Abstract
Tick saliva has potent immunomodulatory properties. In arthropod-borne diseases, this effect is largely used by microorganisms to increase their pathogenicity and to evade host immune responses. We show that in Lyme borreliosis, tick salivary gland extract and a tick saliva protein, Salp15, inhibit in vitro keratinocyte inflammation induced by Borrelia burgdorferi sensu stricto or by the major outer surface lipoprotein of Borrelia, OspC. Chemokines (interleukin-8 [IL-8] and monocyte chemoattractant protein 1 [MCP-1]) and several antimicrobial peptides (defensins, cathelicidin, psoriasin, and RNase 7) were downregulated. Interestingly, antimicrobial peptides (AMPs) transiently inhibited bacterial motility but did not kill the organisms when tested in vitro. We conclude that tick saliva affects the chemotactic properties of chemokines and AMPs on immune cells and has an antialarmin effect on human primary keratinocytes. Alarmins are mediators that mobilize and activate antigen-presenting cells. Inhibition of cutaneous innate immunity and of the migration of immune cells to the site of the tick bite ensures a favorable environment for Borrelia. The bacterium can then multiply locally and, subsequently, disseminate to the target organs, including joints, heart, and the central nervous system.
The cutaneous interface is a key locus in arthropod-borne diseases (14). To analyze the role of resident cells of the skin in the development of such diseases and to study the potential effect of tick saliva on the inflammatory response of human keratinocytes (KCs), we investigated Lyme borreliosis, a zoonosis caused by Borrelia burgdorferi sensu lato and transmitted by Ixodes ticks. Lyme borreliosis occurs throughout the Northern hemisphere and differs in clinical features according to the geographic distribution of the different species of Borrelia that cause Lyme disease. In the United States, for example, Lyme disease is caused by B. burgdorferi sensu stricto and transmitted by Ixodes scapularis, and its main clinical sign is arthritis. In Europe, the disease is transmitted by Ixodes ricinus, with three major Borrelia species being responsible for different clinical symptoms: B. burgdorferi sensu stricto (arthritic signs), B. afzelii (dermatological signs), and B. garinii (neurological signs) (46).
Among arthropod vehicles for pathogens, the saliva from insects and ticks increases the pathogenicity of transmitted microorganisms. This has been well documented for sandflies infected with the flagellate parasite Leishmania and for ticks infected with Borrelia spp. (1, 49, 50). In Lyme disease models, injection of Borrelia together with salivary gland extract (SGE) increased the dissemination and number of bacteria in mice (37, 55), underscoring the importance of tick saliva in the transmission of the bacteria (51). As a blood-sucking arthropod, the tick stays on its host for a period of 3 to 10 days and therefore must modulate vertebrate host immune responses to avoid rejection (13). Ticks act directly on the host's adaptive immunity by downregulating T-cell proliferation (17).
In recent years, studies have been directed toward analyzing the means by which ticks act on innate immunity. A variety of proteins have been identified in tick saliva that inactivate the complement-mediated immune response or modify the oxidative burst of macrophages (8). A pluripotent protein, Salp15, present in both I. scapularis and I. ricinus during engorgement (24), has been identified as a protective protein for B. burgdorferi. Salp15 inhibits T-cell proliferation (2) and dendritic cell activation (25) and protects Borrelia from complement- and antibody-mediated killing by the host (26, 38).
Before the blood meal, bacteria primarily produce OspA, which functions as a ligand for the tick gut receptor TROSPA (35). Once ticks start feeding on the vertebrate host, spirochetes present in the gut migrate to the salivary glands. During this migration, Borrelia cells are subject to major antigenic modifications: OspA is downregulated, whereas OspC is upregulated (31). Two to 3 days after tick attachment, spirochetes present in the salivary glands are injected into the vertebrate host. OspC is essential for Borrelia transmission from the tick to the host and for the multiplication and dissemination of Borrelia in the vertebrate host (19). These Borrelia lipoproteins encompass specific patterns called “pathogen-associated molecular patterns” (PAMPs), which have the capability to stimulate antigen-presenting cells (APCs) via Toll-like receptors (TLRs). The receptor TLR2 forms a heterodimer with TLR1 to recognize triacylated lipopeptides corresponding to B. burgdorferi sensu lato lipoproteins (20, 22, 43). This interaction leads to the induction of different inflammatory chemokines and cytokines. Antimicrobial peptides (AMPs) are also an important part of a vertebrate's innate immunity (6) and play a role in the control of several skin infections (33, 41).
We have previously shown that the interaction of B. burgdorferi sensu stricto with human primary KCs induces the secretion of interleukin-8 (IL-8) and human β-defensin 2 (hBD-2). It is noteworthy that IL-8 secretion was repressed by crude I. ricinus salivary gland extracts (SGE) (29). In the present study, we analyzed more precisely whether the major surface lipoprotein of Borrelia, OspC, might be responsible for KC inflammation. We investigated additional molecules that may be induced during KC inflammation and their possible repression by tick SGE and Salp15 protein. The two major AMPs of the skin, HBD-2 and LL-37, were tested in vitro on B. burgdorferi and shown to have a transient effect on the bacteria. We conclude from these experiments that tick saliva can inhibit AMPs considered as “alarmin molecules” and chemokines (34, 54) secreted by KCs in the skin, thus inhibiting cellular signaling and trafficking and facilitating Borrelia transmission, multiplication, and dissemination in the vertebrate host.
MATERIALS AND METHODS
Spirochetes.
B. burgdorferi strains N40, B31, and 297 were cultured in BSK-H medium (Sigma) at 33°C 4 days before the assay. Prior to in vitro infection of the cells, bacteria were washed twice by centrifugation for 30 min at 5,000 × g at 20°C. Bacteria were used between passages 5 and 8. The virulence of the Borrelia strain was evaluated by transmission to the mice and to the ticks. B. burgdorferi strain 297 and the OspC-deficient mutant have been described previously (36).
Borrelia proteins and other inducers.
The surface proteins, lipidated OspA and OspC (L-OspA and L-OspC), were cloned and purified from B. burgdorferi sensu stricto B31 as previously described (4). They were purified and eluted in 20 mM Tris-Cl (pH 7.5)-0.3% Triton X-100. These proteins were diluted and used at a concentration of 100 ng/ml. The truncated unlipidated OspA (amino acids [aa] 17 to 273) and unlipidated OspC (aa 19 to 210) of B. burgdorferi sensu stricto B31 were used at a concentration of 100 ng/ml. The triacylated lipopeptide Pam3CSK4 (Pam3Cys-EMC Microcollections, Deutschland GmbH) was used at 10 μg/ml. MALP2 (macrophage-activating lipopeptide from Mycoplasma fermentans) was used as an agonist of Toll-like receptor 2 (TLR2) (Axxora, Deutschland GmbH) at a concentration of 200 ng/ml, as described by Schauber et al. (40). The anti-human TLR2 blocking antibody was used at 1.5 μg/ml (eBioscience, Ltd., United Kingdom) and incubated for 30 min at room temperature on KCs. OspC was then added, and the sample was further incubated for 6 h.
Tick salivary glands and Salp15.
I. ricinus originally came from a tick colony in Neuchâtel, Switzerland, and was maintained in Strasbourg, France. For tick salivary gland extract (SGE) isolation, adult ticks were allowed to feed on mice for 3 days. Collected ticks were first washed in ethanol and then in phosphate-buffered saline (PBS), and salivary glands (SGs) were dissected in PBS under a light microscope. The SGE was frozen and thawed three times and centrifuged for 10 min at 10,000 × g. The supernatant was kept frozen at −80°C until use. After protein determination (Bio-Rad), an equivalent of one tick SG (around 20 μg/ml) was used to study its effect on the modulation of inflammation. Salp15 from I. ricinus, cloned and purified from cultured Drosophila melanogaster S2 cells as described by Hovius et al. (25), was used at a concentration of 30 μg/ml. In the inhibitory assays, Salp15 was preincubated with Borrelia or lipidated OspC for 30 min before the interaction with KCs. All recombinant proteins were tested by the Limulus assay to check for the presence of endotoxins and were found to contain <0.3 endotoxin unit for each concentration of recombinant protein tested.
Cell culture of keratinocytes and stimulation.
Human epidermal KCs (Promocell, Germany) were maintained in KGM2 (Promocell) medium with a calcium concentration of 0.15 mM. To stimulate the cells, KCs were seeded at 7.5 × 104 per well in a 24-well plate. At confluence, the calcium concentration was increased to 1.5 mM to induce KC differentiation. Cells were stimulated with different concentrations of B. burgdorferi (multiplicity of infection [MOI] of 100:1 or 10:1). KCs were used at passages 3 to 5. For the assays with tick SGE or with Salp15, B. burgdorferi was preincubated for 30 min with the tick compounds at room temperature, and the preparation was then transferred on cells and further incubated for 6 or 24 h. To measure the induction of LL-37, KCs were preincubated for 24 h with 25 nM vitamin D3 (Cayman Chemical, MI) prior to the addition of B. burgdorferi or the lipoproteins. Before collection of uninfected or infected cells in Trizol, the viability of cells was checked under an inverted microscope and with Trypan blue staining. The presence of Borrelia had no effect on KC viability.
ELISA.
To measure IL-8 and hBD-2 secreted by KCs, enzyme-linked immunosorbent assays (ELISAs) were performed on supernatants of nonactivated and activated cells. Protocols were based on sandwich techniques, as described by the manufacturers: R&D (France) for IL-8 and Preprotech Ec (London, United Kingdom) for hBD-2.
RNA extraction and quantitative real-time RT-PCR.
Supernatants were removed and KCs were directly suspended in Trizol (Invitrogen) for extraction of total RNA according to the manufacturer's protocol. After treatment with DNase (Ambion), 2 μg of total RNA was reverse transcribed with the Superscript II first-strand synthesis system (Invitrogen). Quantitative reverse transcription (qRT)-PCR was done on a LightCycler system 2.0 (Roche, Meylan, France). The primers used are described in Table 1. For quantification of transcript abundance in cells, target gene expression was normalized to the β-actin gene and compared with that of untreated cells.
TABLE 1.
Primers used for the quantitative RT-PCR
| Primer type | Sequence | Source or reference |
|---|---|---|
| Actin | ||
| Forward | 5′ CGT CAC CAA CTG GGA CGA CA 3′ | Designed in this study |
| Reverse | 5′ GGG GTG TTG AAG GTC TCA AA 3′ | |
| Cathelicidin | ||
| Forward | 5′ CTA GAG GGA GGC AGA CA 3′ | 15 |
| Reverse | 5′ GCG GTA GAG GTT AGC AT 3′ | |
| hBD-2 | ||
| Forward | 5′ ATC TCC TCT TCT CGT TCC TC 3′ | 42 |
| Reverse | 5′ ACC TTC TAG GGC AAA AGA CT 3′ | |
| hBD-3 | ||
| Forward | 5′ CTT CTG TTT GCT TTG CTC TTC C 3′ | 9 |
| Reverse | 5′ CCT CTG ACT CTG CAA TAA TA 3′ | |
| Psoriasin | ||
| Forward | 5′ AGA CGT GAT GAC AAG ATT GAC 3′ | 18 |
| Reverse | 5′ TGT CCT TTT TCT CAA AGA CGT C 3′ | |
| RNase 7 | ||
| Forward | 5′ GGA GTC ACA GCA CGA AGA CCA 3′ | 21 |
| Reverse | 5′ CAT GGC TGA GTT GCA TGC TTG 3′ | |
| IL-8 | ||
| Forward | 5′ TCT GCA GCT CTG TGT GAA GGT GCA GTT 3′ | Designed in this study |
| Reverse | 5′ AAC CCT CTG CAC CCA GTT TTC CTT 3′ | |
| MCP-1 | ||
| Forward | 5′ CGC CTC CAG CAT GAA AGT CT 3′ | 56 |
| Reverse | 5′ GGA ATG AAG GTG GCT GCT ATG 3′ |
Antimicrobial assay.
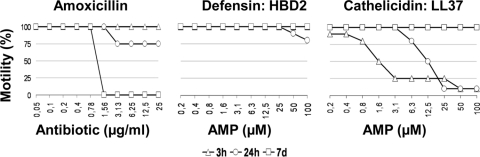
hBD-2 (Innovagen, Sweden) and LL-37 were selected as the two most representative AMPs of the skin. LL-37 was synthesized by solid-phase 9-fluorenylmethoxy carbonyl (Fmoc) chemistry. The assay was performed according to a modified technique described by Lusitani et al. (28). Briefly, in vitro culture of B. burgdorferi sensu stricto N40 was propagated in BSK medium to logarithmic phase and was washed by centrifugation with Hanks' balanced salt solution for 10 min at 3,000 × g at 25°C. The spirochetes were counted under dark-field microscopy and resuspended at the concentration of 5 × 106 Borrelia cells/ml. Bacteria were distributed in a microtiter plate at a concentration of 2.5 × 105/well. AMPs were resuspended in sterile distilled water, and 10 μl of serial dilutions was distributed in each well (range, 100 μM to 0.2 μM). The sample was incubated for 3 and 24 h at 33°C in a microtiter plate. Borrelia cells with AMPs or amoxicillin were then transferred in 5-ml tubes with 3 ml of BSK medium for an additional week. For each time point of the kinetics experiments, 10 μl of bacterial suspension was observed under dark-field microscopy for assessment of Borrelia motility and number. Amoxicillin (Sigma) was used as a positive control with serial dilutions from 25 μg/ml to 0.05 μg/ml.
Statistics.
Each experiment involving cell stimulation with bacteria was carried out at least three times in independent trials. The results are presented as means ± standard deviations (SDs) and were analyzed by Student's t test. Differences in values are considered significant at P < 0.05. For ELISAs, the data are the means ± SDs of triplicate values and are representative of three independent experiments (P < 0.001, P < 0.01, and P < 0.05). For qRT-PCR, the values are normalized to the negative control (medium alone) and shown as the fold number of the control's value. The means ± SDs of three experiments' values were compared between stimulated and unstimulated cells (P < 0.001, P < 0.01, and P < 0.05).
RESULTS
Inhibition of keratinocyte inflammation induced by lipidated outer surface proteins from tick salivary gland extract.
We showed previously that B. burgdorferi sensu stricto N40 induced a strong inflammatory response when coincubated with human primary KCs. This response was inhibited by SGE of I. ricinus (29). We then studied whether OspC (47, 48), the major surface antigen of Borrelia of importance for transmission to the vertebrate host, could be responsible for this inflammation. Because the lipidated Osp antigens we tested were from a different bacterial strain, B. burgdorferi sensu stricto B31, we first tested whether the N40 and B31 strains behave similarly in our in vitro assay. These studies showed that both Borrelia strains induced the chemokine IL-8 and the AMP hBD-2 with the same intensity at all bacterial concentrations tested (Fig. 1 A and B).
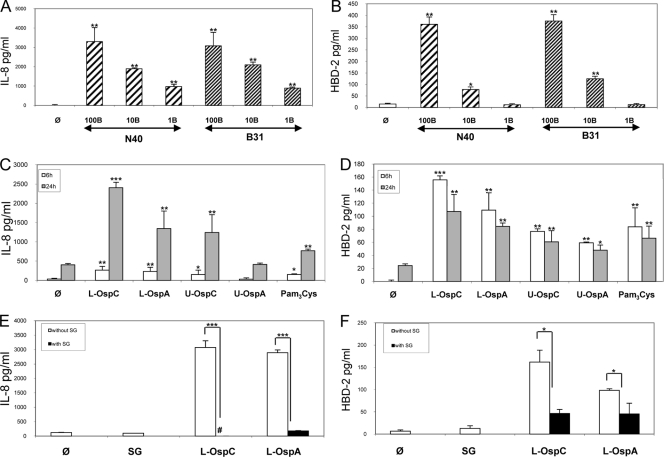
FIG. 1.
Interaction of two B. burgdorferi sensu stricto strains, N40 and B31, and of Osp antigens with human primary keratinocytes (KCs). The secretion of two proteins, the chemokine IL-8, and the AMP hBD-2, was measured by ELISA. (A and B) Comparison of the inflammatory responses of KCs induced by B. burgdorferi strains N40 and B31 for 24 h. Results from titration of Borrelia are shown as follows: 100 Borrelia cells/cell (100B), MOI of 100:1; 10B, MOI of 10:1; and 1B, MOI of 1:1. (C and D) Inflammatory response of KCs stimulated with lipidated (L-) OspC or OspA or unlipidated (U-) OspC or OspA for 6 and 24 h at a concentration of 100 ng/ml and Pam3Cys at a concentration of 10 μg/ml. (E and F) Inhibition by I. ricinus crude salivary gland extract (SG; 20 μg/ml) of the inflammation induced by the lipidated OspC or OspA, measured at 24 h. #, complete inhibition; φ, unstimulated cells; ***, P < 0.001; **, P < 0.01; and *, P < 0.05.
We then tested whether Borrelia lipoproteins were responsible for the KC inflammation induced by whole bacteria. When inoculated by ticks, the outer coats of Borrelia cells are primarily covered by OspC, while OspA is repressed. We performed this study at two separate time points as these specific proinflammatory genes are induced at different times during inflammation. Thus, peak secretion of IL-8 occurs at 24 h, while hBD-2 is secreted at greater amounts from 6 to 24 h of incubation (Fig. 1C and D). These studies showed that lipidated OspC (L-OspC) induced a greater inflammatory response than lipidated OspA (L-OspA). In contrast, unlipidated OspA and OspC exhibited reduced inflammatory responses compared with lipidated OspA and OspC, clearly indicating that the lipid moiety also plays an important role in inflammation. In fact, the lipid moiety alone, Pam3Cys, was shown to induce inflammation. This KC inflammatory response induced by lipidated OspA and OspC is strongly inhibited by tick SGE when KCs are coincubated with lipidated OspC or OspA. The hBD-2 secretion induced by lipidated OspA and OspC is inhibited 60 to 80% by tick SGE at 24 h. IL-8 secretion stimulated by lipidated OspA and OspC is almost 100% inhibited by tick SGE (Fig. 1E and F).
Salp15 inhibition of chemokine and AMP expression by human primary keratinocytes coincubated with whole Borrelia cells.
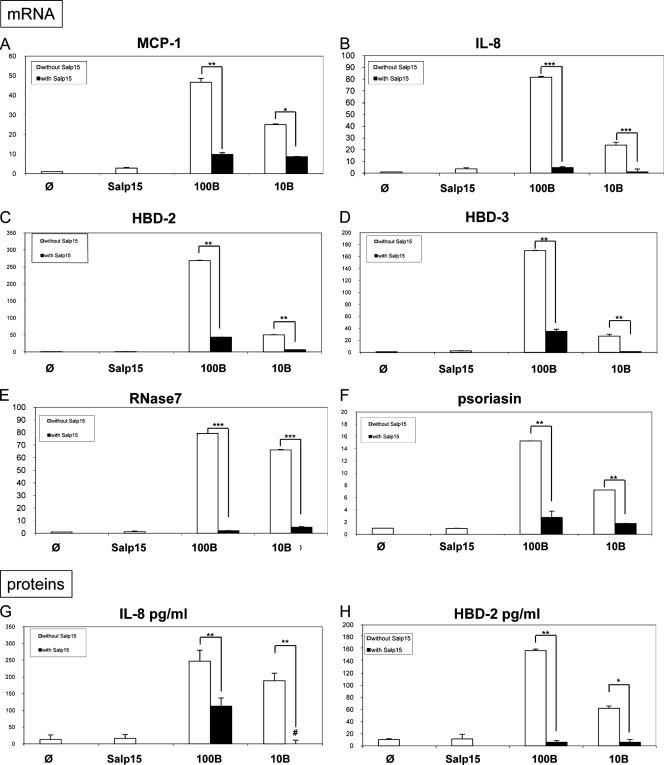
OspC binds to Salp15, thereby facilitating immune evasion by Borrelia spp. (38). To analyze its involvement in skin inflammation, we coincubated Salp15 with whole B. burgdorferi sensu stricto N40 cells and measured the inhibition of gene expression for proinflammatory chemokines. We found that Salp15 inhibited not only the mRNA expression of two chemokines, monocyte chemoattractant protein 1 (MCP-1) and IL-8 (Fig. 2 A and B), but also the expression of the AMPs (hBD-2, hBD-3, RNase 7, and psoriasin) (Fig. 2C to F). Measurements were made at 6 h, as AMPs generally exert their peak activity during the early stages of inflammation (29). The inflammation induced by B. burgdorferi was strongly impaired by the presence of Salp15 no matter which inflammatory gene we assessed at the two Borrelia concentrations tested—100 Borrelia cells (100B) per cell and 10 Borrelia cells (10B) per cell. The presence of Salp15 similarly decreased concentrations of the two proteins tested, IL-8 and hBD-2 (Fig. 2G and H).
FIG. 2.
Inhibition by I. ricinus Salp15 protein of KC inflammation induced by B. burgdorferi sensu stricto N40 strain at 6 h. Shown are the results from measurement by quantitative RT-PCR of the inhibition of mRNA of chemokines MCP-1 (A) and IL-8 (B) and AMPs hBD-2 (C), hBD-3 (D), RNase 7 (E), and psoriasin (F) by Salp15 (30 μg/ml). Panels G and H show measurement by ELISA of the secretion of two selected inflammatory molecules, IL-8 (G) and hBD-2 (H). 100B, 100 Borrelia cells per cell; 10B, 10 Borrelia cells per cell. #, complete inhibition; φ, unstimulated cells; ***, P < 0.001; **, P < 0.01; and *, P < 0.05.
Salp15 inhibition of chemokine and AMP expression by human primary KCs coincubated with OspC.
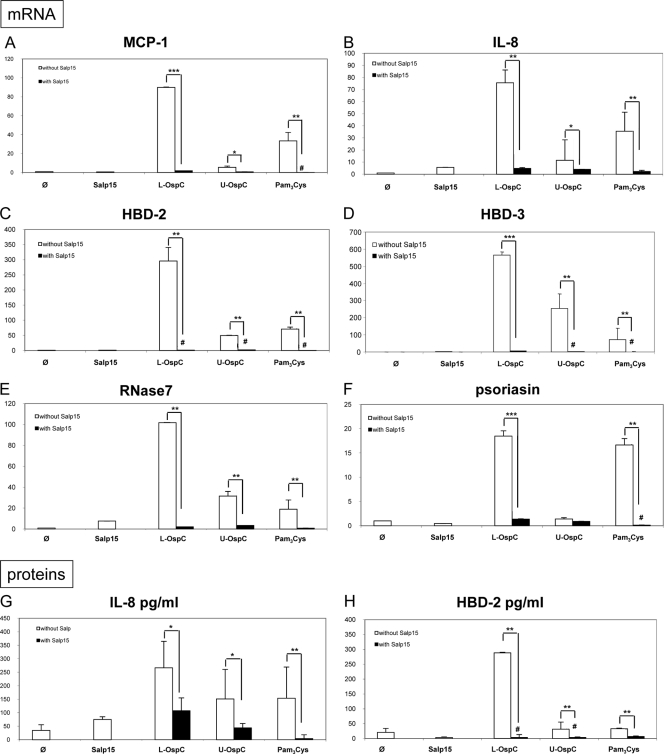
The fact that lipidated OspC-induced inflammation is inhibited by crude SGE prompted us to determine whether the tick saliva protein Salp15 could be responsible for the inhibition of KC inflammation normally induced by OspC. In fact, we found that chemokines (MCP-1 and IL-8) and AMPs (hBD-2, hBD-3, RNAse 7, and psoriasin) were strongly inhibited by Salp15 of I. ricinus at the mRNA level (Fig. 3 A to F) and also at the protein level for the two proteins tested, IL-8 and hBD-2 (Fig. 3G and H). Unlipidated OspC stimulation, as well as stimulation by the lipid moiety alone, was inhibited by Salp15, showing that both the lipid and protein components of OspC are important for the interaction with Salp15.
FIG. 3.
Inhibition by Salp15 of I. ricinus of KC inflammation induced by Borrelia lipoproteins at 6 h. Shown are the results from measurement by quantitative RT-PCR of the inhibition of mRNAs of chemokines MCP-1 (A) and IL-8 (B) and AMPs hBD-2 (C), hBD-3 (D), RNAse7 (E), and psoriasin (F) by Salp15 (30 μg/ml). Panels G and H show measurement by ELISA of the secretion of two selected inflammatory molecules, IL-8 (G) and hBD-2 (H). Lipidated (L-) and unlipidated (U-) OspC proteins were tested at 100 ng/ml, Pam3Cys at 10 μg/ml, and Salp15 at 30 μg/ml. #, complete inhibition; φ, unstimulated cells; ***, P < 0.001; **, P < 0.01; and *, P < 0.05.
Effect of vitamin D3 on the induction of LL-37 during the interaction of Borrelia-KCs and effect of tick SGE or Salp15 on this induction.
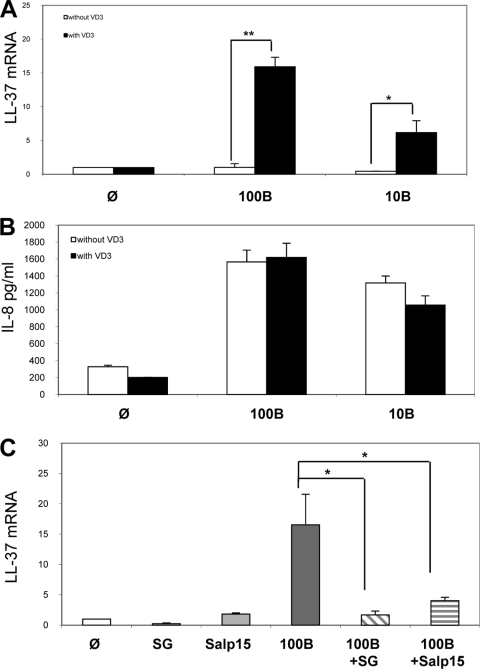
We have previously shown that the interaction of KCs with B. burgdorferi induces hBD-2, although we could not detect mRNA expression of LL-37, whose regulation seems to be more complex than that of the other AMPs (40). We then tested whether the addition of vitamin D3 to our assays affects the secretion of LL-37 or other AMPs, such as IL-8. We found that in the presence of vitamin D3, secretion of LL-37 was induced with different numbers of Borrelia cells coincubated with KCs (Fig. 4A). It is clear that vitamin D3 specifically regulated LL-37 induction, but not IL-8 secretion (Fig. 4B). We also found that LL-37 induction was strongly inhibited by tick SGE or Salp15 (Fig. 4C), which is similar to our results for the other inflammatory genes.
FIG. 4.
Effect of vitamin D3 on induction of LL-37 secreted by KCs. Human primary KCs were preincubated with vitamin D3 (VD3) for 24 h. Cells were then stimulated for 24 h with B. burgdorferi N40 at two concentrations: 100 Borrelia cells per cell (100B) and 10 Borrelia cells per cell (10B). The induction of LL-37 was measured by qRT-PCR (A), and the induction of IL-8 was measured by ELISA (B). The inhibition of LL-37 induction by KCs with crude extract salivary gland (SG) or with Salp15 was measured (C). φ, unstimulated cells; ***, P < 0.001; **, P < 0.01; and *, P < 0.05.
In vitro effect of hBD-2 and cathelicidin on the motility of B. burgdorferi N40.
AMPs have potent antimicrobial activity on a large panel of microorganisms (bacteria, fungi, viruses, and parasites). We tested the antiborrelial activities of the two AMPs most frequently found in several skin inflammations, human cathelicidin (LL-37) and human defensin (hBD-2) (6), through a kinetic study. We examined cultures after 3 h, 24 h, and 7 days under a dark-field microscope to observe the motility, number, and viability of Borrelia cells when cultured in the presence or absence of AMPs or an antibiotic. The effect of the two AMPs was compared to the effect of amoxicillin, the positive control, which completely killed Borrelia after 7 days, starting at a low concentration of 1.56 μg/ml. Defensin had a very slight effect at 24 h, with 80% of Borrelia cells motile. Interestingly, LL-37 severely altered spirochetes, with only 10% of Borrelia cells motile after 3 h and 24 h, and induced bacterial clumps at the highest AMP concentrations tested (Fig. 5). The effects of both AMPS disappeared after 7 days, when 100% Borrelia motility was observed.
FIG. 5.
In vitro effect of hBD-2 and LL-37 on B. burgdorferi sensu stricto motility. Measurement of the activity of hBD-2 (defensin) and LL-37 (human cathelicidin) on the motility of B. burgdorferi N40 strain. Borrelia cells were cultured together with the tested molecule in microtiter plates for 3 and 24 h and then transferred in 3-ml tubes for an additional 7 days. Amoxicillin was used as a positive control. Motility of bacteria was measured by observation of Borrelia under dark-field microscopy at a 200-fold magnification.
Effect of SGE and Salp15 on the interaction between TLR2 and OspC.
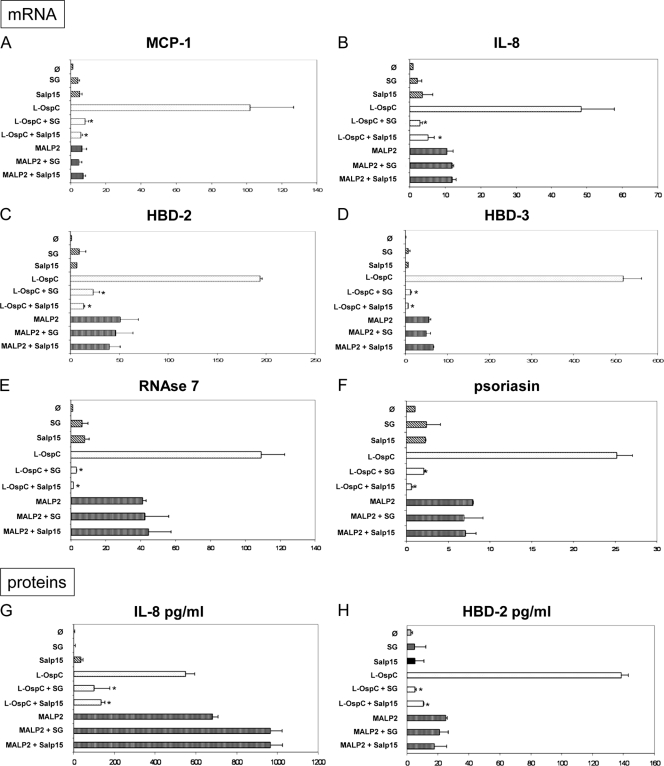
OspC, as a lipoprotein, interacts with TLR2 (13). To further analyze the interaction of OspC with Salp15, we used another TLR2 agonist, MALP2. MALP2 induced the chemokine mRNAs (MCP-1 and IL-8) and the AMP (hBD-2, hBD-3, psoriasin, and RNase 7) mRNAs, as well as IL-8 and hBD-2 proteins (Fig. 6). KC inflammation induced by MALP2 was not as strong as the inflammation induced by OspC. Both tick SGE and Salp15 strongly inhibited the KC inflammation induced by OspC but did not inhibit chemokine or AMP production induced by MALP2-TLR2 interaction, further suggesting that the observed inhibitory effects of KC inflammation are due to interactions of OspC with SGE or OspC with Salp15.
FIG. 6.
Analysis of OspC-Salp15 interaction during KC inflammation. The two TLR-2 agonists, lipidated OspC (L-OspC; 100 ng/ml) and MALP2 (200 ng/ml), were first incubated with salivary gland extract (SG; 20 μg/ml) or Salp15 (30 μg/ml) for 30 min at room temperature. The preparations were then transferred onto human primary KCs and incubated for 6 h. φ, unstimulated cells; *, statistically significant versus L-OspC; ***, P < 0.001; **, P < 0.01; and *, P < 0.05.
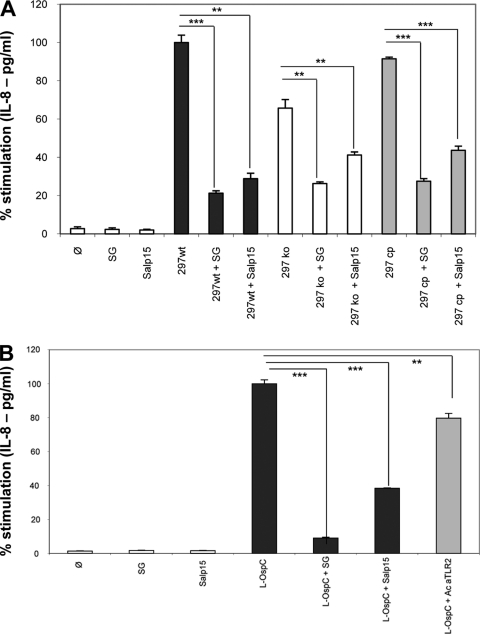
Role of OspC in Borrelia KC inflammation.
In order to measure the importance of OspC in KC inflammation, we tested a knockout Borrelia strain (297 ko) without the OspC protein, comparing the potential of its OspC (297 cp) and that of the wild-type strain B. burgdorferi sensu stricto 297 (36) to induce IL-8 secretion. The 297 ko strain stimulated IL-8 secretion at 65% of the level stimulated by wild-type strain 297 bacteria, while the complemented strain 297 cp stimulated secretion at 91% of the wild-type level (Fig. 7A). The reduction of inflammation by OspC protein suggests that OspC is an important surface antigen for Borrelia KC inflammation. The results also suggest that some other surface antigens are involved in KC inflammation. Interestingly, in the presence of SGE and Salp15, the secretion of IL-8 caused by the OspC mutant still decreased. This suggests that OspC is not the only molecule of Borrelia to interact with tick proteins and that Salp15 and SGE may have additional receptors, either on the bacteria or on the host cell, to control host inflammation.
FIG. 7.
Roles of OspC and Salp15 in KC inflammation. (A) The inflammation (IL-8 secretion in pg/ml) induced by the OspC-deficient mutant (297 ko) was compared to the inflammation induced by the native strain (297 wt) and complemented strain (297 cp). The Borrelia cell/cell ratio was 100:1, and the incubation was for 24 h. SG (20 μg/ml) or Salp15 (30 μg/ml) was preincubated with Borrelia for 30 min at room temperature and then transferred onto KCs. (B) The inhibitory effect of Salp15 on OspC-induced KC inflammation was compared to the blocking effect of an antibody against TLR2. SG and Salp15 were preincubated with L-OspC for 30 min and then transferred onto KCs and further incubated for 6 h. The antibody against TLR2 (Ac aTLR2; 1.5 μg/ml) was incubated for 30 min on KCs, and then the lipidated OspC (L-OspC; 100 ng/ml) was added for 6 h. The level of inhibition was compared to the inhibitory effect of SG (20 μg/ml) or Salp15 (30 μg/ml). ***, P < 0.001; **, P < 0.01; and *, P < 0.05.
We also compared the inhibitory effects of Salp15 and anti-TLR2 antibody on the interaction of OspC with TLR2. Salp15 has a potent inhibitory effect on OspC, with 62% of IL-8 secretion inhibited, while the blocking antibody inhibited only 21% of IL-8 secretion. A titration of the antibody up to 10 μg/ml and a longer incubation (24 h) did not increase further the inhibitory level in our model (data not shown).
DISCUSSION
KCs are the resident skin cells of the epidermis, acting as a physical barrier and as an immune barrier by detecting pathogens and by producing several AMPs, chemokines, and cytokines (6). They express functional Toll-like receptors (TLRs), except TLR7 and TLR8, which recognize a broad spectrum of PAMPs, leading to the activation of the NF-κB pathway. KCs are the main producers of cytokines and chemokines in the skin. Some of these are potent chemoattractants for monocytes, neutrophils, dendritic cells (DCs), T cells, and NK cells (27). Their ability to discriminate among stimuli by generating different patterns of antimicrobial molecules makes KCs important immune cells of skin's innate immunity (30, 44). The function of Langerhans cells is highly influenced by surrounding KCs (3), as the Langerhans cells become activated by inflammatory cytokines derived from KCs. We have previously shown that the interaction of B. burgdorferi sensu stricto with human primary KCs induced a strong inflammatory response that is inhibited by crude SGE of I. ricinus. This pointed out the major role of KCs in an arthropod-borne disease such as Lyme borreliosis.
The inhibition of KC inflammation by tick saliva was previously shown to affect the secretion of IL-8 and hBD-2 (29). We extended the panel of inflammatory molecules studied because the skin expresses several different chemokines and AMPs, of which chemokines have been shown to play an important role in the inflammation induced in Lyme disease (20). Different chemokine-binding proteins have been identified in tick saliva (11, 32) that are neutralized by the saliva, thus avoiding the recruitment of specific immune cells that would kill the inoculated pathogens. In addition to the inhibition of chemokines, we have demonstrated that the AMPs hBD-2, hBD-3, cathelicidin, psoriasin, and RNase 7 are also inhibited by tick saliva. We selected RNAse7 and psoriasin because they are produced by KCs and have potent antimicrobial activity, especially on Gram-positive bacteria (RNase 7) and on Escherichia coli (psoriasin) (39). hBD-2 and hBD-3 are induced differently (45) and also exhibit different antimicrobial activities: hBD-2 has its greatest activity against Gram-negative bacteria, while hBD-3 exhibits a much larger spectrum of antibacterial activity.
In earlier studies, we did not observe the secretion of LL-37 in our model of interaction between KCs and Borrelia (29). That lack of secretion was surprising as LL-37 is involved in numerous cutaneous inflammations (6, 40). Because vitamin D3 enables KCs to recognize pathogens through TLR2, leading to cathelicidin production (40), we preincubated Borrelia with vitamin D3 and observed the induction of LL-37. We then measured the effects of hBD-2 and LL-37, the two most-described AMPs in skin inflammations, on Borrelia. In our in vitro studies, we found that LL-37 has significant antimicrobial activity (90% decrease in Borrelia motility) against B. burgdorferi sensu stricto during the first 24 h, which suggests LL-37 is very fast and effective at inhibition of Borrelia. In contrast to LL-37, we found that hBD-2 had almost no antimicrobial activity against B. burgdorferi sensu stricto. Studies by other investigators have shown that hBD-2 also has no activity against Treponema denticola (7) and that B. burgdorferi is resistant to high levels of LL-37 (39). In our assay, LL-37 transiently affected Borrelia motility, with numerous clumps of bacteria observed in the first hours of incubation, but this effect did not last after a week of incubation.
AMPs are multifunctional molecules that play a central role in several cutaneous inflammations. They neutralize toxins released by bacteria, have potent chemotactic activity, and have angiogenic properties (6, 41). Their overexpression avoids infections in psoriasis, and their absence explains certain infections in atopic dermatitis (33). Our data revealed a potent anti-inflammatory effect of tick saliva on AMPs and chemokines present in the skin. This observation in an in vitro model merits further investigation in an in vivo mouse model.
Another key element in the transmission of Borrelia to the vertebrate host is OspC, the major surface antigen of Borrelia. Studies with OspC mutant spirochetes have shown that this lipoprotein is essential to spirochetal evasion of the host's innate immunity (19, 31, 47). OspC prevents early elimination and promotes dissemination (48). In this study, we showed that OspC is a key element in the induction of KC inflammation and that its lipid moiety, Pam3Cys, is as essential as the protein component in this inflammation. As the tick has been shown to downregulate host immunity, thus facilitating transmission of B. burgdorferi, we tested whether crude SGE and Salp15 can inhibit the inflammatory response of KCs. Our studies demonstrated that SGE inhibited both inflammations: that induced by whole Borrelia cells (29) and that induced by OspC. In tick saliva, Salp15 is a protein specifically induced by Borrelia when it enters the tick during the infective blood meal (36). It is a pluripotent protein: it affects T-cell proliferation by binding to the CD4 coreceptor, inhibits dendritic cell activation by binding to the C-type lectin DC-SIGN, and binds to OspC, protecting Borrelia from antibody-mediated killing (2, 25, 36). In this study, we observed that Salp15 also inhibits KC inflammation.
The OspC lipoprotein is a ligand for TLR2 (43). Initially, the recognition of Borrelia by TLR2 was thought to be mainly due to OspA (22). We have shown that OspC is an important part of the skin inflammation through its interaction with the resident cells of the epidermis, the KCs. The interaction of OspC with tick SGE or with Salp15 is crucial, since the coincubation with another TLR2 agonist, MALP2 (40), did not modify KC inflammation. The interaction of OspC with its receptor is complex, since it interacts as a homodimer with its ligand, and recently a ligand binding domain has been described as being essential for OspC for its adaptation to the mammalian environment (12). However, as shown in this study, OspC is not the only molecule involved in the inflammation process since the OspC-deficient mutant was still able to produce an immune response. OspC is the most abundant protein expressed on the surface of Borrelia during early infection, but this does not exclude that other proteins such as VlsE (variable surface antigen), bbk32 (fibronectin binding protein), or DbpA (decorin binding protein) play a compensatory role in skin inflammation in the absence of OspC (52).
The tick blood meal lasts for several days, and infected ticks do not inoculate Borrelia at the beginning of the blood meal. In the infection model for I. scapularis with B. burgdorferi, the tick inoculates Borrelia into the host skin (23) at approximately day 3, while in the model for I. ricinus with B. afzelii, the transmission occurs earlier (10). During feeding, the tick first dilacerates the epidermis, producing an injury. This injury triggers an inflammatory response with an important secretion of chemokines and AMPs. However, the inhibitory effects of tick saliva may be able to shut down all the danger signals present in the skin before Borrelia injection occurs. In the vertebrate host, Borrelia cells multiply locally before disseminating via the blood to the rest of the body. The inhibitory effect of tick saliva on chemokines and AMPs helps to explain the absence of cellular infiltrate at the site of a tick bite (5, 50).
We propose that tick saliva has a property not previously described: it exhibits an antialarmin effect. Alarmins are secreted by epithelial cells such as KCs and are triggered by endogenous signals or by PAMPs, called “danger-associated molecular patterns” (DAMPs). They recruit and activate antigen-presenting cells, especially dendritic cells, and boost innate and adaptive immune responses (53). In Lyme borreliosis, alarmins induced first by the injury and then by the bacterial inoculation are likely controlled by tick saliva. This property makes the tick saliva a sophisticated regulator of Borrelia infection by acting as a powerful antialarmin and antichemokine factor in the skin (16, 34). Tick saliva is therefore an essential actor in the pathogenesis of skin inflammation.
Acknowledgments
We thank B. Betschart, University of Neuchâtel, for help with maintaining the tick colony, M. H. Metz-Boutigue for LL-37 synthesis, X. Yang for providing the OspC-deficient Borrelia strain, A. Boeuf for technical support, and the REID (Réseau des Interactions Durables) network on ticks for stimulating discussions. We thank Yang Li for editing help.
Claire Marchal was supported by grant 07/908/225 from the Conseil Regional d'Alsace. Aurélie Kern was supported by grant 2009.60.053 from the Conseil Regional d'Alsace and Direction Générale de l'Armement. Part of the research project was supported by the Pasteur Institute, PTR 309 (Programme Transversal de Recherche, Paris, France).
Editor: R. P. Morrison
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Adusumilli, S., C. J. Booth, J. Anguita, and E. Fikrig. 2010. Passage through Ixodes scapularis ticks enhances the virulence of a weakly pathogenic isolate of Borrelia burgdorferi. Infect. Immun. 78:138-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguita, J., et al. 2002. Salp15, an Ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity 16:849-859. [DOI] [PubMed] [Google Scholar]
- 3.Asahina, A., and K. Tamaki. 2006. Role of Langerhans cells in cutaneous protective immunity: is the reappraisal necessary? J. Dermatol. Sci. 44:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Batsford, S., J. Dunn, and M. Mihatsch. 2004. Outer surface lipoproteins of Borrelia burgdorferi vary in their ability to induce experimental joint injury. Arthritis Rheum. 50:2360-2369. [DOI] [PubMed] [Google Scholar]
- 5.Beaufays, J., et al. 2008. Ir-LBP, an Ixodes ricinus tick salivary LTB4-binding lipocalin, interferes with host neutrophil function. PLoS One 3:e3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braff, M. H., and R. L. Gallo. 2006. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr. Top. Microbiol. Immunol. 306:91-110. [DOI] [PubMed] [Google Scholar]
- 7.Brissette, C. A., and S. A. Lukehart. 2007. Mechanisms of decreased susceptibility to beta-defensins by Treponema denticola. Infect. Immun. 75:2307-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brossard, M., and S. K. Wikel. 2004. Tick immunobiology. Parasitology 129(Suppl.):S161-S176. [DOI] [PubMed] [Google Scholar]
- 9.Chong, K. T., et al. 2006. High level expression of human epithelial beta-defensins (hBD-1, 2 and 3) in papillomavirus induced lesions. Virol. J. 3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crippa, M., O. Rais, and L. Gern. 2002. Investigations on the mode and dynamics of transmission and infectivity of Borrelia burgdorferi sensu stricto and Borrelia afzelii in Ixodes ricinus ticks. Vector Borne Zoonotic Dis. 2:3-9. [DOI] [PubMed] [Google Scholar]
- 11.Déruaz, M., et al. 2008. Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J. Exp. Med. 205:2019-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earnhart, C. G., et al. 2010. Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Mol. Microbiol. 76:393-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fikrig, E., and S. Narasimhan. 2006. Borrelia burgdorferi—traveling incognito? Microbes Infect. 8:1390-1399. [DOI] [PubMed] [Google Scholar]
- 14.Frischknecht, F. 2007. The skin as interface in the transmission of arthropod-borne pathogens. Cell. Microbiol. 9:1630-1640. [DOI] [PubMed] [Google Scholar]
- 15.Frohm, M., et al. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258-15263. [DOI] [PubMed] [Google Scholar]
- 16.Gallo, R. L. 2008. Sounding the alarm: multiple functions of host defense peptides. J. Invest. Dermatol. 128:5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg, R., et al. 2006. Cutting edge: CD4 is the receptor for the tick saliva immunosuppressor, Salp15. J. Immunol. 177:6579-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gläser, R., et al. 2005. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. 6:57-64. [DOI] [PubMed] [Google Scholar]
- 19.Grimm, D., et al. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A. 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerau-de-Arellano, M., and B. T. Huber. 2005. Chemokines and Toll-like receptors in Lyme disease pathogenesis. Trends Mol. Med. 11:114-120. [DOI] [PubMed] [Google Scholar]
- 21.Harder, J., and J. M. Schröder. 2002. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 277:46779-46784. [DOI] [PubMed] [Google Scholar]
- 22.Hirschfeld, M., et al. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 23.Hojgaard, A., R. J. Eisen, and J. Piesman. 2008. Transmission dynamics of Borrelia burgdorferi s.s. during the key third day of feeding by nymphal Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 45:732-736. [DOI] [PubMed] [Google Scholar]
- 24.Hovius, J. W., et al. 2008. Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog. 4:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovius, J. W., et al. 2007. Identification of Salp15 homologues in Ixodes ricinus ticks. Vector Borne Zoonotic Dis. 7:296-303. [DOI] [PubMed] [Google Scholar]
- 26.Hovius, J. W., et al. 2008. Preferential protection of Borrelia burgdorferi sensu stricto by a Salp15 homologue in Ixodes ricinus saliva. J. Infect. Dis. 198:1189-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebre, M. C., et al. 2007. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Invest. Dermatol. 127:331-341. [DOI] [PubMed] [Google Scholar]
- 28.Lusitani, D., S. E. Malawista, and R. R. Montgomery. 2002. Borrelia burgdorferi are susceptible to killing by a variety of human polymorphonuclear leukocyte components. J. Infect. Dis. 185:797-804. [DOI] [PubMed] [Google Scholar]
- 29.Marchal, C. M., et al. 2009. Defensin is suppressed by tick salivary gland extract during the in vitro interaction of resident skin cells with Borrelia burgdorferi. J. Invest. Dermatol. 129:2515-2517. [DOI] [PubMed] [Google Scholar]
- 30.Nestle, F. O., P. Di Meglio, J. Z. Qin, and B. J. Nickoloff. 2009. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9:679-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. U. S. A. 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira, C. J., et al. 2008. Tick saliva inhibits the chemotactic function of MIP-1alpha and selectively impairs chemotaxis of immature dendritic cells by down-regulating cell-surface CCR5. Int. J. Parasitol. 38:705-716. [DOI] [PubMed] [Google Scholar]
- 33.Ong, P. Y., et al. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151-1160. [DOI] [PubMed] [Google Scholar]
- 34.Oppenheim, J. J., P. Tewary, G. de la Rosa, and D. Yang. 2007. Alarmins initiate host defense. Adv. Exp. Med. Biol. 601:185-194. [DOI] [PubMed] [Google Scholar]
- 35.Pal, U., et al. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119:457-468. [DOI] [PubMed] [Google Scholar]
- 36.Pal, U., et al. 2004. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J. Clin. Invest. 113:220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pechová, J., G. Stĕpánová, L. Kovár, and J. Kopecký. 2002. Tick salivary gland extract-activated transmission of Borrelia afzelii spirochaetes. Folia Parasitol. 49:153-159. [PubMed] [Google Scholar]
- 38.Ramamoorthi, N., et al. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436:573-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkar, A., et al. 2009. Borrelia burgdorferi resistance to a major skin antimicrobial peptide is independent of outer surface lipoprotein content. Antimicrob. Agents Chemother. 53:4490-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schauber, J., et al. 2007. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 117:803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schröder, J. M., and J. Harder. 2006. Antimicrobial skin peptides and proteins. Cell. Mol. Life Sci. 63:469-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seo, S. J., S. W. Ahn, C. K. Hong, and B. I. Ro. 2001. Expressions of beta-defensins in human keratinocyte cell lines. J. Dermatol. Sci. 27:183-191. [DOI] [PubMed] [Google Scholar]
- 43.Singh, S. K., and H. J. Girschick. 2006. Toll-like receptors in Borrelia burgdorferi-induced inflammation. Clin. Microbiol. Infect. 12:705-717. [DOI] [PubMed] [Google Scholar]
- 44.Sorensen, O. E., et al. 2003. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J. Immunol. 170:5583-5589. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen, O. E., et al. 2005. Differential regulation of beta-defensin expression in human skin by microbial stimuli. J. Immunol. 174:4870-4879. [DOI] [PubMed] [Google Scholar]
- 46.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Invest. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tilly, K., A. Bestor, M. W. Jewett, and P. Rosa. 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect. Immun. 75:1517-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tilly, K., et al. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Titus, R. G., J. V. Bishop, and J. S. Mejia. 2006. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 28:131-141. [DOI] [PubMed] [Google Scholar]
- 50.Wikel, S. K. 1999. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 29:851-859. [DOI] [PubMed] [Google Scholar]
- 51.Xu, Q., S. V. Seemanapalli, K. E. Reif, C. R. Brown, and F. T. Liang. 2007. Increasing the recruitment of neutrophils to the site of infection dramatically attenuates Borrelia burgdorferi infectivity. J. Immunol. 178:5109-5115. [DOI] [PubMed] [Google Scholar]
- 52.Xu, Q., K. McShan, and F. T. Liang. 2008. Essential protective role attributed to the surface lipoproteins of Borrelia burgdorferi against innate defences. Mol. Microbiol. 69:15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 54.Yang, D., G. de la Rosa, P. Tewary, and J. J. Oppenheim. 2009. Alarmins link neutrophils and dendritic cells. Trends Immunol. 30:531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeidner, N. S., B. S. Schneider, M. S. Nuncio, L. Gern, and J. Piesman. 2002. Coinoculation of Borrelia spp. with tick salivary gland lysate enhances spirochete load in mice and is tick species-specific. J. Parasitol. 88:1276-1278. [DOI] [PubMed] [Google Scholar]
- 56.Zhao, Z., B. McCloud, R. Fleming, and M. S. Klempner. 2007. Borrelia burgdorferi-induced monocyte chemoattractant protein-1 production in vivo and in vitro. Biochem. Biophys. Res. Commun. 358:528-533. [DOI] [PubMed] [Google Scholar]