Abstract
Studies have shown that epithelium-expressed antimicrobial peptides (AMPs), e.g., β-defensins, play a role in clearing bacteria from mouse corneas already infected with Pseudomonas aeruginosa. Less is known about the role of AMPs in allowing the cornea to resist infection when healthy. We previously reported that contact lens exposure, a major cause of P. aeruginosa keratitis, can inhibit the upregulation of human β-defensin 2 (hBD-2) by corneal epithelial cells in response to P. aeruginosa antigens in vitro. Here, we studied the role of AMPs in maintaining the corneal epithelial barrier to P. aeruginosa penetration using both in vitro (human) and in vivo (mouse) experiments. Results showed that preexposing human corneal epithelial multilayers to bacterial antigens in a culture supernatant (known to upregulate AMP expression) reduced epithelial susceptibility to P. aeruginosa traversal up to 6-fold (P < 0.001). Accordingly, small interfering RNA (siRNA) knockdown of any one of four AMPs expressed by human epithelia promoted P. aeruginosa traversal by more than 3-fold (P < 0.001). The combination knockdown of AMPs further enhanced susceptibility to bacterial traversal by ∼8-fold (P < 0.001). In vivo experiments showed that the loss of murine β-defensin 3 (mBD-3), a murine ortholog of hBD-2, enhanced corneal susceptibility to P. aeruginosa. The uninjured ocular surface of mBD-3−/− mice showed a reduced capacity to clear P. aeruginosa, and their corneal epithelia were more susceptible to bacterial colonization, even when inoculated ex vivo to exclude tear fluid effects. Together, these in vitro and in vivo data show functional roles for AMPs in normal corneal epithelial cell barrier function against P. aeruginosa.
The opportunistic bacterial pathogen Pseudomonas aeruginosa is capable of infecting numerous tissues in the human body, including the airways (nosocomial/ventilator-associated pneumonia), the urinary tract, and the cornea (24, 34, 53). The pathogenesis of P. aeruginosa infections is complex, but in most instances, P. aeruginosa (like other microbial pathogens) must overcome single or multilayered epithelial cell barriers to establish infection. For example, in the cornea, a multilayered epithelium protects the underlying stroma. Indeed, P. aeruginosa corneal infection does not occur in the absence of full-thickness epithelial injury or contact lens wear (32, 43, 54). For this reason, much of what we understand about host-microbe interactions in vivo has been derived from experimental models that deliberately bypass the epithelial barrier (9, 18). How this multilayered epithelium maintains a barrier to microbial traversal during health has not been well studied. However, there is likely much to learn, considering that these cells are highly vulnerable to P. aeruginosa virulence strategies when grown in vitro (10, 12). An understanding of the molecular details of how epithelial cell barrier function is modulated will provide a foundation for studies aimed at an understanding of how it becomes compromised by contact lens wear or other risk factors. Similar knowledge gaps exist for epithelia that line our other body surfaces.
Factors that might enable otherwise vulnerable epithelial cells to form a resistant barrier in vivo could include extraepithelial molecules (e.g., factors in tear fluid or the basement membrane) or epithelial cell-derived factors differentially expressed in the in vivo environment. Potential candidates include secretory IgA (36) or surfactant proteins, e.g., surfactant protein D (SP-D) (28, 45, 46), mucin glycoproteins (13, 16), tight-junction/epithelial polarity (10, 25, 52), and epithelium-derived antimicrobial peptides (AMPs), which can inhibit or kill microbes, e.g., cationic AMPs, including human β-defensin 1 (hBD-1), hBD-2, hBD-3, and the cathelicidin LL-37 (6, 23, 30, 37, 40). To date, few of these have been directly tested for their involvement in limiting epithelial cell traversal by adherent bacteria. For the multilayered corneal epithelium, we have found that the addition of tear fluid and the growth of the cells on basement membrane proteins are each protective against P. aeruginosa traversal (2, 29). We have also recently found that tissue paper blotting of intact murine corneas allowed corneal adhesion, but not traversal, whereas EGTA treatment to disrupt tight junctions, or SP-D gene knockout, allowed full or partial bacterial traversal, respectively (3). Little else has been reported on this topic for these or other epithelial cell types.
The cornea expresses several AMPs, some expressed constitutively and others upregulated in response to microbial antigens (15, 37, 38, 40). AMPs are known to have diverse functions, including direct antimicrobial activity, phagocyte chemotaxis, and contributions to wound healing. Alone or in combination, they are thought to help protect the cornea from microbial pathogens. However, their relative contributions have been studied only during active infection. For example, for P. aeruginosa infections enabled using a scarification method, previous studies have found that a murine defensin, murine β-defensin 3 (mBD-3) (an ortholog of hBD-2) promoted disease resolution (56), while flagellin-mediated, cathelicidin-related antimicrobial peptide (CRAMP) expression can also reduce disease severity (27).
In this study, we hypothesized that corneal antimicrobial peptides also participate in protecting healthy cornea (i.e., resistant epithelium) against P. aeruginosa. This was tested by using human corneal epithelia grown as multilayers in vitro and also nonscarified mouse corneas.
MATERIALS AND METHODS
Bacteria and preparation of culture supernatant.
Pseudomonas aeruginosa strain PAO1 (12) (expressing green fluorescent protein [GFP] on plasmid pSMC2) was used unless otherwise stated (43). Bacteria were grown on Trypticase soy agar (BD Biosciences, CA) supplemented with carbenicillin (300 μg/ml) at 37°C for ∼16 h and then resuspended in keratinocyte growth medium (KGM-2 medium) without antibiotics at a concentration of ∼108 CFU/ml (optical density [OD] at 650 nm of ∼0.1). This plasmid is stably retained by P. aeruginosa without antibiotic selection even after 48 h in vivo (31). Inocula were then prepared by diluting this suspension in KGM-2 medium to a final concentration of ∼106 CFU/ml for use in most experiments. In vivo experiments involved a higher inoculum of ∼109 CFU in 5 μl (∼1011 CFU/ml). Viable counts were used to confirm inoculum size. In some experiments, corneal epithelial cells were pretreated with a PAO1 culture supernatant prior to inoculation with whole bacteria of strain PAO1. The supernatant was prepared by growing strain PAO1 in Trypticase soy broth (BD Biosciences) with shaking at 250 rpm at 37°C for ∼16 h, subculturing into fresh medium, and allowing growth to the mid-logarithmic phase (OD at 650 nm of ∼0.7). The culture was centrifuged at 10,000 rpm (∼10,000 × g) at 4°C for 50 min, and the supernatant was then removed and filtered through a 0.2-μm membrane filter (Corning, Inc., NY) to remove whole bacteria but leaving pathogen-associated molecular patterns (PAMPs), including lipopolysaccharide (LPS), flagellin, and pilin and other extracellular components (35, 40). The culture supernatant was prepared as a batch and frozen in aliquots (−20°C). When needed for experiments, an aliquot of the supernatant was defrosted and diluted (1:5) in KGM-2 medium.
Cell culture.
Telomerase-immortalized human corneal epithelial (HCE) cells were maintained in KGM-2 medium (Lonza, MD) containing 0.15 mM CaCl2 (low calcium) in T75 tissue flasks (Falcon Labware; BD Biosciences, CA) (in 5% CO2 at 37°C) and passaged when cells reached 80% confluence. Polarized multilayers of these epithelial cells were generated by seeding 5.0 × 104 HCE cells/ml onto 12-well collagen-coated tissue culture inserts (3.0-μm pore size; Corning, Inc., NY) in KGM-2 medium containing 1.15 mM CaCl2 (high calcium) as previously described (50). Briefly, cells were submerged in high-calcium KGM-2 medium on both apical (upper) and basal (lower) compartments. The medium was changed on alternate days for up to 4 days. To induce epithelial cell differentiation, the medium was removed from the apical compartment, exposing cells to an air-liquid interface (air lifting). The medium in the basal compartment was changed every day during air lifting for 7 days. Transepithelial resistance (TER) readings were collected by using an epithelial voltohmmeter (EVOM) (World Precision Instruments, Inc., Sarasota, FL). Cells were used for experiments when the TER measurement was ≥200 Ω/cm2.
In vitro traversal assay.
Traversal assays involved a methodology similar to that described previously (1, 2, 29). Briefly, air-lifted HCE cells were cultured on 3.0-μm-pore-size filters as described above (Fig. 1). PAO1 inocula (106 CFU in 1 ml of medium, unless otherwise stated) were carefully added to the apical compartment and incubated with the cells (in 5% CO2 at 37°C) for up to 8 h. Apical and basal compartments were sampled at 4 h and/or 8 h postinoculation to determine numbers of viable bacteria. In control experiments (not shown), the sampling of the basal compartment at 0 h did not result in a recovery of viable bacteria. Uninfected corneal epithelial cells were sham inoculated as controls. The internalization of PAO1 by the HCE cells was quantified by using gentamicin survival assays by removing medium from both the apical and basal compartments, washing with phosphate-buffered saline (PBS), and then immersing the inserts into high-calcium KGM-2 medium supplemented with gentamicin (200 μg/ml; Lonza, MD) for 1 h to kill extracellular bacteria. The inserts were then washed once with PBS, treated with 0.25% (vol/vol) nonionic surfactant (Triton X-100; Sigma, MO) in PBS for 2 min, and vortexed to further disrupt eukaryotic membranes. Viable counts were performed for the cell lysate. All traversal and internalization assays were performed in triplicate. TER controls included EGTA-treated (disrupted tight junctions) and sham-inoculated (intact tight junctions) cells. In control experiments (not shown), fluorescein isothiocyanate (FITC)-conjugated inulin (3.5 kDa; 100 μg/ml in culture medium) was added to the apical compartment of uninfected epithelial cells during the experimental procedure to measure epithelial cell permeability. Aliquots of basal medium were taken, and fluorescence was measured (absorbance at 490 nm) using a standard enzyme-linked immunosorbent assay (ELISA) plate reader. No significant changes in epithelial cell permeability were observed over an 8-h time period.
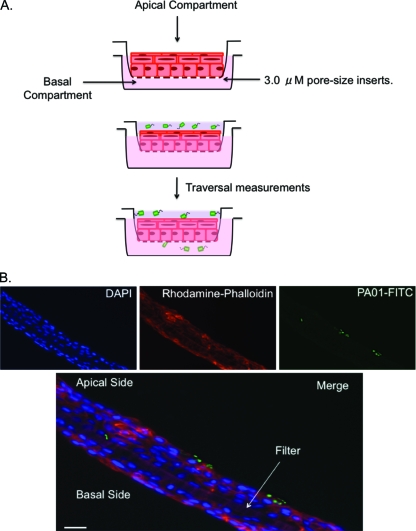
FIG. 1.
Schematic diagram (A) and immunofluorescence microscopy pictures (B) illustrating the in vitro traversal model. Human corneal epithelial cells were grown air lifted on Transwell tissue culture inserts (3-μm pore size) under high-calcium conditions (1.15 mM) to induce a polarized multilayered epithelium. To measure bacterial traversal, bacteria were added only to the apical compartment, and bacteria traversing to the basal compartment were enumerated at various times postinoculation. Fluorescence microscopy using rhodamine-conjugated phalloidin (red) to label the actin cytoskeleton and DAPI (blue) to label cell nuclei showed that the cells formed a multilayered epithelium to which P. aeruginosa (FITC labeled using antibody to PAO1 [green]) can adhere and traverse (B). Magnification, ∼×1,000. Bar, 10 μm.
RNA interference.
The target-specific knockdown of antimicrobial peptides was performed by using small interfering RNA (siRNA) for hBD-1 to hBD-3 or LL-37 with an appropriate scrambled control (Santa Cruz Biotechnology, CA) using Lipofectamine RNAiMAX (Invitrogen, CA) transfection according to the manufacturer's instructions. siRNA transfection was started 2 days prior to bacterial inoculation (traversal assay) by adding siRNA (8 μM) to the apical and basal compartments. On the day of infection 4 μM siRNA was added to the apical compartment only. The knockdown efficiency of each antimicrobial peptide was tested by using Western immunoblotting.
SDS-PAGE and Western immunoblotting.
Corneal epithelial cells collected from the culture inserts were pooled, resuspended in lysis buffer (1% sodium dodecyl sulfate [SDS] and 50 mM Tris-HCl [pH 8.0]), and boiled for 3 min. The culture supernatant was concentrated by using a precipitation method with a 10% trichloroacetic acid (TCA)-acetone solution. The protein concentration was determined for both supernatant and corneal epithelial cells by using a DC protein assay kit (Bio-Rad, CA) with bovine serum albumin as standards. Standardized concentrations of each fraction were added to Tricine sample buffer (Bio-Rad, CA). Total proteins were separated at equal concentrations from scramble control and siRNA-treated hBD-1 to hBD-3 or LL-37 samples and the control peptide (2 μg; Abcam, MA) using a 10 to 20% Tris-Tricine-peptide gel (Bio-Rad, CA) under denaturing conditions. Proteins were transferred onto polyvinylidene difluoride (PVDF) (Bio-Rad, CA) membranes using standard tank blot buffer and standard conditions (100 mV for 2 h). Membranes were then blocked overnight at 4°C using 5% nonfat dry milk prepared in PBS-T (1× PBS containing 0.05% Tween 20) (Fisher Scientific, PA). Membranes were incubated with primary rabbit anti-human hBD-1 to hBD-3 or LL-37 antibodies (diluted 1/500 in PBS-T containing 1% nonfat milk) (Santa Cruz Biotechnology, CA) for 2 h, followed by goat anti-rabbit IgG peroxidase secondary antibody (diluted 1/5,000 in PBS-T containing 1% nonfat milk) (Abcam, MA) for 2 h at 4°C, washed three times for 10 min each with PBS-T, and developed by using Amersham ECL Plus (GE Healthcare, NJ) according to the manufacturer's instructions.
Real-time RT-PCR.
Total RNA was isolated from corneal epithelial cells using Trizol (Invitrogen, CA) according to the manufacturer's instructions. Specific primer sets for target genes were previously described and are shown in Table 1. Total RNA was checked for integrity and the absence of DNA by PCR prior to cDNA synthesis using 1 μg of total RNA and the GeneAmp RNA PCR kit (Applied Biosystems, CA), according to the manufacturer's instructions. mRNA transcript concentrations were measured using SYBR green PCR master mix (Applied Biosystems, CA). Reactions were carried out by using the Step One Plus real-time PCR system (Applied Biosystems, CA) under the following conditions: 95°C for 10 min followed by 40 cycles of 95°C for 30 s, 58°C for 1 min, and 72°C for 30 s. The data acquisition step was set at 58°C, with a final melt-curve analysis to ensure the amplification of a single product. Transcript levels of each gene were normalized to that of the internal control β-actin, and expression was calculated by using the 2−ΔΔCT method (33). The absence of DNA contamination was verified by using negative controls in which reverse transcriptase (RT) was omitted from the master mix.
TABLE 1.
Real-time RT-qPCR primers used in this study
| Gene | Sequence (5′-3′)a | Reference |
|---|---|---|
| hBD-1 | GGAGGGCAATGTCTCATTCTG (F) | 47 |
| CTCTGTAACAGGTGCCTTGAA (R) | ||
| hBD-2 | TCCTCTTCTCGTTCCTCTTCA (F) | 47 |
| AGGGCAAAAGACTGGATGAC (R) | ||
| hBD-3 | CATTATCTTCTGTTTGCTTTGCTC (F) | 47 |
| CGATCTGTTCCTCCTTTGGA (R) | ||
| LL-37 | GAAGACCCAAAGGAATGGCC (F) | 41 |
| CAFAFCCCAGAAGCCTGAGC (R) | ||
| β-Actin | GATTACTGCTCTGGCTCCTAGC (F) | 41 |
| GACTCATCGTACTCCTGCTTGC (R) |
F, forward; R, reverse.
Murine models.
Wild-type C57BL/6 mice (6 to 8 weeks old) and age-matched mBD-3 gene knockout (mBD-3−/−) mice were used. The mBD-3−/− mice were obtained from the Mutant Mouse Regional Resource Center (MMRRC) at the University of California, Davis, CA. The MMRRC provided the control wild-type mice with a genetic background matching that of the mBD-3−/− mice.
To study the impact of AMPs on the defense against epithelial cell traversal by bacteria, eyeballs were removed and immersed in PBS (5 ml) to exclude tear fluid, before superficial blotting with tissue paper (Kimwipe) was performed to enable susceptibility to bacterial adhesion. The eyeballs were then inoculated with 5 μl of a bacterial suspension containing ∼109 CFU for either 4.5 or 7.5 h before processing for confocal microscopy as described below.
The impact of AMPs on bacterial clearance from the living healthy murine ocular surface was quantified by using a null infection model as previously described (43). Briefly, after the induction of anesthesia (intraperitoneal infection with 21 mg/ml ketamine, 2.4 mg/ml xylazine, and 0.3 mg/ml acepromazine), 5 μl of bacterial suspension containing ∼109 CFU was applied onto the healthy ocular surface without prior manipulation of the eye. At 3 h or 6 h postinoculation, ocular surface wash samples were collected (by capillary action) from the lateral canthus after 4 μl of PBS had been added to the surface of the eye. Viable counts were used to determine the number of viable bacteria in the collected samples. All experiments involving animals were conducted under a protocol approved by the Animal Care and Use Committee of the University of California, Berkeley, CA.
Microscopy.
Cultured corneal epithelial cells were imaged by immunofluorescence microscopy with deconvolution. Briefly, Transwell-grown epithelial cells were fixed in neutral-buffered 4% (vol/vol) paraformaldehyde, embedded in Tissue-Tek frozen optimal cutting temperature (OCT) compound (Sakura Finetek, Inc., CA), and frozen in liquid nitrogen. Samples were cross-sectioned at a 10-μm thickness, mounted onto glass microscope slides, and incubated at 37°C overnight to dry the samples. After permeabilization with saponin (0.005%, wt/vol), samples were blocked by using goat serum and bovine serum albumin (BSA) in PBS (3%, wt/vol, each) and then incubated overnight at 4°C with rabbit antisera raised against P. aeruginosa strain PAO1 (49) diluted 1:10,000 in blocking buffer (unless GFP-labeled bacteria were used). Samples were then washed twice in PBS at room temperature for 10 min each, blocked again (5 min), and incubated for 1 h at room temperature with goat polyclonal anti-rabbit IgG (Abcam, MA) diluted 1:5,000 in blocking buffer. After two more PBS washes, samples were labeled with rhodamine-phalloidin according to the manufacturer's instructions (Invitrogen, CA). After two more PBS washes, samples were mounted by using Vectashield 4′,6-diamidino-2-phenylindole (DAPI) embedding medium (Vector Laboratories, Inc., CA) and viewed with an immunofluorescence microscope (Olympus IX-70).
Whole eyes that had been challenged ex vivo with PAO1-GFP were examined by confocal microscopy. After bacterial exposure, eyes were rinsed with PBS three times to remove nonadherent bacteria. Each eyeball was attached to a 12-mm round-glass coverslip with instant glue to maintain an upright position (i.e., cornea facing upwards) and then placed into a 47-mm petri dish filled with tissue culture medium (i.e., KBM medium) to completely cover the ocular surface. Eyes were imaged by using a Zeiss 510 AxioImager Meta/NLO confocal 2-photon microscope equipped with a 63×/0.95 water-dipping objective at the University of California, Berkeley, Molecular Imaging Center. Corneal cells were imaged without chemical fixation or labeling by using a 633-nm laser to obtain confocal images via reflection from the membranes of all cells (live or dead). GFP-expressing bacteria were imaged via fluorescence by excitation at a wavelength of 488 nm. Images were captured with a 2-channel confocal detector and processed with the Zeiss LSM Image Browser. Measurements for the distribution of PAO1-GFP were done by using NIH Image J 1.42q software (http://rsb.info.gov/ij). Three or more random fields of each eye were imaged from the corneal surface through the entire epithelium in 0.5-μm steps (confirmed by the reflection of cells during image acquisition).
Statistical analysis.
Data were expressed as means ± standard deviations (SD) for in vitro traversal assays or medians with upper and lower quartiles for in vivo clearance assays. Statistical significance between groups was determined by using analysis of variance (ANOVA) (with Fisher protected least-significant-difference [PLSD] post hoc analysis) for multiple-group comparisons or an unpaired Student's t test for two groups of normally distributed data. Otherwise, a nonparametric Mann-Whitney test was used to compare two groups. P values of <0.05 were considered significant.
RESULTS
P. aeruginosa traverses telomerase-immortalized multilayered human corneal epithelium without disrupting the TER.
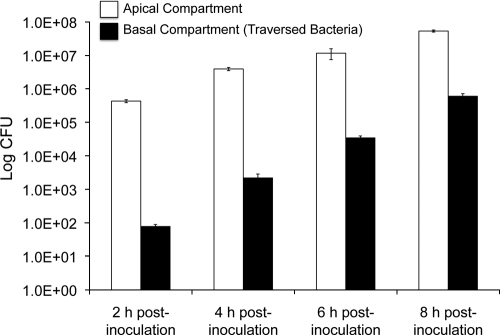
The capacity of P. aeruginosa invasive strain PAO1 to traverse human corneal epithelial cells grown as multilayers on Transwell filters was explored. This was done by performing viable counts of the lower (basal) medium compartment at various times up to 8 h after the addition of bacteria to only the upper (apical) compartment. As expected from previous studies using rabbit corneal epithelia (1, 2, 29), some bacteria traversed the multilayered human corneal epithelia as early as 2 h, with increasing traversal to the basal compartment observed over the remainder of the 8-h period (Fig. 2). As we have previously shown for rabbit corneal epithelial cells (1, 2, 29), P. aeruginosa traversal occurred without a significant reduction of the TER of the cells. After 8 h, the TER of uninfected cells was 231 ± 1 Ω/cm2, compared to 227 ± 4 Ω/cm2 for infected cells (P > 0.05, Student's t test). Control experiments (not shown) indicated that (i) bacterial growth rates in the apical and basal compartments were similar, (ii) bacterial growth in the basal compartment was not sufficient to account for total bacterial numbers at any time in the assay, and (iii) bacteria continued to traverse the epithelia at all times during the assay. However, it is clear that bacterial counts in the basal compartment with time reflect the combination of the continued traversal and growth of previously traversed bacteria.
FIG. 2.
P. aeruginosa traversal of multilayered human corneal epithelial cells in vitro. PAO1 (106 CFU) was added to the apical compartment, and viable counts were taken from the apical (white bar) and basal (black bar) compartments at 2, 4, 6, and 8 h postinoculation. P. aeruginosa traversal was detected at each time point. Data from one of three independent experiments are shown.
Preexposure of multilayered human corneal epithelia to P. aeruginosa culture supernatant reduces bacterial traversal.
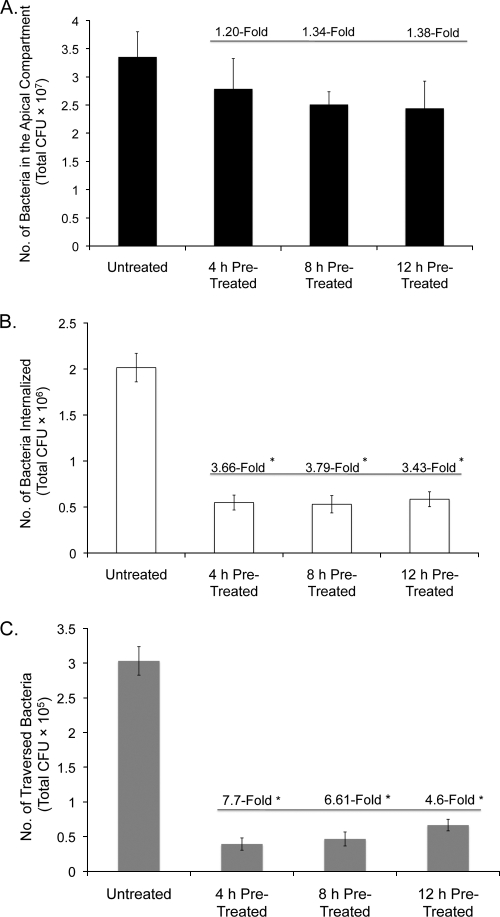
The P. aeruginosa culture supernatant contains bacterial antigens (e.g., LPS, flagellin, and pilin), some of which are known to activate innate host defenses, including the upregulation of antimicrobial peptide expression (15, 35, 40). We explored the impact of culture supernatant preexposure on corneal epithelial susceptibility to P. aeruginosa traversal. Corneal epithelial cells were pretreated with PAO1 culture supernatant (1:5 dilution in KGM-2 tissue culture medium [see Materials and Methods]) for 4, 8, and 12 h and compared to medium-treated controls for susceptibility to bacterial traversal at 8 h postinoculation with ∼106 CFU bacteria (in the continued presence of the supernatant). All pretreatment durations (4, 8, and 12 h) reduced epithelial susceptibility to traversal by up to ∼6.5-fold at 8 h (P = 0.0001 for each comparison of pretreated cells versus cells treated with medium only) (Fig. 3 C). The TER remained stable throughout experiments, remaining within 6% of baseline values. A ∼1.4-fold decrease in bacterial numbers was observed for apical compartments after supernatant treatments, although differences from controls were not significant (Fig. 3A). Bacterial internalization into epithelial cells could provide a possible explanation for decreased PAO1 traversal (1). However, gentamicin survival assays showed that bacterial internalization was also significantly reduced for supernatant-treated cells, by ∼3.5-fold at 8 h (P < 0.001) (Fig. 3B). Similar results were found when this experiment was shortened to 4 h (same pretreatment conditions) except that internalization was not significantly different between samples (data not shown). In each instance, the continued presence of the supernatant was required for these effects. The removal of the supernatant from corneal epithelial cells after pretreatment, and replacement with KGM-2 medium before bacterial inoculation, resulted in the loss of supernatant pretreatment effects (not shown), suggesting that protection against traversal involved supernatant-induced factors secreted from the epithelial cells.
FIG. 3.
P. aeruginosa in vitro traversal assay using multilayered human corneal epithelia that were preexposed to PAO1 culture supernatant (1:5 dilution in KGM-2 medium) for 4, 8, or 12 h before inoculation with 106 CFU of strain PAO1 for 8 h in the continued presence of the supernatant. Controls were treated with KGM-2 medium only. Viable counts were taken from the apical compartment (A), internalized bacteria were assessed by gentamicin survival (B), and viable traversed bacteria (basal compartment) were counted (C). *, P < 0.001 in each instance versus untreated controls (ANOVA with Fisher PLSD post hoc analysis). Data representative of three experiments are shown. Note that the x axis represents different supernatant pretreatment times prior to PAO1 inoculation.
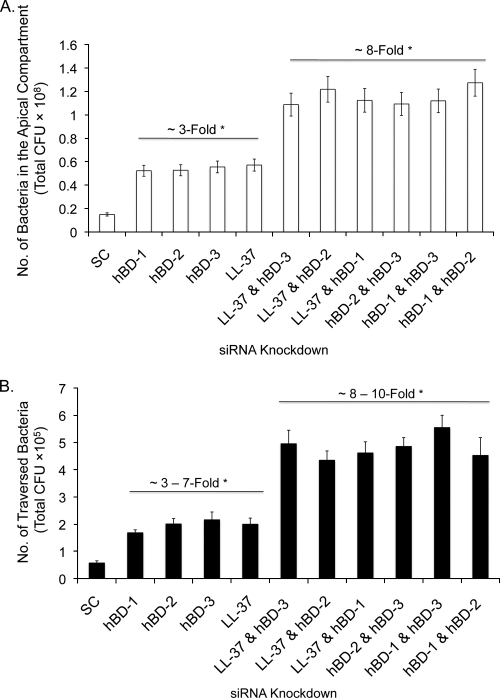
Knockdown of hBD-1, hBD-2, hBD-3, or LL-37 antimicrobial peptides using siRNA enhances P. aeruginosa traversal of multilayered human corneal epithelia.
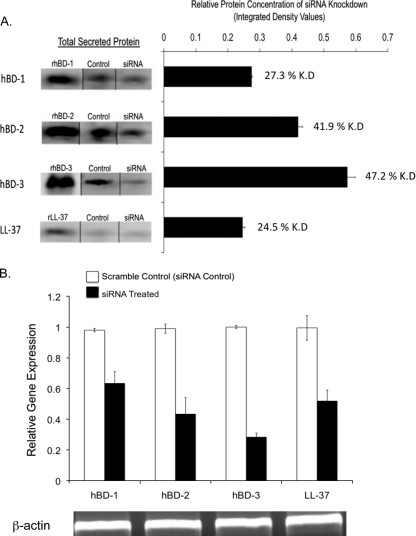
Since AMPs can impact the viability of P. aeruginosa (21), and because preexposure to P. aeruginosa antigens enhances AMP expression by corneal epithelia (15, 35) while increasing resistance to bacterial traversal, we hypothesized that AMPs participate in epithelial defense against bacterial traversal. Small interfering RNA (siRNA) was used to knock down four different AMPs known to be expressed by human corneal epithelium (human β-defensins [hBD-1 to hBD3] and the cathelicidin LL-37). Epithelial cells were then pretreated with bacterial culture supernatant (as described above) for 4 h to provide a stimulus for AMP expression and then inoculated with P. aeruginosa (106 CFU) in the continued presence of the supernatant. After 8 h, Western immunoblotting confirmed the effects of siRNA treatment on reducing AMP peptide expression (Fig. 4A), which was confirmed by real-time RT-quantitative PCR (qPCR) (Fig. 4B). Moreover, bacterial traversal was increased by ∼3-fold in siRNA-treated epithelia compared to scrambled siRNA controls (P < 0.0001 in each instance) (Fig. 5 B). The combination knockdown of multiple antimicrobial peptides resulted in a further enhancement of bacterial traversal by more than 8-fold (P < 0.0001) (Fig. 5B). Importantly, AMP knockdown increased the number of viable bacteria recoverable from the apical compartment, where the inoculum had been added at the beginning of the experiment (P < 0.003 in all instances) (Fig. 5A), showing that AMP knockdown promoted bacterial survival/growth at that location. The magnitudes of the increased apical viable counts were similar to those of increased traversal (basal viable counts) for each sample set, suggesting that the mechanisms were linked. While bacterial traversal caused some reduction of the TER in these experiments (∼9.0% for scrambled siRNA compared to 9.5 to 13% for sequence-specific siRNA), there were no significant effects of siRNA on TER compared to untreated controls either before or after bacteria had traversed the epithelial cells.
FIG. 4.
(A) Immunoblot analysis of secreted hBD-1 to hBD-3 or LL-37 peptide expression by human corneal epithelial cells after siRNA treatment versus scrambled siRNA controls, followed by a 4-h pretreatment with PAO1 culture supernatant (1:5 dilution in KGM-2 medium) and an 8-h exposure to bacteria in a traversal assay in the continued presence of the supernatant. (Left) Two micrograms of recombinant (r) positive-control peptide was included in each experiment. (Right) Relative knockdown (K.D) of each peptide (i.e., the integrated density of immunoblot bands normalized to the scrambled siRNA control for each peptide). (B) Gene expression levels of hBD-1 to hBD-3 or LL-37 in siRNA-treated human corneal epithelial cells (under the same experimental conditions), as measured by RT-qPCR and normalized to β-actin, show successful siRNA knockdown. Data are representative of data from three similar experiments, each using six pooled culture inserts.
FIG. 5.
P. aeruginosa traversal of human corneal epithelia in vitro after siRNA knockdown of AMPs. Cells were treated with siRNA specific to hBD-1 to hBD-3 or LL-37 or with a scrambled siRNA control (SC) stimulated with bacterial culture supernatant (1:5 in KGM-2 medium) for 4 h prior to inoculation with 106 CFU of PAO1 in the continued presence of the supernatant. Viable counts of the apical (A) and basal (B) compartments showed significant increases in bacterial survival in the apical compartment, and in bacterial traversal to the basal compartment, in siRNA-treated cells versus the scrambled control at 8 h postinoculation. Effects on bacterial survival and traversal were greater when AMPs were knocked down in combination. *, P < 0.001 (by a Student's t test) in each instance, compared to scrambled siRNA controls. Results represent data from one of three independent experiments.
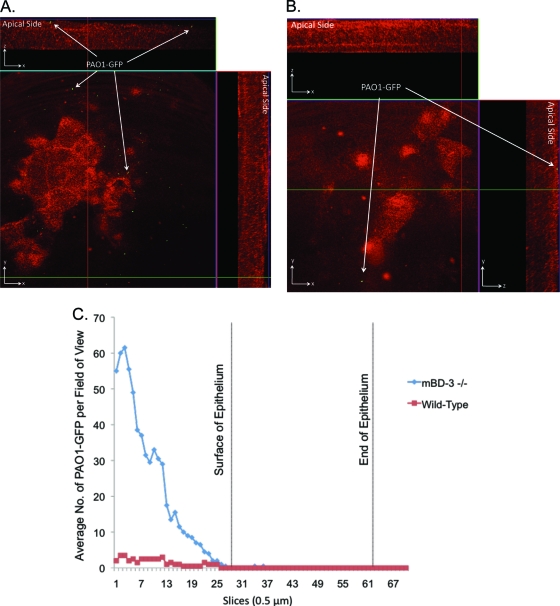
P. aeruginosa colonizes the corneas of mBD-3-deficient, but not wild-type, mice ex vivo.
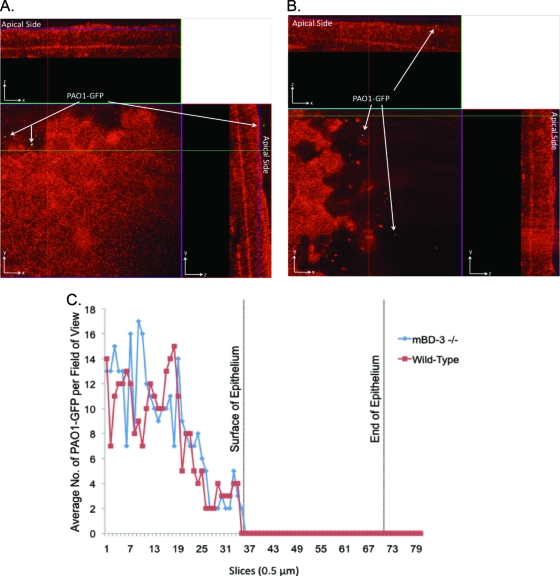
Since in vitro studies showed a role(s) for AMPs in preventing P. aeruginosa traversal of corneal epithelia, we began to test the role of AMPs in preventing bacterial colonization of nonscarified corneas in vivo, using AMP gene knockout mice. To ensure that bacterial inocula were matched for wild-type and AMP mutant corneas throughout the assays, epithelial cell susceptibility to bacteria was examined by using eyeballs ex vivo to exclude tear fluid. This was done because some tear fluid AMPs could impact bacterial viability or the ability to adhere/traverse. This also served to exclude known effects of tear fluid on AMP efficacy (22). mBD-3 (a murine ortholog of hBD-2) was chosen for study given its recently reported contributions to the resolution of P. aeruginosa corneal infections established by scratch injury (56). Enucleated whole eyeballs of mBD-3−/− C57BL/6 mice, and wild-type controls, were challenged with 5 μl of a bacterial suspension containing ∼109 CFU of PAO1-GFP for 4.5 or 7.5 h. Corneal epithelia were imaged without fixation or labeling using confocal microscopy (see Materials and Methods). Ex vivo, the mBD-3−/− mice showed increased susceptibility to P. aeruginosa adherence at 4.5 h postinoculation (Fig. 6A) compared to the wild type, with the latter showing little or no attachment (Fig. 6B). Adherent bacteria did not penetrate the corneal epithelium of either mBD-3−/− or wild-type mice (Fig. 6A and B). Differences in adherence, and the lack of epithelial cell traversal, were clearly seen when bacterial numbers on inoculated corneas were quantified (Fig. 6C). Adherent bacteria still did not penetrate the corneal epithelium of either mutant or wild-type eyes after 7.5 h (Fig. 7). At this later time point, differences in bacterial adherence between mBD-3−/− and wild-type mice were no longer apparent, with wild-type corneas also showing susceptibility to bacterial adhesion (Fig. 7).
FIG. 6.
(A and B) Confocal reflection microscopy of mBD-3−/− (A) or wild-type (B) C57BL/6 mouse eyes at 4.5 h after inoculation ex vivo with ∼109 CFU P. aeruginosa strain PAO1-GFP (green). Eyeballs were carefully enucleated, rinsed with PBS, and then tissue paper blotted before bacterial challenge (see Materials and Methods). z-stack images were split into an orthogonal view to show x, y, and z planes of the corneal epithelium. Bacteria showed greater adherence to mBD-3−/− corneas (red) but did not traverse the epithelium. (C) These differences were clearly shown by quantifying the bacterial distribution over the corneal epithelium (red indicates the reflection of corneal epithelial cells at 633 nm). Magnification, ×∼1,000.
FIG. 7.
(A and B) Confocal reflection microscopy of mBD-3−/− (A) or wild-type (B) C57BL/6 mouse eyes at 7.5 h after inoculation ex vivo with ∼109 CFU P. aeruginosa strain PAO1-GFP (green). Eyeballs were carefully enucleated, rinsed with PBS, and then tissue paper blotted before bacterial challenge (see Materials and Methods). z-stack images were split into an orthogonal view to show x, y, and z planes of the intact corneal epithelium. After 7.5 h, bacteria showed equal levels of adherence to mBD-3−/− and wild-type corneas but still did not traverse the epithelium. (C) These data were confirmed by quantifying the bacterial distribution over the corneal epithelium (red indicates the reflection of corneal epithelial cells at 633 nm). Magnification, ×∼1,000.
mBD-3−/− mice show a delayed clearance of P. aeruginosa from the ocular surface in vivo.
The ability of mBD-3−/− mice to clear PAO1 from the healthy ocular surface after in vivo inoculation (i.e., in the presence of tear fluid and other in vivo factors) was explored by using a null infection model which utilizes an uninjured cornea (43). As shown in Fig. 8, the ocular surfaces of mBD-3−/− mice were colonized by significantly more viable bacteria at both 3 and 6 h postinoculation with ∼109 CFU of P. aeruginosa (∼190-fold at 3 h and 32-fold at 6 h; P < 0.03 for both comparisons).
FIG. 8.
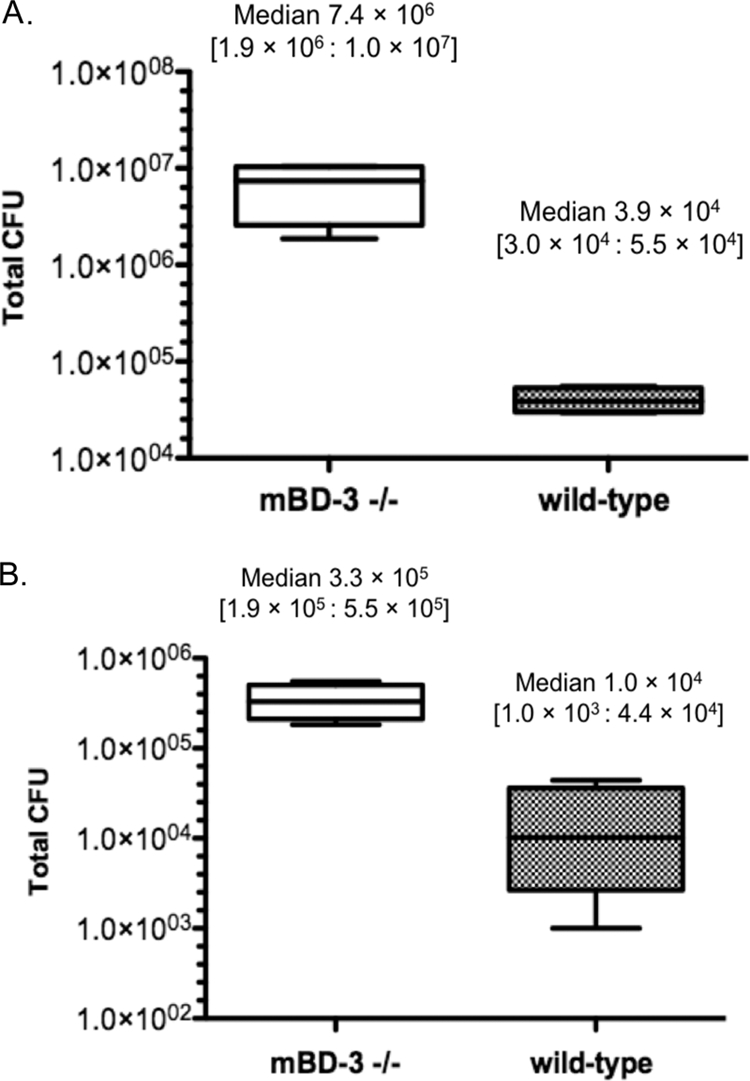
Viable P. aeruginosa strain PAO1 in the tear fluid of mBD-3−/− versus wild-type C57BL/6 mice at 3 h (A) or 6 h (B) postinoculation with ∼109 CFU bacteria in vivo using the null infection clearance model (n = 6 to 8 mice per group) (43). Data are expressed as a median (lower quartile:upper quartile). *, P < 0.03 by a Mann-Whitney test. Data from one of three independent experiments are shown.
DISCUSSION
The results of this study show that antimicrobial peptides (AMPs), specifically human β-defensins (hBD-1, -2, and -3) and the human cathelicidin LL-37, contribute to the ability of human corneal epithelial cells to resist traversal by P. aeruginosa in vitro. They also show that the murine β-defensin mBD-3 (an ortholog of human hBD-2) contributes to the clearance of P. aeruginosa from the healthy mouse eye in vivo and additionally limits P. aeruginosa adhesion to the corneal epithelial surface. These data suggest that in addition to their role in innate defense responses to ocular injury and infection (27, 56), AMPs are also involved in maintaining normal resistance to infection when the tissue is healthy.
Previous studies investigating mechanisms of P. aeruginosa traversal have been done mostly using epithelial cell monolayers grown on permeable filters, e.g., MDCK cells (4, 5, 19, 20), and some have a found concomitant loss of TER, suggesting that a disruption of epithelial tight junctions is involved. The rhamnolipid-mediated disruption of tight junctions was associated with the P. aeruginosa traversal of primary cultured multilayered human airway epithelia (58), as was the type III secreted effector ExoS for monolayers of airway epithelia (51). However, our results with corneal epithelial cell multilayers differ in that they show P. aeruginosa traversal can occur without TER disruption. This is consistent with our previous studies using rabbit corneal epithelial cells (1, 29). Thus, reduced TER is not a prerequisite for allowing P. aeruginosa to traverse cell multilayers. Moreover, bacterial traversal does not necessarily result in a loss of TER.
It is possible that P. aeruginosa-mediated disruptions in epithelial cell polarity involving the expression of basolateral membrane proteins on apical cell surfaces (26) could impact the traversal of P. aeruginosa observed in the present study. P. aeruginosa-induced cell polarity changes, which we have shown can occur without a disruption of tight junctions (26), would promote the internalization of bacteria into epithelial cells (10). Growth factor-mediated polarity changes could have the same effect (11). We have previously shown that the traversal of multilayered rabbit corneal epithelia by invasive P. aeruginosa involves twitching motility, which we also found to be involved in the exit of epithelial cells after internalization (1). How cell internalization (and therefore cellular exit) participates in cellular traversal mechanisms of P. aeruginosa has not yet been elucidated. However, it is of interest that our data continue to show correlations between internalization and traversal; i.e., preexposure to P. aeruginosa antigens reduced susceptibility to both phenomena at 8 h (Fig. 3B and C). Considering how the number of intracellular bacteria was impacted, the data suggest either that cells became more resistant to bacterial entry or that they were more efficient at killing intracellular bacteria. However, since this recovery of viable intracellular bacteria by gentamicin assays depends on how many bacteria are internalized and not just how many survive after internalization, both could be simultaneously impacted, with their effects potentially masking one another. Our observation that antigen pretreatment could reduce P. aeruginosa traversal after 4 h without impacting internalization suggests that these processes can be separated, although the above-described caveats regarding gentamicin survival assays could also apply. Further studies will be needed to discern the relative contribution(s) of internalization to traversal, but the data continue to suggest a role for internalization in the P. aeruginosa traversal of multilayered epithelia that could include contributions to, and defense against, traversal.
It is of interest that the siRNA knockdown of any one of several different AMPs in vitro could promote P. aeruginosa traversal. This finding would suggest that AMP defense against traversal is a multifactorial process involving additive or synergistic effects between peptides, which is partially compromised by the removal of any single contributing peptide. Additive or synergistic effects for antimicrobial peptides have been shown with respect to their antimicrobial activity (8, 44) and in their contributions to the resolution of murine P. aeruginosa corneal infections (56). While it is possible that traversal effects of individual siRNAs involved some secondary effects on other AMPs, our data from the combination siRNA knockdown of dual AMPs clearly supports the suggestion that AMPs exert additive or synergistic effects on traversal.
Since AMPs can have broad-spectrum antimicrobial activity, which includes activity against P. aeruginosa (14, 22, 37, 42), it is likely that at least part of the mechanism for AMP protection against P. aeruginosa traversal involves the killing of bacteria in the in vitro assays. Indeed, the data showed increased bacterial viability at the apical site of inoculation after AMP knockdown in vitro and knockout in vivo. However, AMPs could also impact traversing intercellular bacteria or the processes of internalization and/or intracellular survival (see above). Autocrine and paracrine effects of AMPs on mammalian cells can influence epithelial wound healing and cytokine expression (15, 37, 42). Thus, it remains possible that the role of AMPs in protection against P. aeruginosa traversal in vitro also involves other indirect effects via other epithelium-derived factors or multilayer integrity.
Our data demonstrated a role for mBD-3, a murine ortholog of hBD-2, in clearing bacteria from the healthy mouse eye in vivo. This is of interest considering that tear fluid has been shown to interfere with the antimicrobial function of its ortholog hBD-2 albeit in vitro (22). In a previous study, we found that SP-D can also contribute to bacterial clearance from the healthy ocular surface (43), yet it has weak antimicrobial activity against the strains tested. However, it is likely that many factors involved in clearance at the ocular surface work additively or synergistically to increase their antimicrobial activity against P. aeruginosa (22, 55), thereby also countering the negative impact of potential modulators such as tear fluid.
The pretreatment of cells with bacterial culture supernatant, containing multiple bacterial factors with the potential to activate Toll-like receptor (TLR)-mediated signaling (7, 48), reduced cell susceptibility to P. aeruginosa traversal. We and others have shown that AMP expression in epithelial cells can be upregulated by this supernatant or by purified factors contained within it (15, 35, 40, 57). The siRNA data presented in this study show that multiple epithelially expressed AMPs participate in protection against traversal. Thus, it is likely that the culture supernatant induces a defense against traversal via AMP upregulation. However, the culture supernatant could have a plethora of other effects on the innate immunity of these cells beyond AMP upregulation, and some of these might also modulate their susceptibility to P. aeruginosa traversal. A potential candidate includes surfactant protein D, which we previously showed to be upregulated by either P. aeruginosa LPS or flagellin. SP-D inhibits P. aeruginosa invasion of corneal epithelial cells (45, 46) and helps defend against P. aeruginosa traversal of the corneal epithelium in vivo (3). Other candidates include mucin glycoproteins, which can also be upregulated by bacterial antigens and can prevent P. aeruginosa attachment to epithelial surfaces (13, 17, 39).
While knocking down hBD-2 in human corneal epithelial cells in vitro promoted bacterial traversal, the knockout of mBD-3 (an ortholog of hBD-2) in mice promoted bacterial adherence but not bacterial traversal. While this could be because mBD-3 plays no role in preventing bacterial traversal after adherence, it is more likely to reflect functional redundancy with other traversal defense factors. Indeed, the siRNA data showed additive effects among AMPs for cells grown in vitro, suggesting that functional redundancy exists. There may also be a compensatory upregulation of other protective factors in the mBD-3 gene knockout animals; e.g., CRAMP has also been shown to have a protective role in P. aeruginosa infection (27). While we were unable to prove directly that mBD-3 protects mouse corneas against bacterial traversal, other data did show that it participates in maintaining ocular surface resistance to bacteria during health, i.e., in clearing bacteria from the healthy ocular surface and protecting the corneal epithelium against bacterial adherence, even when experiments were done ex vivo to compensate for differences in clearance. The mechanism for these protective roles in vivo could involve the killing of approaching bacterial cells (22), but effects on the epithelial cell expression of other antimicrobial/antiadherence factors, or even on resident dendritic cells of the cornea, cannot be ruled out.
In summary, the capacity to cross the epithelial cell multilayers is likely to be a critical virulence strategy for both opportunistic and outright pathogens that infect body surfaces in the absence of overt tissue injury. However, much of what has been learned about epithelial cell defense against microbes has been derived from one-on-one bacterium-cell interaction studies using epithelial cells grown as monolayers on plastic or glass, with relevance to barrier function only assumed. This study adds to a growing body of literature suggesting that a complex set of events occurs when microbes attempt to cross the epithelial cell multilayers. Further studies to elucidate the mechanisms involved in epithelial cell traversal by bacteria, the barriers that normally protect us against it during health, and how these are compromised during susceptibility, using models that enable this critical step in pathogenesis to be studied directly, will enhance our understanding of microbial keratitis and of other epithelium-associated infections.
Acknowledgments
This work was supported by NIH research grant EY011221 (S.M.J.F.) and Ruth L. Kirschstein National Research Service award EY019456 (D.K.A.).
We thank Gerald B. Pier (Harvard Medical School) for providing antibody against P. aeruginosa strain PAO1.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 29 November 2010.
REFERENCES
- 1.Alarcon, I., D. J. Evans, and S. M. Fleiszig. 2009. The role of twitching motility in Pseudomonas aeruginosa exit from and translocation of corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 50:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alarcon, I., L. Kwan, C. Yu, D. J. Evans, and S. M. Fleiszig. 2009. Role of the corneal epithelial basement membrane in ocular defense against Pseudomonas aeruginosa. Infect. Immun. 77:3264-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alarcon, I., et al. Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Invest. Ophthalmol. Vis. Sci., in press. [DOI] [PMC free article] [PubMed]
- 4.Azghani, A. O. 1996. Pseudomonas aeruginosa and epithelial permeability: role of virulence factors elastase and exotoxin A. Am. J. Respir. Cell Mol. Biol. 15:132-140. [DOI] [PubMed] [Google Scholar]
- 5.Azghani, A. O., L. D. Gray, and A. R. Johnson. 1993. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect. Immun. 61:2681-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bals, R., et al. 1999. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect. Immun. 67:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton, G. M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 300:1524-1525. [DOI] [PubMed] [Google Scholar]
- 8.Chen, X., et al. 2005. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J. Dermatol. Sci. 40:123-132. [DOI] [PubMed] [Google Scholar]
- 9.Evans, D. J., N. A. McNamara, and S. M. Fleiszig. 2007. Life at the front: dissecting bacterial-host interactions at the ocular surface. Ocul. Surf. 5:213-227. [DOI] [PubMed] [Google Scholar]
- 10.Fleiszig, S. M., et al. 1997. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65:2861-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleiszig, S. M., et al. 1998. Susceptibility of epithelial cells to Pseudomonas aeruginosa invasion and cytotoxicity is upregulated by hepatocyte growth factor. Infect. Immun. 66:3443-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleiszig, S. M., et al. 1997. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleiszig, S. M., T. S. Zaidi, R. Ramphal, and G. B. Pier. 1994. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect. Immun. 62:1799-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 15.Gao, N., A. Kumar, J. Jyot, and F. S. Yu. Flagellin-induced corneal antimicrobial peptide production and wound repair involve a novel NF-kappaB-independent and EGFR-dependent pathway. PLoS One 5:e9351. [DOI] [PMC free article] [PubMed]
- 16.Gipson, I. K., and P. Argueso. 2003. Role of mucins in the function of the corneal and conjunctival epithelia. Int. Rev. Cytol. 231:1-49. [DOI] [PubMed] [Google Scholar]
- 17.Hauber, H. P., et al. 2007. LPS-induced mucin expression in human sinus mucosa can be attenuated by hCLCA inhibitors. J. Endotoxin Res. 13:109-116. [DOI] [PubMed] [Google Scholar]
- 18.Hazlett, L. D. 2004. Corneal response to Pseudomonas aeruginosa infection. Prog. Retin. Eye Res. 23:1-30. [DOI] [PubMed] [Google Scholar]
- 19.Hirakata, Y., et al. 2000. Penetration of clinical isolates of Pseudomonas aeruginosa through MDCK epithelial cell monolayers. J. Infect. Dis. 181:765-769. [DOI] [PubMed] [Google Scholar]
- 20.Hirakata, Y., et al. 2002. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J. Exp. Med. 196:109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, L. C., D. Jean, R. J. Proske, R. Y. Reins, and A. M. McDermott. 2007. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr. Eye Res. 32:595-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, L. C., R. L. Redfern, S. Narayanan, R. Y. Reins, and A. M. McDermott. 2007. In vitro activity of human beta-defensin 2 against Pseudomonas aeruginosa in the presence of tear fluid. Antimicrob. Agents Chemother. 51:3853-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, L. C., R. Y. Reins, R. L. Gallo, and A. M. McDermott. 2007. Cathelicidin-deficient (Cnlp−/−) mice show increased susceptibility to Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 48:4498-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim, Y. W., D. L. Boase, and I. A. Cree. 2009. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br. J. Ophthalmol. 93:1319-1324. [DOI] [PubMed] [Google Scholar]
- 25.Kazmierczak, B. I., K. Mostov, and J. N. Engel. 2001. Interaction of bacterial pathogens with polarized epithelium. Annu. Rev. Microbiol. 55:407-435. [DOI] [PubMed] [Google Scholar]
- 26.Kierbel, A., et al. 2007. Pseudomonas aeruginosa exploits a PIP3-dependent pathway to transform apical into basolateral membrane. J. Cell Biol. 177:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar, A., N. Gao, T. J. Standiford, R. L. Gallo, and F. S. Yu. 2010. Topical flagellin protects the injured corneas from Pseudomonas aeruginosa infection. Microbes Infect. 12:978-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuroki, Y., M. Takahashi, and C. Nishitani. 2007. Pulmonary collectins in innate immunity of the lung. Cell. Microbiol. 9:1871-1879. [DOI] [PubMed] [Google Scholar]
- 29.Kwong, M. S., D. J. Evans, M. Ni, B. A. Cowell, and S. M. Fleiszig. 2007. Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infect. Immun. 75:2325-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laube, D. M., S. Yim, L. K. Ryan, K. O. Kisich, and G. Diamond. 2006. Antimicrobial peptides in the airway. Curr. Top. Microbiol. Immunol. 306:153-182. [DOI] [PubMed] [Google Scholar]
- 31.Lee, E. J., B. A. Cowell, D. J. Evans, and S. M. Fleiszig. 2003. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest. Ophthalmol. Vis. Sci. 44:3892-3898. [DOI] [PubMed] [Google Scholar]
- 32.Lee, E. J., D. J. Evans, and S. M. Fleiszig. 2003. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Invest. Ophthalmol. Vis. Sci. 44:5220-5227. [DOI] [PubMed] [Google Scholar]
- 33.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 34.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 35.Maltseva, I. A., et al. 2007. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human beta-defensin-2 in response to antigens of Pseudomonas aeruginosa. Exp. Eye Res. 85:142-153. [DOI] [PubMed] [Google Scholar]
- 36.Masinick, S. A., C. P. Montgomery, P. C. Montgomery, and L. D. Hazlett. 1997. Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Invest. Ophthalmol. Vis. Sci. 38:910-918. [PubMed] [Google Scholar]
- 37.McDermott, A. M. 2009. The role of antimicrobial peptides at the ocular surface. Ophthalmic Res. 41:60-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDermott, A. M., et al. 2003. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 44:1859-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNamara, N., and C. Basbaum. 2001. Signaling networks controlling mucin production in response to Gram-positive and Gram-negative bacteria. Glycoconj. J. 18:715-722. [DOI] [PubMed] [Google Scholar]
- 40.McNamara, N. A., R. Van, O. S. Tuchin, and S. M. Fleiszig. 1999. Ocular surface epithelia express mRNA for human beta defensin-2. Exp. Eye Res. 69:483-490. [DOI] [PubMed] [Google Scholar]
- 41.Mendez-Samperio, P., E. Miranda, and A. Trejo. 2008. Expression and secretion of cathelicidin LL-37 in human epithelial cells after infection by Mycobacterium bovis bacillus Calmette-Guerin. Clin. Vaccine Immunol. 15:1450-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menendez, A., and B. B. Finlay. 2007. Defensins in the immunology of bacterial infections. Curr. Opin. Immunol. 19:385-391. [DOI] [PubMed] [Google Scholar]
- 43.Mun, J. J., et al. 2009. Clearance of Pseudomonas aeruginosa from a healthy ocular surface involves surfactant protein D and is compromised by bacterial elastase in a murine null-infection model. Infect. Immun. 77:2392-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagaoka, I., S. Hirota, S. Yomogida, A. Ohwada, and M. Hirata. 2000. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm. Res. 49:73-79. [DOI] [PubMed] [Google Scholar]
- 45.Ni, M., et al. 2005. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect. Immun. 73:2147-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ni, M., et al. 2008. Expression of surfactant protein D in human corneal epithelial cells is upregulated by Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 54:177-184. [DOI] [PubMed] [Google Scholar]
- 47.Paris, S., M. Wolgin, A. M. Kielbassa, A. Pries, and A. Zakrzewicz. 2009. Gene expression of human beta-defensins in healthy and inflamed human dental pulps. J. Endod. 35:520-523. [DOI] [PubMed] [Google Scholar]
- 48.Pearlman, E., et al. 2008. Toll-like receptors at the ocular surface. Ocul. Surf. 6:108-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Priebe, G. P., G. J. Meluleni, F. T. Coleman, J. B. Goldberg, and G. B. Pier. 2003. Protection against fatal Pseudomonas aeruginosa pneumonia in mice after nasal immunization with a live, attenuated aroA deletion mutant. Infect. Immun. 71:1453-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robertson, D. M., et al. 2005. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest. Ophthalmol. Vis. Sci. 46:470-478. [DOI] [PubMed] [Google Scholar]
- 51.Soong, G., D. Parker, M. Magargee, and A. S. Prince. 2008. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J. Bacteriol. 190:2814-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sosnova-Netukova, M., P. Kuchynka, and J. V. Forrester. 2007. The suprabasal layer of corneal epithelial cells represents the major barrier site to the passive movement of small molecules and trafficking leukocytes. Br. J. Ophthalmol. 91:372-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stapleton, F., et al. 2008. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology 115:1655-1662. [DOI] [PubMed] [Google Scholar]
- 54.Tam, C., J. J. Mun, D. J. Evans, and S. M. J. Fleiszig. 2010. The impact of inoculation parameters on the pathogenesis of contact lens-related infectious keratitis. Invest. Ophthalmol. Vis. Sci. 51:3100-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, H., et al. 2003. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J. Clin. Invest. 111:1589-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu, M., S. A. McClellan, R. P. Barrett, Y. Zhang, and L. D. Hazlett. 2009. Beta-defensins 2 and 3 together promote resistance to Pseudomonas aeruginosa keratitis. J. Immunol. 183:8054-8060. [DOI] [PubMed] [Google Scholar]
- 57.Yu, F. S., et al. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J. Immunol. 185:1142-1149. [DOI] [PMC free article] [PubMed]
- 58.Zulianello, L., et al. 2006. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect. Immun. 74:3134-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]