Abstract
Invasive aspergillosis is a major threat for patients suffering from chronic granulomatous disease (CGD). Although Aspergillus fumigatus is the most commonly encountered Aspergillus species, the presence of A. nidulans appears to be disproportionately high in CGD patients. The purpose of this study was to investigate the involvement of the NADPH oxidase and the resulting reactive oxygen species (ROS) in host defense against fungi and to clarify their relationship toward A. nidulans. Murine CGD alveolar macrophages (AM) and polymorphonuclear leukocytes (PMN) and peripheral blood mononuclear cells (PBMC) from healthy controls and CGD patients were challenged with either A. fumigatus or A. nidulans. Analysis of the antifungal effects of ROS revealed that A. nidulans, in contrast to A. fumigatus, is not susceptible to ROS. In addition, infection with live A. nidulans did not result in any measurable ROS release. Remarkably, human CGD PMN and PBMC and murine CGD AM were at least equipotent at arresting conidial germination compared to healthy controls. Blocking of the NADPH oxidase resulted in significantly reduced damage of A. fumigatus but did not affect A. nidulans hyphae. Furthermore, the microbicidal activity of CGD PMN was maintained toward A. nidulans but not A. fumigatus. In summary, antifungal resistance to A. nidulans is not directly ROS related. The etiology of A. nidulans infections in CGD cannot be explained by the simple absence of the direct microbicidal effect of ROS. In vivo, the NADPH oxidase is a critical regulator of innate immunity whose unraveling will improve our understanding of fungal pathogenesis in CGD.
Chronic granulomatous disease (CGD) is a rare (prevalence, 1/250,000 individuals) inherited immunodeficiency disorder of the NADPH oxidase in which phagocytes fail to generate the microbicidal reactive oxidant superoxide anion and its metabolites due to a mutation in any of the five structural components of the NADPH oxidase complex. As a result, CGD patients are characterized by recurrent life-threatening bacterial and fungal infections as well as chronic inflammatory diseases due to dysregulated inflammatory pathways. The lifetime incidence of invasive aspergillosis (IA) in children with CGD varies between 25% and 40%, and IA has been the main cause of death (35%) (7, 24).
The etiology of IA differs remarkably between patients with CGD and those with other underlying immunodeficiencies. Although Aspergillus fumigatus is by far the most frequently encountered pathogen, A. nidulans causes up to 50% of cases of osteomyelitis and is overrepresented in cases of invasive aspergillosis in CGD patients, with a 5- to 10-fold higher mortality rate (8, 22). Differences in either fungal virulence factors or antifungal mechanisms mounted by the host to kill these pathogens could explain why A. nidulans causes such a disproportionately high infection rate in CGD patients versus other immunocompromised patients (1, 2, 5, 14, 31).
Most studies focusing on innate immune responses against Aspergillus spp. have used A. fumigatus; knowledge of the host response against other Aspergillus species is scarce, in particular with respect to A. nidulans. The almost exclusive contribution of NADPH oxidase to microbial killing has been debated, with studies showing contradictory results. On the one hand, the absence of NADPH oxidase in CGD cells indicates its essential role in effective microbial killing. Investigators have focused attention on the products of the oxidase themselves as the microbicidal agents. In addition, clinical epidemiological data for infections occurring in CGD patients suggest a direct link between catalase-positive microorganisms as causative organisms and the absence of reactive oxygen species (ROS) in CGD patients. Species such as Staphylococcus aureus, Burkholderia cepacia, Serratia marcescens, Aspergillus species, and Nocardia species are able to destroy their own hydrogen peroxide radicals. This makes microbe-produced H2O2 unavailable to the phagocytic cells for conversion into more potent and potentially toxic microbicidal reactive oxygen intermediates. Several in vitro studies have proposed a direct link between an increased oxidative response and enhanced fungal damage (19, 20).
On the other hand, in vivo and in vitro studies have reported unaltered virulence of catalase-deficient A. nidulans and S. aureus in the CGD host (5, 16), and experimental studies with alveolar macrophages (AM) from C57BL/6 and gp91phox-deficient mice showed that conidial germination was equally inhibited in vitro. These results suggest that NADPH oxidase-independent mechanisms in murine alveolar macrophages contribute to the inhibition of conidial germination (6).
In order to unravel the unique interaction of the CGD host and A. nidulans and to evaluate the role of the NADPH oxidase complex and ROS in resistance to Aspergillus spp., we investigated the ability of various phagocytic cells to generate ROS in response to fungal invasion. This was carried out with the CGD-specific pathogen A. nidulans and compared to the more general pathogen A. fumigatus.
MATERIALS AND METHODS
Human PBMC and PMN.
Venous blood was drawn from healthy volunteers and two gp91phox-deficient CGD patients after informed consent. Peripheral blood mononuclear cells (PBMC) and polymorphonuclear leukocytes (PMN) were isolated from peripheral blood by use of Lymphoprep (Axis-Shield) and density gradient centrifugation. Briefly, blood was anticoagulated by use of lithium heparin (BD Vacutainer) and then diluted with an equal volume of phosphate-buffered saline (PBS). The diluted blood was carefully added to the top of the Lymphoprep and centrifuged at 800 × g to separate the plasma from the PBMC fraction and the PMN. PBMC were harvested, washed twice in PBS, and counted by use of a hemocytometer. To remove the erythrocytes from the PMN, the pellet was shocked at least twice with an ice-cold lysing reagent (NH4Cl, Na2EDTA, KHCO3). Subsequently, cells were washed and resuspended in PBS. When the experiment required cells in Hanks’ buffered salt solution (HBSS; Invitrogen Life Technologies) or culture medium (CM), the last step was adjusted with the corresponding medium. RPMI 1640 GlutaMAX-I medium (Invitrogen Life Technologies) plus 10% heat-inactivated fetal calf serum (FCS) was used as CM for all cell experiments.
Animal handling and AM sample collection.
Wild-type 8- to 16-week-old male and female C57BL/6 mice and CYBB/C57BL/6 mice, which have a homozygous mutation in the CYBB gene, were used. The animals were maintained in sterilized microisolator cages in a pathogen-free environment at the animal facility of the National Institute of Allergy and Infectious Diseases, Bethesda, MD. AM were isolated from bronchoalveolar lavage fluid (17). Lavage was performed by using 0.75 ml warm PBS (37°C) plus 3 mM EDTA. In total, 10 lavages were done for each mouse to increase the yield of AM. The bronchoalveolar lavage fluid was spun down at 400 × g for 12 min and washed once with warm PBS. If many red blood cells were observed, a lysis buffer was added and an extra washing step was performed. The AM were resuspended in warm RPMI plus 10% fetal bovine serum (FBS) in a total volume of 1 ml. The cells were counted with the use of a Bürker chamber and adjusted to a concentration of 5 × 105/ml. Cell viability was checked by trypan blue exclusion. Aliquots of 100 μl/well (5 × 104 AM) were added to a 96-well plate and incubated overnight at 37°C. After 18 h, the nonadherent cells were washed away and the medium (RPMI plus 10% FBS) was refreshed.
Fungal strains.
In all experiments, we used fully molecularly characterized A. nidulans and A. fumigatus strains originally isolated from patients with CGD who were suffering from invasive aspergillosis. The cell-free assay and the assays for the assessment of the interaction with human PMN and PBMC were performed with A. fumigatus strain V45-07 and A. nidulans strain V44-46. A. fumigatus strain B5233 and A. nidulans strain RYCB were used for the animal experiments. The growth and killing assays of the Aspergillus isolates were prepared in a lipopolysaccharide (LPS)-free fashion. A. fumigatus strains were grown on Sabouraud glucose agar supplemented with chloramphenicol for 4 to 7 days at 37°C. Abundant conidia were produced under these conditions. Conidia were harvested by gently scraping the surfaces of agar slants and suspending the conidia in PBS with 0.05% Tween 80. To remove hyphal debris, the conidial suspension was filtered twice through five layers of sterile gauze. Conidia were washed twice in PBS, resuspended in HBSS without phenol red, calcium, or magnesium [HBSS(−)], and stored in individual aliquots of 1 × 108/ml at −80°C. Due to the tendency of A. nidulans strains to produce cleistothecia in their sexual cycle, the growth conditions used for A. fumigatus had to be modified. After growth on Sabouraud glucose agar, conidia were plated on water-agar for 3 days, followed by incubation on malt extract agar for an additional 3 days. Under these conditions, pure asexual growth was obtained. The harvest protocol was identical to that described for A. fumigatus.
To collect hyphal fragments, conidia were added to 5 ml of yeast nitrogen base medium (YNB) (Difco) at a final concentration of 1 × 106/ml. After 18 h of incubation at 37°C, the cultures were centrifuged at 3,000 × g for 15 min, and the pellet, containing almost exclusively mycelia, was washed twice in PBS and resuspended in 1 ml of HBSS(−).
Aliquots of the conidial suspension were heat killed for 15 min at 121°C. Nonviable conidia were centrifuged at 3,000 × g for 15 min, resuspended, and vortexed vigorously. Finally, the suspension was washed three times with HBSS(−) to remove released Aspergillus components and then kept frozen at −80°C until they were used. The viability of the fungi was checked by culture at 37°C on Sabouraud glucose agar. No growth was observed following heat treatment.
Cell-free effect of reactive oxygen species.
The antifungal activity of ROS was evaluated by the growth rate and the amount of cell-free hydrogen peroxide-induced damage. Resting conidia, swollen conidia, or hyphae (1 × 106/ml) were incubated at 37°C in the presence of hydrogen peroxide at three different concentrations, namely, 10−1 M, 10−3 M, and 10−5 M H2O2. Swollen conidia were obtained by incubation in YNB at a density of 1 × 106 conidia/ml. Growth was measured for 48 h by spectrophotometry (optical density at 450 nm [OD450]) and compared to that of the negative control. The cultures were observed under a microscope during the first 5 h. All experiments were performed in triplicate under the same conditions and were repeated twice.
Measurement of superoxide production/reactive oxygen intermediates.
Oxygen radical production was evaluated by luminol-enhanced chemiluminescence, as measured in an automated LB96V Microlumat Plus luminometer (EG&G Berthold, Bad Wildberg, Germany). Luminol (Sigma-Aldrich) is an organic dye which reacts with both extracellular and intracellular oxygen species. As a result, the luminol molecule is excited, and when the luminol returns from the excited state to the steady state, the energy is released in the form of light, which can be detected by a luminometer. Briefly, 100 μl of PBMC or PMN suspension (2 × 106 cells/ml) in HBSS(−) was added to a 96-well nontransparent white microtiter plate. The cells were then challenged with 0.5 μg/ml phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich), 1 mg/ml opsonized zymosan (Sigma-Aldrich), or fungal cells. Either resting or heat-killed conidia of A. nidulans or A. fumigatus were tested at a cell-to-conidium ratio of 1:20 (100 μl of 4 × 107 cells/ml per well). Control wells received 100 μl of medium. Luminol (10−2 M [1.77 mg/ml] in dimethyl sulfoxide [DMSO]) was diluted 10-fold in HBSS-0.5% bovine serum albumin (BSA) prior to use. The reaction was started by the addition of 20 μl of luminol solution. Each measurement was performed in quintuplicate. Chemiluminescence was measured using a 1-s integration time at intervals of 228 s over a 3-hour incubation period at 37°C and a 0.5-s integration time over a 10-hour incubation. Luminescence was expressed as relative light units per second (RLU/s). The area under the concentration-time curve (AUC), representing the overall oxygen radical production during incubation, was calculated. To evaluate the effect of gamma interferon (IFN-γ), conidia were added with or without 100 U or 500 U of commercially available human IFN-γ/ml (Immukine; Boehringer Ingelheim BV). Blocking of the NADPH complex was performed by use of diphenyleneiodonium chloride (DPI; Sigma-Aldrich).
XTT assay.
The XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)2H-tetrazolium-5-carboxanilide sodium salt] assay of fungal viability described by Meletiadis et al. (15) was modified as follows. Each well of a 96-well plate was filled with 100 μl of live resting conidia of A. nidulans or A. fumigatus at 1 × 106/ml of CM. Hyphae were obtained directly in the test well by overnight incubation (13 h) of the conidia (1 × 106/ml) at 37°C. Subsequently, CM was refreshed, and 100 μl of isolated PMN or PBMC was added at a concentration of 5 × 106/ml. Conidial viability was assayed after 18 h of incubation at 37°C in 5% CO2, while hyphal viability was assayed after 90 min of incubation to avoid overgrowth of hyphae. At the indicated times, the plates were centrifuged at 3,000 × g for 10 min and the supernatant was removed. Cells were lysed by addition of ice-cold MilliQ with 0.025% Triton for 5 to 10 min on ice, after which each well was washed to remove cell debris. One hundred microliters of PBS and 100 μl of XTT-menadione solution was added to each well in order to obtain a final concentration of 200 μg/ml XTT (Sigma-Aldrich) and 25 μM (4.3 μg/ml) menadione (Sigma-Aldrich). Incubation was continued at 37°C for at least 2 h in the dark to allow conversion of XTT to its formazan derivative. Control wells containing PBMC, PMN, or AM alone were used as negative controls, while wells containing conidia or hyphae alone were used as positive controls. Plates were centrifuged, and the absorbance of the supernatant was read at 450 nm in a spectrophotometer. Fungal damage was defined as the percent reduction in metabolic activity compared to that of controls and was calculated as follows: [1 − (X/C)] × 100, where X is the mean absorbance in the test wells and C is the mean absorbance in the control wells. In addition, the potential enhancement of the antifungal effect by IFN-γ was tested at 2 different concentrations: 100 U and 500 U of IFN-γ/ml. This process was followed as indicated above. Ninety-six-well clear-bottom plates were used for the killing experiments with healthy host cells. To minimize loss of fungi, the killing experiments with and without DPI and the comparison between healthy and CGD cells were performed with a 96-well 0.45-μm-filter-bottom plate (Millipore Multiscreen-HV).
Statistical analysis.
Each experiment was performed with the cells of one particular donor and by their use in triplicate (growth rate), quadruplicate (XTT assay), or quintuplicate (chemiluminescence) wells. The mean value for these replicate wells was taken as the value for the particular donor and/or experiment. All cell-mediated experiments were performed in quadruplicate unless otherwise indicated. The means were then used in the data analysis to calculate the means ± standard errors of the means (SEM) for all of the experiments under the same conditions. Comparisons between two means were tested by t tests, calculated using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA). P values of <0.05 were considered statistically significant. Comparisons between more than two means were analyzed by analysis of variance (ANOVA), using the statistical software SPSS 16.0. When the ANOVA result was significant, pairwise comparisons were performed using a Bonferroni-adjusted level of significance.
RESULTS
Swollen A. nidulans conidia are resistant to H2O2.
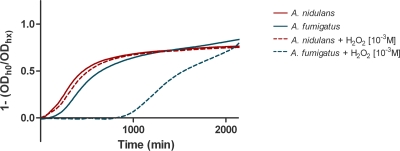
We initially investigated the link between the products of the NADPH oxidase themselves and the death of the microbe by assessing the direct antifungal effect of ROS. Therefore, growth characteristics of resting conidia, swollen conidia, and hyphae were evaluated in the presence of H2O2 at three different concentrations, namely, 10−1 M, 10−3 M, and 10−5 M H2O2. There was a direct correlation in the dose-response relationship between H2O2 and growth inhibition of both species of Aspergillus. The 10−3 M H2O2-induced damage to swollen A. fumigatus conidia was significantly greater than that to A. nidulans conidia and was sustained for up to 18 h of incubation (P < 0.01). Growth curves for A. nidulans at 10−3 M H2O2 were similar to those for their negative controls, suggesting resistance of swollen A. nidulans conidia to H2O2 (Fig. 1). No differences in growth inhibition were found between resting conidia or hyphae of both species at either 10−3 M H2O2 or 10−1 M H2O2 (complete inhibition) or at 10−5 M H2O2 (no inhibition).
FIG. 1.
Cell-free damage of swollen conidia by H2O2. The growth curve for A. nidulans at 10−3 M H2O2 (dashed line) was similar to that for the negative control (solid line), reflecting complete H2O2 resistance of swollen A. nidulans conidia.
Live and heat-killed A. nidulans conidia are poor stimulators of ROS.
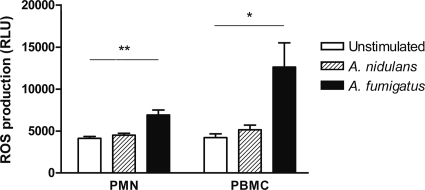
Since phagocytosis is associated with the activation of NADPH oxidase, resulting in the release of ROS, the release of ROS was assessed in real time and during the whole process of phagocytosis by use of luminol-enhanced chemiluminescence. Human polymorphonuclear cells and PBMC produced ROS in response to live resting A. fumigatus conidia. In contrast, no significant NADPH oxidase activity could be measured in response to live resting A. nidulans conidia, either in PBMC or in PMN. Furthermore, the global respiratory burst activities of the PBMC and PMN during stimulation with live A. nidulans conidia (5,161 ± 547 RLU/s and 4,512 ± 227 RLU/s, respectively) were similar to those of unstimulated cells (4,224 ± 428 RLU/s and 4,129 ± 225 RLU/s, respectively) (Fig. 2).
FIG. 2.
Live resting A. nidulans conidia are extremely poor stimulators of ROS. Data represent mean relative light units (RLU) per second, as measured by luminescence. Bar heights indicate the mean areas under the concentration-time curve (AUC) for 5 healthy donors. Error bars are SEM for the 5 results. *, P < 0.05; **, P < 0.01.
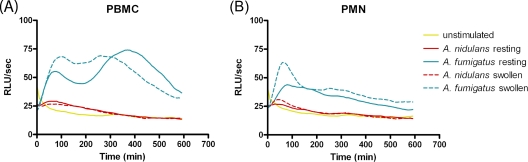
To determine whether the lack of ROS production after A. nidulans conidial stimulation was dependent on the metabolic activity of the fungus, we exposed the PMN and PBMC to swollen conidia (4 h of incubation prior to stimulation). However, no additional germination-induced ROS production was observed (data not shown). Increasing the time of measurement for up to 10 h, thereby covering the entire process of germination, from resting conidia to swollen conidia and then to hyphae, confirmed that infection with live A. nidulans did not result in any significant increase of ROS production by phagocytic cells. In contrast, live A. fumigatus conidia were able to generate a secondary peak during germination, but this was observed only for PBMC (Fig. 3). The fungal morphology was checked microscopically, and the viability of PMN and PBMC was tested by trypan blue exclusion at the 10-h time point. Both cell types reached similar levels of viability.
FIG. 3.
Germinated A. nidulans conidia do not stimulate the respiratory burst. Data from a representative experiment with each cell type are shown. ROS produced by unstimulated cells and resting or swollen A. nidulans- or A. fumigatus-stimulated PBMC (A) and PMN (B) were measured by luminol-enhanced chemiluminescence for 10 h.
To determine whether the lack of ROS production in response to live A. nidulans was due to active processes suppressing the respiratory burst or, alternatively, to a failure to stimulate the phagocytic cells, we exposed PMN and PBMC to heat-killed A. fumigatus and A. nidulans conidia. No significant difference in ROS induction between heat-killed and live A. fumigatus stimulation was observed for either cell type (7,338 ± 452 RLU/s and 6,914 ± 590 RLU/s, respectively, for PMN; and 15,477 ± 2,869 RLU/s and 12,632 ± 2,869 RLU/s, respectively, for PBMC). Although PMN were unable to respond to live A. nidulans, they did respond to heat-killed A. nidulans conidia (P < 0.05), suggesting some active response to the killed conidia (4,512 ± 227 RLU/s versus 6,558 ± 463 RLU/s). The cells were still able to mount an ROS reaction in response to PMA or zymosan after incubation with the conidia, indicating a maintained cell viability and NADPH activity.
Since both species have a slightly different green color, it is possible that differences in light absorption between the species could interfere with chemiluminescence. Therefore, live and heat-killed A. nidulans or A. fumigatus was incubated in a cell-free ROS generation system, ammonium persulfate (APS) (Bio-Rad), and light emission was measured by luminol-enhanced luminescence. Only live A. fumigatus conidia absorbed light significantly more than their negative controls (40% ± 3%), further accentuating the observed differences between live A. nidulans and A. fumigatus.
Live A. nidulans hyphae are extremely poor stimulators of ROS.
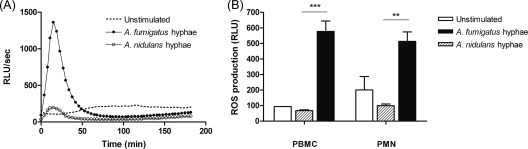
Whether ROS are important for conidial host defense is somewhat controversial, but it is widely accepted that once the conidia germinate into hyphae, degranulation of PMN and release of ROS induce hyphal damage. Interestingly, after incubation of A. nidulans hyphae with healthy PMN, no significant amount of ROS could be measured compared to that in unstimulated cells (9,922 ± 1,131 RLU/s versus 20,100 ± 8,616 RLU/s). In contrast, A. fumigatus hyphae induced a rapid and robust response (51,360 ± 6,083 RLU/s), with a peak at approximately 20 min and completion within 1 h. PBMC were also able to mount a significant respiratory burst in response to A. fumigatus hyphae. Interestingly, although the reaction was much less pronounced than that observed in PMN, it lasted longer (180 min). As seen with the PMN, ROS were not detected after stimulation of PBMC with A. nidulans hyphae. For both cell types, the differences between A. fumigatus and A. nidulans were significant (P < 0.01 and P < 0.001 for PMN and PBMC, respectively) (Fig. 4).
FIG. 4.
Oxidative burst activity in phagocytes. (A) Data for one representative experiment with PMN. (B) Overall ROS production. Data represent mean relative light units (RLU) per second, as measured by luminescence. Bar heights indicate the mean AUC (n = 4). **, P < 0.01; ***, P < 0.001.
Germination of A. nidulans is reduced significantly compared to that of A. fumigatus.
In order to assess the relationship between the NADPH oxidase activity and the antifungal activity of the cell, the capacity of healthy PMN and PBMC to arrest conidial growth was assessed by XTT assay. Interestingly, the degree of PMN-induced fungal damage, defined as the percent reduction in metabolic activity compared to that of the negative controls, was significantly higher for A. nidulans (63% ± 2%) than for A. fumigatus (30% ± 4%) (P < 0.001). Also, the PBMC-induced antifungal activity to A. nidulans was higher than that to A. fumigatus (74% ± 9% versus 52% ± 16%), but it did not achieve significance.
PBMC and PMN cause more damage to A. nidulans hyphae than to A. fumigatus hyphae.
Hyphae were obtained directly in the test well by overnight incubation of resting conidia. Subsequently, CM was refreshed and cells were added at a 5:1 cell-to-conidium ratio. After 90 min of incubation with PMN, hyphal damage toward A. nidulans reached 67% (±2%), compared to 21% (±7%) for A. fumigatus (P < 0.001). PBMC-mediated hyphal damage was found to be 50% (±3%) for A. nidulans, compared to 32% (±6%) for A. fumigatus (P < 0.05).
Murine gp91phox-deficient AM inhibit germination of A. nidulans more efficiently than do control AM.
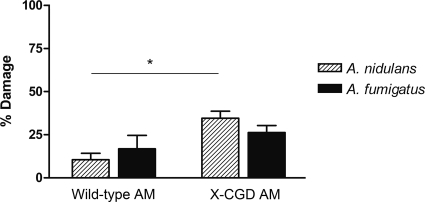
Since Aspergillus species are airborne, the most common route of infection is the lung. As a consequence, the first line of defense consists of resident alveolar macrophages, the main phagocytic cells of the lung. Establishment of invasive aspergillosis occurs in immunocompromised patients as the fungus escapes from AM control and invades the tissue. We investigated the ability of AM from gp91phox-deficient mice (CYBB/C57BL/6 mice) to inhibit germination of A. nidulans and A. fumigatus and compared it to that for AM of healthy mice (C57BL/6 mice). A cell-to-conidium ratio of 1:5 was used. Interestingly, the growth inhibition of A. nidulans and A. fumigatus by murine gp91phox-deficient AM (35% ± 4% and 26% ± 4%, respectively) was higher than that by healthy AM (11% ± 4% and 17% ± 8%, respectively) (P < 0.05) (Fig. 5).
FIG. 5.
Alveolar macrophage-mediated antifungal activity assessed by XTT assay. X-CGD murine AM induced fungal damage toward A. fumigatus and A. nidulans compared to wild-type AM. Data were derived from four separate experiments. X-CGD murine AM inhibited A. nidulans conidia significantly better than wild-type AM did (*, P < 0.05).
Blocking of NADPH oxidase does not affect germination and hyphal damage of A. nidulans.
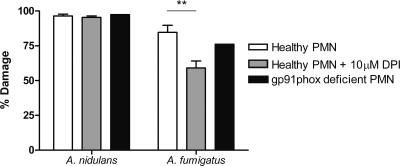
To further investigate the antifungal effect of the NADPH oxidase on A. fumigatus and A. nidulans, we assessed the fungal damage to A. nidulans induced by PMN and PBMC in the presence of the NADPH oxidase inhibitor DPI (10 μM). Inhibition of NADPH oxidase activity was controlled by serum-opsonized zymosan stimulation of the phagocytic cells after 1 h of DPI preincubation. NADPH oxidase blocking by DPI resulted in a 98% reduction of superoxide production by granulocytes, as measured by chemiluminescence. Since DPI has an inhibitory effect on fungal germination, PMN and PBMC were preincubated with DPI for 1 h at 37°C prior to addition of fungi. Cells were washed, resuspended in fresh CM, and incubated with the hyphae. No differences were found in the inhibition of germination, either toward A. nidulans or toward A. fumigatus. Accordingly, A. nidulans hyphae were equally damaged by PMN in the absence and presence of the NADPH oxidase blocker DPI (96% ± 1% versus 95% ± 1%). However, in contrast to the case for A. nidulans, the hyphal damage of A. fumigatus was significantly (P < 0.01) lower after blocking of NADPH oxidase (85% ± 5% versus 59% ± 5%) (Fig. 6).
FIG. 6.
Percent hyphal damage by wild-type PMN with (gray bars) and without (white bars) NADPH oxidase blocked by 10 μM DPI and by gp91phox-deficient PMN (black bars) for A. nidulans and A. fumigatus hyphae. Bar heights indicate % fungal damage. Error bars indicate SEM for 4 healthy controls. **, P < 0.01.
gp91phox-deficient PMN and PBMC inhibit conidial germination as efficiently as control cells do.
CGD patients having a defect in the gp91phox component of the NADPH complex are unable to produce microbicidal superoxide anion and its metabolites. In order to test the hypothesis that CGD patients suffer from invasive aspergillosis due to the absence of microbicidal ROS, we analyzed the antifungal capacity of human CGD cells toward A. fumigatus and A. nidulans. PBMC and PMN were isolated from patients with a defect in the gp91phox component of the NADPH complex (X-CGD PBMC and PMN) and were incubated with either conidia or hyphae. The capacity of X-CGD PMN to inhibit germination of either A. nidulans conidia (72% ± 3% versus 67% ± 2%) or A. fumigatus conidia (65% ± 4% versus 60% ± 2%) did not show a significant difference compared to that of healthy control cells. Accordingly, no differences were found in the ability of X-CGD and healthy PBMC to cope with either A. nidulans (86% ± 3% versus 95% ± 2%) or A. fumigatus (74% ± 9% versus 73% ± 16%).
Furthermore, to test whether IFN-γ could affect ROS production or enhance antifungal capacity, we added IFN-γ (Immukine; Boehringer Ingelheim BV) to reaction mixtures to assess both processes. Neither of the tested concentrations (100 U/ml and 500 U/ml) enhanced the antifungal effect or the respiratory burst activity.
Antifungal activity of gp91phox-deficient PMN toward A. nidulans hyphae is maintained.
Having characterized ROS release after stimulation of phagocytic cells with either A. fumigatus or A. nidulans hyphae as well as the antifungal activity after blocking of the NADPH oxidase by DPI, we tested the extent to which PMN of a gp91phox-deficient patient were able to damage either A. nidulans or A. fumigatus. Interestingly, in line with previous results obtained after blocking the NADPH complex with DPI, in vitro X-CGD cells were perfectly able to damage A. nidulans hyphae (97% versus 95% ± 1% for CGD cells with and without NADPH blocking, respectively). In contrast, the ability of the gp91phox-deficient PMN to induce hyphal damage to A. fumigatus was between that of healthy controls and that after NADPH blocking (Fig. 6).
DISCUSSION
We investigated the effect of the NADPH oxidase and its products on fungal host defense. Specifically, we attempted to clarify the host resistance of the unique CGD fungal pathogen A. nidulans. We observed striking differences between reactivities toward A. nidulans and A. fumigatus. Unexpectedly, we concluded that human phagocytes appear to kill A. nidulans by NADPH-independent mechanisms. This surprising conclusion is based on the following observations: (i) A. nidulans is not susceptible to the direct effect of ROS, (ii) A. nidulans does not induce a measurably different ROS release by PMN or PBMC of healthy donors, (iii) human gp91phox-deficient PMN and PBMC and murine gp91phox-deficient AM inhibit conidial germination as efficiently as healthy cells do, (iv) blocking of the NADPH oxidase complex results in a significantly reduced ability to damage A. fumigatus but does not affect the damage to A. nidulans hyphae, and (v) the antifungal activity of human gp91phox-deficient PMN toward A. nidulans hyphae is maintained.
Roilides et al. (19, 20) showed that superoxide is important for efficient host defense and is produced through NADPH-dependent mechanisms. Stimulation of PMN and PBMC with either conidia or hyphae of A. fumigatus resulted in increased ROS production, leading to inhibition of germination and to fungicidal activity. However, we found A. nidulans to be a weak inducer of ROS in both cell types. Surprisingly, even in the absence of any NADPH activity, as in CGD cells, A. nidulans growth was inhibited just as in normal cells, indicating that mechanisms other than ROS must be responsible for the predilection of CGD patients for A. nidulans infection. Since CGD phagocytes lack the ability to produce ROS, recent studies have focused on the role of ROS-independent factors in arresting the growth of Aspergillus conidia (9, 27, 32). Zarember et al. demonstrated that neutrophils from healthy as well as CGD donors were able to arrest conidial growth with equal efficiencies and that lactoferrin, a major protein of neutrophil granules, contributes to reduced conidial growth (32). These data were subsequently confirmed by transcriptional responses of Aspergillus conidia to healthy and CGD PMN (27). The role of ROS-independent antifungal mechanisms was underscored by Shibuya et al. (25). They found that the mycelium of the double cat1 cat2 mutant of A. fumigatus, which has no catalase activity at all, has only a slightly increased sensitivity to H2O2 and is as sensitive to killing by PMN as the wild-type strain but shows delayed infection in an experimental rat model of invasive aspergillosis. Chang et al. (5) showed previously that the virulence of catalase-deficient A. nidulans was unimpaired in p47phox−/− mice, indicating that the catalase activity of fungi is not a virulence factor for CGD pathogenicity.
Cornish et al. showed that the in vitro inhibition of germination of A. fumigatus conidia by C57BL/6 and gp91phox-deficient mouse AM was comparable. Furthermore, they could not find evidence of the importance of NADPH oxidase in AM by using transcriptional microarray analysis, luminometry, or nitroblue tetrazolium (NBT) staining (6). Instead, they found that the most strongly upregulated transcripts in vivo encoded chemokines and additional factors that play critical roles in neutrophil and monocyte recruitment. Indirect evidence of NADPH-independent resistance in AM was also found by Shibuya et al., who found that killing of catA conidia by AM and conidial virulence in animals were similar to those with wild-type conidia (25). In contrast, AM from p47phox−/− mice were unable to kill A. fumigatus conidia in vivo, as shown by Philippe et al. (18). In addition, inhibitors of NADPH oxidase that decreased the production of reactive oxidant intermediates inhibited the killing of A. fumigatus conidia in vitro, suggesting an NADPH oxidase dependence. However, the inoculation time was limited to 6 h, in contrast to the 24-h inoculation time in vivo, probably reflecting a more fungistatic effect than a fungicidal effect. Our XTT assays with murine AM underlined the ability of conserved primary defense mechanisms toward Aspergillus conidia. Even in the absence of a functional NADPH oxidase, germination of both A. fumigatus and A. nidulans was inhibited, with the latter inhibited even better by gp91phox-deficient AM than by those derived from C57BL/6 mice.
With respect to the antifungal host defense mechanisms against hyphae, several studies have investigated the ability of Aspergillus species to modulate ROS in phagocytes relative to their fungicidal activity (1, 10). Akpogheneta et al. (1) evaluated the activity of human PMN and mononuclear phagocytes against hyphae of non-A. fumigatus Aspergillus species and compared it to that against A. fumigatus hyphae. They concluded that nonopsonized hyphae suppress the oxidative burst of PMN and that these cells respond with a less vigorous oxidative burst in the presence of serum-opsonized hyphae of non-fumigatus Aspergillus species. Similarly, PMN induced less hyphal damage to non-fumigatus species, particularly A. flavus and A. nidulans, than to A. fumigatus, as assessed by XTT colorimetric assay. They concluded that non-fumigatus aspergilli are generally more resistant to mononuclear and polymorphonuclear phagocytes than A. fumigatus aspergilli. These results suggest a direct link between the induced oxidative response and hyphal damage. In contrast, we found that nonopsonized hyphae of A. fumigatus, being potent ROS inducers, were less damaged than those of A. nidulans, which were not able to induce any measurable respiratory burst activity. Akpogheneta et al. monitored the ROS at the 1-h time point and concluded that nonopsonized hyphae suppress the oxidative burst compared to that in nonstimulated cells. By using luminol-enhanced chemiluminescence, which gave us the ability to follow the amount of ROS produced over time, we observed a high peak of ROS, with a maximum at 20 min and completion at 60 min. We could indeed confirm that the NADPH oxidase activity at 60 min decreased below the basal activity.
The unique compositions of the cell walls of A. fumigatus and A. nidulans might be responsible for the observed differences with respect to ROS stimulation and fungicidal activity of host immune cells. Melanin is known to be an important virulence factor by protecting fungal cells from ROS produced by phagocytic cells (13, 28) and by modulating the host immune response (4). The biosynthesis of melanin, and consequently its composition, differs between A. nidulans and A. fumigatus (3, 29). Furthermore, during germination, the hydrophobic rodlet layer is removed and fungal pathogen-associated molecular patterns (PAMPs) are exposed, mediating the immune response. Girardin et al. (11) found clear physiochemical differences in the composition of the cell wall between A. nidulans and A. fumigatus after they removed this rodlet layer. Analysis and comparison of the chemical characteristics of both species would definitively give new insights into this complex interface and are the subjects of future studies.
The question remains of whether the pathogenicity of aspergillosis in CGD patients is due to the loss of a direct antifungal effect of superoxide and its metabolites or to the loss of more downstream effects of NADPH activation. We attempted to clarify some of these points by examining the opportunistic pathogen A. nidulans, which is found almost exclusively in patients suffering from CGD. Our findings are robust but unexpected: first, A. nidulans is not susceptible to the direct fungicidal effect of ROS; second, gp91phox-deficient AM are able to inhibit A. nidulans more efficiently than controls; third, PMN and PBMC from healthy donors inhibit germination and induce hyphal damage to A. nidulans, even in the absence of significant ROS production, distinct from A. fumigatus; fourth, blocking the NADPH oxidase does not affect the ability to kill A. nidulans hyphae, in stark contrast to the killing of A. fumigatus; and fifth, gp91phox-deficient neutrophils, which have complete NADPH deficiency, kill A. nidulans adequately in vitro, suggesting that at least the early antifungal effector activity of the innate response is maintained in the absence of a functional NADPH oxidase.
Despite these robust findings, CGD patients are the most susceptible hosts for invasive aspergillosis, in particular that caused by A. nidulans. The central role of neutrophils in optimal microbial killing and regulation of tissue damage has been discussed extensively by Smith (26). Smith describes the complex function and balance of stimulatory and inhibitory pathways in the neutrophil, which are regulated by a plethora of local and systemic mediators. Superoxide is an important signal transduction intermediate (12). Romani et al. (21) indicated that the absence of ROS leads to defective tryptophan catabolism, dominant overproduction of interleukin-17 (IL-17), defective regulatory T-cell activity, and excessive inflammation. The role of ROS as mediators/regulators in inflammation has also been underscored by van de Veerdonk et al. (30). They showed that ROS appear to dampen inflammasome activation. As a consequence, the absence of ROS in CGD monocytes may partly explain the inflammatory complications seen in CGD patients. A recent study by Segal et al. (23) supports the occurrence of NADPH oxidase-dependent, redox-mediated signaling which is critical for termination of lung inflammation. By challenging NADPH oxidase-deficient p47phox−/− mice and gp91phox-deficient mice with intratracheal zymosan, they showed that NADPH oxidase limits lung inflammation by attenuating the proinflammatory transcription factor NF-κβ and by activating Nrf2, a key redox-sensitive anti-inflammatory regulatory transcription factor. Results for X-linked CGD patients were consistent with these findings (26). It may well be that the increased inflammation in CGD patients leads to the observed pathogenicity of Aspergillus spp. instead of it being the result of direct deficient innate immune responses against these fungi.
In summary, these in vitro studies do not support a direct role of ROS in the antifungal resistance of A. nidulans and cannot explain the etiology of A. nidulans infections in CGD patients by the simple absence of the direct microbicidal reactive oxidant superoxide anion and its metabolites. The NADPH oxidase is a critical regulator of certain limbs of innate immunity, and its unraveling will improve our understanding of fungal pathogenesis in CGD.
Acknowledgments
This work was supported by a European Society of Pediatric Infectious Disease/Wyeth fellowship grant (2008 to 2010) and by the Division of Intramural Research, NIAID, NIH.
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 15 November 2010.
REFERENCES
- 1.Akpogheneta, O., C. Gil-Lamaignere, A. Maloukou, and E. Roilides. 2003. Antifungal activity of human polymorphonuclear and mononuclear phagocytes against non-fumigatus Aspergillus species. Mycoses 46:77-83. [DOI] [PubMed] [Google Scholar]
- 2.Bignell, E., et al. 2005. Virulence comparisons of Aspergillus nidulans mutants are confounded by the inflammatory response of p47phox−/− mice. Infect. Immun. 73:5204-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brakhage, A. A., K. Langfelder, G. Wanner, A. Schmidt, and B. Jahn. 1999. Pigment biosynthesis and virulence. Contrib. Microbiol. 2:205-215. [DOI] [PubMed] [Google Scholar]
- 4.Chai, L. Y., et al. 2010. Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 215:915-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, Y. C., B. H. Segal, S. M. Holland, G. F. Miller, and K. J. Kwon-Chung. 1998. Virulence of catalase-deficient Aspergillus nidulans in p47(phox)−/− mice. Implications for fungal pathogenicity and host defense in chronic granulomatous disease. J. Clin. Invest. 101:1843-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornish, E. J., et al. 2008. Reduced nicotinamide adenine dinucleotide phosphate oxidase-independent resistance to Aspergillus fumigatus in alveolar macrophages. J. Immunol. 180:6854-6867. [DOI] [PubMed] [Google Scholar]
- 7.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 8.Dotis, J., and E. Roilides. 2004. Osteomyelitis due to Aspergillus spp. in patients with chronic granulomatous disease: comparison of Aspergillus nidulans and Aspergillus fumigatus. Int. J. Infect. Dis. 8:103-110. [DOI] [PubMed] [Google Scholar]
- 9.Gallin, J. I., and K. Zarember. 2007. Lessons about the pathogenesis and management of aspergillosis from studies in chronic granulomatous disease. Trans. Am. Clin. Climatol. Assoc. 118:175-185. [PMC free article] [PubMed] [Google Scholar]
- 10.Gil-Lamaignere, C., A. Maloukou, J. L. Rodriguez-Tudela, and E. Roilides. 2001. Human phagocytic cell responses to Scedosporium prolificans. Med. Mycol. 39:169-175. [DOI] [PubMed] [Google Scholar]
- 11.Girardin, H., S. Paris, J. Rault, M. N. Bellon-Fontaine, and J. P. Latge. 1999. The role of the rodlet structure on the physicochemical properties of Aspergillus conidia. Lett. Appl. Microbiol. 29:364-369. [DOI] [PubMed] [Google Scholar]
- 12.Gwinn, M. R., and V. Vallyathan. 2006. Respiratory burst: role in signal transduction in alveolar macrophages. J. Toxicol. Environ. Health B Crit. Rev. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 13.Jahn, B., et al. 1997. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect. Immun. 65:5110-5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki, L., D. Wysong, R. Diamond, and J. Aguirre. 1997. Two divergent catalase genes are differentially regulated during Aspergillus nidulans development and oxidative stress. J. Bacteriol. 179:3284-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meletiadis, J., et al. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messina, C. G., E. P. Reeves, J. Roes, and A. W. Segal. 2002. Catalase negative Staphylococcus aureus retain virulence in mouse model of chronic granulomatous disease. FEBS Lett. 518:107-110. [DOI] [PubMed] [Google Scholar]
- 17.Montagnoli, C., et al. 2006. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J. Immunol. 176:1712-1723. [DOI] [PubMed] [Google Scholar]
- 18.Philippe, B., et al. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71:3034-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roilides, E., A. Dimitriadou-Georgiadou, T. Sein, I. Kadiltsoglou, and T. J. Walsh. 1998. Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect. Immun. 66:5999-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roilides, E., K. Uhlig, D. Venzon, P. A. Pizzo, and T. J. Walsh. 1993. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect. Immun. 61:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romani, L., et al. 2008. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451:211-215. [DOI] [PubMed] [Google Scholar]
- 22.Segal, B. H., et al. 1998. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore) 77:345-354. [DOI] [PubMed] [Google Scholar]
- 23.Segal, B. H., et al. 2010. NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One 5:e9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segal, B. H., T. L. Leto, J. I. Gallin, H. L. Malech, and S. M. Holland. 2000. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 79:170-200. [DOI] [PubMed] [Google Scholar]
- 25.Shibuya, K., et al. 2006. Catalases of Aspergillus fumigatus and inflammation in aspergillosis. Nippon Ishinkin Gakkai Zasshi 47:249-255. [DOI] [PubMed] [Google Scholar]
- 26.Smith, J. A. 1994. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukoc. Biol. 56:672-686. [DOI] [PubMed] [Google Scholar]
- 27.Sugui, J. A., et al. 2008. Genes differentially expressed in conidia and hyphae of Aspergillus fumigatus upon exposure to human neutrophils. PLoS One 3:e2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai, H. F., Y. C. Chang, R. G. Washburn, M. H. Wheeler, and K. J. Kwon-Chung. 1998. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J. Bacteriol. 180:3031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai, H. F., M. H. Wheeler, Y. C. Chang, and K. J. Kwon-Chung. 1999. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 181:6469-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Veerdonk, F., et al. 2010. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl. Acad. Sci. U. S. A. 107:3030-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warris, A., et al. 2005. Cytokine responses and regulation of interferon-gamma release by human mononuclear cells to Aspergillus fumigatus and other filamentous fungi. Med. Mycol. 43:613-621. [DOI] [PubMed] [Google Scholar]
- 32.Zarember, K. A., J. A. Sugui, Y. C. Chang, K. J. Kwon-Chung, and J. I. Gallin. 2007. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J. Immunol. 178:6367-6373. [DOI] [PubMed] [Google Scholar]