Abstract
Bacterial lipoproteins are a set of membrane proteins with many different functions. Due to this broad-ranging functionality, these proteins have a considerable significance in many phenomena, from cellular physiology through cell division and virulence. Here we give a general overview of lipoprotein biogenesis and highlight examples of the roles of lipoproteins in bacterial disease caused by a selection of medically relevant Gram-negative and Gram-positive pathogens: Mycobacterium tuberculosis, Streptococcus pneumoniae, Borrelia burgdorferi, and Neisseria meningitidis. Lipoproteins have been shown to play key roles in adhesion to host cells, modulation of inflammatory processes, and translocation of virulence factors into host cells. As such, a number of lipoproteins have been shown to be potential vaccines. This review provides a summary of some of the reported roles of lipoproteins and of how this knowledge has been exploited in some cases for the generation of novel countermeasures to bacterial diseases.
Lipid modification of bacterial proteins facilitates the anchoring of hydrophilic proteins to hydrophobic surfaces through the hydrophobic interaction of the attached acyl groups to the cell wall phospholipids. The addition of acyl moieties effectively provides a membrane anchor allowing the protein to function effectively in the aqueous environment (86). Bacterial lipoproteins have been shown to perform various roles, including nutrient uptake, signal transduction, adhesion, conjugation, and sporulation, and participate in antibiotic resistance, transport (such as ABC transporter systems), and extracytoplasmic folding of proteins (2, 91, 105, 131, 170). In the case of pathogens, lipoproteins have been shown to play a direct role in virulence-associated functions, such as colonization, invasion, evasion of host defense, and immunomodulation (75, 82, 88). In Gram-negative bacteria, two of the three lipoprotein biosynthetic enzymes appear to be essential for viability (55, 63, 123, 177), while in some Gram-positive bacteria, they have been shown to be dispensable (95). Consequently, mutations in enzymes involved in the pathway of lipoprotein processing are lethal in Gram-negative bacteria, but many Gram-positive bacteria tolerate these mutations, exhibiting only slight growth defects (12, 64, 133, 177). One reason for the viability of Gram-positive mutants could be that the precursors of some essential lipoproteins have the same functionality as the mature lipoproteins (95, 187). However, in many Gram-positive bacteria, the presence of lipoproteins is necessary for virulence (106, 175). Previous reviews of lipoprotein biosynthesis and/or their roles (75, 170) emphasize that there is still much to be discovered concerning the biosynthetic pathways and functions of lipoproteins. Since the latter reviews, there has been a significant increase in the number of proteins reported to be lipoproteins, directly from biochemical studies, and predicted to be lipoproteins, indirectly from sequenced genomes. Further study of this group of bacterial proteins will contribute to a better understanding of their roles and mechanisms of action, supporting their use in the development of countermeasures against bacterial pathogens.
LIPOPROTEIN BIOSYNTHESIS
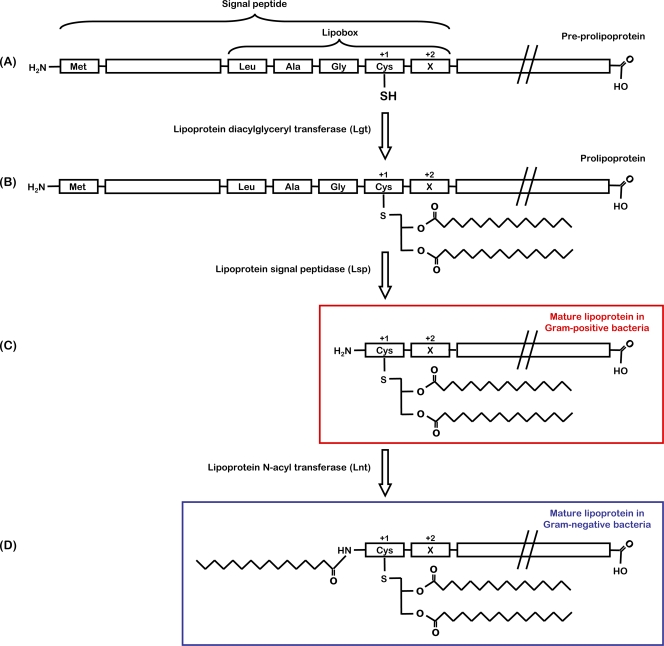
In both Gram-negative and Gram-positive bacteria, lipoproteins are initially translated as preprolipoproteins (Fig. 1), which possess an N-terminal signal peptide of around 20 amino acids with typical characteristic features of the signal peptides of secreted proteins (76). A conserved sequence at the C region of the signal peptides, referred to as lipobox, [LVI][ASTVI][GAS]C, is modified through the covalent attachment of a diacylglycerol moiety to the thiol group on the side chain of the indispensable cysteine residue (7). This modification is catalyzed by the enzyme lipoprotein diacylglyceryl transferase (Lgt), resulting in a prolipoprotein (Fig. 1) consisting of a diacylglycerol moiety linked by a thioester bond to the protein. Lgt is a basic (pKa of ∼10) protein and transfers negatively charged phospholipids, phosphatidylglycerol in particular, as its lipid substrate (151). Bacterial lipid modification is suggested to be initiated at the cytoplasmic side of the membrane rather than in the cytoplasm (159), but due to insufficient information about Lgt, this is only speculation. Lgt is a critical enzyme in the generation of bacterial lipoproteins, although most of its characteristics are still poorly understood. Comparisons of the Lgt sequences from phylogenetically distantly related microorganisms revealed several highly conserved regions that might be important for function (140). In site-directed mutagenesis studies, His-103 and Tyr-235 were found to be essential for Lgt activity (150). Although lgt is found as a single gene in most bacterial genomes, there are two putative lgt paralogues carried in some bacteria, such as Coxiella burnetti, Bacillus cereus, Clostridium perfringens, and Streptomyces coelicolor (75), but the reason why multiple Lgt enzymes are present in these strains is unknown.
FIG. 1.
Biosynthesis of bacterial lipoproteins. (A to C) Two-step biosynthetic pathway in Gram-positive bacteria. (A to D) Three-step biosynthetic pathway in Gram-negative bacteria. (A) The precursor of lipoproteins is the preprolipoprotein, with an N-terminal signal peptide possessing a characteristic consensus sequence of the lipobox. (B) During lipoprotein maturation, the thiol group of the invariant cysteine in the lipobox is modified by a diacylglyceryl moiety by lipoprotein diacylglyceryl transferase (Lgt), which serves as a membrane anchor. (C) After lipidation, lipoprotein signal peptidase (Lsp) cleaves the signal peptide, leaving the cysteine as the new amino-terminal residue forming the mature lipoprotein in Gram-positive bacteria. (D) In Gram-negative and some Gram-positive bacteria, the mature lipoprotein has an additional amide-linked fatty acid at the N-terminal cysteine residue attached by lipoprotein N-acyl transferase (Lnt). Amino acid residues at position +2, +3, and +4 have a role in membrane localization of Gram-negative bacterial lipoproteins.
After lipidation, lipoprotein signal peptidase (Lsp or SPase II) is responsible for cleaving the signal sequence of the lipidated prolipoprotein and leaves the cysteine of the lipobox as the new amino-terminal residue (181). This transmembrane enzyme (108) has five conserved sequence regions (178) and six functionally important residues (Asp-14, Asn-99, Asp-102, Asn-126, Ala-128, and Asp-129) and is a member of the aspartic protease family (40). Application of the reversible, noncompetitive inhibitor of Lsp, globomycin, has been important in studying the activity and function of Lsp enzymes (39), and a mechanism for cleavage of target proteins by Lsp has been proposed by Tjalsma et al. (178). Similarly to the case with lgt, only a single lsp gene is present in most bacteria, although some organisms possess two putative lsp paralogues (for example, Listeria monocytogenes and Nocardia farcinica). However, the roles of these paralogues are unknown (75, 144). Interestingly, a second peptidase, called Eep (enhanced expression of pheromone), a metallopeptidase, has been found in Enterococcus faecalis and is involved in the cleaving process of signal peptides of some lipoproteins (3). Lsp was originally thought to cleave only lipid-modified precursors. In a selection of Eubacteria (e.g., L. monocytogenes and Streptococcus agalactiae), however, it has been demonstrated that lipidation is not a prerequisite for the activity of Lsp (12, 70).
In Gram-negative bacteria and some Gram-positive bacteria, the cleaved prolipoprotein undergoes an additional modification by attachment of an amide-linked acyl group to the N-terminal cysteine residue by lipoprotein N-acyl transferase (Lnt) (63). Lnt has been shown to be able to utilize all available phospholipids from phosphatidylglycerol through phosphatidylethanolamine to cardiolipin as acyl donors (77). BLAST search analysis for homologues of Escherichia coli Lnt revealed that Lnt is widely present in Gram-negative bacteria. Some indications for N acylation in low-GC Gram-positive bacteria have been reported (89), but there is no evidence for the presence of Lnt in this group, raising the question of which enzyme catalyzes the N acylation of lipoproteins in these bacteria or if it indeed occurs. In contrast, lnt homologues have been identified in all classes of high-GC Gram-positive bacteria (189). Recently, Lnt activity was identified in mycobacteria, and the responsible genes were also assigned (183). There are two Lnt homologues in Streptomyces coelicolor, but their genes failed to rescue an E. coli lnt-deficient strain (189), and the function of these enzymes thus remains unclear. The previously held notion that lipoproteins of Gram-positive bacteria are diacylated and those of Gram-negative bacteria are triacylated has therefore ceased to be so clear-cut. Indeed, the situation may be more diverse in that a single species may contain both forms of lipoproteins, since it has been shown that Mycoplasma gallisepticum possesses both di- and triacylated proteins (80-81). In common with the aminoacylation of the amino-terminal cysteine of bacterial lipoproteins, it has been demonstrated that the eukaryotic signaling protein Sonic hedgehog is also acylated at its amino-terminal cysteine. Notwithstanding the characteristics of this modification relative to that performed by Lnt, i.e., formation of an acyl-amine linkage, the eukaryotic enzyme that facilitates this amino-terminal acylation has no similarity to its prokaryotic counterpart (25).
Both the diacylglyceryl group and the amino-terminal acyl group are derived from membrane phospholipids and provide tight anchorage of the lipoprotein to the membrane (66). Although the structure of the acyl chains is thought to be predominantly from membrane phospholipids, there are reports on variations in the structure of these acyl chains (22). Furthermore, in Borrelia burgdorferi, an unusual lipid component has been found in lipoproteins where one of the ester-linked fatty acids is replaced by an acetyl group (13). Since some lipoproteins are surface exposed, this raises the possibility that the acyl moieties and modifications may play more of a role than just anchoring the lipoproteins. The importance of bacterial lipoproteins, especially with respect to disease, is emphasized in the following sections of this review, which highlight the localization of lipoproteins and their effects on host cell interaction. As such, the enzymes involved in the biosynthesis of these posttranslationally modified proteins have been proposed to be targets for the development of novel antibacterial agents. This is in part due to the absence of any homologues in eukaryotes. However, lipidation of some proteins is observed in eukaryotic cells. These modifications are predominantly of two types, posttranslational palmitoylation of cysteine via a thioester linkage and the cotranslational amide-linked myristoylation of amino-termini glycine residues (49, 97), though the enzymes that mediate this are distinct from the enzymes that mediate bacterial lipidation.
LIPOPROTEIN LOCALIZATION
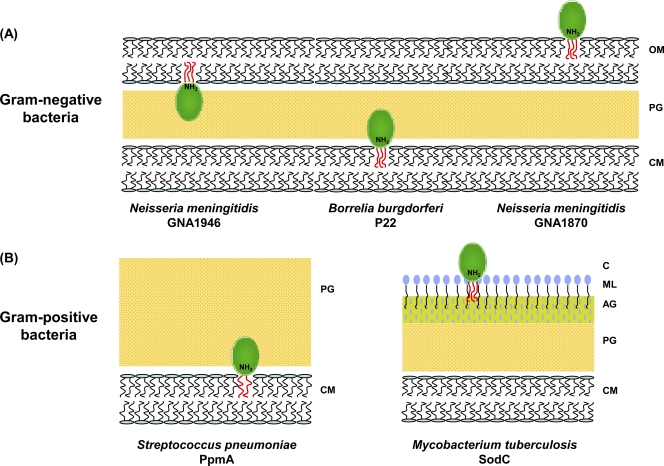
Following signal peptide-dependent translocation across the cytoplasmic membrane, lipoproteins can localize at various places in the cell (Fig. 2). It has been reported that Escherichia coli has more than 90 lipoproteins and that the majority of these are located at the periplasmic face of the outer membrane, with others present at the periplasmic face of the inner membrane (111). Although the lipid moiety is proposed to be responsible for attaching the protein to the membrane through hydrophobic interactions, whether inner membrane periplasmic, outer membrane periplasmic, or outer membrane external, in some cases it is not only the interaction of the acyl component that contributes to anchorage. The peptidoglycan-associated lipoprotein (Pal) of Gram-negative bacteria possesses a region that forms a binding pocket for the m-Dap residue (meso-diaminopimelate) of peptidoglycan. This binding pocket has conserved surface residues that interact with the peptide portion of peptidoglycan with the m-Dap residue, forming hydrogen bond and hydrophobic contacts to Pal (127). Pal is transcribed as part of an operon that also encodes the proteins TolB and TolA, which interact with the outer membrane-anchored Pal, forming a network linking the peptidoglycan layer with the inner and outer membranes (60).
FIG. 2.
Localization of bacterial lipoproteins. (A) In Gram-negative bacteria, lipoproteins are attached to the cytoplasmic membrane, the extracellular or peripheral side of the outer membrane. (B) In Gram-positive bacteria, lipoproteins are anchored to the extracellular surface of the cytoplasmic membrane and also to the unique mycolate-based lipid layer of the cell wall of Mycobacterium tuberculosis. OM, outer membrane; PG, peptidoglycan; CM, cytoplasmic membrane; AG, arabino-galactan; ML, mycolic acid layer; C, capsule-like material; N, N-terminal.
The most abundant lipoprotein of Gram-negative bacteria, and the first discovered lipoprotein, Braun's lipoprotein, is the only known lipoprotein that is covalently linked to the cell wall. It has an unusual structure, with three monomers forming a coiled-coil trimer and the N-terminal domain embedding into the outer membrane. The protein binds to peptidoglycan via a peptide bond between the ɛ-amino group of the C-terminal lysine residue of the Braun lipoprotein and the α-carbonyl of m-Dap present in peptidoglycan (43). The Pal and Braun lipoproteins interact with each other and together contribute to the integrity of the cell wall.
In Gram-negative bacteria, mature lipoproteins are localized to various sites within the cell wall (Fig. 2). They are targeted to the periplasmic face of the inner or outer membranes by the lipoprotein localization machinery (Lol), which consists of a transmembrane protein complex (LolCDE), a periplasmic chaperone (LolA), and an outer-membrane receptor (LolB) (180). Lipoproteins destined for the outer membrane are translocated by the LolCDE complex, an ATP-binding cassette (ABC) transporter, and the periplasmic chaperone, LolA. The determining factor as to whether a lipoprotein is directed to the outer membrane by the Lol machinery or is retained at the inner membrane was initially reported to be the identity of the amino acid adjacent to the conserved cysteine, known as the “+2” rule (200). This rule essentially requires an aspartate residue to be present at the +2 position for a protein to be retained at the inner membrane, while substitution with a different amino acid at this position results in translocation by the Lol machinery to the outer membrane. However, this rule is far from universal (162), with amino acid residues at positions +3 and +4 also having been shown to have a role in outer membrane localization in a number of Gram-negative bacteria(112, 163). Usually, LolCDE does not release lipoproteins, which lack an amino-linked acyl chain (54, 145). However, in a temperature-sensitive lnt mutant of Salmonella enterica serovar Typhimurium, lipoproteins without an amino-linked acyl chain are localized to the outer membrane, though how this occurs remains unknown (63).
Although in E. coli all of the known lipoproteins face the periplasm (21), in some Gram-negative bacteria, including pathogenic spirochetes, lipoproteins are present on the outer leaflet of the outer membrane (Fig. 2). However, little is known about the exact mechanism of how they are translocated across the outer membrane. In contrast to E. coli and many other Gram-negative bacteria, Neisseria meningitidis and several spirochetes do not possess the tol-pal gene cluster, nor do they have a complete Lol apparatus. It has been postulated that the lack of a complete Lol system in the spirochete Borrelia burgdorferi may be a cause of localization of several lipoproteins to the outer leaflet of the outer membrane of this bacterium (155). More recently, work on lipoproteins of Borrrelia has led to the proposal that release from the inner membrane and outer membrane translocation are separate events and that there may be other amino-terminal determinants that direct outer membrane translocation relative to those that direct inner membrane retention or release (154). The role of the amino terminus of lipoproteins may be more than just to direct outer membrane localization. A recent study used nuclear magnetic resonance (NMR) to demonstrate that the amino terminus of the Neisseria meningitidis lipoprotein, LP2086, serves as an extended linker to display the protein at the extracellular surface (102-103).
In Gram-positive bacteria, the issue of localization is conceptually simpler due to their lack of an outer membrane, and it has been reviewed in detail by Hutchings et al. (75). The preprolipoprotein precursor is translocated across the cytoplasmic membrane of bacteria, directed by the N-terminal signal peptide sequence, where it is modified by the biosynthetic enzymes discussed above. The resulting lipoproteins are thus being exposed to the extracellular environment, anchored to the outer leaflet of the plasma membrane via hydrophobic interactions. The majority of preprolipoproteins are exported in an unfolded conformation by the universally conserved Secretory (Sec) pathway (44) and obtain tertiary structure entirely after the Sec pore. In addition to the Sec pathway, many prokaryotic organisms possess other protein export pathways, including the twin-arginine translocation (Tat) pathway, which is responsible for the transport of folded proteins across the membrane (16). Translocation of lipoproteins by the Tat system in the high-GC Gram-positive bacteria (Actinomycetes) has also been indicated (195). Similarly, Giménez et al. represented the first experimental demonstration of a lipoprotein Tat substrate in the archaeon Haloferax volcanii (58). Tat-mediated transport of lipoproteins across the cytoplasmic membrane of Gram-negative bacteria has also been reported (186).
The cell wall of Mycobacterium tuberculosis contains an atypical outer lipid layer, consisting predominantly of mycolic acids, which serves as a potential anchoring point for lipoproteins (Fig. 2). This is supported by immunogold labeling experiments that demonstrate that mycobacterial lipoproteins are surface exposed (198). There is evidence for glycosylation of a number of mycobacterial lipoproteins (90, 107), although the role of glycosylation is not clear. It might contribute to maintaining the membrane association of the protein or protect against proteolytic cleavage (72). Finally, lipoproteins can be shed from the cell and may play an extracellular role, extending their ability to function farther away from the cell (196).
As described above, the pathway for lipoprotein biogenesis consists of three steps catalyzed by lipoprotein diacylglyceryl transferase (Lgt), lipoprotein or type II signal peptidase (Lsp or SPase II), and lipoprotein N-acyl transferase (Lnt). The accurate processing of lipoproteins is likely to be carried out by a close interaction between Lgt, Lsp, and the protein translocation apparatus. How the biosynthetic pathway couples to the translocation machinery nonetheless remains to be resolved (75). Moreover, recent publications regarding the existence of Tat translocation of lipoproteins reveal that linear lipoprotein precursors can not only be lipidated but also folded, which makes the understanding of the mode of modification even more unclear.
BIOLOGICAL PROPERTIES OF LIPOPROTEINS ASSOCIATED WITH VIRULENCE
Lipoproteins are required for virulence in some bacteria playing a variety of roles in host-pathogen interaction, from surface adhesion and initiation of inflammatory processes through to translocation of virulence factors into the host cytoplasm (Table 1). These roles of lipoproteins in virulence have typically been revealed in lgt and lsp mutants. In order to investigate the role of lipoproteins in virulence, studies relating to pathogenesis on lgt or lsp mutant bacteria have been carried out (35). For example, a reduction in fibronectin and laminin binding of Lsp mutants of Streptococcus pyogenes has been demonstrated, with the mutant showing reduced levels of adhesion and internalization (47). In many cases, mutations have led to attenuation of virulence in animal models of infection. For example, an lgt mutant of Streptococcus pneumoniae (133) was avirulent in a mouse model of infection, while disruption of lgt resulted in moderately attenuated virulence of Streptococcus equi (64), with 3 of 30 mice challenged with the Δlgt strain exhibiting signs of disease. Loss of Lsp markedly reduced virulence of M. tuberculosis, (149), but no effect has been found on the virulence of Streptococcus suis (38). An lsp mutant of L. monocytogenes showed reduced virulence; the 50% lethal dose (LD50) value of the mutant was 10-fold lower than that of the wild-type (144). Importantly, an lgt mutant of Staphylococcus aureus showed a hypervirulent phenotype in a mouse infection model, accompanied with attenuated growth (168). An explanation for this increase in virulence could be that the nonlipidated lipoproteins are not recognized by the immune system and are thus unable to activate Toll-like receptor 2 (TLR2)-mediated signaling, a component of the innate immune response (24, 168). Bacterial lipopeptides are recognized by TLR2 in a complex manner. Initially, it was reported that diacylated lipoproteins were recognized by a heterodimer of both TLR2 and TLR6 and that triacylated lipopeptides, typically produced by Gram-negative bacteria, are recognized by a heterodimer of both TLR2 and TLR1 (171-172). However, in addition to the recent findings that some lipoproteins from Gram-positive bacteria are triacylated, it has also been shown that these triacylated proteins were native ligands of TLR2 that did not require either TLR6 or TLR1 (89). Although it has been shown that it is the acyl moiety of lipoproteins that interacts with TLR2, the question remains as to how these structures interact if the acyl chains are interacting hydrophobically with the bacterial membrane (83). Notwithstanding this, the discovery that bacterial lipoproteins are potent inducers of the host inflammatory responses adds a novel dimension to their role in pathogenesis, highlighting their potential to affect a wide range of mechanisms in virulence. The following section looks in detail at the roles of lipoproteins of four pathogens where the acyl-modified proteins of these bacteria have been shown to have several roles in pathogenesis.
TABLE 1.
Representative biological activities elicited by lipoproteins from bacterial pathogens
| Role or function | Lipoprotein(s) | Strain | Reference(s) |
|---|---|---|---|
| Antigenicity | LpqH, LprG, PstS-1 | M. tuberculosis | 41, 51, 56, 73, 114, 148, 166, 179 |
| PsaA, PiaA, PiuA | S. pneumoniae | 85, 125 | |
| VlsE, Osp | B. burgdorferi | 10, 199 | |
| fHbp, LbpB, P47, GNA2132 | N. meningitidis | 5, 32, 100, 147, 160 | |
| Involved in colonization | PsaA, PpmA, SlrA | S. pneumoniae | 34, 71, 101, 117 |
| DbpA, OspB, BmpA | B. burgdorferi | 18, 113, 188 | |
| Components of transport systems | PstS-1, FecB | M. tuberculosis | 182, 192 |
| PsaA, PiaA, PiuA, PspA | S. pneumoniae | 23, 42, 65 | |
| OppA, OspB | B. burgdorferi | 96, 113 | |
| LpbB, GNA1946, Tpb2 | N. meningitidis | 94, 134, 190, 201 | |
| Required for growth | ModA, SubI, LpqH | M. tuberculosis | 28, 153, 166 |
| PsaA | S. pneumoniae | 101 | |
| OspB | B. burgdorferi | 113 | |
| DsbA, GNA33 | N. meningitidis | 1, 176 | |
| Protein folding | PpmA, SlrA | S. pneumoniae | 71, 121 |
| DsbA | N. meningitidis | 176 | |
| Signal transduction | LprF, LprJ | M. tuberculosis | 167 |
| Role in antibiotic resistance | PsaA | S. pneumoniae | 117 |
Lipoproteins of Mycobacterium tuberculosis.
The M. tuberculosis genome encodes 99 putative lipoproteins, ca. 2.5% of the predicted proteome, with many different functions verified either experimentally or by bioinformatical tools or both (29, 169). Some lipoproteins are solute binding proteins (SBPs), contributing to virulence as components of ABC transporters (PstS and ModA) (28, 191) or play a role in growth (SubI and GlnH) (153). PstSs are phosphate-binding receptors of a phosphate-specific transport system, and PstS-3 and PstS-1 have been shown to be promising vaccine candidates (37, 173). PstS-1 is a 38-kDa lipoglycoprotein involved in phosphate transport and has been demonstrated to be an apoptogenic molecule. It induces both the intrinsic and extrinsic pathway of the caspase cascade (148). In addition to PstS-1, three other lipoprotein antigens, namely, LpqH, LprG, and LprA, have also been shown to contribute to virulence by induction of either immunosuppressive responses and/or humoral and cellular responses. ModA functions as a molybdenum transporter and is potentially involved in virulence (28). A redox enzyme, the SodC (Cu, Zn superoxide dismutase) antioxidant, may participate in inhibition of the immune response in the phagolysosomal oxidative environment, and there is evidence of its lipid modification (36). The Mce family of proteins, whose members enable M. tuberculosis to invade mammalian cells, contains a high proportion of lipoproteins (33). However, how these lipoproteins mediate entry has not yet been established.
Similar to PstS, the 19-kDa antigen of M. tuberculosis is also a lipoglycoprotein and has been the focus of several studies because of its pleiotropic effects on the immune response. Glycosylation was found to be important for retaining the protein within the cell in association with the acyl component (197). The 19-kDa antigen is recognized by TLR2, activating immune responses at early time points of infection with an associated increase in the production of cytokines (e.g., interleukin 1β [IL-1β], IL-12p40, and tumor necrosis factor alpha [TNF-α] secretion) (197) and toxic intermediates, such as reactive oxygen radicals and reactive nitrogen intermediates (20). In contrast, in chronic infection, the 19-kDa antigen has been shown to inhibit antimicrobial mechanisms, including inhibition of gamma interferon (IFN-γ)-dependent responses, such as antigen processing (122). Following antigen presentation by major histocompatibility complex (MHC) class II molecules, CD4+ T cells become activated and secrete cytokines, including IFN-γl which plays a central role in the host defense against tuberculosis by inducing transcription of more than 200 genes essential for antimicrobial mechanisms (51). Prolonged exposure of macrophages to the 19-kDa lipoprotein inhibits transcription of some genes regulated by IFN-γ, including MHC class II transactivator (CIITA), MHC II, interferon regulatory transcription factor I (IRF-I), and type I receptor for the Fc domain of IgG (CD64) (51, 130). Inhibition of these pathways is an efficient way to make macrophages unable to eradicate M. tuberculosis, and it is likely that it is not solely the 19-kDa lipoprotein of M. tuberculosis that is able to do so. With this mechanism, the 19-kDa lipoprotein evades immune responses by inhibiting MHC-II expression and antigen presentation, allowing the pathogen to persist in macrophages and maintain a chronic infection.
Although CD4+ T cells are the more dominant participants of immunity against tuberculosis, the 19-kDa antigen can also modulate CD8+ T cell responses, even if the mechanism of inhibition is different. In macrophages, the alternate MHC class I antigen presentation of M. tuberculosis occurs in a vacuole, not in the cytoplasm, consisting of phagosomal degradation of the antigen and then probably phagosomal binding of the antigenic peptides to MHC class I, leading to lysis of infected cells or cytokine production by the CD8+ T cells. It has been shown that the 19-kDa antigen does not affect the levels of MHC-I and MHC-I mRNA but inhibits phagosome maturation and lysosomal protease delivery, resulting in decreased catabolism and presentation of antigens, resulting in inhibition of IFN-γ signaling (179).
Apoptosis is an important element of host defense mechanisms to kill intracellular pathogens. However, it remains unclear whether the pathogen or, rather, the host benefits from the apoptosis of infected cells. Apoptosis has been thought of as a silent event, although it has been shown that infection-induced apoptosis is accompanied by inflammation through production of proinflammatory mediators (e.g., IL-1β, which attracts neutrophil granulocytes to the site of infection), providing further immune reactions. It was shown that mycobacterial antigens carried by released apoptotic vesicles are presented by bystander dendritic cells through MHC-I molecules, leading to cross-priming of CD8+ T cells (19). The 19-kDa antigen is involved in apoptosis, acting as a proapoptotic factor in monocytes and macrophages. The signaling is TLR2 mediated, dependent on caspase-8 but independent of caspase-9 (98).
The 19-kDa lipoprotein also stimulates neutrophil activation as determined by a decreased expression of L-selectin and an increased expression of CR3 integrin and CR1. These phenotypic changes modulate functional changes in neutrophils, including adhesion and diapedesis abilities, and binding of opsonins. Furthermore, the 19-kDa antigen is indirectly involved in the late events of neutrophil activation, such as increased production of reactive oxygen species (ROS). While it does not have the capacity to induce the oxidative burst by itself, the 19-kDa antigen can enhance the production of ROS indirectly through priming for subsequent activation by n-formyl-Met-Leu-Phe (fMLP) (114).
Lipoproteins of Streptococcus pneumoniae.
Five of the 42 predicted lipoproteins among the numerous virulence determinants (capsule, surface, and subsurface proteins) of Streptococcus pneumoniae are immunogenic and are suggested to be involved in virulence (15). Two of them, namely, PiaA (pneumococcal iron acquisition) and PiuA (pneumococcal iron uptake) (formerly Pit1A and Pit2A), are encoded on a pathogenicity island and are present in all typical S. pneumoniae strains (194). PiaA and PiuA are homologous ABC iron transporters (23), and anti-PiaA and -PiuA antibodies have been shown to promote opsonophagocytosis of Streptococcus pneumoniae (85). All serotypes of S. pneumoniae express PsaA (pneumococcal surface adhesion A), which has been demonstrated to play a major role in the pathogenesis of S. pneumoniae infection (17), while immunization with PsaA has been shown to be protective against carriage of S. pneumoniae but not against systemic disease (84). PpmA (putative proteinase maturation protein A) and SlrA (streptococcal lipoprotein rotamase A) are lipoproteins belonging to a family of peptidyl isomerases (PPIases) and have been demonstrated to contribute to the virulence of S. pneumoniae by promoting colonization (34, 71).
Similarly to the 19-kDa antigen of M. tuberculosis, PsaA also has pleiotropic effects on cellular physiology and might thus be a promising vaccine target. This 37-kDa antigen belongs to the lipoprotein receptor-associated antigen I (LraI) family and was previously thought to be an adhesin, although it is more likely to be involved in the regulation of adherence rather than being an adhesin itself. This question concerning the adhesin characteristics of PsaA has come from the finding that initial identification of some other proteins (FimA of Streptococcus parasanguinis and ScaA of Streptococcus gordonii) (4, 50, 64) from the LraI family of adhesins has seemed to be false (53, 79). However, in support of its role as an adhesin, application of antibodies against PsaA reduced the ability of pneumococci to adhere to nasopharyngeal epithelial cells (146), and in another study, upregulation of psaA has been found during attachment to pharyngeal epithelial cells (120). Consistent with these observations, PsaA mutants of S. pneumoniae showed impaired adherence to type II pneumocytes, and these strains were avirulent in vivo (17). Furthermore, PsaA exhibits invasion-like effects, and E-cadherin has been shown to be a potential eukaryotic receptor for PsaA (141). Contradictory to these findings is the fact that the structure of PsaA (93) and its membrane anchoring prevent the protein from reaching out of the at least 40-nm-thick Gram-positive bacterial cell wall and capsule (57, 84). The suggested regulatory role of PsaA in adhesion is supported by a study where the production of the adhesin, CbpA, was completely absent in a PsaA-deficient mutant (117). The psaA gene is found in the psa operon along with genes of other components of the transport system (ATP-binding protein and integral membrane protein) and encodes an ABC-type permease for Mn2+ (117), which makes the indirect role of the protein in adherence more relevant. Although a dual function of this lipoprotein is still not conclusively disproved, monofunctionality seems to be more accepted.
The cellular physiology of many pathogens relies on Fe(II), although some bacteria can grow independently of iron or require Mn(II) for both growth and pathogenesis (42, 78, 84, 116, 138). The Mn(II) concentration within the host is very low, and high-affinity manganese transporters are required for survival. In S. pneumoniae, PsaA might be the transporter which fulfils this function. Studies with bacteria containing mutations in their manganese transporters proved them to be highly sensitive to oxidative stress, thereby indicating that manganese is a key metal in protection from the oxidative environment (87, 185). Inactivation of the psaA gene makes S. pneumoniae hypersensitive to oxidative stress (184). H2O2 is produced by S. pneumoniae (165) and is thought to have role in virulence since H2O2 is toxic to alveolar epithelial cells (45). Another benefit of H2O2 apart from this is that it can eradicate other competing microorganisms in the upper respiratory tract (132). S. pneumoniae lacks catalase and protects itself from internally generated hydrogen peroxide and reactive radicals, produced by the Fenton reaction, which cause cellular damage (135) by detoxifying mechanisms such as those performed by SodA (superoxide dismutase), which requires Mn(II) (203). Virulence studies of sodA and psa mutant S. pneumoniae showed that a lack of SodA causes only partially impaired virulence while a lack of Psa makes the strain avirulent (101, 203), supporting the pleiotropic effect of Psa's complex role in adherence and detoxification.
PsaA stimulates immune responses through the production of antibodies (IgA, IgG, and IgM) by B cells (206) and by activation of CD4+ T cells, resulting in expression of IL-4, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, and TNF-α (125). In addition, in vivo survival studies and human antibody (Ab) responses to pneumococcal infection revealed great immunogenicity of PsaA (141). In addition to being immunogenic, PsaA has been shown to contribute to the virulence of S. pneumoniae by being involved in adherence, growth, protection against oxidative stress, and competence by providing transport of the essential manganese. Vaccines against S. pneumoniae contain a mixture of serotype-specific capsule polysaccharides, but it is not possible to cover all serotypes given that new ones emerge from time to time. Being highly conserved among the S. pneumoniae serotypes from all over the world, PsaA may provide protection against a larger number of serotypes. Furthermore, when combined with another species-common immunogenic protein (PspA), its immunogenicity was shown to be enhanced (118).
Lipoproteins of Borrelia burgdorferi.
The Gram-negative spirochete Borrelia burgdorferi is the causative agent of the emerging Lyme disease. Borrelia is transmitted to humans by the bite of infected ticks belonging to a few species of the genus Ixodes (26). Initial analysis of the genome sequence of B. burgdorferi identified 105 putative lipoproteins as predicted by the presence of a consensus lipobox in the first 30 amino acids (52). These represented more than 8% of the coding sequences, compared to 2.3% for Neisseria meningitidis (126). However, given the plasticity of the consensus motif (lipobox) for acyl modification in spirochetes, a novel algorithm was generated that, when applied to the B. burgdofreri genome, identified 120 lipoproteins (161).
Intriguingly, for a bacterium that has a lifestyle that encounters diverse physiological niches, B. burgdorferi has no identifiable machinery for the synthesis of amino acids. It does, however, possess the oligopeptide ABC transporter (opp operon), which exhibits broad substrate specificity, probably compensating for its restricted coding potential by producing proteins that can import a wide variety of solutes (52). This peptide transport system is a member of the superfamily of ABC transporters and has a high degree of similarity to the oligopeptide permease (Opp) system of Escherichia coli (129). In E. coli, this system consists of a peptide binding protein (OppA), two transmembrane proteins (OppB and OppC), and two ATP binding proteins (OppD and OppF). In B. burgdorferi, the opp operon encodes two additional OppA-like proteins (OppA-2 and OppA-3). These oppA genes are part of a seven-gene operon that completes the components of a predicted peptide transport system. The three oppA homologues all encode lipoproteins, and this arrangement of proximal multiple tandem genes for peptide-binding proteins is unique among operons that encode peptide transporters (129). In addition to these three chromosomally encoded oppA genes, there are two further plasmid-carried homologues whose products, OppA-4 and OppA-5, are also lipoproteins (96). The observation that the five periplasmic binding proteins (OppA1 to -5) are lipoproteins suggests that they may be anchored to the outer surface of the cytoplasmic membrane as in Gram-positive bacteria, rather than being localized in the periplasmic space. The functional significance of this remains to be determined, although it is interesting to speculate that this may in some way be involved with the atypical architecture of the spirochete cell wall.
The ability of this spirochete to infect different hosts requires it to express different molecules at various stages of its life cycle, depending on its current environment. The differential expression of lipoproteins by B. burgdorferi is a characteristic of its transition to life inside the mammalian host (27). The 6.6-kDa lipoprotein of B. burgdorferi is one such protein that has been shown to be involved in the formation of outer membrane protein complexes and is consistently expressed within ticks (139). This pattern of expression is analogous that of to other Borrelia lipoproteins important in the vector, such as OspA and OspB (202). These findings suggest that the function of selected lipoproteins is likely to be important for the spirochete life cycle in ticks. In contrast, in murine models of host infection, these and other lipoproteins have been shown to be downregulated (156).
In addition to their variable expression, one of these lipoproteins, VlsE, is capable of antigenic variation and is a key virulence determinant in persistence of disease. The vls locus is comprised of an expression site (vlsE) encoding the 35-kDa lipoprotein VlsE and an operon of 15 unexpressed cassettes which have homology to the central region of VlsE. These cassette segments recombine with the expression locus in order to produce large numbers of distinct antigenic variants during infection (205). Recently, studies on a mutant strain of B. burgdorferi deficient for VlsE expression have demonstrated that while the strain is unable to persist in immunocompetent mice, it is able to persist in immunodeficient SCID mice, thus suggesting that VlsE is not required for persistent infection in the absence of an adaptive immune response and supporting the hypothesis that vls recombination occurs to evade the humoral immune response in the murine host (10). Other immunogenic lipoproteins of B. burgdorferi include the decorin binding protein DbpA, and this prompts the question as to why antibodies raised against this surface lipoprotein are not bactericidal. Nevertheless, it is clear that lipoproteins play a key role in evading the humoral immune response.
Lipoproteins of Neisseria meningitidis.
Analysis of the genome sequences of the pathogen Neisseria meningitidis Z2491 (126), a serogroup A strain, and of the closely related meningococcal strains MC58 and FAM18 (serogroups B and C, respectively) (14, 174) reveals that all strains contain the genes encoding LolABCD (NMA0830, -1091, -1403, and -1402), a complex essential for targeting lipoproteins to the outer membrane (119). The annotation of N. meningitidis Z2491 (126) predicts that this genome encodes for 53 lipoproteins based on the presence of a signal peptide (115) and the presence of a prokaryotic membrane lipoprotein lipid attachment site (Prosite PS51527) (74). The following discusses the role of some of these lipoproteins in meningococcal disease.
Meningococcal lipoproteins that may play a role, although indirect, in the virulence of this pathogen include components of the type IV pili and those involved in the generation of bacterial disulfide bridges. The pathophysiology of meningitis caused by N. meningitidis involves the bacteria crossing the oropharyngeal mucosal barrier, surviving and replicating in the bloodstream, and finally crossing the blood-cerebrospinal fluid (CSF) barrier. Ex vivo, type IV pili have been shown to mediate adhesion of N. meningitidis to human endothelium and to the meninges, suggesting that they play a key role in the adherence of this pathogen in disease (67). The N. meningitidis lipoprotein PilP (NMA0651) is integral to the generation of type IV pili, since mutation of pilP results in a nonpiliated strain of meningococcus (8). In addition to pili, many pathogenic bacteria express a broad array of disulfide-bonded virulence factors, including secreted toxins and surface components. The formation of these bonds is mediated by the Dsb system, of which DsbA is the component that oxidizes the cysteine residues of the substrate molecule. N. meningitidis is unique in its possession of three homologues of this protein, two of which are lipoproteins. Deletion of any one of these homologues is compensated by the others, but deletion of the two lipoproteins results in the loss of functionality of the type IV pili (176).
Currently, vaccines against N. meningitidis are based on the polysaccharides of the O antigens of serogroups A, C, W-135, and Y, with glycoconjugate vaccines having these carbohydrates conjugated to a modified diphtheria toxin (164). However, such an approach is not applicable in the generation of a vaccine to serogroup B strains since their polysaccharide is nonimmunogenic because of its chemical identity to human neural surface antigens. Initial approaches to vaccines against N. meningitidis serogroup B focused on outer-membrane vesicles (OMVs) and iron-regulated outer membrane proteins (9, 30). These studies led to the identification of several immunogenic lipoproteins, including the antigen P47 (NMB0035) (5), lactoferrin binding protein B (LbpB) (NMB1541) (134), and transferrin binding protein B (Tbp2) (NMB0460) (94). All of these proteins elicit an immune response, and sera against them are bactericidal in vitro. In alternative approaches to the development of a vaccine against N. meningitidis serogroup B, a reverse vaccinology approach identified a novel surface-exposed lipoprotein as genome-derived neisserial antigen GNA1870, or rLP2086 (NMA0586), which is conserved across many of the serogroups and is currently in phase III clinical trials as a component of a pentavalent vaccine, 5CVMB, for serogroup B meningitis (59, 104). Its inclusion in this vaccine is due to the profound effect this lipoprotein has on protecting the meningococcus from the immune system.
Complement is an important arm of innate immune defenses against invading pathogens. Complement activation leads to the deposition of C3 fragments (C3b and iC3b), which can enhance opsonophagocytosis of microbes. N. meningitidis binds to factor H, the main inhibitor of the alternative complement pathway, which enhances their ability to evade complement-dependent killing. This binding of factor H is mediated by the lipoprotein GNA1870 (also called factor H binding protein [fHBP]) (100). This was demonstrated by insertional inactivation of gna1870, which resulted in an abrogation of factor H binding directly to the bacterial surface, with a subsequent increased sensitivity of the meningococcus to complement-dependent killing in a serum bactericidal assay. This phenomenon mirrors the function of the B. burgderfori OspE lipoprotein, which also binds factor H (69). The ability to colonize the nasopharynx efficiently is also dependent on the ability of the meningococcus to evade host killing mechanisms, such as antimicrobial peptides. LL-37, one such peptide, is produced by upper-airway epithelial cells and has been shown to play a role in mucosal immunity against oral microflora (109). GNA1870 was found to counteract the antimicrobial effects of LL-37, probably through electrostatic interactions that prevent the peptide from accessing the bacterial surface (157). These observations demonstrate that this factor H binding protein from N. meningitidis plays a key role in evading attack from the innate immune system.
More recent bioinformatic approaches used to identify potential novel protein vaccine candidates against serogroup B N. meningitidis have identified several more lipoproteins (NMA1123, -1134, and -1091 [LolB] and NMB0033, -1163, -1946, and -2132 [sometimes NMB is replaced with GNA] [124, 137]) that are immunogenic and generate bactericidal antibodies as determined by a serum bactericidal assay. The functions of many of these proteins are unknown, though LolB is recognized by the immune system and was initially identified through early immunological screens of meningococcal libraries with a monoclonal antibody, H.8, that had been developed to selectively recognize pathogenic neisserial strains (31, 61). More recently, NMB2132 (renamed neisserial heparin binding antigen (NHBA), has been shown to bind heparin and induce bactericidal antibodies in humans (160).
SUMMARY
This review highlights the diverse roles that lipoproteins of bacterial pathogens play in infection, and as such, it can be postulated that there is much more to be elucidated about the potential role of these molecules as targets for the generation of novel antibacterial agents. Lipopeptides were first demonstrated to have a role in stimulating cytotoxic T-lymphocyte production around 20 years ago (110), and since then, lipoproteins of numerous pathogens have been promoted as vaccine candidates. In addition to the well-characterized lipoprotein vaccine antigens of Neisseria discussed above, bacterial lipoproteins have been shown to affect both the innate and acquired immune systems via such mechanisms as TLR2 signaling and the generation of cytotoxic T lymphocytes and bactericidal antibodies (11, 104, 204). As such, a number of lipoproteins from many pathogens have been evaluated as vaccine candidates (6, 48, 99, 136, 143, 152, 193). Many of these, however, have been administered in recombinant form, making it difficult to ascertain the contribution of the native acyl moiety to protection. There are two notable instances where lipoproteins have been shown to have a deleterious effect on protection. These are two immunodominant lipoproteins of M. tuberculosis, the 19-kDa and 27-kDa antigens, which, when overexpressed in Mycobacterium bovis BCG, cause a deleterious effect on protection (73, 142). In the case of the 19-kDa antigen, the overexpression is likely to be the cause of the deleterious phenotype, since immunization of mice with BCG, or purified 19-kDa antigen, leads to a protective phenotype, but whether it is overexpression of the lipid portion remains to be determined. In the case of the 27-kDa antigen, it is the protein rather than the lipid portion that is responsible for the antiprotective effect, since immunization with a nonacylated form of the protein led to an increase in M. tuberculosis multiplication following challenge relative to unimmunized mice. However, the acylated amino terminus of the 19-kDa antigen of M. tuberculosis has been used to create heterologous antigens that, when expressed in BCG, confer protection against pathogens such as Listeria monocytogenes, Streptococcus pneumoniae, Borrelia burgdorferi, and Escherichia coli (46, 62, 68, 92, 158). Thus, these immunogenic surface-exposed lipoproteins undoubtedly have an important role to play in the future development of vaccines against bacterial pathogens. Furthermore, the essentiality of certain lipoproteins and the enzymes involved in their biosynthesis and localization indicates that these proteins may be targets for the generation of novel antibacterials. This last point is highlighted by the finding that a novel screening approach of small molecules identified an inhibitor of LolA that may lead to a new generation of Gram-negative-specific antimicrobials (128) (128). Further understanding of the roles and mechanisms of synthesis of bacterial lipoproteins will aid these avenues of research.
Editor: H. L. Andrews-Polymenis
Footnotes
Published ahead of print on 25 October 2010.
REFERENCES
- 1.Adu-Bobie, J., P. Lupetti, B. Brunelli, D. Granoff, N. Norais, G. Ferrari, G. Grandi, R. Rappuoli, and M. Pizza. 2004. GNA33 of Neisseria meningitidis is a lipoprotein required for cell separation, membrane architecture, and virulence. Infect. Immun. 72:1914-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alloing, G., P. de Philip, and J. P. Claverys. 1994. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of the gram-positive Streptococcus pneumoniae. J. Mol. Biol. 241:44-58. [DOI] [PubMed] [Google Scholar]
- 3.An, F. Y., M. C. Sulavik, and D. B. Clewell. 1999. Identification and characterization of a determinant (eep) on the Enterococcus faecalis chromosome that is involved in production of the peptide sex pheromone cAD1. J. Bacteriol. 181:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen, R. N., N. Ganeshkumar, and P. E. Kolenbrander. 1993. Cloning of the Streptococcus gordonii PK488 gene, encoding an adhesin which mediates coaggregation with Actinomyces naeslundii PK606. Infect. Immun. 61:981-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arenas, J., A. Abel, S. Sanchez, B. Alcala, M. T. Criado, and C. M. Ferreiros. 2006. Locus NMB0035 codes for a 47-kDa surface-accessible conserved antigen in Neisseria. Int. Microbiol. 9:273-280. [PubMed] [Google Scholar]
- 6.Ayalew, S., D. L. Step, M. Montelongo, and A. W. Confer. 2009. Intranasal vaccination of calves with Mannheimia haemolytica chimeric protein containing the major surface epitope of outer membrane lipoprotein PlpE, the neutralizing epitope of leukotoxin, and cholera toxin subunit B. Vet. Immunol. Immunopathol. 132:295-302. [DOI] [PubMed] [Google Scholar]
- 7.Babu, M. M., M. L. Priya, A. T. Selvan, M. Madera, J. Gough, L. Aravind, and K. Sankaran. 2006. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J. Bacteriol. 188:2761-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balasingham, S. V., R. F. Collins, R. Assalkhou, H. Homberset, S. A. Frye, J. P. Derrick, and T. Tonjum. 2007. Interactions between the lipoprotein PilP and the secretin PilQ in Neisseria meningitidis. J. Bacteriol. 189:5716-5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee-Bhatnagar, N., and C. E. Frasch. 1990. Expression of Neisseria meningitidis iron-regulated outer membrane proteins, including a 70-kilodalton transferrin receptor, and their potential for use as vaccines. Infect. Immun. 58:2875-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bankhead, T., and G. Chaconas. 2007. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol. Microbiol. 65:1547-1558. [DOI] [PubMed] [Google Scholar]
- 11.Bastian, M., T. Braun, H. Bruns, M. Rollinghoff, and S. Stenger. 2008. Mycobacterial lipopeptides elicit CD4+ CTLs in Mycobacterium tuberculosis-infected humans. J. Immunol. 180:3436-3446. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner, M., U. Karst, B. Gerstel, M. Loessner, J. Wehland, and L. Jansch. 2007. Inactivation of Lgt allows systematic characterization of lipoproteins from Listeria monocytogenes. J. Bacteriol. 189:313-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beermann, C., G. Lochnit, R. Geyer, P. Groscurth, and L. Filgueira. 2000. The lipid component of lipoproteins from Borrelia burgdorferi: structural analysis, antigenicity, and presentation via human dendritic cells. Biochem. Biophys. Res. Commun. 267:897-905. [DOI] [PubMed] [Google Scholar]
- 14.Bentley, S. D., G. S. Vernikos, L. A. Snyder, C. Churcher, C. Arrowsmith, T. Chillingworth, A. Cronin, P. H. Davis, N. E. Holroyd, K. Jagels, M. Maddison, S. Moule, E. Rabbinowitsch, S. Sharp, L. Unwin, S. Whitehead, M. A. Quail, M. Achtman, B. Barrell, N. J. Saunders, and J. Parkhill. 2007. Meningococcal genetic variation mechanisms viewed through comparative analysis of serogroup C strain FAM18. PLoS Genet. 3:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergmann, S., and S. Hammerschmidt. 2006. Versatility of pneumococcal surface proteins. Microbiology 152:295-303. [DOI] [PubMed] [Google Scholar]
- 16.Berks, B. C., T. Palmer, and F. Sargent. 2005. Protein targeting by the bacterial twin-arginine translocation (Tat) pathway. Curr. Opin. Microbiol. 8:174-181. [DOI] [PubMed] [Google Scholar]
- 17.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blevins, J. S., K. E. Hagman, and M. V. Norgard. 2008. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bocchino, M., D. Galati, A. Sanduzzi, V. Colizzi, E. Brunetti, and G. Mancino. 2005. Role of mycobacteria-induced monocyte/macrophage apoptosis in the pathogenesis of human tuberculosis. Int. J. Tuberc. Lung Dis. 9:375-383. [PubMed] [Google Scholar]
- 20.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 21.Bos, M. P., V. Robert, and J. Tommassen. 2007. Biogenesis of the gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61:191-214. [DOI] [PubMed] [Google Scholar]
- 22.Bouchon, B., M. Klein, R. Bischoff, A. Van Dorsselaer, and C. Roitsch. 1997. Analysis of the lipidated recombinant outer surface protein A from Borrelia burgdorferi by mass spectrometry. Anal. Biochem. 246:52-61. [DOI] [PubMed] [Google Scholar]
- 23.Brown, J. S., S. M. Gilliland, and D. W. Holden. 2001. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40:572-585. [DOI] [PubMed] [Google Scholar]
- 24.Bubeck Wardenburg, J., W. A. Williams, and D. Missiakas. 2006. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 103:13831-13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buglino, J. A., and M. D. Resh. 2008. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J. Biol. Chem. 283:22076-22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 27.Caimano, M. J., R. Iyer, C. H. Eggers, C. Gonzalez, E. A. Morton, M. A. Gilbert, I. Schwartz, and J. D. Radolf. 2007. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65:1193-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camacho, L. R., D. Ensergueix, E. Perez, B. Gicquel, and C. Guilhot. 1999. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol. Microbiol. 34:257-267. [DOI] [PubMed] [Google Scholar]
- 29.Camus, J. C., M. J. Pryor, C. Medigue, and S. T. Cole. 2002. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology 148:2967-2973. [DOI] [PubMed] [Google Scholar]
- 30.Cannon, J. G. 1989. Conserved lipoproteins of pathogenic Neisseria species bearing the H.8 epitope: lipid-modified azurin and H.8 outer membrane protein. Clin. Microbiol. Rev. 2(Suppl.):S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon, J. G., W. J. Black, I. Nachamkin, and P. W. Stewart. 1984. Monoclonal antibody that recognizes an outer membrane antigen common to the pathogenic Neisseria species but not to most nonpathogenic Neisseria species. Infect. Immun. 43:994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantini, F., D. Veggi, S. Dragonetti, S. Savino, M. Scarselli, G. Romagnoli, M. Pizza, L. Banci, and R. Rappuoli. 2009. Solution structure of the factor H-binding protein, a survival factor and protective antigen of Neisseria meningitidis. J. Biol. Chem. 284:9022-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casali, N., and L. W. Riley. 2007. A phylogenomic analysis of the Actinomycetales mce operons. BMC Genomics 8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cron, L. E., H. J. Bootsma, N. Noske, P. Burghout, S. Hammerschmidt, and P. W. Hermans. 2009. Surface-associated lipoprotein PpmA of Streptococcus pneumoniae is involved in colonization in a strain-specific manner. Microbiology 155:2401-2410. [DOI] [PubMed] [Google Scholar]
- 35.Das, S., T. Kanamoto, X. Ge, P. Xu, T. Unoki, C. L. Munro, and T. Kitten. 2009. Contribution of lipoproteins and lipoprotein processing to endocarditis virulence in Streptococcus sanguinis. J. Bacteriol. 191:4166-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'orazio, M., S. Folcarelli, F. Mariani, V. Colizzi, G. Rotilio, and A. Battistoni. 2001. Lipid modification of the Cu,Zn superoxide dismutase from Mycobacterium tuberculosis. Biochem. J. 359:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Souza, S., V. Rosseels, O. Denis, A. Tanghe, N. De Smet, F. Jurion, K. Palfliet, N. Castiglioni, A. Vanonckelen, C. Wheeler, and K. Huygen. 2002. Improved tuberculosis DNA vaccines by formulation in cationic lipids. Infect. Immun. 70:3681-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Greeff, A., A. Hamilton, I. C. Sutcliffe, H. Buys, L. Van Alphen, and H. E. Smith. 2003. Lipoprotein signal peptidase of Streptococcus suis serotype 2. Microbiology 149:1399-1407. [DOI] [PubMed] [Google Scholar]
- 39.Dev, I. K., R. J. Harvey, and P. H. Ray. 1985. Inhibition of prolipoprotein signal peptidase by globomycin. J. Biol. Chem. 260:5891-5894. [PubMed] [Google Scholar]
- 40.Dev, I. K., and P. H. Ray. 1984. Rapid assay and purification of a unique signal peptidase that processes the prolipoprotein from Escherichia coli B. J. Biol. Chem. 259:11114-11120. [PubMed] [Google Scholar]
- 41.Diaz-Silvestre, H., P. Espinosa-Cueto, A. Sanchez-Gonzalez, M. A. Esparza-Ceron, A. L. Pereira-Suarez, G. Bernal-Fernandez, C. Espitia, and R. Mancilla. 2005. The 19-kDa antigen of Mycobacterium tuberculosis is a major adhesin that binds the mannose receptor of THP-1 monocytic cells and promotes phagocytosis of mycobacteria. Microb. Pathog. 39:97-107. [DOI] [PubMed] [Google Scholar]
- 42.Dintilhac, A., G. Alloing, C. Granadel, and J. P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25:727-739. [DOI] [PubMed] [Google Scholar]
- 43.Dramsi, S., S. Magnet, S. Davison, and M. Arthur. 2008. Covalent attachment of proteins to peptidoglycan. FEMS Microbiol. Rev. 32:307-320. [DOI] [PubMed] [Google Scholar]
- 44.Driessen, A. J., and N. Nouwen. 2008. Protein translocation across the bacterial cytoplasmic membrane. Annu. Rev. Biochem. 77:643-667. [DOI] [PubMed] [Google Scholar]
- 45.Duane, P. G., J. B. Rubins, H. R. Weisel, and E. N. Janoff. 1993. Identification of hydrogen peroxide as a Streptococcus pneumoniae toxin for rat alveolar epithelial cells. Infect. Immun. 61:4392-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edelman, R., K. Palmer, K. G. Russ, H. P. Secrest, J. A. Becker, S. A. Bodison, J. G. Perry, A. R. Sills, A. G. Barbour, C. J. Luke, M. S. Hanson, C. K. Stover, J. E. Burlein, G. P. Bansal, E. M. Connor, and S. Koenig. 1999. Safety and immunogenicity of recombinant bacille Calmette-Guerin (rBCG) expressing Borrelia burgdorferi outer surface protein A (OspA) lipoprotein in adult volunteers: a candidate Lyme disease vaccine. Vaccine 17:904-914. [DOI] [PubMed] [Google Scholar]
- 47.Elsner, A., B. Kreikemeyer, A. Braun-Kiewnick, B. Spellerberg, B. A. Buttaro, and A. Podbielski. 2002. Involvement of Lsp, a member of the LraI-lipoprotein family in Streptococcus pyogenes, in eukaryotic cell adhesion and internalization. Infect. Immun. 70:4859-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erdile, L. F., M. A. Brandt, D. J. Warakomski, G. J. Westrack, A. Sadziene, A. G. Barbour, and J. P. Mays. 1993. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect. Immun. 61:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farazi, T. A., G. Waksman, and J. I. Gordon. 2001. The biology and enzymology of protein N-myristoylation. J. Biol. Chem. 276:39501-39504. [DOI] [PubMed] [Google Scholar]
- 50.Fenno, J. C., D. J. LeBlanc, and P. Fives-Taylor. 1989. Nucleotide sequence analysis of a type 1 fimbrial gene of Streptococcus sanguis FW213. Infect. Immun. 57:3527-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fortune, S. M., A. Solache, A. Jaeger, P. J. Hill, J. T. Belisle, B. R. Bloom, E. J. Rubin, and J. D. Ernst. 2004. Mycobacterium tuberculosis inhibits macrophage responses to IFN-gamma through myeloid differentiation factor 88-dependent and -independent mechanisms. J. Immunol. 172:6272-6280. [DOI] [PubMed] [Google Scholar]
- 52.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fuji, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 53.Froeliger, E. H., and P. Fives-Taylor. 2000. Streptococcus parasanguis FimA does not contribute to adherence to SHA. J. Dent. Res. 79:337. [Google Scholar]
- 54.Fukuda, A., S. Matsuyama, T. Hara, J. Nakayama, H. Nagasawa, and H. Tokuda. 2002. Aminoacylation of the N-terminal cysteine is essential for Lol-dependent release of lipoproteins from membranes but does not depend on lipoprotein sorting signals. J. Biol. Chem. 277:43512-43518. [DOI] [PubMed] [Google Scholar]
- 55.Gan, K., S. D. Gupta, K. Sankaran, M. B. Schmid, and H. C. Wu. 1993. Isolation and characterization of a temperature-sensitive mutant of Salmonella typhimurium defective in prolipoprotein modification. J. Biol. Chem. 268:16544-16550. [PubMed] [Google Scholar]
- 56.Gehring, A. J., K. M. Dobos, J. T. Belisle, C. V. Harding, and W. H. Boom. 2004. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J. Immunol. 173:2660-2668. [DOI] [PubMed] [Google Scholar]
- 57.Giesbrecht, P., T. Kersten, H. Maidhof, and J. Wecke. 1998. Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin. Microbiol. Mol. Biol. Rev. 62:1371-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gimenez, M. I., K. Dilks, and M. Pohlschroder. 2007. Haloferax volcanii twin-arginine translocation substates include secreted soluble, C-terminally anchored and lipoproteins. Mol. Microbiol. 66:1597-1606. [DOI] [PubMed] [Google Scholar]
- 59.Giuliani, M. M., J. Adu-Bobie, M. Comanducci, B. Arico, S. Savino, L. Santini, B. Brunelli, S. Bambini, A. Biolchi, B. Capecchi, E. Cartocci, L. Ciucchi, F. Di Marcello, F. Ferlicca, B. Galli, E. Luzzi, V. Masignani, D. Serruto, D. Veggi, M. Contorni, M. Morandi, A. Bartalesi, V. Cinotti, D. Mannucci, F. Titta, E. Ovidi, J. A. Welsch, D. Granoff, R. Rappuoli, and M. Pizza. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. U. S. A. 103:10834-10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Godlewska, R., K. Wisniewska, Z. Pietras, and E. K. Jagusztyn-Krynicka. 2009. Peptidoglycan-associated lipoprotein (Pal) of Gram-negative bacteria: function, structure, role in pathogenesis and potential application in immunoprophylaxis. FEMS Microbiol. Lett. 298:1-11. [DOI] [PubMed] [Google Scholar]
- 61.Gotschlich, E. C., M. S. Blake, J. M. Koomey, M. Seiff, and A. Derman. 1986. Cloning of the structural genes of three H8 antigens and of protein III of Neisseria gonorrhoeae. J. Exp. Med. 164:868-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grode, L., M. Kursar, J. Fensterle, S. H. Kaufmann, and J. Hess. 2002. Cell-mediated immunity induced by recombinant Mycobacterium bovis bacille Calmette-Guerin strains against an intracellular bacterial pathogen: importance of antigen secretion or membrane-targeted antigen display as lipoprotein for vaccine efficacy. J. Immunol. 168:1869-1876. [DOI] [PubMed] [Google Scholar]
- 63.Gupta, S. D., K. Gan, M. B. Schmid, and H. C. Wu. 1993. Characterization of a temperature-sensitive mutant of Salmonella typhimurium defective in apolipoprotein N-acyltransferase. J. Biol. Chem. 268:16551-16556. [PubMed] [Google Scholar]
- 64.Hamilton, A., C. Robinson, I. C. Sutcliffe, J. Slater, D. J. Maskell, N. Davis-Poynter, K. Smith, A. Waller, and D. J. Harrington. 2006. Mutation of the maturase lipoprotein attenuates the virulence of Streptococcus equi to a greater extent than does loss of general lipoprotein lipidation. Infect. Immun. 74:6907-6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hammerschmidt, S., G. Bethe, P. H. Remane, and G. S. Chhatwal. 1999. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 67:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hantke, K., and V. Braun. 1973. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur. J. Biochem. 34:284-296. [DOI] [PubMed] [Google Scholar]
- 67.Hardy, S. J., M. Christodoulides, R. O. Weller, and J. E. Heckels. 2000. Interactions of Neisseria meningitidis with cells of the human meninges. Mol. Microbiol. 36:817-829. [DOI] [PubMed] [Google Scholar]
- 68.Hayward, C. M., P. O'Gaora, D. B. Young, G. E. Griffin, J. Thole, T. R. Hirst, L. R. Castello-Branco, and D. J. Lewis. 1999. Construction and murine immunogenicity of recombinant bacille Calmette Guerin vaccines expressing the B subunit of Escherichia coli heat labile enterotoxin. Vaccine 17:1272-1281. [DOI] [PubMed] [Google Scholar]
- 69.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 70.Henneke, P., S. Dramsi, G. Mancuso, K. Chraibi, E. Pellegrini, C. Theilacker, J. Hubner, S. Santos-Sierra, G. Teti, D. T. Golenbock, C. Poyart, and P. Trieu-Cuot. 2008. Lipoproteins are critical TLR2 activating toxins in group B streptococcal sepsis. J. Immunol. 180:6149-6158. [DOI] [PubMed] [Google Scholar]
- 71.Hermans, P. W., P. V. Adrian, C. Albert, S. Estevao, T. Hoogenboezem, I. H. Luijendijk, T. Kamphausen, and S. Hammerschmidt. 2006. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J. Biol. Chem. 281:968-976. [DOI] [PubMed] [Google Scholar]
- 72.Herrmann, J. L., P. O'Gaora, A. Gallagher, J. E. Thole, and D. B. Young. 1996. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 15:3547-3554. [PMC free article] [PubMed] [Google Scholar]
- 73.Hovav, A. H., L. Davidovitch, G. Nussbaum, J. Mullerad, Y. Fishman, and H. Bercovier. 2004. Mitogenicity of the recombinant mycobacterial 27-kilodalton lipoprotein is not connected to its antiprotective effect. Infect. Immun. 72:3383-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hulo, N., A. Bairoch, V. Bulliard, L. Cerutti, B. A. Cuche, E. de Castro, C. Lachaize, P. S. Langendijk-Genevaux, and C. J. Sigrist. 2008. The 20 years of PROSITE. Nucleic Acids Res. 36:D245-D249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hutchings, M. I., T. Palmer, D. J. Harrington, and I. C. Sutcliffe. 2009. Lipoprotein biogenesis in Gram-positive bacteria: knowing when to hold 'em, knowing when to fold 'em. Trends Microbiol. 17:13-21. [DOI] [PubMed] [Google Scholar]
- 76.Inouye, S., S. Wang, J. Sekizawa, S. Halegoua, and M. Inouye. 1977. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc. Natl. Acad. Sci. U. S. A. 74:1004-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackowski, S., and C. O. Rock. 1986. Transfer of fatty acids from the 1-position of phosphatidylethanolamine to the major outer membrane lipoprotein of Escherichia coli. J. Biol. Chem. 261:11328-11333. [PubMed] [Google Scholar]
- 78.Jakubovics, N. S., and H. F. Jenkinson. 2001. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147:1709-1718. [DOI] [PubMed] [Google Scholar]
- 79.Jakubovics, N. S., A. W. Smith, and H. F. Jenkinson. 2000. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol. Microbiol. 38:140-153. [DOI] [PubMed] [Google Scholar]
- 80.Jan, G., C. Brenner, and H. Wroblewski. 1996. Purification of Mycoplasma gallisepticum membrane proteins p52, p67 (pMGA), and p77 by high-performance liquid chromatography. Protein Expr. Purif. 7:160-166. [DOI] [PubMed] [Google Scholar]
- 81.Jan, G., C. Fontenelle, M. Le Henaff, and H. Wroblewski. 1995. Acylation and immunological properties of Mycoplasma gallisepticum membrane proteins. Res. Microbiol. 146:739-750. [DOI] [PubMed] [Google Scholar]
- 82.Jenkinson, H. F. 1994. Cell surface protein receptors in oral streptococci. FEMS Microbiol. Lett. 121:133-140. [DOI] [PubMed] [Google Scholar]
- 83.Jin, M. S., S. E. Kim, J. Y. Heo, M. E. Lee, H. M. Kim, S. G. Paik, H. Lee, and J. O. Lee. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130:1071-1082. [DOI] [PubMed] [Google Scholar]
- 84.Johnston, J. W., L. E. Myers, M. M. Ochs, W. H. Benjamin, Jr., D. E. Briles, and S. K. Hollingshead. 2004. Lipoprotein PsaA in virulence of Streptococcus pneumoniae: surface accessibility and role in protection from superoxide. Infect. Immun. 72:5858-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jomaa, M., J. Yuste, J. C. Paton, C. Jones, G. Dougan, and J. S. Brown. 2005. Antibodies to the iron uptake ABC transporter lipoproteins PiaA and PiuA promote opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 73:6852-6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kamalakkannan, S., V. Murugan, M. V. Jagannadham, R. Nagaraj, and K. Sankaran. 2004. Bacterial lipid modification of proteins for novel protein engineering applications. Protein Eng. Des. Sel. 17:721-729. [DOI] [PubMed] [Google Scholar]
- 87.Kehres, D. G., M. L. Zaharik, B. B. Finlay, and M. E. Maguire. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085-1100. [DOI] [PubMed] [Google Scholar]
- 88.Khandavilli, S., K. A. Homer, J. Yuste, S. Basavanna, T. Mitchell, and J. S. Brown. 2008. Maturation of Streptococcus pneumoniae lipoproteins by a type II signal peptidase is required for ABC transporter function and full virulence. Mol. Microbiol. 67:541-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurokawa, K., H. Lee, K. B. Roh, M. Asanuma, Y. S. Kim, H. Nakayama, A. Shiratsuchi, Y. Choi, O. Takeuchi, H. J. Kang, N. Dohmae, Y. Nakanishi, S. Akira, K. Sekimizu, and B. L. Lee. 2009. The triacylated ATP binding cluster transporter substrate-binding lipoprotein of Staphylococcus aureus functions as a native ligand for Toll-Like receptor 2. J. Biol. Chem. 284:8406-8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lagoumintzis, G., M. Christofidou, G. Dimitracopoulos, and F. Paliogianni. 2003. Pseudomonas aeruginosa slime glycolipoprotein is a potent stimulant of tumor necrosis factor alpha gene expression and activation of transcription activators nuclear factor kappa B and activator protein 1 in human monocytes. Infect. Immun. 71:4614-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lampen, J. O., and J. B. Nielsen. 1984. N-terminal glyceride-cysteine modification of membrane penicillinases in gram-positive bacteria. Methods Enzymol. 106:365-368. [DOI] [PubMed] [Google Scholar]
- 92.Langermann, S., S. R. Palaszynski, J. E. Burlein, S. Koenig, M. S. Hanson, D. E. Briles, and C. K. Stover. 1994. Protective humoral response against pneumococcal infection in mice elicited by recombinant bacille Calmette-Guerin vaccines expressing pneumococcal surface protein A. J. Exp. Med. 180:2277-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. M. Berry, A. D. Ogunniyi, and J. C. Paton. 1998. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 6:1553-1561. [DOI] [PubMed] [Google Scholar]
- 94.Legrain, M., V. Mazarin, S. W. Irwin, B. Bouchon, M. J. Quentin-Millet, E. Jacobs, and A. B. Schryvers. 1993. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene 130:73-80. [DOI] [PubMed] [Google Scholar]
- 95.Leskela, S., E. Wahlstrom, V. P. Kontinen, and M. Sarvas. 1999. Lipid modification of prelipoproteins is dispensable for growth but essential for efficient protein secretion in Bacillus subtilis: characterization of the Lgt gene. Mol. Microbiol. 31:1075-1085. [DOI] [PubMed] [Google Scholar]
- 96.Lin, B., S. A. Short, M. Eskildsen, M. S. Klempner, and L. T. Hu. 2001. Functional testing of putative oligopeptide permease (Opp) proteins of Borrelia burgdorferi: a complementation model in opp(−) Escherichia coli. Biochim. Biophys. Acta 1499:222-231. [DOI] [PubMed] [Google Scholar]
- 97.Linder, M. E., and R. J. Deschenes. 2007. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8:74-84. [DOI] [PubMed] [Google Scholar]
- 98.Lopez, M., L. M. Sly, Y. Luu, D. Young, H. Cooper, and N. E. Reiner. 2003. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J. Immunol. 170:2409-2416. [DOI] [PubMed] [Google Scholar]
- 99.Luo, D., F. Xue, D. M. Ojcius, J. Zhao, Y. Mao, L. Li, X. Lin, and J. Yan. 2009. Protein typing of major outer membrane lipoproteins from Chinese pathogenic Leptospira spp. and characterization of their immunogenicity. Vaccine 28:243-255. [DOI] [PubMed] [Google Scholar]
- 100.Madico, G., J. A. Welsch, L. A. Lewis, A. McNaughton, D. H. Perlman, C. E. Costello, J. Ngampasutadol, U. Vogel, D. M. Granoff, and S. Ram. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177:501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marra, A., S. Lawson, J. S. Asundi, D. Brigham, and A. E. Hromockyj. 2002. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology 148:1483-1491. [DOI] [PubMed] [Google Scholar]
- 102.Mascioni, A., B. E. Bentley, R. Camarda, D. A. Dilts, P. Fink, V. Gusarova, S. K. Hoiseth, J. Jacob, S. L. Lin, K. Malakian, L. K. McNeil, T. Mininni, F. Moy, E. Murphy, E. Novikova, S. Sigethy, Y. Wen, G. W. Zlotnick, and D. H. Tsao. 2009. Structural basis for the immunogenic properties of the meningococcal vaccine candidate LP2086. J. Biol. Chem. 284:8738-8746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mascioni, A., F. J. Moy, L. K. McNeil, E. Murphy, B. E. Bentley, R. Camarda, D. A. Dilts, P. S. Fink, V. Gusarova, S. K. Hoiseth, K. Malakian, T. Mininni, E. Novikova, S. Lin, S. Sigethy, G. W. Zlotnick, and D. H. Tsao. 2010. NMR dynamics and antibody recognition of the meningococcal lipidated outer membrane protein LP2086 in micellar solution. Biochim. Biophys. Acta 1798:87-93. [DOI] [PubMed] [Google Scholar]
- 104.Masignani, V., M. Comanducci, M. M. Giuliani, S. Bambini, J. Adu-Bobie, B. Arico, B. Brunelli, A. Pieri, L. Santini, S. Savino, D. Serruto, D. Litt, S. Kroll, J. A. Welsch, D. M. Granoff, R. Rappuoli, and M. Pizza. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mathiopoulos, C., J. P. Mueller, F. J. Slack, C. G. Murphy, S. Patankar, G. Bukusoglu, and A. L. Sonenshein. 1991. A Bacillus subtilis dipeptide transport system expressed early during sporulation. Mol. Microbiol. 5:1903-1913. [DOI] [PubMed] [Google Scholar]
- 106.Mei, J. M., F. Nourbakhsh, C. W. Ford, and D. W. Holden. 1997. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol. Microbiol. 26:399-407. [DOI] [PubMed] [Google Scholar]
- 107.Michell, S. L., A. O. Whelan, P. R. Wheeler, M. Panico, R. L. Easton, A. T. Etienne, S. M. Haslam, A. Dell, H. R. Morris, A. J. Reason, J. L. Herrmann, D. B. Young, and R. G. Hewinson. 2003. The MPB83 antigen from Mycobacterium bovis contains O-linked mannose and (1→3)-mannobiose moieties. J. Biol. Chem. 278:16423-16432. [DOI] [PubMed] [Google Scholar]
- 108.Munoa, F. J., K. W. Miller, R. Beers, M. Graham, and H. C. Wu. 1991. Membrane topology of Escherichia coli prolipoprotein signal peptidase (signal peptidase II). J. Biol. Chem. 266:17667-17672. [PubMed] [Google Scholar]
- 109.Murakami, M., T. Ohtake, R. A. Dorschner, and R. L. Gallo. 2002. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J. Dent. Res. 81:845-850. [DOI] [PubMed] [Google Scholar]
- 110.Nardelli, B., P. B. Haser, and J. P. Tam. 1994. Oral administration of an antigenic synthetic lipopeptide (MAP-P3C) evokes salivary antibodies and systemic humoral and cellular responses. Vaccine 12:1335-1339. [DOI] [PubMed] [Google Scholar]
- 111.Narita, S., S. Matsuyama, and H. Tokuda. 2004. Lipoprotein trafficking in Escherichia coli. Arch. Microbiol. 182:1-6. [DOI] [PubMed] [Google Scholar]
- 112.Narita, S., and H. Tokuda. 2007. Amino acids at positions 3 and 4 determine the membrane specificity of Pseudomonas aeruginosa lipoproteins. J. Biol. Chem. 282:13372-13378. [DOI] [PubMed] [Google Scholar]
- 113.Neelakanta, G., X. Li, U. Pal, X. Liu, D. S. Beck, K. DePonte, D. Fish, F. S. Kantor, and E. Fikrig. 2007. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog. 3:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neufert, C., R. K. Pai, E. H. Noss, M. Berger, W. H. Boom, and C. V. Harding. 2001. Mycobacterium tuberculosis 19-kDa lipoprotein promotes neutrophil activation. J. Immunol. 167:1542-1549. [DOI] [PubMed] [Google Scholar]
- 115.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 116.Niven, D. F., A. Ekins, and A. A. al-Samaurai. 1999. Effects of iron and manganese availability on growth and production of superoxide dismutase by Streptococcus suis. Can. J. Microbiol. 45:1027-1032. [DOI] [PubMed] [Google Scholar]
- 117.Novak, R., J. S. Braun, E. Charpentier, and E. Tuomanen. 1998. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex Psa. Mol. Microbiol. 29:1285-1296. [DOI] [PubMed] [Google Scholar]
- 118.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okuda, S., and H. Tokuda. 2009. Model of mouth-to-mouth transfer of bacterial lipoproteins through inner membrane LolC, periplasmic LolA, and outer membrane LolB. Proc. Natl. Acad. Sci. U. S. A. 106:5877-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orihuela, C. J., J. N. Radin, J. E. Sublett, G. Gao, D. Kaushal, and E. I. Tuomanen. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 72:5582-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Overweg, K., C. D. Pericone, G. G. Verhoef, J. N. Weiser, H. D. Meiring, A. P. De Jong, R. De Groot, and P. W. Hermans. 2000. Differential protein expression in phenotypic variants of Streptococcus pneumoniae. Infect. Immun. 68:4604-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pai, R. K., M. Convery, T. A. Hamilton, W. H. Boom, and C. V. Harding. 2003. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 171:175-184. [DOI] [PubMed] [Google Scholar]
- 123.Paitan, Y., E. Orr, E. Z. Ron, and E. Rosenberg. 1999. A nonessential signal peptidase II (Lsp) of Myxococcus xanthus might be involved in biosynthesis of the polyketide antibiotic TA. J. Bacteriol. 181:5644-5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pajon, R., D. Yero, O. Niebla, Y. Climent, G. Sardinas, D. Garcia, Y. Perera, A. Llanes, M. Delgado, K. Cobas, E. Caballero, S. Taylor, C. Brookes, and A. Gorringe. 2009. Identification of new meningococcal serogroup B surface antigens through a systematic analysis of neisserial genomes. Vaccine 28:532-541. [DOI] [PubMed] [Google Scholar]
- 125.Palaniappan, R., S. Singh, U. P. Singh, S. K. Sakthivel, E. W. Ades, D. E. Briles, S. K. Hollingshead, J. C. Paton, J. S. Sampson, and J. W. Lillard, Jr. 2005. Differential PsaA-, PspA-, PspC-, and PdB-specific immune responses in a mouse model of pneumococcal carriage. Infect. Immun. 73:1006-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M. A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 127.Parsons, L. M., F. Lin, and J. Orban. 2006. Peptidoglycan recognition by Pal, an outer membrane lipoprotein. Biochemistry 45:2122-2128. [DOI] [PubMed] [Google Scholar]
- 128.Pathania, R., S. Zlitni, C. Barker, R. Das, D. A. Gerritsma, J. Lebert, E. Awuah, G. Melacini, F. A. Capretta, and E. D. Brown. 2009. Chemical genomics in Escherichia coli identifies an inhibitor of bacterial lipoprotein targeting. Nat. Chem. Biol. 5:849-856. [DOI] [PubMed] [Google Scholar]
- 129.Payne, J. W., and M. W. Smith. 1994. Peptide transport by micro-organisms. Adv. Microb. Physiol. 36:1-80. [DOI] [PubMed] [Google Scholar]
- 130.Pennini, M. E., R. K. Pai, D. C. Schultz, W. H. Boom, and C. V. Harding. 2006. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-gamma-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. J. Immunol. 176:4323-4330. [DOI] [PubMed] [Google Scholar]
- 131.Perego, M., C. F. Higgins, S. R. Pearce, M. P. Gallagher, and J. A. Hoch. 1991. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol. Microbiol. 5:173-185. [DOI] [PubMed] [Google Scholar]
- 132.Pericone, C. D., K. Overweg, P. W. Hermans, and J. N. Weiser. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 68:3990-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Petit, C. M., J. R. Brown, K. Ingraham, A. P. Bryant, and D. J. Holmes. 2001. Lipid modification of prelipoproteins is dispensable for growth in vitro but essential for virulence in Streptococcus pneumoniae. FEMS Microbiol. Lett. 200:229-233. [DOI] [PubMed] [Google Scholar]
- 134.Pettersson, A., T. Prinz, A. Umar, J. van der Biezen, and J. Tommassen. 1998. Molecular characterization of LbpB, the second lactoferrin-binding protein of Neisseria meningitidis. Mol. Microbiol. 27:599-610. [DOI] [PubMed] [Google Scholar]
- 135.Pierre, J. L., and M. Fontecave. 1999. Iron and activated oxygen species in biology: the basic chemistry. Biometals 12:195-199. [DOI] [PubMed] [Google Scholar]
- 136.Pimenta, F. C., E. N. Miyaji, A. P. Areas, M. L. Oliveira, A. L. de Andrade, P. L. Ho, S. K. Hollingshead, and L. C. Leite. 2006. Intranasal immunization with the cholera toxin B subunit-pneumococcal surface antigen A fusion protein induces protection against colonization with Streptococcus pneumoniae and has negligible impact on the nasopharyngeal and oral microbiota of mice. Infect. Immun. 74:4939-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 138.Posey, J. E., and F. C. Gherardini. 2000. Lack of a role for iron in the Lyme disease pathogen. Science 288:1651-1653. [DOI] [PubMed] [Google Scholar]
- 139.Promnares, K., M. Kumar, D. Y. Shroder, X. Zhang, J. F. Anderson, and U. Pal. 2009. Borrelia burgdorferi small lipoprotein Lp6.6 is a member of multiple protein complexes in the outer membrane and facilitates pathogen transmission from ticks to mice. Mol. Microbiol. 74:112-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qi, H. Y., K. Sankaran, K. Gan, and H. C. Wu. 1995. Structure-function relationship of bacterial prolipoprotein diacylglyceryl transferase: functionally significant conserved regions. J. Bacteriol. 177:6820-6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rajam, G., J. M. Anderton, G. M. Carlone, J. S. Sampson, and E. W. Ades. 2008. Pneumococcal surface adhesin A (PsaA): a review. Crit. Rev. Microbiol. 34:131-142. [DOI] [PubMed] [Google Scholar]
- 142.Rao, V., N. Dhar, H. Shakila, R. Singh, A. Khera, R. Jain, M. Naseema, C. N. Paramasivan, P. R. Narayanan, V. D. Ramanathan, and A. K. Tyagi. 2005. Increased expression of Mycobacterium tuberculosis 19 kDa lipoprotein obliterates the protective efficacy of BCG by polarizing host immune responses to the Th2 subtype. Scand. J. Immunol. 61:410-417. [DOI] [PubMed] [Google Scholar]
- 143.Rao, V., N. Dhar, and A. K. Tyagi. 2003. Modulation of host immune responses by overexpression of immunodominant antigens of Mycobacterium tuberculosis in bacille Calmette-Guerin. Scand. J. Immunol. 58:449-461. [DOI] [PubMed] [Google Scholar]
- 144.Reglier-Poupet, H., C. Frehel, I. Dubail, J. L. Beretti, P. Berche, A. Charbit, and C. Raynaud. 2003. Maturation of lipoproteins by type II signal peptidase is required for phagosomal escape of Listeria monocytogenes. J. Biol. Chem. 278:49469-49477. [DOI] [PubMed] [Google Scholar]
- 145.Robichon, C., D. Vidal-Ingigliardi, and A. P. Pugsley. 2005. Depletion of apolipoprotein N-acyltransferase causes mislocalization of outer membrane lipoproteins in Escherichia coli. J. Biol. Chem. 280:974-983. [DOI] [PubMed] [Google Scholar]
- 146.Romero-Steiner, S., T. Pilishvili, J. S. Sampson, S. E. Johnson, A. Stinson, G. M. Carlone, and E. W. Ades. 2003. Inhibition of pneumococcal adherence to human nasopharyngeal epithelial cells by anti-PsaA antibodies. Clin. Diagn. Lab. Immunol. 10:246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roussel-Jazede, V., I. Jongerius, M. P. Bos, J. Tommassen, and P. van Ulsen. 2010. NalP-mediated proteolytic release of lactoferrin-binding protein B from the meningococcal cell surface. Infect. Immun. 78:3083-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanchez, A., P. Espinosa, M. A. Esparza, M. Colon, G. Bernal, and R. Mancilla. 2009. Mycobacterium tuberculosis 38-kDa lipoprotein is apoptogenic for human monocyte-derived macrophages. Scand. J. Immunol. 69:20-28. [DOI] [PubMed] [Google Scholar]
- 149.Sander, P., M. Rezwan, B. Walker, S. K. Rampini, R. M. Kroppenstedt, S. Ehlers, C. Keller, J. R. Keeble, M. Hagemeier, M. J. Colston, B. Springer, and E. C. Bottger. 2004. Lipoprotein processing is required for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 52:1543-1552. [DOI] [PubMed] [Google Scholar]
- 150.Sankaran, K., K. Gan, B. Rash, H. Y. Qi, H. C. Wu, and P. D. Rick. 1997. Roles of histidine-103 and tyrosine-235 in the function of the prolipoprotein diacylglyceryl transferase of Escherichia coli. J. Bacteriol. 179:2944-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sankaran, K., and H. C. Wu. 1994. Lipid modification of bacterial prolipoprotein. Transfer of diacylglyceryl moiety from phosphatidylglycerol. J. Biol. Chem. 269:19701-19706. [PubMed] [Google Scholar]
- 152.Sardinas, G., Y. Climent, Y. Rodriguez, S. Gonzalez, D. Garcia, K. Cobas, E. Caballero, Y. Perez, C. Brookes, S. Taylor, A. Gorringe, M. Delgado, R. Pajon, and D. Yero. 2009. Assessment of vaccine potential of the Neisseria-specific protein NMB0938. Vaccine 27:6910-6917. [DOI] [PubMed] [Google Scholar]
- 153.Sassetti, C. M., D. H. Boyd, and E. J. Rubin. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48:77-84. [DOI] [PubMed] [Google Scholar]
- 154.Schulze, R. J., S. Chen, O. S. Kumru, and W. R. Zuckert. 2010. Translocation of Borrelia burgdorferi surface lipoprotein OspA through the outer membrane requires an unfolded conformation and can initiate at the C-terminus. Mol. Microbiol. 76:1266-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schulze, R. J., and W. R. Zuckert. 2006. Borrelia burgdorferi lipoproteins are secreted to the outer surface by default. Mol. Microbiol. 59:1473-1484. [DOI] [PubMed] [Google Scholar]
- 156.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Seib, K. L., D. Serruto, F. Oriente, I. Delany, J. Adu-Bobie, D. Veggi, B. Arico, R. Rappuoli, and M. Pizza. 2009. Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infect. Immun. 77:292-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Seixas, F. K., C. H. Fernandes, D. D. Hartwig, F. R. Conceicao, J. A. Aleixo, and O. A. Dellagostin. 2007. Evaluation of different ways of presenting LipL32 to the immune system with the aim of developing a recombinant vaccine against leptospirosis. Can. J. Microbiol. 53:472-479. [DOI] [PubMed] [Google Scholar]
- 159.Selvan, A. T., and K. Sankaran. 2008. Localization and characterization of prolipoprotein diacylglyceryl transferase (Lgt) critical in bacterial lipoprotein biosynthesis. Biochimie 90:1647-1655. [DOI] [PubMed] [Google Scholar]
- 160.Serruto, D., T. Spadafina, L. Ciucchi, L. A. Lewis, S. Ram, M. Tontini, L. Santini, A. Biolchi, K. L. Seib, M. M. Giuliani, J. J. Donnelly, F. Berti, S. Savino, M. Scarselli, P. Costantino, J. S. Kroll, C. O'Dwyer, J. Qiu, A. G. Plaut, R. Moxon, R. Rappuoli, M. Pizza, and B. Arico. 2010. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc. Natl. Acad. Sci. U. S. A. 107:3770-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Setubal, J. C., M. Reis, J. Matsunaga, and D. A. Haake. 2006. Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seydel, A., P. Gounon, and A. P. Pugsley. 1999. Testing the ‘+2 rule’ for lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol. Microbiol. 34:810-821. [DOI] [PubMed] [Google Scholar]
- 163.Silva-Herzog, E., F. Ferracci, M. W. Jackson, S. S. Joseph, and G. V. Plano. 2008. Membrane localization and topology of the Yersinia pestis YscJ lipoprotein. Microbiology 154:593-607. [DOI] [PubMed] [Google Scholar]
- 164.Snape, M. D., K. P. Perrett, K. J. Ford, T. M. John, D. Pace, L. M. Yu, J. M. Langley, S. McNeil, P. M. Dull, F. Ceddia, A. Anemona, S. A. Halperin, S. Dobson, and A. J. Pollard. 2008. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA 299:173-184. [DOI] [PubMed] [Google Scholar]
- 165.Spellerberg, B., D. R. Cundell, J. Sandros, B. J. Pearce, I. Idanpaan-Heikkila, C. Rosenow, and H. R. Masure. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803-813. [DOI] [PubMed] [Google Scholar]
- 166.Stewart, G. R., K. A. Wilkinson, S. M. Newton, S. M. Sullivan, O. Neyrolles, J. R. Wain, J. Patel, K. L. Pool, D. B. Young, and R. J. Wilkinson. 2005. Effect of deletion or overexpression of the 19-kilodalton lipoprotein Rv3763 on the innate response to Mycobacterium tuberculosis. Infect. Immun. 73:6831-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Steyn, A. J., J. Joseph, and B. R. Bloom. 2003. Interaction of the sensor module of Mycobacterium tuberculosis H37Rv KdpD with members of the Lpr family. Mol. Microbiol. 47:1075-1089. [DOI] [PubMed] [Google Scholar]
- 168.Stoll, H., J. Dengjel, C. Nerz, and F. Gotz. 2005. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect. Immun. 73:2411-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sutcliffe, I. C., and D. J. Harrington. 2004. Lipoproteins of Mycobacterium tuberculosis: an abundant and functionally diverse class of cell envelope components. FEMS Microbiol. Rev. 28:645-659. [DOI] [PubMed] [Google Scholar]
- 170.Sutcliffe, I. C., and R. R. Russell. 1995. Lipoproteins of gram-positive bacteria. J. Bacteriol. 177:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 172.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 173.Tanghe, A., P. Lefevre, O. Denis, S. D'Souza, M. Braibant, E. Lozes, M. Singh, D. Montgomery, J. Content, and K. Huygen. 1999. Immunogenicity and protective efficacy of tuberculosis DNA vaccines encoding putative phosphate transport receptors. J. Immunol. 162:1113-1119. [PubMed] [Google Scholar]
- 174.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 175.Tidhar, A., Y. Flashner, S. Cohen, Y. Levi, A. Zauberman, D. Gur, M. Aftalion, E. Elhanany, A. Zvi, A. Shafferman, and E. Mamroud. 2009. The NlpD lipoprotein is a novel Yersinia pestis virulence factor essential for the development of plague. PLoS One 4:e7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tinsley, C. R., R. Voulhoux, J. L. Beretti, J. Tommassen, and X. Nassif. 2004. Three homologues, including two membrane-bound proteins, of the disulfide oxidoreductase DsbA in Neisseria meningitidis: effects on bacterial growth and biogenesis of functional type IV pili. J. Biol. Chem. 279:27078-27087. [DOI] [PubMed] [Google Scholar]
- 177.Tjalsma, H., V. P. Kontinen, Z. Pragai, H. Wu, R. Meima, G. Venema, S. Bron, M. Sarvas, and J. M. van Dijl. 1999. The role of lipoprotein processing by signal peptidase II in the Gram-positive eubacterium bacillus subtilis. Signal peptidase II is required for the efficient secretion of alpha-amylase, a non-lipoprotein. J. Biol. Chem. 274:1698-1707. [DOI] [PubMed] [Google Scholar]
- 178.Tjalsma, H., G. Zanen, G. Venema, S. Bron, and J. M. van Dijl. 1999. The potential active site of the lipoprotein-specific (type II) signal peptidase of Bacillus subtilis. J. Biol. Chem. 274:28191-28197. [DOI] [PubMed] [Google Scholar]
- 179.Tobian, A. A., N. S. Potter, L. Ramachandra, R. K. Pai, M. Convery, W. H. Boom, and C. V. Harding. 2003. Alternate class I MHC antigen processing is inhibited by Toll-like receptor signaling pathogen-associated molecular patterns: Mycobacterium tuberculosis 19-kDa lipoprotein, CpG DNA, and lipopolysaccharide. J. Immunol. 171:1413-1422. [DOI] [PubMed] [Google Scholar]
- 180.Tokuda, H. 2009. Biogenesis of outer membranes in Gram-negative bacteria. Biosci. Biotechnol. Biochem. 73:465-473. [DOI] [PubMed] [Google Scholar]
- 181.Tokunaga, M., H. Tokunaga, and H. C. Wu. 1982. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc. Natl. Acad. Sci. U. S. A. 79:2255-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Torres, A., M. D. Juarez, R. Cervantes, and C. Espitia. 2001. Molecular analysis of Mycobacterium tuberculosis phosphate specific transport system in Mycobacterium smegmatis. Characterization of recombinant 38 kDa (PstS-1). Microb. Pathog. 30:289-297. [DOI] [PubMed] [Google Scholar]
- 183.Tschumi, A., C. Nai, Y. Auchli, P. Hunziker, P. Gehrig, P. Keller, T. Grau, and P. Sander. 2009. Identification of apolipoprotein N-acyltransferase (Lnt) in mycobacteria. J. Biol. Chem. 284:27146-27156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tseng, H. J., A. G. McEwan, J. C. Paton, and M. P. Jennings. 2002. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect. Immun. 70:1635-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tseng, H. J., Y. Srikhanta, A. G. McEwan, and M. P. Jennings. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40:1175-1186. [DOI] [PubMed] [Google Scholar]
- 186.Valente, F. M., P. M. Pereira, S. S. Venceslau, M. Regalla, A. V. Coelho, and I. A. Pereira. 2007. The [NiFeSe] hydrogenase from Desulfovibrio vulgaris Hildenborough is a bacterial lipoprotein lacking a typical lipoprotein signal peptide. FEBS Lett. 581:3341-3344. [DOI] [PubMed] [Google Scholar]
- 187.Venema, R., H. Tjalsma, J. M. van Dijl, A. de Jong, K. Leenhouts, G. Buist, and G. Venema. 2003. Active lipoprotein precursors in the Gram-positive eubacterium Lactococcus lactis. J. Biol. Chem. 278:14739-14746. [DOI] [PubMed] [Google Scholar]
- 188.Verma, A., C. A. Brissette, A. Bowman, and B. Stevenson. 2009. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect. Immun. 77:4940-4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vidal-Ingigliardi, D., S. Lewenza, and N. Buddelmeijer. 2007. Identification of essential residues in apolipoprotein N-acyl transferase, a member of the CN hydrolase family. J. Bacteriol. 189:4456-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vonder Haar, R. A., M. Legrain, H. V. Kolbe, and E. Jacobs. 1994. Characterization of a highly structured domain in Tbp2 from Neisseria meningitidis involved in binding to human transferrin. J. Bacteriol. 176:6207-6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vyas, N. K., M. N. Vyas, and F. A. Quiocho. 2003. Crystal structure of M tuberculosis ABC phosphate transport receptor: specificity and charge compensation dominated by ion-dipole interactions. Structure 11:765-774. [DOI] [PubMed] [Google Scholar]
- 192.Wagner, D., F. J. Sangari, A. Parker, and L. E. Bermudez. 2005. fecB, a gene potentially involved in iron transport in Mycobacterium avium, is not induced within macrophages. FEMS Microbiol. Lett. 247:185-191. [DOI] [PubMed] [Google Scholar]
- 193.Weimer, E. T., S. E. Ervin, D. J. Wozniak, and S. B. Mizel. 2009. Immunization of young African green monkeys with OprF epitope 8-OprI-type A- and B-flagellin fusion proteins promotes the production of protective antibodies against nonmucoid Pseudomonas aeruginosa. Vaccine 27:6762-6769. [DOI] [PubMed] [Google Scholar]
- 194.Whalan, R. H., S. G. Funnell, L. D. Bowler, M. J. Hudson, A. Robinson, and C. G. Dowson. 2006. Distribution and genetic diversity of the ABC transporter lipoproteins PiuA and PiaA within Streptococcus pneumoniae and related streptococci. J. Bacteriol. 188:1031-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Widdick, D. A., K. Dilks, G. Chandra, A. Bottrill, M. Naldrett, M. Pohlschroder, and T. Palmer. 2006. The twin-arginine translocation pathway is a major route of protein export in Streptomyces coelicolor. Proc. Natl. Acad. Sci. U. S. A. 103:17927-17932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wiker, H. G. 2009. MPB70 and MPB83—major antigens of Mycobacterium bovis. Scand. J. Immunol. 69:492-499. [DOI] [PubMed] [Google Scholar]
- 197.Wilkinson, K. A., S. M. Newton, G. R. Stewart, A. R. Martineau, J. Patel, S. M. Sullivan, J. L. Herrmann, O. Neyrolles, D. B. Young, and R. J. Wilkinson. 2009. Genetic determination of the effect of post-translational modification on the innate immune response to the 19 kDa lipoprotein of Mycobacterium tuberculosis. BMC Microbiol. 9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wu, C. H., J. J. Tsai-Wu, Y. T. Huang, C. Y. Lin, G. G. Lioua, and F. J. Lee. 1998. Identification and subcellular localization of a novel Cu,Zn superoxide dismutase of Mycobacterium tuberculosis. FEBS Lett. 439:192-196. [DOI] [PubMed] [Google Scholar]
- 199.Xu, Q., K. McShan, and F. T. Liang. 2008. Essential protective role attributed to the surface lipoproteins of Borrelia burgdorferi against innate defences. Mol. Microbiol. 69:15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yamaguchi, K., F. Yu, and M. Inouye. 1988. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell 53:423-432. [DOI] [PubMed] [Google Scholar]
- 201.Yang, X., Z. Wu, X. Wang, C. Yang, H. Xu, and Y. Shen. 2009. Crystal structure of lipoprotein GNA1946 from Neisseria meningitidis. J. Struct. Biol. 168:437-443. [DOI] [PubMed] [Google Scholar]
- 202.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yesilkaya, H., A. Kadioglu, N. Gingles, J. E. Alexander, T. J. Mitchell, and P. W. Andrew. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 68:2819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zahringer, U., B. Lindner, S. Inamura, H. Heine, and C. Alexander. 2008. TLR2—promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213:205-224. [DOI] [PubMed] [Google Scholar]
- 205.Zhang, J. R., and S. J. Norris. 1998. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect. Immun. 66:3698-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang, Q., S. Choo, and A. Finn. 2002. Immune responses to novel pneumococcal proteins pneumolysin, PspA, PsaA, and CbpA in adenoidal B cells from children. Infect. Immun. 70:5363-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]