Abstract
Purified protein derivative (PPD) is a widely used reagent for the diagnosis of Mycobacterium tuberculosis infection. Recently, the molecular composition of PPD was defined, with hundreds of mycobacterial protein representatives making up PPD. Which, if any, of these specific products drive the potency of PPD remains in question. In this study, two proteins (DnaK and GroEL2) previously identified as dominant proteins in PPD were tested for the capacity to induce delayed-type hypersensitivity (DTH) responses in H37Rv-infected or BCG-vaccinated guinea pigs. These two proteins were used in pull-down assays to identify interacting PPD products. Six proteins were identified as interacting partners with DnaK and GroEL2, i.e., Rv0009, Rv0475, Rv0569, Rv0685, Rv2626c, and Rv2632c. These six proteins were tested alone and in combination with DnaK and GroEL2 for the capacity to induce a DTH response in the guinea pig model. From these studies, two cocktails, DnaK/GroEL2/Rv0009 and DnaK/GroEL2/Rv0685, were found to induce DTH responses in H37Rv-infected or BCG-vaccinated guinea pigs that were indistinguishable from DTH responses driven by a PPD injection. The mechanism by which DTH responses were induced was elucidated by histologic examination, analysis of activated CD4+/CD8+ T cells, and cytokine mRNA expression at the site of the DTH response. PPD and the protein cocktails tested induced strong DTH responses in H37Rv-infected guinea pigs. Ex vivo phenotyping of T cells at the DTH site indicated that this response is mediated by activated CD4+ and CD8+ T cells, with increases in gamma interferon and tumor necrosis factor alpha, but not interleukin-10, at the site of the DTH response. Our results demonstrate for the first time that the PPD response can be mimicked at the molecular level with defined protein cocktails. The use of this defined product will allow a more thorough understanding of the DTH response and may provide a platform for more rapid and sensitive second-generation skin test reagents for the diagnosis of M. tuberculosis infection.
Tuberculosis remains one of the largest single causes of disease and death from an infectious agent, particularly in developing nations. With nearly 2 million deaths each year and an estimated 8 to 9 million new cases annually, tuberculosis is the second leading cause of death worldwide among communicable diseases (8). Further, it is estimated that one-third of the world's population is latently infected with Mycobacterium tuberculosis (9). The epidemic of human immunodeficiency virus infection (27) and the increase in the incidence of multidrug-resistant M. tuberculosis (10) have further complicated tuberculosis control efforts.
The most common way persons infected with M. tuberculosis are identified is through use of the tuberculin skin test (TST). Over the last century, the TST has been proven to be an important diagnostic tool for tuberculosis infection (25, 35). It is also an important component of epidemiologic studies to evaluate the prevalence of latent tuberculosis in various populations.
The TST, which is also called the Mantoux test after the French physician Charles Mantoux (1877 to 1947), was developed by Florence B. Seibert (1897 to 1991) (33). The TST is a modification of Koch's old tuberculin, resulting in purified protein derivative (PPD). Several PPD standards exist, including PPD-S (1, 16), the first FDA standard; PPD-RT23 (7, 30), the standard produced by the Statens Serum Institut; and PPD-S2 (37), the new FDA standard of PPD. The basic methods used to produce these PPD standards are similar. Proteins are purified by repeated precipitation with trichloroacetic acid, ammonium sulfate, or both after cultures of M. tuberculosis are grown to stationary phase and sterilized (34). The preparations are dominated by M. tuberculosis proteins and peptides and contain few polysaccharides, nucleic acids, and lipids. The elucidation of the molecular composition of PPD demonstrated that PPD is a complex mixture of hundreds of denatured antigens (3, 19; Y. S. Cho et al., unpublished data). It is not surprising, then, that little is known about the active components of PPD responsible for its potency. The presence of so many antigens likely contributes to the poor specificity observed with PPD testing; it cannot differentiate active tuberculosis from latent infection or vaccination, complicating TST use as a tool for epidemiologic studies and diagnosis of tuberculosis. PPD from different sources may vary in the delayed-type hypersensitivity (DTH) response because there is no way to standardize the hundreds of antigenic components in PPD (23). In addition to issues arising from the number of proteins present in PPD, three of the most dominant proteins in PPD, Rv3418 (GroES), Rv0440 (GroEL2), and Rv0350 (DnaK) (3, 19; Cho et al., unpublished), are highly conserved chaperones; reactivity to these proteins may also explain the diminished specificity of PPD. Lastly, the complexity of PPD limits our capacity to tease out the molecular interactions involved in the DTH response and, in particular, understand which of these interactions, if any, discern chronic infections from protective immunity. Skin test reagents using defined antigens may be able to be used to address these questions.
Over the past 20 years, major efforts have focused on the identification of highly purified M. tuberculosis recombinant proteins that may be used to develop a more specific diagnostic skin test (31). Some protein candidates, including ESAT-6 (28), CFP10 (39), MPT64 (24), DPPD (6), and Rv0934 (17), were tested and found to induce strong DTH reactions in guinea pigs, but none of them were developed as second-generation skin test reagents. Several studies indicate that a combination of several purified antigens (antigen cocktails) or a combination of several DTH-inducing epitopes may be required for second-generation skin test reagents to effectively replace PPD (21, 24, 32).
The definition of the molecular composition of PPD allows for the development of a more refined product. Recently, four heat shock proteins (GroES, GroEL2, HspX, and DnaK) were identified as dominant products in PPD-S2, the U.S. FDA standard PPD (Cho et al., unpublished). In this study, DnaK and GroEL2, two of the major chaperones in PPD-S2 (Cho et al., unpublished) and bovine and avian PPD (3), were tested for the capacity to induce a DTH response and were found to elicit DTH responses in H37Rv-infected and BCG-vaccinated guinea pigs. As these proteins are conserved among other mycobacteria, their use in a second-generation TST may be limited. Thus, these proteins were used to pull out additional protein representatives from PPD, resulting in the identification of six highly interacting proteins. When these proteins were tested in various formulations for the potential to elicit a DTH response, two cocktails, DnaK/GroEL2/Rv0685 and DnaK/GroEL2/Rv0009, were found to induce DTH responses that were indistinguishable from that induced by PPD in the guinea pig model of tuberculosis, as determined by skin test responses and at the molecular level.
MATERIALS AND METHODS
Expression of recombinant proteins in Escherichia coli.
The four chaperones, DnaK (Rv0350), GroEL2 (Rv0440), GroES (Rv3418c), and HspX (Rv2031c), and M. tuberculosis protein Rv2626c were expressed using plasmids available from the Tuberculosis Vaccine Testing and Research Material contract (HHSN266200400091C; http://www.cvmbs.colostate.edu/mip/tb/recombinant.htm).
Gateway cloning-based (Invitrogen, Carlsbad, CA) entry plasmids containing the open reading frames for M. tuberculosis proteins Rv0009, Rv0475, Rv0569, Rv0685, Rv2626c, and Rv2632c were obtained from the Pathogen Functional Genomics Resource Center (http://pfgrc.jcvi.org/fir_index.php/fir/available_libraries.html). LR Clonase II Enzyme Mix (Invitrogen, Carlsbad, CA) was used to catalyze homologous recombination between each entry plasmid and the pDEST17 destination vector (Invitrogen), thus creating an expression plasmid for the gene of interest. The resulting expression clones were transformed to DH5α competent cells (Invitrogen). Plasmids were prepared using a QIAprep Spin Miniprep kit (Qiagen, Valencia, CA). The sequence fidelity of expression clones was confirmed by DNA sequencing (Proteomics and Metabolomics Facility, Colorado State University).
E. coli BL21(DE3)pLysS or BL21(DE3) host cells (Invitrogen) were transformed with the expression clones. The pDEST17 destination vector contains an N-terminal His tag allowing for affinity purification of expressed proteins. Recombinant His-tagged proteins were expressed following induction with IPTG (isopropyl-β-d-thiogalactopyranoside; Calbiochem, Darmstadt, Germany) and then purified by affinity chromatography using nickel (Ni)-charged His-bind resin (Novagen, Darmstadt, Germany). Resin containing bound recombinant protein was washed with 10 mM Tris-HCl containing 0.5% ASB-14 (Calbiochem, Darmstadt, Germany) to remove endotoxin prior to elution of proteins with imidazole. Purified proteins were dialyzed against 10 mM ammonium bicarbonate overnight at 4°C. The purity of recombinant proteins was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by silver staining. The identification of His-tagged recombinant proteins was performed by Western blotting with mouse monoclonal IgG1 anti-DnaK and anti-GroEL2 primary antibodies (IT41 and IT13, respectively, provided by the Tuberculosis Vaccine Testing and Research Materials contract) or mouse monoclonal IgG1 Penta-His antibody (Qiagen, Valencia, CA). The reaction was detected with anti-mouse antibody conjugated to allophycocyanin, followed by development with 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium (Sigma, St. Louis, MO). The concentration of recombinant proteins was determined with the bicinchoninic acid protein assay (Pierce, Rockford, IL). The endotoxin level for all the proteins was <10 ng−1 ml−1 mg−1 protein, as determined by the Limulus amebocyte lysate QCL-1000 kit (Cambrex, Walkersville, MD), performed according to the manufacturer's instructions.
Identification of GroEL2 and DnaK interacting proteins.
Purified recombinant DnaK or GroEL2 or a mixture of the two proteins was used in pull-down assays to identify protein representatives in PPD which bound to DnaK and GroEL2. Briefly, 0.5 mg of recombinant DnaK, GroEL2, or a 1:1 mixture of DnaK and GroEL2 was incubated with 5.0 mg of PPD overnight at 4°C. The protein-PPD mixture was then passed separately over Ni2+ affinity resin (His band Quick 300 cartridges; Novagen, Darmstadt, Germany) to capture the recombinant protein and any PPD products bound to it, the resin was washed extensively with 10 mM Tris-HCl to remove nonspecifically bound material, and the bound material was eluted with imidazole as described above.
Mass spectrometry.
The pull-down assay-eluted samples were reduced, alkylated, trypsin digested, and dissolved in 5% acetonitrile-0.1% acetic acid for protein identification by liquid chromatography-mass spectrometry (MS) as described previously (22). Tandem mass spectra were extracted, charge state deconvoluted, and deisotoped by BioWorks version 3.2. All tandem MS (MS/MS) samples were analyzed using Sequest (version 2.7; ThermoFinnigan, San Jose, CA) set up to search the M. tuberculosis database (GenBank accession no. AL123456, R9, 3913 entries) assuming the digestion enzyme trypsin and a maximum of four missed cleavages. Sequest was searched with a fragment ion mass tolerance of 1.00 Da and a parent ion tolerance of 2.5 Da. Scaffold (version Scaffold_2_05_02; Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm. Protein identifications were accepted if they could be established at greater than 90.0% probability and contained at least two identified peptides.
Guinea pig model.
Specific-pathogen-free, outbred Hartley guinea pigs (450 to 500 g at the beginning of the experiment) from Charles River Laboratories (Wilmington, MA) were maintained under biosafety level 3 conditions. Guinea pigs were randomly assigned to one of three groups: BCG vaccinated, H37Rv infected, or negative control. All animal experiment procedures were approved by the Animal Care and Use Committee of Colorado State University.
BCG vaccination and aerosol challenge.
Vaccinations were performed by subcutaneous injection of 0.1 ml (103 CFU) of BCG Pasteur.
Guinea pigs were infected using a Madison chamber aerosol generation device to deliver M. tuberculosis H37Rv at a low aerosol dose of 20 bacilli. Subcutaneous injection with 100 μl endotoxin-free phosphate-buffered saline (PBS) was used as a negative control.
Skin testing and biopsy.
BCG-vaccinated or H37Rv-infected guinea pigs were examined for a DTH response induced by different formulations (PPD-S2, DnaK, GroEL2, GroES, HspX, DnaK/GroEL2, DnaK/GroEL2/Rv0009, DnaK/GroEL2/Rv0475, DnaK/GroEL2/Rv0569, DnaK/GroEL2/Rv0685, DnaK/GroEL2/Rv2626c, DnaK/GroEL2/Rv2632c, and PBS as a negative control). The skin test, according to the Mantoux technique, was performed 10 weeks after aerosol infection or BCG vaccination. The hair on the ventral side was shaved off, and the guinea pigs were injected intradermally with 0.1 ml antigen solution diluted in endotoxin-free PBS. The PPD-S2 and formulations containing single proteins were used at a dose of 0.4 μg. The DnaK/GroEL2 and three-protein formulations all used 0.13 μg of each protein.
Erythema and induration were measured with a micrometer after 24 and 48 h. The diameter (in millimeters) was defined as the mean of vertical and transverse measurements.
Skin samples were obtained from infected group guinea pigs. The guinea pigs were euthanized by injection with sodium pentobarbital (Sleepaway; Fort Dodge Laboratories, Inc., Fort Dodge, IA) 48 h after antigen stimulation. The injection site skin was rinsed with 70% ethanol, removed, and then fixed with 10% formalin for histological examination. Skin samples were prepared for RNA extraction (30- to 50-mg skin sample in 0.6 ml RNAlater [Qiagen]) or cell isolation (the same area of skin sample in 1.5 ml of incomplete RPMI medium). The samples for RNA extraction were homogenized in RNAlater and immediately stored at −20°C. The samples for cell isolation were kept on ice and used immediately after biopsy.
Histological examination.
Following fixation in formalin, the skin tissues were routinely processed for paraffin embedding. Sections were cut at a 4- to 5-μm thickness, stained with hematoxylin and eosin (H&E), and examined on a Nikon Eclipse 50i microscope. Microphotographs were taken with a DS-Fi1 camera and associated software (Nikon, Japan).
Flow cytometric analysis of cell surface markers.
Flow cytometry was performed to investigate the subgroups of T cells in infected guinea pigs stimulated with different formulations. Briefly, after the removal of subcutaneous fat and muscle tissues by scraping, the skin samples were minced into small pieces and digested for 1 h at 37°C with 2 mg/ml collagenase type IV and 1.2 U/ml dispase (Worthington, Lakewood, NJ). The digested skin samples were further disrupted by gently pushing the tissue through a 70-μm cell strainer (BD Biosciences, San Jose, CA). Red blood cells were lysed with ACK buffer (0.14 M NH4Cl, 1.0 mM KHCO3, pH 7.2 to 7.4) and then washed and resuspended in complete RPMI medium. The viability of digested skin cells was determined by trypan blue staining. Single-cell suspensions from each individual guinea pig were stained with fluorescently labeled monoclonal antibodies against CD4 (clone FITC CT7; Serotec Inc., Raleigh, NC) (36), CD8 (clone FITC CT6; Serotec Inc.) (36), pan-T cells (clone APC CT5; Serotec Inc.) (36), and CD62L (clone PE lam1-116; Santa Cruz Biotechnology, Santa Cruz, CA) (13) at 4°C for 30 min. Antibodies were used at 0.2 μg/106 cells. Cells were gated on lymphocytes by forward and side scatter according to their characteristic scatter profiles and further gated based on pan-T and CD4 or CD8 expression.
All analyses were performed with an acquisition of at least 100,000 events on a Becton Dickinson FACScalibur flow cytometer (BD Biosciences, San Jose, CA), and the data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Total RNA isolation and real-time quantitative reverse transcription (RT)-PCR assay.
To determine the quantities of cytokine mRNA in skin samples, real-time RT-PCR was performed for the H37Rv-infected guinea pig group. First, the skin samples were homogenized and released skin RNA was extracted using the RNeasy mini kit with columns (Qiagen, Valencia, CA). Contaminating DNA was removed by on-column treatment with RNase-free DNase (Qiagen, Valencia, CA) according to the manufacturer's instructions. The yield and quality of total RNA were examined using an ND1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). The RNA was then stored at −80°C until required for gene expression studies. RT was performed using an iScript cDNA synthesis kit (Qiagen, Valencia, CA). Real-time RT-PCR, performed with iQ CYBR green supermix (Bio-Rad, Hercules, CA), was carried out using primers for guinea pig β-actin, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-10 (IL-10) as described previously (15). Results were recorded as relative gene expression after normalization for β-actin threshold cycle (CT) values by the 2−ΔΔCT method (20). All real-time RT-PCR data are presented such that the values for stimulated samples using different formulations are expressed relative to the value for the PBS-stimulated site on the same animal.
Statistical analysis.
Descriptive analyses were carried out for histopathological features. All other data were expressed as the mean ± the SEM (standard error of the mean). The DTH response data were analyzed by one-tailed Student t test. The Mann-Whitney test was used to characterize changes in cytokine mRNA expression and cell surface markers between groups of guinea pigs. All statistical comparisons were carried out using GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA). P values of <0.05 were considered statistically significant.
RESULTS
DTH responses of the dominant chaperones in PPD.
Four chaperones (DnaK, GroEL2, GroES, and HspX) were expressed as recombinant proteins in E. coli BL21(DE3)pLysS cells and purified using Ni-charged His-bind resin (data not shown). M. tuberculosis-infected, BCG-vaccinated, and naïve guinea pigs were used to evaluate DTH responses to these chaperones, with PPD and saline as positive and negative controls, respectively. The results demonstrated that among the four recombinant proteins used, only DnaK and GroEL2 elicited DTH responses ranging from weak to significant (one study demonstrated mean responses of 3.36 ± 4.53 and 10.04 ± 2.29 mm for DnaK and GroEL2, respectively). All four chaperones were combined in various formulations and tested for the capacity to induce a DTH response. Only the combination of DnaK and GroEL2 augmented the DTH response in infected and vaccinated animals (one study demonstrated mean responses of 10.68 ± 2.32 mm for DnaK/GroEL2 versus 9.46 ± 1.83 mm for PPD). None of the naïve guinea pigs developed a reaction to any of the antigens (data not shown). Dose-response assays were performed with injection doses ranging from 0.1 to 1.2 μg total protein/site. Positive DTH responses were observed for all doses, with 0.25 to 0.4 μg total protein/site being the optimum dose (data not shown).
Pull-down assays.
Ni chromatography was used to pull down protein representatives and peptides in PPD that bound to recombinant DnaK and GroEL2. From this, DnaK and GroEL2 were found to interact with each other, and six additional protein representatives were identified in the pull-down assay elution buffer, indicating an interaction with these two chaperones (Table 1). All six proteins are small-size proteins with native molecular masses ranging from 9.5 to 22 kDa, except Rv0685, with a molecular mass of 44 kDa. As expected from previous SDS-PAGE analyses of PPD (3; Cho et al., unpublished), only recombinant DnaK and GroEL2 and none of the six new products were observed as discrete protein bands upon SDS-PAGE analysis of eluted samples (data not shown), due to the fact that PPD is composed mostly of protein representatives or aggregates of partially denatured proteins and peptides rather than discrete protein molecules (3; Cho et al., unpublished).
TABLE 1.
Proteins identified in PPD-S2 through DnaK and GroEL2 pull-down assay
| Accession no. | Product | Molecular mass (kDa) | Binds DnaK, GroEL2, or both |
|---|---|---|---|
| Rv0009 | Probable iron-regulated peptidyl-prolyl cis-trans isomerase A PpiA | 19.2 | Both |
| Rv0350 | Probable chaperone protein DnaK | 66.8 | GroEL2 |
| Rv0440 | 60-kDa chaperone protein GroEL2 | 56.7 | DnaK |
| Rv0475 | Iron-regulated heparin-binding hemagglutinin HbhA | 21.5 | Both |
| Rv0569 | Hypothetical protein | 9.5 | Both |
| Rv0685 | Elongation factor Tu | 43.6 | Both |
| Rv2626c | Hypothetical protein | 15.5 | Both |
| Rv2632c | Hypothetical protein | 10.6 | Both |
DTH responses to protein cocktails.
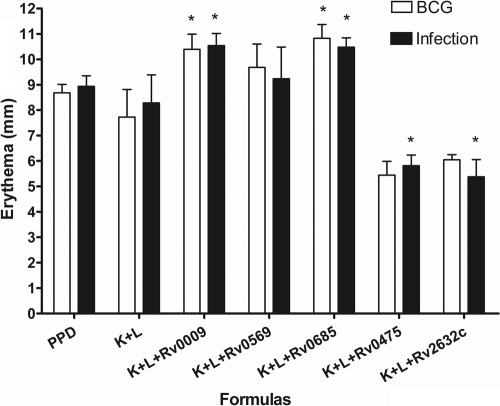
All of the proteins identified as interacting partners in the pull-down assay were expressed and purified as soluble recombinant products, except Rv0685, which was purified from inclusion bodies. To establish the diagnostic potential of these proteins, we evaluated DTH responses to these proteins, individually and combined with DnaK and GroEL2, in M. tuberculosis H37Rv-infected, BCG-vaccinated, and naïve guinea pigs. None of the naïve guinea pigs developed a reaction to any of the formulations (data not shown). The results showed that there was no DTH response when the six proteins were injected individually (data not shown); however, distinct DTH responses could be measured when proteins were combined with DnaK and GroEL2 (Fig. 1). As expected, PPD did not discriminate between the infected and BCG-vaccinated guinea pigs, with DTH responses of 8.68 ± 0.34 mm (mean responses of erythema ± SEM) and 8.94 ± 0.4 mm, respectively. Like PPD, all of the other formulations tested elicited almost identical DTH reactions in M. tuberculosis-infected and BCG-vaccinated guinea pigs. However, both DnaK/GroEL2/Rv0009 and DnaK/GroEL2/Rv0685 induced stronger DTH responses than did the DnaK/GroEL2 formulation alone (P < 0.05); the DTH response to DnaK/GroEL2/Rv0569 was equivalent to that to DnaK/GroEL2 alone. Interestingly, the DnaK/GroEL2/Rv0475, DnaK/GroEL2/Rv2626c, and DnaK/GroEL2/Rv2632c formulations weakened the DTH responses in both groups (P < 0.05) compared to that elicited by DnaK/GroEL2 (data not shown for DnaK/GroEL2/Rv2626c).
FIG. 1.
Skin test responses in M. tuberculosis H37Rv-infected or BCG-vaccinated guinea pigs following intradermal injection with PPD (0.4 μg/site), DnaK/GroEL2 (0.13 μg of each protein/site), DnaK/GroEL2/Rv0009 (0.13 μg of each protein/site), DnaK/GroEL2/Rv0569 (0.13 μg of each protein/site), DnaK/GroEL2/Rv0685 (0.13 μg of each protein/site), DnaK/GroEL2/Rv0475 (0.13 μg of each protein/site), DnaK/GroEL2/Rv2632c (0.13 μg of each protein/site), and PBS (data not shown). The 48-h DTH (skin erythema measurement) response is shown. No DTH reactions were found in naïve guinea pigs stimulated with any of the antigens or in any animals stimulated with PBS alone. The results shown are from one representative experiment (eight animals per group; all experiments were duplicated) and are expressed as the mean and SEM. *, P < 0.05 (compared to the DnaK/GroEL2 group).
Histological examination of DTH sites from infected guinea pigs.
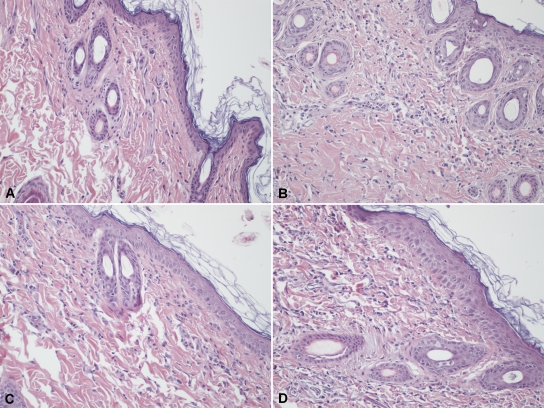
To assess if the same type of cellular and inflammatory response was induced by using different formulations and PPD, microscopic examination of H&E-stained sections was performed on skin test sites from infected guinea pigs (Fig. 2). This revealed that the different protein formulations induced pathological changes similar to that of PPD. In all cases, the inflammation in the dermis was characterized by vasodilation, edema, degranulation of mast cells, and infiltration of granulocytes, mainly neutrophils, as well as lymphocytes and monocytes, at the site of antigen injection. These results suggest that the different formulations induced a DTH response that closely mimics that of PPD. No significant response was observed at the site of the saline injection in infected animals.
FIG. 2.
Microphotographs of inflammatory changes at injection sites in skin from guinea pigs infected with M. tuberculosis H37Rv. Skin biopsy specimens were taken 48 h following intradermal injection of PPD, protein cocktails, and PBS as a negative control. (A) No changes were apparent at the PBS injection site. (B to D) Following injection of PPD (0.4 μg protein/site) (B), DnaK/GroEL2 (0.13 μg each protein/site) (C), or DnaK/GroEL2/Rv0685 (0.13 μg each protein/site) (D), there was vasodilation, mild edema, and dermal infiltration of neutrophils, lymphocytes, and monocyte/macrophages. Final magnification, ×200.
Flow cytometry.
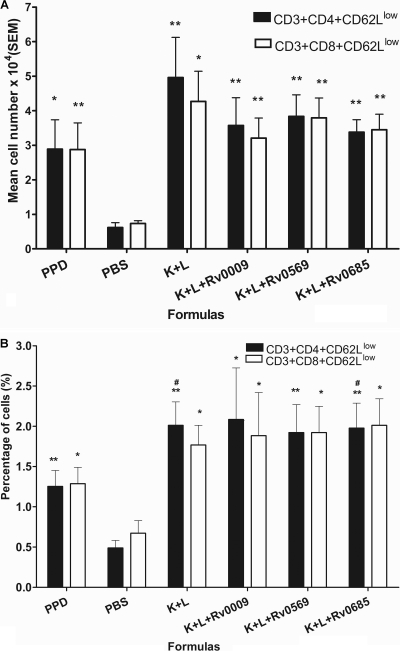
To determine the phenotype of the T cells recruited to the DTH reaction site, flow cytometry was performed on skin samples from infected guinea pigs stimulated with four different protein cocktails and PPD. The PBS injection site was used to measure nonspecific T-cell accumulation. The absolute number and percentage of T subgroup cells positive for each marker studied was determined by comparison to a sample stained with an isotype control antibody. We observed a 3- to 4-fold increase in both the absolute number and the percentage of CD3+ CD4+ CD62Llow and CD3+ CD8+ CD62Llow T cells in animals treated with the different formulations compared to those of animals treated with the PBS control (P < 0.05 or P < 0.001, Fig. 3 A and B). An increase was also observed in terms of the percentage of CD3+ CD4+ CD62Llow T cells in the DnaK/GroEL2 and DnaK/GroEL2/Rv0685 groups compared to that in the PPD group (P < 0.05, Fig. 3B); however, there were no differences between different formulations when the data on the absolute numbers of CD3+ CD4+ CD62Llow and CD3+ CD8+ CD62Llow T cells in the samples were analyzed (Fig. 3A).
FIG. 3.
Flow cytometry of the DTH response in M. tuberculosis-infected guinea pigs. Skin samples were obtained from M. tuberculosis H37Rv-infected guinea pigs stimulated with PPD (0.4 μg protein/site), DnaK/GroEL2 (0.13 μg each protein/site), DnaK/GroEL2/Rv0009 (0.13 μg each protein/site), DnaK/GroEL2/Rv0569 (0.13 μg each protein/site), DnaK/GroEL2/Rv0685 (0.13 μg each protein/site), and PBS. Proportions of T cells and their subsets were determined by flow cytometry analysis after staining the cells with monoclonal antibodies directed against the surface T cells (MCA751APC), CD4+ T cells (MCA749F and MCA749PE), CD8+ T cells (MCA752F), and CD62L cells (SC-13505PE). Results are expressed as the mean number of cells of each sample ± the SEM (A) and the mean percentage of cells ± the SEM (B). Eight animals were used for each assay. *, P < 0.05; **, P < 0.001 (compared to the PBS group).
Cytokine expression.
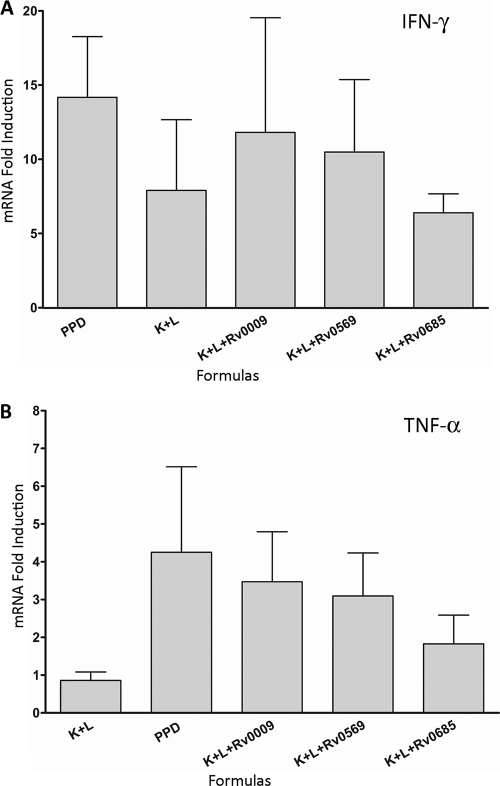
To investigate further the potential differences in the cytokine secretion pattern of skin cells at the DTH sites, we analyzed cytokine expression by real-time RT-PCR in infected animals using four protein cocktails and PPD. The results demonstrate that IFN-γ and TNF-α were significantly upregulated in response to all of the formulations over PBS (Fig. 4). While there were no significant differences in cytokine induction between different formulations, there was a trend of higher TNF-α expression in three of the protein cocktails and PPD versus the cocktail containing DnaK and GroEL2 only. The results also showed an absence of detectable IL-10 mRNA in any of the samples.
FIG. 4.
Cytokine mRNA expression at the site of the DTH response in M. tuberculosis-infected guinea pigs. Skin cells were obtained from M. tuberculosis H37Rv-infected guinea pigs stimulated with PPD (0.4 μg protein/site), DnaK/GroEL2 (0.13 μg each protein/site), DnaK/GroEL2/Rv0009 (0.13 μg each protein/site), DnaK/GroEL2/Rv0569 (0.13 μg each protein/site), DnaK/GroEL2/Rv0685 (0.13 μg each protein/site), or PBS. IFN-γ (A), TNF-α (B), and IL-10 (data not shown) mRNA expression was quantified by real-time RT-PCR. The n-fold induction of mRNA was calculated from the CT values normalized to β-actin CT values and then to PBS-stimulated skin samples. The results are expressed as the mean and SEM. Five animals were used for each assay.
DISCUSSION
In this study, we tested four dominant proteins found in PPD for their potential to induce a DTH response in M. tuberculosis-infected or BCG-vaccinated guinea pigs. A baseline DTH response was established for two of these proteins, DnaK and GroEL2. Products in PPD that interacted with these two chaperones were identified by pull-down assays and mass spectrometry, produced as recombinant antigens, and tested alone or in combination with DnaK and GroEL2 for the capacity to induce a DTH response. Three cocktails, each containing three proteins, were as potent as PPD in the guinea pig model of tuberculosis. The primary molecular mechanisms responsible for inducing a DTH response were indistinguishable when differences between these cocktails and PPD were evaluated. Thus, the biological potency of PPD can be reproduced using a simplified, three-protein cocktail mixture of DnaK/GroEL2/Rv0009, DnaK/GroEL2/Rv0569, or DnaK/GroEL2/Rv0685. These well-defined cocktails can be used to further define the molecular mechanisms of the DTH response in tuberculosis, including the kinetics of the response and the role of sensitization. PPD contains hundreds of antigens that cumulatively induce the DTH response (3; Cho et al., unpublished). Prior to this study, it was unknown which, or which combination, of these products, were responsible for the specificity or sensitivity of the DTH response. This is further complicated by the abundance of denatured, aggregated, and lysed proteinaceous products in PPD, complicating analysis of the PPD proteome, such that only two publications describe the PPD proteome in detail (3; Cho et al., unpublished). Both the work by McFadden and our own identified the chaperones DnaK, GroEL2, and GroES as dominant products in PPD. In this study, these three chaperones and an additional chaperone, HspX, were tested using the guinea pig model of M. tuberculosis infection. DTH responses similar to that induced by PPD were observed when DnaK and GroEL2 were used separately or in combination in M. tuberculosis-infected or BCG-vaccinated guinea pigs. HspX and GroES, on the other hand, were negative or gave a poor response, whether injected separately or as a cocktail, in the guinea pig model of tuberculosis. Based on the relative abundance of DnaK and GroEL2 in PPD, we hypothesize that these two proteins significantly contribute to both the potency and lack of specificity of PPD in the DTH response.
Because both DnaK and GroEL2 are chaperone proteins, it was proposed that other proteins in PPD may be interacting with DnaK and GroEL2, and addition of these may enhance and engender a more specific DTH response than the use of these two proteins alone. To test this hypothesis, a pull-down experiment was performed to identify these proteins in PPD. Six novel proteins in PPD were identified by this method (Rv0009, Rv0475, Rv0569, Rv0685, Rv2626c, Rv2632c). None of these proteins induced a DTH response when tested individually. However, the addition of some of these proteins to DnaK and GroEL2 elicited a potent DTH response. Specifically, the protein cocktails DnaK/GroEL2/Rv0009 and DnaK/GroEL2/Rv0685 induced stronger DTH responses in H37Rv-infected and BCG-vaccinated guinea pigs than did DnaK/GroEL2 only, while the addition of other proteins to the DnaK/ GroEL2 mixture resulted in either an equally active or a weakened DTH response. This illustrates that protein-protein interactions either enhance or abrogate the DTH response. In addition, it is possible that the proteins contributing to a weakened DTH response may be inducing an alternative type of immune response. An anti-inflammatory or regulatory T-cell-type response may be occurring, leading to an overall weakening of the DTH-mediated immune response. Combined, these factors explain, in part, the variability seen in DTH responses, further demonstrating the challenges faced when defining the DTH response in M. tuberculosis infection or after BCG vaccination. The specific cellular attributes responsible for the DTH response in this model of tuberculosis are currently being studied using these defined protein cocktails. Discovery of these essential components during infection with M. tuberculosis or protection by BCG vaccination may lead to the development of rapid, inexpensive second-generation skin test antigens for tuberculosis.
Many protein candidates have been described as tuberculosis-specific skin test antigens over the past 2 decades (5, 6, 11, 24); however, none have reached an optimal diagnostic sensitivity. In this study, the protein mixtures described elicited a response that was equal to or greater than the response to PPD and was indistinguishable from PPD at the molecular level. While these cocktails did not demonstrate significantly different responses between infected and vaccinated animals, they do provide a tool to define the DTH response during the course of infection. Once defined, additional protein formulations that induce a strong and tuberculosis-specific DTH response can be rapidly tested.
To elucidate the mechanism of the DTH response in M. tuberculosis-infected guinea pigs, histological examination and cellular analyses were performed at PPD or protein formulation injection sites on the skin of infected animals. The analysis of the flow cytometry data demonstrated that almost the same number of activated CD4+ and CD8+ T cells are involved in the DTH responses. Previous studies identified more CD4+ T cells than CD8+ T cells in DTH sites (14, 29, 38). There are two probable reasons for the discrepancy. One is the differences between the human experience and the guinea pig model of tuberculosis. The other is that, in this study, activated CD4+ and CD8+ T-cell populations were measured directly at the site of the DTH response. To the best of our knowledge, this study is the first in which flow cytometry was used to look at DTH responses in the guinea pig model and may more accurately reflect the essential mechanisms of a productive DTH response.
DTH is classically defined as the recruitment of T cells to be activated by antigen-presenting cells, mainly Langerhans cells in skin, to produce cytokines that mediate local inflammation. Although the role of CD8+ T cells was previously poorly recognized, there is now considerable evidence that during the process of DTH, the antigen is processed and presented to both CD4+ and CD8+ T cells (2). It is well known that phagocytosed antigens enter the exogenous pathway and are processed for presentation on major histocompatibility complex (MHC) class II molecules to CD4+ T cells, while cytoplasmic antigens are processed by the endogenous pathway for presentation on MHC class I molecules to CD8+ T cells. However, exogenous antigen can also enter the endogenous pathway to be presented to CD8+ T cells (18). Our data demonstrate the activation of CD8+ T cells in DTH responses using PPD and different formulations, indicating that after proteins are digested in vivo, the specific peptides are processed and presented on both MHC class I and II molecules. The expression of cytokines during the DTH response was determined by measuring specific mRNA expression levels at the sites of the DTH response in infected guinea pigs. Our data demonstrated that IFN-γ and TNF-α are actively expressed at the DTH site, and the same immune response pattern is induced by defined protein cocktails and PPD. Further, the T-cell response appears to be biased toward a Th1 T-cell response, based on the increases in the Th1 cytokines IFN-γ and TNF-α and the absence of IL-10 at the DTH sites. Injection of PPD into the footpads of H37Rv-infected mice is also characterized by high expression of Th1 cytokines like IL-2 and IFN-γ and the absence of Th2 cytokines such as IL-4 detected by RT-PCR (26). Similarly, the expression of IFN-γ, the major cytokine produced by Th1 cells at sites of DTH responses, has been confirmed by others (4, 12) and is consistent with our results. Real-time RT-PCR can thus be used for validation of candidate PPD alternatives.
In summary, in our study, DnaK/GroEL2/Rv0009 and DnaK/GroEL2/Rv0685 were found to induce stronger responses than single protein or DnaK/GroEL2 protein cocktail injections and DTH responses indistinguishable from that elicited by PPD in the guinea pig model of tuberculosis. This is the first time that mimicking the PPD response has been shown at the molecular level with defined protein cocktails. The use of defined formulations will not only help provide a more thorough understanding of the DTH response but also provide a platform for standardized, defined second-generation skin test reagents for the diagnosis of M. tuberculosis infection and possibly novel skin test reagents for the evaluation of vaccine efficacy.
Acknowledgments
This work was supported by the Tuberculosis Vaccine Testing and Research Materials contract (HHSN266200400091C) from the NIH.
We thank Linda Izzo for technical assistance with real-time PCR assays.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Affronti, L. F. 1959. Purified protein derivatives (PPD) and antigens prepared from atypical acid-fast bacilli and Nocardia asteroides. Am. Rev. Tuberc. 79:284-295. [DOI] [PubMed] [Google Scholar]
- 2.Black, C. A. 1999. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol. Online J. 5:7. [PubMed] [Google Scholar]
- 3.Borsuk, S., J. Newcombe, T. A. Mendum, O. A. Dellagostin, and J. McFadden. 2009. Identification of proteins from tuberculin purified protein derivative (PPD) by LC-MS/MS. Tuberculosis (Edinb.) 89:423-430. [DOI] [PubMed] [Google Scholar]
- 4.Cher, D. J., and T. R. Mosmann. 1987. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J. Immunol. 138:3688-3694. [PubMed] [Google Scholar]
- 5.Colangeli, R., et al. 2000. MTSA-10, the product of the Rv3874 gene of Mycobacterium tuberculosis, elicits tuberculosis-specific, delayed-type hypersensitivity in guinea pigs. Infect. Immun. 68:990-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coler, R. N., et al. 2000. Cloning of a Mycobacterium tuberculosis gene encoding a purified protein derivative protein that elicits strong tuberculosis-specific delayed-type hypersensitivity. J. Infect. Dis. 182:224-233. [DOI] [PubMed] [Google Scholar]
- 7.Comstock, G. W., L. B. Edwards, R. N. Philip, and W. A. Winn. 1964. A comparison in the United States of America of two tuberculins, Ppd-S and Rt 23. Bull. World Health Organ. 31:161-170. [PMC free article] [PubMed] [Google Scholar]
- 8.Donald, P. R., and P. D. van Helden. 2009. The global burden of tuberculosis—combating drug resistance in difficult times. N. Engl. J. Med. 360:2393-2395. [DOI] [PubMed] [Google Scholar]
- 9.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 10.Dye, C., B. G. Williams, M. A. Espinal, and M. C. Raviglione. 2002. Erasing the world's slow stain: strategies to beat multidrug-resistant tuberculosis. Science 295:2042-2046. [DOI] [PubMed] [Google Scholar]
- 11.Elhay, M. J., T. Oettinger, and P. Andersen. 1998. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect. Immun. 66:3454-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong, T. A., and T. R. Mosmann. 1989. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J. Immunol. 143:2887-2893. [PubMed] [Google Scholar]
- 13.Fryer, A. D., et al. 1997. Antibody to VLA-4, but not to l-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J. Clin. Invest. 99:2036-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs, J. H., et al. 1984. Histometric study of the localisation of lymphocyte subsets and accessory cells in human Mantoux reactions. J. Clin. Pathol. 37:1227-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grover, A., et al. 2009. Kinetics of the immune response profile in guinea pigs after vaccination with Mycobacterium bovis BCG and infection with Mycobacterium tuberculosis. Infect. Immun. 77:4837-4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guld, J., et al. 1958. Standardization of a new batch of purified tuberculin (PPD) intended for international use. Bull. World Health Organ. 19:845-951. [PMC free article] [PubMed] [Google Scholar]
- 17.He, X. Y., et al. 2008. Clinical evaluation of the recombinant 38 kDa protein of Mycobacterium tuberculosis. Scand. J. Infect. Dis. 40:121-126. [DOI] [PubMed] [Google Scholar]
- 18.Kalish, R. S., and P. W. Askenase. 1999. Molecular mechanisms of CD8+ T cell-mediated delayed hypersensitivity: implications for allergies, asthma, and autoimmunity. J. Allergy Clin. Immunol. 103:192-199. [DOI] [PubMed] [Google Scholar]
- 19.Klausen, J., M. Magnusson, A. B. Andersen, and C. Koch. 1994. Characterization of purified protein derivative of tuberculin by use of monoclonal antibodies: isolation of a delayed-type hypersensitivity reactive component from M. tuberculosis culture filtrate. Scand. J. Immunol. 40:345-349. [DOI] [PubMed] [Google Scholar]
- 20.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 21.Lyashchenko, K., et al. 1998. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect. Immun. 66:3606-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehaffy, C., et al. 2010. Descriptive proteomic analysis shows protein variability between closely related clinical isolates of Mycobacterium tuberculosis. Proteomics 10:1966-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mustafa, A. S. 2001. Biotechnology in the development of new vaccines and diagnostic reagents against tuberculosis. Curr. Pharm. Biotechnol. 2:157-173. [DOI] [PubMed] [Google Scholar]
- 24.Oettinger, T., A. Holm, I. M. Mtoni, A. B. Andersen, and K. Hasloov. 1995. Mapping of the delayed-type hypersensitivity-inducing epitope of secreted protein MPT64 from Mycobacterium tuberculosis. Infect. Immun. 63:4613-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pai, M., K. Dheda, J. Cunningham, F. Scano, and R. O'Brien. 2007. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect. Dis. 7:428-438. [DOI] [PubMed] [Google Scholar]
- 26.Pais, T. F., R. A. Silva, B. Smedegaard, R. Appelberg, and P. Andersen. 1998. Analysis of T cells recruited during delayed-type hypersensitivity to purified protein derivative (PPD) versus challenge with tuberculosis infection. Immunology 95:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillay, T., M. Khan, J. Moodley, M. Adhikari, and H. Coovadia. 2004. Perinatal tuberculosis and HIV-1: considerations for resource-limited settings. Lancet Infect. Dis. 4:155-165. [DOI] [PubMed] [Google Scholar]
- 28.Pollock, J. M., et al. 2003. Specific delayed-type hypersensitivity responses to ESAT-6 identify tuberculosis-infected cattle. J. Clin. Microbiol. 41:1856-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poulter, L. W., G. J. Seymour, O. Duke, G. Janossy, and G. Panayi. 1982. Immunohistological analysis of delayed-type hypersensitivity in man. Cell. Immunol. 74:358-369. [DOI] [PubMed] [Google Scholar]
- 30.Rangel-Frausto, M. S., S. Ponce-De-Leon-Rosales, C. Martinez-Abaroa, and K. Haslov. 2001. Tuberculosis and tuberculin quality: best intentions, misleading results. Infect. Control Hosp. Epidemiol. 22:481-484. [DOI] [PubMed] [Google Scholar]
- 31.Reed, S. G., and A. Campos-Neto. 2003. Vaccines for parasitic and bacterial diseases. Curr. Opin. Immunol. 15:456-460. [DOI] [PubMed] [Google Scholar]
- 32.Rhodes, S. G., et al. 2000. Antigen specificity in experimental bovine tuberculosis. Infect. Immun. 68:2573-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seibert, F. B. 1934. The isolation and properties of the purified protein derivative of tuberculin. Am. Rev. Tuberc. 30:713-720. [Google Scholar]
- 34.Seibert, F. B., and J. T. Glen. 1941. Tuberculin purified protein derivative: preparation and analyses of a large quantity of standard. Am. Rev. Tuberc. 44:9-24. [Google Scholar]
- 35.Shingadia, D., and V. Novelli. 2008. The tuberculin skin test: a hundred, not out? Arch. Dis. Child. 93:189-190. [DOI] [PubMed] [Google Scholar]
- 36.Tan, B. T., et al. 1985. Production of monoclonal antibodies defining guinea pig T-cell surface markers and a strain 13 Ia-like antigen: the value of immunohistological screening. Hybridoma 4:115-124. [DOI] [PubMed] [Google Scholar]
- 37.Villarino, M. E., et al. 2000. Comparison testing of current (PPD-S1) and proposed (PPD-S2) reference tuberculin standards. Am. J. Respir. Crit. Care Med. 161:1167-1171. [DOI] [PubMed] [Google Scholar]
- 38.Vukmanovic-Stejic, M., J. R. Reed, K. E. Lacy, M. H. Rustin, and A. N. Akbar. 2006. Mantoux test as a model for a secondary immune response in humans. Immunol. Lett. 107:93-101. [DOI] [PubMed] [Google Scholar]
- 39.Weldingh, K., and P. Andersen. 2008. ESAT-6/CFP10 skin test predicts disease in M. tuberculosis-infected guinea pigs. PLoS One 3:e1978. [DOI] [PMC free article] [PubMed] [Google Scholar]