Abstract
Yersinia pestis is a highly pathogenic Gram-negative organism and the causative agent of bubonic and pneumonic plague. Y. pestis is capable of causing major epidemics; thus, there is a need for vaccine targets and a greater understanding of the role of these targets in pathogenesis. Two prime Y. pestis vaccine candidates are the usher-chaperone fimbriae Psa and Caf. Herein we report that Y. pestis requires, in a nonredundant manner, both PsaA and Caf1 to achieve its full pathogenic ability in both pneumonic and bubonic plague in C57BL/6J mice. Deletion of psaA leads to a decrease in the organ bacterial burden and to a significant increase in the 50% lethal dose (LD50) after subcutaneous infection. Deletion of caf1 also leads to a significant decrease in the organ bacterial burden but more importantly leads to a significantly greater increase in the LD50 than was observed for the ΔpsaA mutant strain after subcutaneous infection of C57BL/6J mice. Furthermore, the degree of attenuation of the Δcaf1 mutant strain is mouse background dependent, as the Δcaf1 mutant strain was attenuated to a lesser degree in BALB/cJ mice by the subcutaneous route than in C57BL/6J mice. This observation that the degree of requirement for Caf1 is dependent on the mouse background indicates that the virulence of Y. pestis is dependent on the genetic makeup of its host and provides further support for the hypothesis that PsaA and Caf1 have different targets.
Yersinia pestis, the causative agent of both bubonic and pneumonic plague (29), is a highly virulent organism that has been responsible for three major pandemics in history, each with a devastating morbidity rate (28). This medieval disease is still pertinent today, as pockets of infected animals exist throughout the world and human outbreaks still occur. After transmission to a mammalian host from an infected flea, Y. pestis is able to escape the host innate immune response and colonize the proximal lymph node. If left untreated, the organism will proliferate in the lymph nodes, leading to swollen and inflamed lymph nodes called buboes, a hallmark of bubonic plague. From the lymph nodes, the organism is able to disseminate and colonize the liver, spleen, and lungs. Colonization of the lungs can develop into a secondary pneumonia and in some instances leads to person-to-person transmission (primary pneumonia) (28).
Early in infection, Y. pestis is thought to enter cells and replicate intracellularly; however, as infection progresses, Y. pestis is predominantly an extracellular organism (31). To sustain itself extracellularly in the mammalian host, Y. pestis has evolved factors that enable it to withstand the innate immune system and inhibit phagocytosis (7-9, 39). Two of these antiphagocytic factors that inhibit the internalization of Y. pestis by macrophages are surface-exposed capsular antigen fraction 1 (F1 or Caf1) and pH 6 antigen (Psa) fimbriae (14, 22). These fimbriae appear to use different strategies to inhibit internalization. Caf1 is thought to prevent adhesion to the epithelial cells and thereby prevent internalization (25). In contrast, Psa fimbriae promote adherence to cells but this interaction does not lead to phagocytosis, possibly through interactions with an unknown host receptor to block internalization (25).
Caf and Psa fimbriae have similar biogenesis mechanisms. Both fimbriae use the chaperone/usher machinery to export the subunits (Caf1 or PsaA) to the bacterial surface, where they associate in a homopolymeric form (24, 46). In spite of similar biogenesis mechanisms, both fimbriae produce unique structures on the bacterial surface. By electron microscopy, the PsaA fimbriae form individual strands, aggregates, or bundles that protrude from the bacterial cell envelope (6, 24). In contrast, Caf1 forms an envelope or gel-like capsule (F1 antigen) on the outer surface of the bacteria, which upon closer inspection consists of linear fibers that are loosely associated with the bacteria (6, 34, 35, 46). Caf1 can also be detected in the supernatants of cultures and in tissues of infected animals, indicating that large quantities of the subunit are shed from the bacterial surface (5, 11). The expression of both fimbrial operons is highly upregulated in the mammalian host and responds to a temperature shift from 26°C to 37°C (21, 23, 26, 30).
As one of the most abundant surface-exposed epitopes during mammalian infection, F1 is a prime vaccine candidate. Immunization with different F1 constructs has been shown to be protective against bubonic and pneumonic plague in mice and is therefore included in several second-generation vaccines (37). The low frequency of caf1-negative natural isolates and infections in humans and animals suggests a selective pressure for F1/Caf in nature (27, 44). This, together with the increase in caf1 gene expression during mammalian infection, implies an important role for F1 in the mammalian phase of infection. This is supported by several publications showing a requirement for F1 by Y. pestis for successful pathogenicity (2, 13, 17, 41, 43). However, recent work by independent groups has shown that there is no increase in the 50% lethal dose (LD50) with F1 mutant strains after infection via the subcutaneous (s.c.) route in either BALB/cAnNCrl or Swiss-Webster mice (10, 32, 36); this finding is supported by earlier studies that investigated the role of F1 in other mammalian species (11, 17, 42, 45). The F1 antigen has been shown to be required for successful transmission from the flea vector to the mammalian host, as a caf1-negative strain was not as readily transmitted from fleas to Swiss-Webster mice as the parental strain (36). This finding suggests that flea-to-mammal transmission could exert selective pressure for maintenance of the caf locus. Combined, these conflicting data indicate that the exact requirement of F1 in the mammalian phase of infection remains to be elucidated.
In the light of recent in vitro data that PsaA and F1 have redundant (or partially redundant) functions (25), it is worth revisiting the role of F1 in a mammalian infection. Thus, the phenotypes of ΔpsaA and Δcaf1 single mutants, as well as a Δpsa Δcaf1 double mutant, in our mouse models of infection were investigated. The results suggest that both fimbriae are required for pathogenesis during bubonic and pneumonic infections and that their functions are not completely redundant. Finally, we also demonstrate that the degree of the contribution of F1 to Y. pestis pathogenesis is mouse background dependent.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Wild-type Y. pestis CO92 (naturally polymyxin B resistant) is a clinical isolate from a pneumonic infection provided by the U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD (12). The ΔpsaA (YP16) mutant strain was constructed and described previously (3). Strains were cultivated at 26°C on brain heart infusion (BHI; Difco, Sparks, MD) plates for 48 h. Liquid cultures of CO92 were grown for 15 h with aeration at 26°C in BHI or at 37°C in BHI supplemented with 2.5 mM CaCl2, unless otherwise noted. When needed, antibiotics were used at the following concentrations: kanamycin, 50 μg/ml; polymyxin B, 25 μg/ml; ampicillin, 50 μg/ml.
Δcaf1 and ΔpsaA Δcaf1 plasmid and mutant strain construction.
A deletion of the caf1 gene in wild-type CO92 and in the ΔpsaA mutant strain (YP16) was generated using a modified form of λ Red recombination as previously described (3). In brief, the 500 bp upstream and the 500 bp downstream of caf1 were independently amplified by PCR with primers caf1-5′-501 (5′-CAT CAC CAA TTA TGG TTA CTT AAC CCC TTA-3′) and caf1-5′+512-P4 (5′-GGT CGA CGG ATC CCC GGA ATA ATC CAT ATA GAT AAT AGA TAA AGG AGG GCT-3′) for the upstream fragment and caf1-3′+3-P1 (5′-GAA GCA GCT CCA GCC TAC ACC ATA TAT TAC CTC TAT CGA ATA ATC CAA TCC ACG-3′) and caf1-3′+1004 (5′-CTC TTG CTC TCC TCA AGA GGG TC-3′) for the downstream region. The resulting products were gel purified and combined with a kan cassette flanked by FRT sites (previously amplified from plasmid pKD13 by PCR) in a second PCR amplification using primers caf1-5′-501 and caf1-3′+1004. The resulting fragment then was used to generate the Δcaf1 (YP229) and ΔpsaA Δcaf1 (YP292) mutant strains with the modified form of λ Red recombination as previously described (3).
To restore caf1 in the Δcaf1 mutant strain in its native position, the entire open reading frame of caf1 and the 500 to 800 bp flanking caf1 were amplified by PCR using DNA purified from CO92 as the template and primers F-caf1comp-5′-Sal (5′-GCG TCG ACG TGT ACG GTG AAG CCT TTG ATC-3′) and R-caf1comp3′ 5693Not (5′-ATA AGA ATG CGG CCG CAG TTG CAT GGA TGA TGG ATA CC-3′). The PCR fragment was digested with SalI and NotI and cloned into the same sites of suicide vector pSR47S (40). The resulting plasmid, pGK511, was confirmed by sequencing. Plasmid pGK511 was conjugated into the Δcaf1 mutant strain, and recombination of caf1 into the native site was confirmed by PCR. The resulting Kans caf1+ strain was designated YP306.
Animal infections.
All animal experiments were approved by either the Washington University Animal Studies Committee (protocol 20050189) or the University of North Carolina IACUC (protocol 2008-144). Five- to 7-week-old female C57BL/6J mice from The Jackson Laboratory (Bar Harbor, ME) were maintained in the Washington University barrier facility with sterilized food and water ad libitum. Five- to 7-week-old female BALB/cJ mice from The Jackson Laboratory (Bar Harbor, ME) were maintained in the University of North Carolina animal facility with food and water ad libitum. Before infection, all mice were anesthetized with a mixture of ketamine HCl (100 mg/ml) and xylazine HCl (20 mg/ml) mixed 1:1 and delivered at a dose of 0.5 ml/kg of body weight by intraperitoneal (i.p.) injection. Mice were euthanized by i.p. injection of pentobarbital (Nembutal) sodium at a dose of 0.5 mg/kg of body weight.
Virulence assays were performed as described previously (3). In brief, groups of mice were infected for each time point examined with either wild-type strain CO92 or a mutant by s.c. injection of 100 μl (∼2 × 102 CFU) into the neck or 20 μl intranasal (i.n.) inoculation (∼1 × 104 CFU). At various times after inoculation, mice were euthanized and their lungs, spleens, and superficial cervical lymph nodes were removed. The bacterial load of each organ was determined by plating dilutions of the macerated tissues onto BHI agar and reported as the number of CFU per gram of tissue. Infections were repeated in at least two independent experiments. To determine the LD50, four groups of 10 mice were infected with serial 10-fold dilutions of the bacterial suspension (105 to 1 CFU for s.c. inoculations). Mice were checked every 12 h for 7 days, and the LD50 was calculated as previously described (33). For survival analysis, groups of 10 mice were infected i.n. with ∼1 × 104 CFU. Mice were checked every 12 h for 7 days, and the mean number of days to death (MDD) was determined.
Statistical analysis.
Statistical analysis was performed using Prism software from GraphPad Software, Inc. Statistical significance was determined using the Mann-Whitney t test with a two-tailed nonparametric analysis. When comparing data points with a large quantity of undetectable samples, a contingency analysis was performed using the Fisher exact test. Statistical analysis of the survival curves was determined using the log rank test.
RESULTS
Both PsaA and Caf1 are required for bubonic infection.
PsaA and Caf1 are members of the same protein family and form fimbrial structures on the surface of Y. pestis. Deletion of either type of fimbriae increases uptake of Y. pestis by human respiratory tract epithelial cells in vitro, and this phenotype is enhanced in a double mutant (25). We previously demonstrated that a ΔpsaA mutant is defective for colonization in a bubonic plague model (3), suggesting that F1 cannot completely compensate for the absence of PsaA during bubonic infection. To further investigate the individual roles of PsaA and Caf1 during bubonic infection, C57BL/6J mice were infected with ∼200 CFU of Y. pestis strains with psaA, caf1, or both genes (ΔpsaA Δcaf1) deleted to determine the impact of these deletions on the progression of infection.
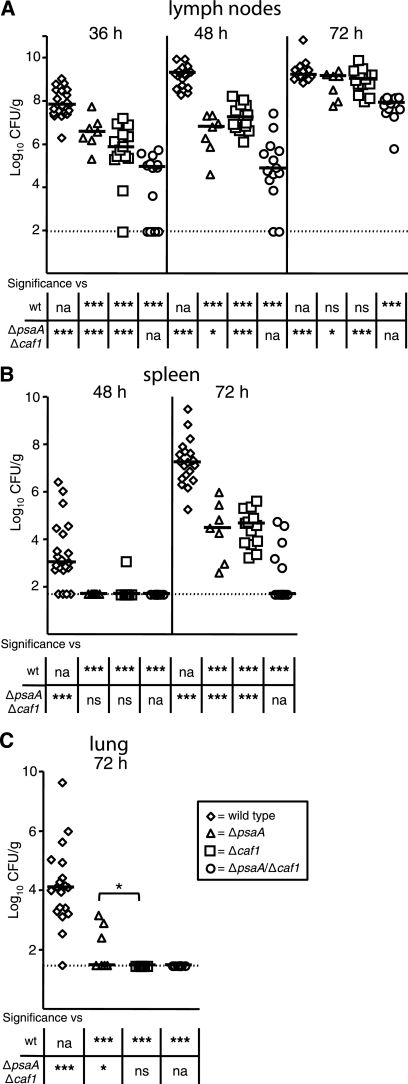
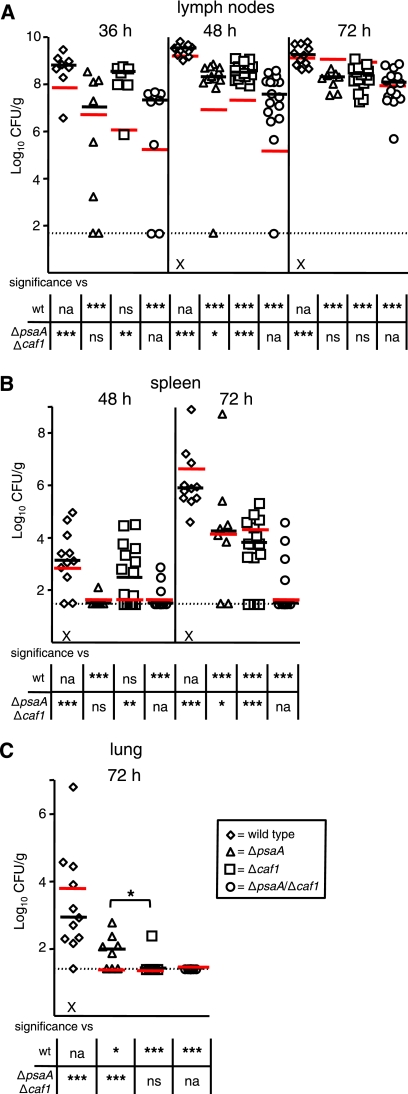
At designated time points, cervical lymph nodes, spleens, and lungs were harvested to determine the number of CFU/g in these tissues. At 36 h postinfection, 108 CFU/g were recovered from the cervical lymph nodes of the wild-type-infected mice, whereas both the ΔpsaA and Δcaf1 mutant strains showed diminished colonization, with ∼106 to 107 CFU/g recovered (Fig. 1A). The ΔpsaA Δcaf1 double mutant displayed an even greater reduction in colonization of the lymph node. The bacterial load in these mice was ∼3 logs lower than in wild-type-infected tissues and ∼1 to 2 logs lower than in tissues from mice infected with the single mutants. However, it should be noted that a substantial number of the ΔpsaA Δcaf1 double mutant-infected mice (64%) did not have detectable bacteria at that time. By 48 h postinfection, all of the mutants were still significantly attenuated compared to the wild type. However, while the wild-type and Δcaf1 mutant bacteria proliferated in the lymph nodes, neither the ΔpsaA nor the ΔpsaA Δcaf1 mutant strain showed expansion of CFU at that time (Fig. 1A). The bacterial loads in the single mutant- and double mutant-infected mice were significantly lower than that in wild-type-infected mice by ∼3 and ∼5 logs, respectively. At 72 h, the number of CFU/g recovered from wild-type-infected mice remained at about 109, whereas the ΔpsaA and Δcaf1 mutant strains proliferated after 48 h, also reaching ∼109 CFU/g. The ΔpsaA Δcaf1 double mutant strain also expanded, reaching ∼5 × 107 CFU/g. However, this level is nearly 2 logs lower than that in the wild-type- and single mutant-infected mice.
FIG. 1.
Colonization and dissemination of ΔpsaA, Δcaf1, and ΔpsaA Δcaf1 mutant Y. pestis strains in C57BL/6J mice during bubonic plague. Mice were infected s.c. in the neck with ∼102 CFU of the wild-type (wt, diamonds) or ΔpsaA (triangles), Δcaf1 (squares), or ΔpsaA Δcaf1 (circles) mutant strain. Mice were sacrificed at 36, 48, and 72 h postinfection, and colonization of the superficial cervical lymph nodes (A), spleen (B), and lungs (C) was determined. Each symbol represents an individual animal. Black bars correspond to the median number of CFU/g of tissue for each group. The dashed line indicates the limit of detection. Asterisks in the graph indicate a significant difference between the median number of CFU/g of tissue between the ΔpsaA and Δcaf1 groups, whereas asterisks in the table indicate a significant difference between the median number of CFU/g of tissue compared to either the wild-type or the ΔpsaA Δcaf1 mutant strain (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005; na, not applicable; ns, not significant). The data represent three independent experiments. The numbers of tissue samples harvested for the wild-type and ΔpsaA, Δcaf1, and ΔpsaA Δcaf1 mutant strains, respectively, were 20, 7, 14, and 14 at 36 h; 21, 7, 14, and 14 at 48 h; and 19, 7, 14, and 14 at 72 h.
To investigate the impact of the psaA and caf1 deletions on dissemination, we enumerated the CFU/g in the spleens at 48 and 72 h and in the lungs at 72 h postinfection. After 48 h, we were unable to recover organisms from most of the spleens from any of the mutant-infected mice, whereas all of the wild-type-infected mice were colonized (median bacterial load, ∼103 CFU/g) (Fig. 1B). By 72 h postinfection, the spleens from mice infected with the single mutants were colonized, but at significantly lower numbers than those of mice infected with the wild type. In the case of the double mutant, only 36% of the infected mice had colonized spleens in which the bacterial numbers were significantly lower than the wild-type- or single mutant-infected spleens (>5 logs and >2 logs lower, respectively) (Fig. 1B). All of the mutant strains were also deficient in the ability to disseminate to the lungs. While all of the wild-type-infected mice had bacteria in their lungs at 72 h (median, 104 CFU/g), only 43% of the mice infected with the ΔpsaA mutant were colonized and no mice infected with the Δcaf1 or ΔpsaA Δcaf1 mutant strain had detectable bacteria in their lungs (Fig. 1C).
To confirm that the phenotypes observed in the experiments described above were due to the deletion of the respective genes, an independent kinetic study with the wild type, the mutants, and the single mutants with a native copy of their respective genes restored, was performed in the same manner as the above-described experiments. Complementation of the single mutants with a native copy of their respective genes completely restored pathogenesis (psaA [3], caf1 [data not shown]). These data indicate that the ΔpsaA, Δcaf1, and ΔpsaA Δcaf1 mutant strains all have an attenuated ability to colonize the lymph nodes and disseminate. Furthermore, the double mutant is more attenuated than either single mutant, suggesting that Y. pestis has nonredundant requirements for both PsaA and Caf1 in bubonic plague in C57BL/6J mice.
Loss of PsaA and Caf1 result in an increase in the LD50 and survival during bubonic plague.
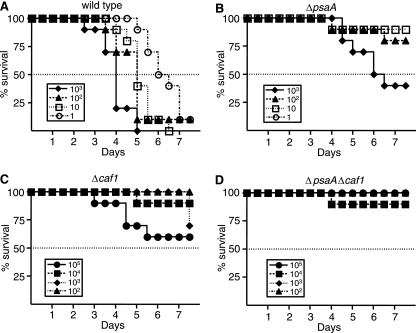
The significant delay in the colonization and dissemination of the ΔpsaA and Δcaf1 mutants suggested that mutant-infected mice might survive longer than wild-type-infected animals or a larger number of mutant bacteria would be required for a lethal infection. To test this hypothesis, C57BL/6J mice were infected with 10-fold dilutions of the wild-type or the ΔpsaA, Δcaf1, or ΔpsaA Δcaf1 mutant strain and monitored for disease progression every 12 h for 7 days. Mice were infected with doses ranging between 1 and 103 organisms of the wild-type and ΔpsaA mutant strains, and doses ranging between 100 and 105 organisms of the Δcaf1 and ΔpsaA Δcaf1 mutant strains. The LD50 of the wild-type strain in C57BL/6J infected by the s.c. route was determined to be ∼1 CFU, which is consistent with previous reports (Fig. 2A) (3). The LD50 of the ΔpsaA mutant strain was 400-fold higher than that of the wild type, with 40%, 80%, and 90% of the mice surviving doses of 1,000, 100, and 10 CFU, respectively (Fig. 2B). The Δcaf1 mutant strain was more attenuated, and in fact, we were unable to calculate an LD50 of this mutant, as 60% of the mice infected with the highest dose (105 CFU) survived the course of the infection. We also were unable to determine an LD50 of the ΔpsaA Δcaf1 mutant strain, as all but one mouse survived all of the doses tested. Therefore, the LD50 was significantly greater than 105 CFU, which is similar to the LD50 of the Δcaf1 mutant strain, and a significant increase over that of both the wild-type and ΔpsaA mutant strains. This LD50 experiment was repeated independently for all four strains, with similar results (data not shown). Although we were unable to calculate an LD50 of the Δcaf1 or ΔpsaA Δcaf1 mutant, the data suggest that there may be a difference in virulence between the Δcaf1 and ΔpsaA Δcaf1 mutant strains. Whereas 20% of the mice infected with all of the doses of the Δcaf1 mutant strain combined succumbed to the infection, only 3% of the ΔpsaA Δcaf1 mutant-infected mice succumbed.
FIG. 2.
Survival and LD50 analysis in C57BL/6J mice infected with the wild-type and ΔpsaA, Δcaf1, and ΔpsaA Δcaf1 mutant strains of Y. pestis. Groups of 10 mice were infected s.c. in the neck with serial 10-fold dilutions of the wild-type (A) or ΔpsaA (B), Δcaf1 (C), or ΔpsaA Δcaf1 (D) mutant strain. Shown is a representative experiment of two independent experiments.
Because it appeared that, in the LD50 experiment, the ΔpsaA Δcaf1 mutant strain was more attenuated than the Δcaf1 mutant strain, we sought to determine if there was any difference in organ burden in these mice. At 7 days postinfection, a subset of the surviving mice infected with 100 CFU (low dose) or 105 CFU (high dose) of the Δcaf1 or the ΔpsaA Δcaf1 mutant strain from the two independent LD50 experiments (Fig. 2) were sacrificed. The bacterial burdens recovered at 7 days postinfection from the lymph nodes and spleens of mice given the low and high doses of the Δcaf1 mutant strain were significantly greater than those recovered from mice infected with the equivalent infectious doses of the ΔpsaA Δcaf1 mutant strain (P ≤ 0.05 for the lymph nodes, P ≤ 0.005 for the spleen) (data not shown). In the majority of the mice infected with the low dose of either strain, we were unable to recover live organisms, especially in the lungs. However, at the high dose, sufficient mice were colonized that a difference in bacterial burden could be observed in the lungs of the Δcaf1 and ΔpsaA Δcaf1 mutant strain-infected mice, as 78% and 36%, respectively, of the mice had detectable levels of bacteria (P ≤ 0.05) (data not shown). Overall, both the Δcaf1 and ΔpsaA mutant strains had a significantly attenuated ability to produce bubonic plague in C57BL/6J mice, and deletion of both genes led to an additional increase in attenuation.
Both PsaA and Caf1 are required for pneumonic infection.
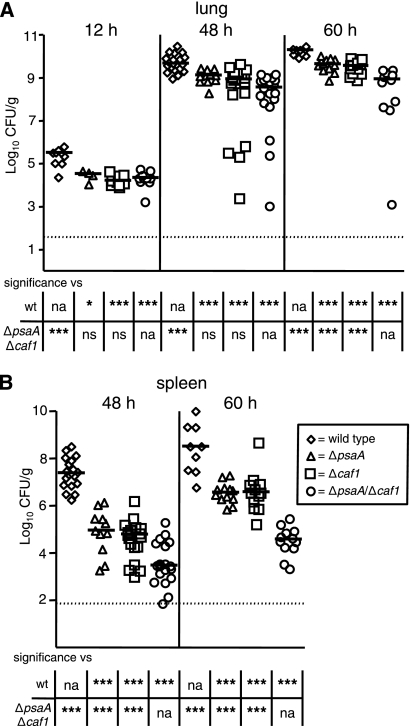
While our data strongly suggest that both PsaA and F1 are required for bubonic infection, several laboratories have reported that F1 is not required for bubonic or pneumonic plague in mice (10, 32, 36). We therefore set out to determine if these proteins impact pneumonic infection in our model. Toward this end, mice were infected i.n. with the ΔpsaA, Δcaf1, and ΔpsaA Δcaf1 mutant strains and colonization and dissemination were compared to those in wild-type-infected animals. At 12 h postinfection, all three mutant strains displayed a modest but significant reduction (∼1 log) in bacterial counts in the lungs compared to wild-type-infected mice and this decreased bacterial burden was constant in the lungs throughout the course of the experiment (Fig. 3A). By 60 h postinfection, there was a significant difference in bacterial counts between the single mutants and the double mutant; the median bacterial loads in the single mutant-infected mice were approximately 10-fold less than those in wild-type-infected animals, compared to an approximately 100-fold difference for the double mutant-infected animals. Dissemination of the mutant bacteria to the spleen was also greatly diminished, and the double mutant appeared even more defective than the individual mutants (Fig. 3B). By 48 h postinfection, the bacterial numbers in the spleens of mice infected with the single mutants were already 2 to 3 logs lower than those in wild-type-infected mice. Deletion of both psaA and caf1 enhanced this dissemination defect, as the ΔpsaA Δcaf1 mutant strain was significantly more attenuated than the wild-type strain (∼4 to 5 logs) and each single mutant (∼2 to 3 logs). This dissemination defect was also observed at 60 h postinfection, as the bacterial loads recovered from the spleens of mice infected with the single and double deletion strains were ∼2 logs and ∼4 logs lower, respectively, than those recovered from wild-type-infected mice. Taken together, these data suggest that, in addition to playing a role in lung colonization in pneumonic plague, both PsaA and F1 are required for efficient dissemination to the spleen.
FIG. 3.
Colonization by and dissemination of ΔpsaA, Δcaf1, and ΔpsaA Δcaf1 mutant Y. pestis strains in C57BL/6J mice during pneumonic plague. Mice were infected i.n. with ∼104 CFU of the wild-type (diamonds) or ΔpsaA (triangles), Δcaf1 (squares), or ΔpsaA Δcaf1 (circles) mutant strain. Mice were sacrificed at 12, 48, and 60 h postinfection, and colonization of the lungs (A) and spleen (B) was determined. Each symbol represents an individual animal. Black bars correspond to the median number of CFU/g of tissue for each group. The dashed line indicates the limit of detection. Asterisks in the table indicate a significant difference between the median number of CFU/g of tissue compared to either the wild-type or the ΔpsaA Δcaf1 mutant strain (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005; na, not applicable; ns, not significant). The data are a combination of three independent experiments. The numbers of tissue samples harvested for the wild-type and ΔpsaA, Δcaf1, and ΔpsaA Δcaf1 mutant strains, respectively, were 9, 4, 10, and 10 at 12 h; 19, 11, 19, and 19 at 48 h; and 9, 12, 12, and 11 at 60 h.
To determine if the defects in tissue colonization of the ΔpsaA, Δcaf1, and ΔpsaA Δcaf1 mutant strains during pneumonic infection result in a delay of death, a survival analysis was performed. We infected C57BL/6J mice i.n. with 104 CFU of the wild-type or the ΔpsaA, Δcaf1, or ΔpsaA Δcaf1 mutant strain and monitored their progress every 12 h for 7 days (Table 1). The MDD for the wild-type-infected mice was calculated at 72 h postinfection. For the ΔpsaA and Δcaf1 mutant strains, the MDD was 84 h, a significant increase of 12 h over the wild-type-infected mice (P = 0.0013 and (P < 0.0001, respectively). An even greater delay of 24 h was determined for the MDD of the ΔpsaA Δcaf1 mutant-infected mice (MDD = 96 h); this is a significant increase over that observed in the wild-type or ΔpsaA mutant strain-infected mice (P < 0.0001 and P = 0.0096, respectively).
TABLE 1.
Analysis of Y. pestis wild-type and ΔpsaA, Δcaf1, ΔpsaA Δcaf1, and Δcaf1 caf1+ mutant strain survival in C57BL/6J mice during pneumonic infection
| Genotype | No. of surviving mice/total at postinfectiona time (h): |
Mean time to death (h) | Significant differenceb (P value) vs: |
|||||
|---|---|---|---|---|---|---|---|---|
| 60 | 72 | 84 | 96 | 108 | Wild type | ΔpsaA mutant | ||
| Wild type | 9/10 | 0/10 | 0/10 | 0/10 | 0/10 | 72 | NAc | 0.0013 |
| ΔpsaA | 10/10 | 7/10 | 1/10 | 0/10 | 0/10 | 84 | 0.0013 | NA |
| Δcaf1 | 10/10 | 10/10 | 5/10 | 0/10 | 0/10 | 84 | <0.0001 | 0.0215 |
| ΔpsaA Δcaf1 | 10/10 | 10/10 | 6/10 | 1/10 | 0/10 | 96 | <0.0001 | 0.0096 |
| Δcaf1caf1+ | 10/10 | 3/10 | 2/10 | 0/10 | 0/10 | 72 | 0.0446 | NSd |
Mice were infected intranasally with ∼1 × 104 CFU.
Statistical comparison of survival curves using the log rank test.
NA, not applicable.
NS, not significant.
To verify that the phenotypes for the Δcaf1 mutant strain are due to the deletion of caf1 and not to a secondary mutation, we restored caf1 to its original position, resulting in strain YP306. This strain was tested in the same survival experiment described above and displayed an MDD time frame similar to that of the wild type (72 h), but due the fact that a few mice infected with the complemented strain survived longer than the wild-type-infected mice, a small but significant difference was still present (P = 0.0446). However, a significant restoration of the Δcaf1 mutant phenotype was observed (P = 0.029), indicating that the phenotype in the Δcaf1 mutant is due the deletion of caf1 and not a secondary mutation (Table 1). These data are consistent with the colonization and dissemination data.
The Δcaf1 mutant strain is attenuated to a lesser degree in bubonic plague in BALB/cJ mice than in C57BL/6J mice.
In contrast to recent reports suggesting that deletion of caf1 does not dramatically affect the virulence of Y. pestis during pneumonic infection (10, 32, 36), our data indicate that F1 is required during plague infection. However, closer inspection of the data of Quenee et al. and Cornelius et al. suggests that there is a difference in survival after s.c. infection (10, 32). One possible explanation for the differences in the degrees of attenuation observed with the Δcaf1 mutant strains between these reports and our data is that all of our experiments were performed with C57BL/6J mice, whereas BALB/cAnNCrl mice were used in some of the other studies. To determine if the mouse background influences the ability of Y. pestis mutants to cause disease, we infected BALB/cJ mice s.c. with the wild-type or ΔpsaA, Δcaf1, or ΔpsaA Δcaf1 mutant strain using the same conditions as described for the C57BL/6J experiments (Fig. 1).
The wild-type and ΔpsaA mutant strains displayed similar abilities to colonize the lymph nodes and disseminate to the spleen and lungs in BALB/cJ mice compared to those observed in C57BL/6J mice (Fig. 4A). The ΔpsaA mutant strain was recovered at significantly lower levels from the lymph nodes throughout the course of infection and also showed a delay in dissemination to the spleen in BALB/cJ mice similar to that in C57BL/6J mice. The ΔpsaA mutant was not detected in spleens at 48 h but was detected at 72 h at a level ∼2 logs lower than that in wild-type-infected mice (Fig. 4B). The ΔpsaA mutant strain also showed diminished dissemination to the lungs, as only 63% of the ΔpsaA-infected mice had detectable bacteria (Fig. 4C).
FIG. 4.
Colonization by and dissemination of ΔpsaA, Δcaf1, and ΔpsaA Δcaf1 mutant strains of Y. pestis in BALB/cJ mice during bubonic plague. Mice were infected s.c. in the neck with ∼102 CFU of the wild-type (diamonds) or ΔpsaA (triangles), Δcaf1 (squares), or ΔpsaA Δcaf1 (circles) mutant strain. Mice were sacrificed at 36, 48, and 72 h postinfection, and colonization of the superficial cervical lymph nodes (A), spleen (B), and lungs (C) was determined. Each symbol represents an individual animal. Black bars correspond to the median number of CFU/g of tissue for each group in BALB/cJ mice. Red bars correspond to the median number of CFU/g of tissue for each group in C57BL/6J mice (from Fig. 1). The dashed line indicates the limit of detection. The symbol × indicates that a mouse was either euthanized or found dead at that time point. An asterisk in a graph indicates a significant difference in the median number of CFU/g of tissue between the ΔpsaA and Δcaf1 mutant-infected groups, whereas asterisks in the tables indicate significant differences in the median numbers of CFU/g of tissue compared to either the wild-type or the ΔpsaA Δcaf1 mutant strain (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.005; na, not applicable; ns, not significant). The data are a combination of three independent experiments. The numbers of tissue samples harvested for the wild-type and ΔpsaA, Δcaf1, and ΔpsaA Δcaf1 mutant strains, respectively, were 8, 8, 7, and 8 at 36 h; 11, 12, 16, and 16 at 48 h; and 11, 8, 15, and 15 at 72 h.
Unlike the psaA mutant, the Δcaf1 mutant strain was recovered at wild-type levels at 36 h postinfection from the lymph nodes of BALB/cJ mice. The Δcaf1 mutant strain did not show any expansion of the number of CFU/g between 36 and 72 h, and the median lymph node burden in the Δcaf1 mutant strain-infected mice was ∼1 to 2 logs lower than in the wild-type-infected mice and similar to those of ΔpsaA-infected mice at both 48 h and 72 h. The Δcaf1 mutant strain was recovered at the same levels as the wild-type strain from spleens at 48 h postinfection, whereas the bacterial load was ∼3 logs lower at 72 h postinfection. The Δcaf1 mutant strain appears to be unable to disseminate to the lungs by 72 h postinfection, as we were unable to recover any live bacteria from the BALB/cJ-infected mice at that time.
The decrease in the colonization of the lymph nodes of ΔpsaA Δcaf1-infected mice at 36 h appears to be mainly due to the loss of ΔpsaA, as equal numbers of CFU of both strains were recovered at that time, leading to a bacterial load ∼2 logs lighter than that in wild-type- and Δcaf1 mutant-infected mice. At 48 h, the ΔpsaA Δcaf1 mutant was recovered from the lymph nodes at a level slightly lower than that of either of the single mutants but ∼3 logs lower than that in wild-type-infected mice. At 72 h, the ΔpsaA Δcaf1 mutant strain was recovered at levels equivalent to those of the single mutants but ∼2 logs lower than that of the wild-type strain. The ability of the ΔpsaA Δcaf1 mutant strain to disseminate to the spleen and lungs in BALB/cJ mice seems to be severely impacted, as we were unable to recover any live bacteria from the spleens or lungs of the majority of the ΔpsaA Δcaf1-infected BALB/cJ mice. Taken together, these data show that PsaA and F1 also contribute to pathogenicity in BALB/cJ mice, but F1 appears to impact bubonic infection to a lesser degree in BALB/cJ mice.
DISCUSSION
Y. pestis is still a major threat to humans, due to endemic pockets of Y. pestis-infected animals and fleas and potential bioterrorism use. There is a need for a greater understanding of the disease process, as well as the development of new vaccine and molecular therapy candidates, and a greater understanding of the roles and functions of these candidates in Y. pestis pathogenesis. One such candidate is the chaperone/usher fimbria Psa, which recently was shown to provide incomplete but significant protection against an attenuated Y. pestis strain (20). The operon encoding this fimbria is expressed at the flea vector temperature of 26°C, but a large increase in expression is observed in the mammalian host and in vitro under mammalian host-like conditions such as a temperature shift and a low pH (24, 30). This large increase in expression during the mammalian phase of infection suggests an important role for PsaA during infection. In vivo evidence supports this, as recent work in our laboratory and others has shown that PsaA is required during bubonic plague (3, 23).
Regardless of the route of infection, our data indicate that psaA is required for the full pathogenicity of Y. pestis, independent of the mouse background. The kinetic analyses of the ΔpsaA mutant strain by either i.n. or s.c. infection revealed that deletion of psaA leads to a reduction in the ability of Y. pestis to colonize the initial site of infection (lung/lymph node) and disseminate to the spleen. This reduction in colonization and dissemination translates into an increase in the LD50, as we observed a 400-fold increase in the LD50 of the ΔpsaA mutant strain by the s.c. route over that of the wild-type strain.
These results for PsaA reaffirm our previously published kinetic data (3) and also are in line with the LD50 of a strain lacking RovA (3), a positive regulator of psaA (4). It furthermore is strengthened by data of an independent group which observed a significant increase in the LD50 by the intravenous route using psa mutants in the pgm mutant parental strain background (23). This finding stands in contrast to a recent report indicating that PsaA is not required for virulence in BALB/c mice (1). The difference in results is most likely not due to the mouse background used, as we observed a similar attenuation of the ΔpsaA mutant strain in both C57BL/6J mice and BALB/cJ mice. One possible explanation for the disparity in results could be the difference in the Y. pestis parent strains used in the studies. A second possibility is that the wild-type and mutant strains in the other study were animal passaged several times before use in their experiments. This potentially could select for isolates that have adapted to loss of the psa locus by changes in their expression profile, leading to compensation for the loss of PsaA and suppression of the attenuation caused by the psaA mutation.
A second usher/chaperone fimbria that is a plague vaccine and molecular therapy candidate is the Caf1 fimbria, also known as the F1 antigen or capsule. Although the F1 antigen has been shown to be required for successful transmission from the flea to the mammalian host (36), contradicting observations have led to questions regarding its requirement in mammalian infection. The Java9 strain, a natural isolate from Indonesian rats that lacks the caf locus, was determined to be fully virulent in Swiss-Webster mice by the s.c. route but displayed attenuation in Hartley guinea pigs by the aerosol route (16). These data, together with independent reports on other mammalian hosts, indicate that at least some caf-negative strains can be fully virulent in specific host backgrounds (10, 11, 17, 32, 36, 42, 45). However, this finding is counterbalanced by the observation that the caf locus displays a strong upregulation in the mammalian host, combined with the observation that most natural isolates contain the caf locus (27, 44). These observations, along with several reports indicating a requirement of the caf locus for full virulence, suggest a role for the F1 antigen during mammalian infection (2, 13, 17, 41, 43). How can these various observations be reconciled? A starting point is to test well-defined mutants under controlled experimental conditions in inbred hosts.
Our data presented here show that F1 is required for Y. pestis to achieve its full pathogenicity in both pneumonic and bubonic plague in C57BL/6J mice. During pneumonic infection of mice, the Δcaf1 mutant strain displayed a modest but significant attenuation in the ability to colonize the lungs and a greater decrease in the ability to disseminate to the spleen compared to that of the wild-type strain. The kinetic analyses of the Δcaf1 mutant strain in the bubonic infection model revealed that deletion of caf1 leads to a dramatic reduction in the ability of Y. pestis to colonize the lymph node and disseminate to the spleen and lung. In fact, in this model, the Δcaf1 mutant strain was more attenuated than the ΔpsaA mutant strain, as the LD50 of the Δcaf1 mutant strain is more than 100,000-fold greater than that of the wild type, which is significantly greater than the LD50 of the ΔpsaA mutant strain.
Evidence from in vitro studies suggests that Psa and F1 likely have both overlapping and distinct functions (19, 25). Both have been shown to have antiphagocytic properties, and PsaA also has been shown to function as an adhesin for cells in culture (14, 15, 18, 22). Thus, we investigated the potential redundancy of these two fimbriae in vivo and found that PsaA and Caf1 are both required for full pathogenicity in pneumonic plague of C57BL/6J mice, as both single deletion strains displayed a modest but significant attenuation of the ability to colonize the lungs and a decrease in the ability to disseminate to the spleen. Moreover, Y. pestis lacking both fimbriae is more attenuated than either of the single mutants for both colonization of the lungs and dissemination to (or survival in) the spleen, and it is more attenuated in the pneumonic mouse model in terms of mouse survival than either of the single mutants.
Similar results were observed for the ΔpsaA Δcaf1 mutant in the bubonic model of infection of C57BL/6J mice. Deletion of both fimbriae leads to a greater reduction in the ability of Y. pestis to colonize the lymph node and disseminate to the spleen and lungs compared to either single mutant. Analogous to the Δcaf1 mutant strain, we were unable to calculate an exact LD50 of the ΔpsaA Δcaf1 mutant strain but could extrapolate from the data that its LD50 was more than 100,000-fold greater than that of the wild type. These data suggest that Caf1 and PsaA are both required for Y. pestis pathogenesis in C57BL/6J mice and are both required regardless of the route of infection.
The requirement for Caf1 in both pneumonic and bubonic plague infections in our experiments at first appeared to be in conflict with reports that suggest that the F1 antigen is not required for virulence (10, 11, 17, 32, 36, 42, 45). However, while no change in LD50 was observed by Quenee et al. and Cornelius et al. during pneumonic infection, their data from a bubonic infection indicated a change in the time to death (10, 32). The most notable difference between our data and these reports was the background of mice used in the studies; BALB/cAnNCrl mice were used by Quenee et al. (32) and Cornelius et al. (10), whereas we used C57BL/6J mice. Thus, we tested the wild-type strain and the Δcaf1 mutant in BALB/cJ mice and compared this to infection of C57BL/6J mice. Indeed, we found that the wild type behaved similarly in the two strains of mice but that the Δcaf1 mutant was more attenuated in C57BL/6J mice than in BALB/cJ mice. Our data support previous studies using the BALB/cAnNCrl mouse background in which the Δcaf1 mutant had no increase in LD50 but did show a significant increase in MDD (10, 32). Though it was not to the same degree as in C57BL/6J mice, we observed some attenuation of our Δcaf1 mutant strain in lymph node colonization and dissemination to the deeper tissues in BALB/cJ mice in the s.c. kinetic experiments, which would explain the delay in death in the previous studies (10, 32). These results may shed light on the contradictory reports on the role of F1 in the mammalian phase of infection of Y. pestis, as these data indicate that the level of contribution of F1 is dependent on the host genetic background. The fact that we observed different degrees of attenuation in the two different mouse backgrounds when using the same Δcaf1 mutant strain could explain the differences observed in previous reports for caf1 deletion strains, as those reports used different mouse backgrounds. Furthermore, our findings may also help explain differences in F1 requirements for infections in different species, as the degree of F1 requirement could be both intra- and interspecies dependent.
The precise mechanism of attenuation of the Δcaf1 mutant strain and the functional role of the F1 antigen in the mammalian host are unclear; however, in vitro evidence suggests that the F1 antigen plays a role in preventing phagocytosis (14). The interplay of Caf1 with the innate immune systems of genetically distinct mice could result in a different outcome for the Δcaf1 mutant strain. One of the known differences between C57BL/6J and BALB/cJ mice is that C57BL/6J mice display a Th1 cytokine profile whereas BALB/cJ mice display a Th2 cytokine profile. This difference is also reflected in their macrophage activation state. C57BL/6J mice have M1 macrophages, which are more readily activated than the M2 macrophages found in BALB/cJ mice. One could postulate that if Caf1 is required to resist macrophage activation, then Caf1 would be more important for infection of mice with M1 macrophages (C57Bl/6J) than of mice with M2 macrophages (BALB/cJ mice) that are activated less readily.
This work presents the first comprehensive analysis of both wild-type and mutant Y. pestis strains in two different mouse backgrounds and highlights the importance of the mouse backgrounds used in the analysis of virulence factors of Y. pestis. We observed no overt differences in wild-type Y. pestis s.c. infection between the two mouse backgrounds, and our results with BALB/cJ mice are similar to those of other groups using BALB/cAnNCrl mice with wild-type Y. pestis (10, 32). This is in contrast to a report that BALB/cJ mice are highly resistant to Y. pestis, compared to C57BL/6J mice (38). Given the observations presented here that there is a differential effect in these mouse backgrounds with various mutants but not with the wild type, the most likely explanation for these differences is that in that study an attenuated strain of Y. pestis (pgm mutant) was used, whereas in our and other studies (10, 32) a fully virulent strain was utilized. The wild-type fully virulent strain most likely is able to overcome the difference between these mouse backgrounds, as the disease progression is so rapid and acute. In contrast, in infection with attenuated strains, disease progression is slower, potentially allowing subtle differences between mouse backgrounds to become apparent. Given the observation that Y. pestis is able to withstand the innate immune response of the host, by using numerous virulence factors to downplay this response or skew this response in its own favor, it is perhaps not surprising that the analysis and interpretation of the role of these potential virulence factors of Y. pestis are influenced by the host genetic background. Our results suggest that caution should be used when drawing conclusions about infections of a single mouse background with mutant strains of Y. pestis.
Acknowledgments
We thank Kimberly A. Walker for critical review of the manuscript and helpful discussion.
This study was supported by National Institutes of Health grants AI52167 and AI53298 awarded to V.L.M. J.S.C. was supported by Lucille P. Markey Special Emphasis Pathway in Human Biology and Training Program in Cellular and Molecular Biology grant T32-GM07067. G.K. was supported by Pediatric Gastroenterology Research training grant T32DO77653-16. P.P. was supported by Training Program in Cellular and Molecular Biology grant T32-GM07067.
Editor: A. Camilli
Footnotes
Published ahead of print on 29 November 2010.
REFERENCES
- 1.Anisimov, A. P., et al. 2009. The subcutaneous inoculation of pH 6 antigen mutants of Yersinia pestis does not affect virulence and immune response in mice. J. Med. Microbiol. 58:26-36. [DOI] [PubMed] [Google Scholar]
- 2.Burrows, T. W. 1957. Virulence of Pasteurella pestis. Nature 179:1246-1247. [DOI] [PubMed] [Google Scholar]
- 3.Cathelyn, J. S., S. D. Crosby, W. W. Lathem, W. E. Goldman, and V. L. Miller. 2006. RovA, a global regulator of Yersinia pestis, specifically required for bubonic plague. Proc. Natl. Acad. Sci. U. S. A. 103:13514-13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cathelyn, J. S., D. W. Ellison, S. J. Hinchliffe, B. W. Wren, and V. L. Miller. 2007. The RovA regulons of Yersinia enterocolitica and Yersinia pestis are distinct: evidence that many RovA-regulated genes were acquired more recently than the core genome. Mol. Microbiol. 66:189-205. [DOI] [PubMed] [Google Scholar]
- 5.Chen, T. H., T. T. Crocker, and K. F. Meyer. 1956. Electron microscopic study of the extracellular materials of Pasteurella pestis. J. Bacteriol. 72:851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, T. H., and S. S. Elberg. 1977. Scanning electron microscopic study of virulent Yersinia pestis and Yersinia pseudotuberculosis type 1. Infect. Immun. 15:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis, G. R. 2002. The Yersinia Ysc-Yop virulence apparatus. Int. J. Med. Microbiol. 291:455-462. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelis, G. R., et al. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelius, C. A., L. E. Quenee, D. Elli, N. A. Ciletti, and O. Schneewind. 2009. Yersinia pestis IS1541 transposition provides for escape from plague immunity. Infect. Immun. 77:1807-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis, K. J., et al. 1996. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopithecus aethiops). Arch. Pathol. Lab. Med. 120:156-163. [PubMed] [Google Scholar]
- 12.Doll, J. M., et al. 1994. Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am. J. Trop. Med. Hyg. 51:109-114. [DOI] [PubMed] [Google Scholar]
- 13.Donavan, J. E., D. Ham, G. M. Fukui, and M. J. Surgalla. 1961. Role of the capsule of Pasteurella pestis in bubonic plague in the guinea pig. J. Infect. Dis. 109:154-157. [DOI] [PubMed] [Google Scholar]
- 14.Du, Y., R. Rosqvist, and A. Forsberg. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 70:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felek, S., T. M. Tsang, and E. S. Krukonis. 2010. Three Yersinia pestis adhesins facilitate Yop delivery to eukaryotic cells and contribute to plague virulence. Infect. Immun. 78:4134-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filippov, A. A., N. S. Solodovnikov, L. M. Kookleva, and O. A. Protsenko. 1990. Plasmid content in Yersinia pestis strains of different origin. FEMS Microbiol. Lett. 55:45-48. [DOI] [PubMed] [Google Scholar]
- 17.Friedlander, A. M., et al. 1995. Relationship between virulence and immunity as revealed in recent studies of the F1 capsule of Yersinia pestis. Clin. Infect. Dis. 21(Suppl. 2):S178-S181. [DOI] [PubMed] [Google Scholar]
- 18.Galván, E. M., H. Chen, and D. M. Schifferli. 2007. The Psa fimbriae of Yersinia pestis interact with phosphatidylcholine on alveolar epithelial cells and pulmonary surfactant. Infect. Immun. 75:1272-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galván, E. M., M. A. Lasaro, and D. M. Schifferli. 2008. Capsular antigen fraction 1 and Pla modulate the susceptibility of Yersinia pestis to pulmonary antimicrobial peptides such as cathelicidin. Infect. Immun. 76:1456-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galván, E. M., M. K. Nair, H. Chen, F. Del Piero, and D. M. Schifferli. 2010. Biosafety level 2 model of pneumonic plague and protection studies with F1 and Psa. Infect. Immun. 78:3443-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han, Y., et al. 2004. Microarray analysis of temperature-induced transcriptome of Yersinia pestis. Microbiol. Immunol. 48:791-805. [DOI] [PubMed] [Google Scholar]
- 22.Huang, X. Z., and L. E. Lindler. 2004. The pH 6 antigen is an antiphagocytic factor produced by Yersinia pestis independent of Yersinia outer proteins and capsule antigen. Infect. Immun. 72:7212-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindler, L. E., M. S. Klempner, and S. C. Straley. 1990. Yersinia pestis pH 6 antigen: genetic, biochemical, and virulence characterization of a protein involved in the pathogenesis of bubonic plague. Infect. Immun. 58:2569-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindler, L. E., and B. D. Tall. 1993. Yersinia pestis pH 6 antigen forms fimbriae and is induced by intracellular association with macrophages. Mol. Microbiol. 8:311-324. [DOI] [PubMed] [Google Scholar]
- 25.Liu, F., H. Chen, E. M. Galván, M. A. Lasaro, and D. M. Schifferli. 2006. Effects of Psa and F1 on the adhesive and invasive interactions of Yersinia pestis with human respiratory tract epithelial cells. Infect. Immun. 74:5636-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, H., et al. 2009. Transcriptional profiling of a mice plague model: insights into interaction between Yersinia pestis and its host. J. Basic Microbiol. 49:92-99. [DOI] [PubMed] [Google Scholar]
- 27.Meka-Mechenko, T. V. 2003. F1-negative natural Y. pestis strains. Adv. Exp. Med. Biol. 529:379-381. [DOI] [PubMed] [Google Scholar]
- 28.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prentice, M. B., and L. Rahalison. 2007. Plague. Lancet 369:1196-1207. [DOI] [PubMed] [Google Scholar]
- 30.Price, S. B., M. D. Freeman, and K. S. Yeh. 1995. Transcriptional analysis of the Yersinia pestis pH 6 antigen gene. J. Bacteriol. 177:5997-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pujol, C., and J. B. Bliska. 2005. Turning Yersinia pathogenesis outside in: subversion of macrophage function by intracellular yersiniae. Clin. Immunol. 114:216-226. [DOI] [PubMed] [Google Scholar]
- 32.Quenee, L. E., C. A. Cornelius, N. A. Ciletti, D. Elli, and O. Schneewind. 2008. Yersinia pestis caf1 variants and the limits of plague vaccine protection. Infect. Immun. 76:2025-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 34.Rowland, S. 1914. LXVI. The morphology of the plague bacillus. J. Hyg. (Lond.) 13(Suppl.):418-422. [PMC free article] [PubMed] [Google Scholar]
- 35.Runco, L. M., S. Myrczek, J. B. Bliska, and D. G. Thanassi. 2008. Biogenesis of the fraction 1 capsule and analysis of the ultrastructure of Yersinia pestis. J. Bacteriol. 190:3381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sebbane, F., C. Jarrett, D. Gardner, D. Long, and B. J. Hinnebusch. 2009. The Yersinia pestis caf1M1A1 fimbrial capsule operon promotes transmission by flea bite in a mouse model of bubonic plague. Infect. Immun. 77:1222-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Titball, R. W., and E. D. Williamson. 2001. Vaccination against bubonic and pneumonic plague. Vaccine 19:4175-4184. [DOI] [PubMed] [Google Scholar]
- 38.Turner, J. K., M. M. McAllister, J. L. Xu, and R. I. Tapping. 2008. The resistance of BALB/cJ mice to Yersinia pestis maps to the major histocompatibility complex of chromosome 17. Infect. Immun. 76:4092-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69-89. [DOI] [PubMed] [Google Scholar]
- 40.Walker, K. A., and V. L. Miller. 2004. Regulation of the Ysa type III secretion system of Yersinia enterocolitica by YsaE/SycB and YsrS/YsrR. J. Bacteriol. 186:4056-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welkos, S. L., G. P. Andrews, L. E. Lindler, N. J. Snellings, and S. D. Strachan. 2004. Mu dI1(Ap lac) mutagenesis of Yersinia pestis plasmid pFra and identification of temperature-regulated loci associated with virulence. Plasmid 51:1-11. [DOI] [PubMed] [Google Scholar]
- 42.Welkos, S. L., K. M. Davis, L. M. Pitt, P. L. Worsham, and A. M. Freidlander. 1995. Studies on the contribution of the F1 capsule-associated plasmid pFra to the virulence of Yersinia pestis. Contrib. Microbiol. Immunol. 13:299-305. [PubMed] [Google Scholar]
- 43.Williams, J. E., and D. C. Cavanaugh. 1984. Potential for rat plague from nonencapsulated variants of the plague bacillus (Yersinia pestis). Experientia 40:739-740. [DOI] [PubMed] [Google Scholar]
- 44.Winter, C. C., W. B. Cherry, and M. D. Moody. 1960. An unusual strain of Pasteurella pestis isolated from a fatal human case of plague. Bull. World Health Organ. 23:408-409. [PMC free article] [PubMed] [Google Scholar]
- 45.Worsham, P. L., M. P. Stein, and S. L. Welkos. 1995. Construction of defined F1 negative mutants of virulent Yersinia pestis. Contrib. Microbiol. Immunol. 13:325-328. [PubMed] [Google Scholar]
- 46.Zavialov, A. V., et al. 2003. Structure and biogenesis of the capsular F1 antigen from Yersinia pestis: preserved folding energy drives fiber formation. Cell 113:587-596. [DOI] [PubMed] [Google Scholar]