A., P. Valverde, X. Han, and T. Kawai. 2005. Immune response: the key to bone resorption in periodontal disease. J. Periodontol. 76:2033-2041. [DOI] [PubMed] [Google Scholar]
- 29.Taubman, M. A., H. Yoshie, J. L. Ebersole, D. J. Smith, and C. L. Olson. 1984. Host response in experimental periodontal disease. J. Dent. Res. 63:455-460. [DOI] [PubMed] [Google Scholar]
- 30.Theill, L. E., W. J. Boyle, and J. M. Penninger. 2002. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 20:795-823. [DOI] [PubMed] [Google Scholar]
- 31.Valverde, P., T. Kawai, and M. A. Taubman. 2004. Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J. Bone Miner. Res. 19:155-164. [DOI] [PubMed] [Google Scholar]
- 32.Wilson, M. E., and R. G. Hamilton. 1995. Immunoglobulin G subclass response of juvenile periodontitis subjects to principal outer membrane proteins of Actinobacillus actinomycetemcomitans. Infect. Immun. 63:1062-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita, K., J. W. Eastcott, M. A. Taubman, D. J. Smith, and D. S. Cox. 1991. Effect of adoptive transfer of cloned Actinobacillus actinomycetemcomitans-specific T helper cells on periodontal disease. Infect. Immun. 59:1529-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zadeh, H. H., F. C. Nichols, and K. T. Miyasaki. 1999. The role of the cell-mediated immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontitis. Periodontol. 2000 20:239-288. [DOI] [PubMed] [Google Scholar]