Abstract
Induction of strong immune responses against a vectored antigen in hosts immunized with live attenuated Salmonella vaccines is related in part to the amount of antigen delivered and the overall fitness of the Salmonella vector in relation to its ability to stimulate the host immune system. Constitutive high-level antigen synthesis causes a metabolic burden to the vaccine vector strain that can reduce the vaccine strain's ability to interact with host lymphoid tissues, resulting in a compromised immune response. A solution to this problem is the use of systems that regulate antigen gene expression, permitting high levels of antigen synthesis only after the vaccine strain has reached its target tissues. In vivo-inducible promoters (IVIPs) are often used to accomplish this. We recently developed an alternative strategy, a regulated delayed antigen synthesis (RDAS) system, in which the LacI-repressible Ptrc promoter controls antigen gene expression by adding arabinose. In this paper, we compared the RDAS system with two commonly used IVIPs, PssaG and PpagC. Three nearly identical plasmids, differing only in the promoter used to direct transcription of the pneumococcal pspA gene, Ptrc, PssaG, or PpagC, were constructed and introduced into isogenic Salmonella vaccine strains with or without arabinose-inducible LacI synthesis. Mice immunized with the RDAS strain developed slightly higher titers of mucosal and serum anti-PspA antibodies than PpagC-immunized mice, while titers in mice immunized with the PssaG strain were 100-fold lower. Both the RDAS and PpagC strains conferred similar levels of protection against Streptococcus pneumoniae challenge, significantly greater than those for the PssaG strain or controls. Thus, RDAS provides another choice for inclusion in the live vaccine design to increase immunogenicity.
Attenuated live bacteria have been widely used as vaccine and vaccine vector systems to deliver antigens or plasmids encoding antigen genes for prophylaxis and therapy purposes (56, 65, 93). One of the most important factors that affect the immune response is the level of antigen synthesis (11). To achieve high levels of antigen synthesis, strong promoters driving antigen gene expression from multicopy plasmids have been used. One problem with this strategy is that high levels of antigen synthesis can result in a metabolic burden to the vaccine strain, leading to a number of unwanted effects, including hyperattenuation, loss of viability, loss of plasmid, modified or poorly expressed antigen genes, and reduction in colonizing ability, ultimately resulting in poor immunogenicity (31). To circumvent this problem, a number of different strategies have been proposed, such as reducing the level of protein synthesis by expressing the antigen gene from the vaccine strain chromosome (41, 82) or using a low-copy-number plasmid (34), the use of secretion signals to export the antigen out of the cell (34, 86), and the use of runaway vectors (68, 85). Induction of gene expression from an arabinose-inducible promoter by injecting immunized animals with arabinose has also been explored (56). One of the most popular solutions is using in vivo-inducible promoters (IVIPs) to drive antigen gene expression (13). In Salmonella, the IVIPs chosen as candidates for vaccine development drive expression genes that are poorly expressed in common laboratory media but exhibit elevated expression levels in cultured macrophages or in host tissues (36).
PnirB was the first in vivo-inducible promoter introduced for use in live bacterial vaccines and has been tested in mice and in a phase one clinical trial (13, 58, 83). Subsequently, many IVIPs have been evaluated in live bacterial vaccines, driving antigen gene expression from multicopy plasmids or from the bacterial chromosome (1, 2, 9, 11, 29, 39, 44, 69, 70, 77, 84, 96). Two commonly used IVIPs are PpagC and PssaG. PagC is an outer membrane protein important for survival in macrophages (64), and SsaG is a component of secretion apparatus in the Salmonella pathogenicity island 2 (SPI-2) type III secretion system (12). Synthesis of both proteins is upregulated in macrophages (23, 25, 62, 73, 87). PpagC has been shown to function in different species (88). While PnirB has been used to express a number of different antigen genes in live attenuated Salmonella vaccines and other species (15, 28, 43, 57, 63, 90, 94), PpagC has emerged as a favorable choice in several studies, including studies that directly compare the two promoters (9, 10, 14, 22, 39, 79). In studies comparing a number of promoters, PpagC was found to have the greatest activity in murine tissues (11, 22). This attribute, combined with its low in vitro activity, has made it an attractive choice for driving antigen expression. The ssaG gene is also highly induced during macrophage infection (23, 87) and under in vitro conditions when Salmonella encounters an acidic pH combined with low levels of phosphate and magnesium (21, 76). The PssaG promoter has been used successfully to drive antigen gene transcription from the bacterial chromosome in both Salmonella enterica serovar Typhimurium and S. enterica serovar Typhi, generating anti-antigen antibody responses in immunized mice (62, 82). An S. Typhi strain, in which PssaG drives transcription of eltB, encoding the Escherichia coli heat-labile toxin subunit B, has been evaluated in a phase 1 clinical trial (48). Thus, these two promoters were chosen for comparison with our regulated delayed antigen synthesis (RDAS) system (Fig. 1).
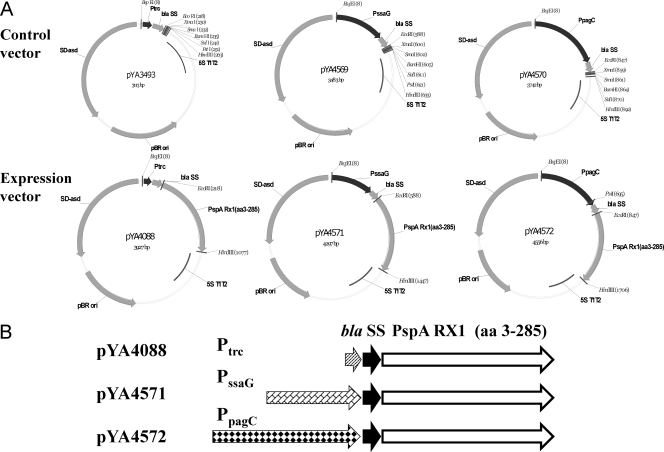
FIG. 1.
Recombinant plasmids for pspA expression. (A) Maps of recombinant plasmids pYA3493 (Ptrc), pYA4569 (PssaG), pYA4570 (PpagC), pYA4088 (Ptrc pspA), pYA4571 (PssaG pspA), and pYA4572 (PpagC pspA). (B) Schematic diagram of the promoter, secretion signal, and antigens. Black arrows, β-lactamase secretion signal; white arrows, pspA (amino acids 3 to 285); other arrows, indicated promoters.
In our laboratory, we typically use the LacI-repressible Ptrc promoter directing transcription of antigen genes (46, 66, 78). Transcription from Ptrc is constitutive due to the absence of LacI in Salmonella. The Ptrc promoter is more transcriptionally active both anaerobically and aerobically than the nirB promoter (13, 22). In one study comparing Ptrc, PpagC, and PnirB driving tetC expression in an attenuated Salmonella strain, all mice immunized with the PpagC strain developed high anti-TetC serum IgG titers (22). Four of five mice immunized with the Ptrc strain developed high anti-TetC IgG titers, although the titers in these mice were lower than those of the mice immunized with the PpagC strain. Mice immunized with the PnirB strain did not develop detectable anti-TetC IgG serum antibody. The mice in all three groups developed similar anti-lipopolysaccharide (LPS) IgG titers, indicating that each of the vaccine strains were capable of stimulating the host immune system. The reason for the differences observed between Ptrc and PpagC in this study may be a reflection of the inability of strains with unregulated antigen gene expression from the Ptrc promoter to reliably induce a robust immune response in immunized animals.
In an effort to reduce the metabolic burden imposed by constitutive antigen gene expression and to permit the initiation of antigen synthesis when the bacterium reaches an immunocompetent site in the host, we developed an RDAS system (91). In this system, LacI represses transcription from Ptrc during in vitro cultivation, with gradual derepression as a consequence of cell divisions occurring during colonization of internal lymphoid tissues following immunization. The arabinose-regulated promoter PBAD drives lacI transcription such that when a vaccine strain is grown in vitro in the presence of arabinose, the LacI synthesized binds to Ptrc, resulting in a reduction in antigen gene expression. Once the Salmonella vaccine invades host tissues, where free arabinose is not available, lacI expression ceases and antigen gene expression increases (91). In our initial analysis using a plasmid carrying the pneumococcal gene pspA under transcriptional control of Ptrc, mice immunized with RDAS strains developed higher anti-PspA IgG and mucosal IgA titers and provided significantly greater protection against pneumococcal challenge than mice immunized with an isogenic strain without the RDAS system (no lacI) (91).
In this study, we compared strains with the RDAS system expressing pspA from Ptrc to strains with and without the RDAS system expressing pspA from the IVIPs PpagC and PssaG. Our results show that RDAS strains colonized spleen tissues and induced anti-PspA antibody responses as well or better than strains expressing pspA from PpagC or PssaG. Immunization with either the RDAS or PpagC strain elicited similar levels of protective immunity that were significantly greater than that of the PssaG strain or controls.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Salmonella Typhimurium vaccine strains were derived from the highly virulent parent strain UK-1 (19). The attenuated strains all carry ΔpabA ΔpabB mutations. pabA and pabB are two unlinked genes that encode 4-amino-4-deoxy-chorismate synthase, required for the production of folic acid in Salmonella. Mutations in either of these genes are attenuating, due to the fact that Salmonella cannot assimilate folic acid from the environment (81). Growth of strains with araC PBAD-regulated genes in the presence of arabinose results in acid production that can cause cessation of growth. We have therefore included the ΔaraBAD23 mutation, which prevents use of arabinose. The ΔasdA16 mutation was introduced to support the use of the asd balanced-lethal plasmid maintenance system (66). S. Typhimurium cultures were grown at 37°C in LB broth (5) or on LB agar with or without 0.05% arabinose. The MgM medium was used as an induction medium (6, 16). Bacterial growth curves were obtained using optical density measurements with a Genesys 10 spectrophotometer (Thermo Scientific) and by plating serial dilutions of bacterial culture on LB agar. Diaminopimelic acid (DAP) was added (50 μg/ml) for the growth of Δasd mutant strains (66). Streptococcus pneumoniae WU2 was cultured on brain heart infusion agar containing 5% sheep blood or in Todd-Hewitt broth plus 0.5% yeast extract (8).
TABLE 1.
Strains and plasmids used in this research
| Strain or plasmid | Relevant characteristic(s)/genotype | Source or reference |
|---|---|---|
| Strains | ||
| S. enterica serovar Typhimurium UK-1 | ||
| χ3761 | Wild-type UK-1 | 19 |
| χ8276 | ΔasdA16 | 46 |
| χ8916 | ΔasdA16 ΔphoP233 | 50 |
| χ9555 | ΔpabA1516 ΔpabB232 ΔasdA16 ΔaraBAD23 ΔrelA1123 | 91 |
| χ9241 | ΔpabA1516 ΔpabB232 ΔasdA16 ΔaraBAD23 ΔrelA198::araC PBADlacI TT | 92 |
| S. pneumoniae WU2 | Wild-type virulent, encapsulated type 3 | 8 |
| Plasmids | ||
| pYA3342 | Asd+ vector, Ptrc, pBR ori | 46 |
| pYA3493 | Asd+ vector with β-lactamase N-terminal signal sequence, Ptrc, pBR ori | 46 |
| pYA4088 | Asd+ vector with β-lactamase N-terminal secretion signal specifying PspA Rx1 (amino acids 3-285) in pYA3493, Ptrc, pBR ori | 91 |
| pYA4569 | Asd+ vector with β-lactamase N-terminal secretion signal, PssaG, pBR ori | pYA3342 |
| pYA4570 | Asd+ vector with β-lactamase N-terminal secretion signal, PpagC, pBR ori | pYA3342 |
| pYA4571 | Asd+ vector with β-lactamase N-terminal secretion signal specifying PspA Rx1 (amino acids 3-285) in pYA4569, PssaG, pBR ori | pYA4569 |
| pYA4572 | Asd+ vector with β-lactamase N-terminal secretion signal specifying PspA Rx1 (amino acids 3-285) in pYA4570, PpagC, pBR ori | pYA4570 |
Construction of plasmids and strains.
To construct plasmid pYA4569, the ssaG promoter was amplified by primer pairs PssaG-BspEI-S (5′-ATATTCCGGATATTGCCATCGCGGATGTC-3′) and PssaG-NcoIEcoRI-a (5′-GGAATTCCCATGGTGCTTTTCCTTAAAATAAATACATC-3′) using S. Typhimurium strain χ3761 genomic DNA as a template. The bla N-terminal sequence was amplified by primer pairs BlaN-S (5′-AGTATTCAACATTTCCGTGTCGC-3′) and BlaN-(C)-EcoRI-a (5′-GCGCGCGAATTCTTCAGCATCTTTTACTTTC-3′) by using plasmid pYA3493 as a template. The two resulting PCR fragments were cloned into plasmid pYA3342 cut with BspEI/EcoRI to generate plasmid pYA4569. The pspA gene encoding amino acids 3 to 285 was cloned from plasmid pYA4088 using EcoRI/HindIII and ligated into pYA4569 cut with the same enzymes to yield plasmid pYA4571.
The plasmid pYA4570 was constructed essentially as described above, except that the PpagC promoter was substituted for the PssaG promoter. The PpagC promoter was amplified by primer pairs PpagC-BspEI-S (5′-AGTGTCCGGAGTTAACCACTCTTAATAATAATG-3′) and PpagC-NcoIEcoRI-a (5′-GGAATTCCCATGGCAACTCCTTAATACTAC-3′) using χ3761 genomic DNA as a template. The bla N-terminal sequence was amplified as described above, and the resulting two PCR fragments were cloned into pYA3342 cut with BspEI/EcoRI to generate plasmid pYA4570. The pspA gene was inserted as described above to generate plasmid pYA4572. All inserts were verified by DNA sequencing. Plasmids pYA4569, pYA4570, pYA4571, and pYA4572 were then transferred into S. Typhimurium strains χ9555 and χ9241.
SDS-PAGE and immunoblot analyses.
Vaccine strains from static cultures grown overnight at 37°C in LB broth without (for IVIPs) or with (for RDAS) 0.05% arabinose were diluted 1:100 into the same medium. The culture was grown with aeration at 37°C to an optical density at 600 nm (OD600) of 0.85 to 0.9. Equal numbers of cells were collected as preinduction samples. The cultures were washed three times with MgM medium, and growth was continued in MgM medium for 4 h to induce the IVIPs (6, 16). Equal numbers of cells were collected from each culture. All samples were subjected to SDS-PAGE and Western blot analysis as previously described (91).
Immunization of mice.
All animal procedures were approved by the Arizona State University Animal Care and Use Committees. Female BALB/c mice, 6 to 8 weeks old, were obtained from Charles River Laboratories (Wilmington, MA). Mice were acclimated for 7 days after arrival before starting the experiments.
Static overnight cultures of vaccine strains were diluted 1:100 into LB broth at 37°C. LB was supplemented with 0.05% arabinose for growth of RDAS strains. Each culture was grown with aeration at 37°C to an OD600 of 0.85 to 0.9. Procedures for cell collection, immunization, and blood and vaginal-wash sample collection and storage have been described (91). Groups of mice were orally inoculated with approximately 1 × 109 CFU of vaccine strains on day 0 and again on day 42.
To evaluate colonization, five animals per group were euthanized on days 3 and 7 after inoculation. Spleen, liver, Peyer's patches (PPs), and mesenteric lymph nodes (MLNs) were collected and weighed, and BSG buffer (33) was added to a final volume of 1 ml. Samples were homogenized and plated onto MacConkey agar and LB agar to determine the number of viable bacteria. The detection limit was 2 CFU. For representation in graphic and statistical analysis, log10 was applied to the values, and recovery of 0 CFU was reported as 1 CFU/g.
Antigen preparation and ELISA.
Recombinant PspA (rPspA) protein and Salmonella serovar Typhimurium outer membrane proteins (SOMPs) were purified as described previously (46). S. Typhimurium LPS was obtained from Sigma. The rPspA clone was a kind gift from Susan Hollingshead at the University of Alabama at Birmingham. An enzyme-linked immunosorbent assay (ELISA) was used to assay antibodies to S. Typhimurium LPS and rPspA in serum and to rPspA in vaginal washes as described previously (91).
IL-4 and IFN-γ ELISPOT assays.
On days 7, 14, and 49, spleen and MLN cells from three mice were harvested from each group. Enzyme-linked immunospot (ELISPOT) assays were performed as previously described (44). Briefly, polyvinylidene difluoride membrane plates (Millipore, Bedford, MA) were coated with 100 μl of anti-interleukin-4 (IL-4) or anti-gamma interferon (IFN-γ) monoclonal antibodies (MAb) (BD Pharmingen, San Diego, CA) at 5 μg/ml in phosphate-buffered saline (PBS) overnight at 4°C. The wells were washed with PBS and blocked with RPMI medium with 10% fetal calf serum (FCS). Next, 100 μl of RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 100 IU/ml penicillin, 100 IU/ml streptomycin, and 1% HEPES, with or without rPspA at 5 μg/ml, and approximately 1,000,000 spleen cells in 100 μl of cell medium were added per well in duplicate and incubated overnight in 5% CO2 at 37°C. The next day, the cell suspensions were discarded and the plates washed with PBS. Biotinylated anti-IL-4 or anti-IFN-γ MAb (BD Pharmingen) at 0.5 μg/ml in PBS with 1% FCS was added and incubated at room temperature for 2 h. After the plates were washed with PBS, 100 μl/well of avidin peroxidase diluted 1:1,000 (vol/vol) in PBS-Tween 20 containing 1% FCS was added and was followed by incubation for 1 h at room temperature. 3-Amino-9-ethylcarbazole substrate (Vector Laboratories, Burlingame, CA) was prepared according to the manufacturer's specifications, and 100 μl of substrate was added per well. Spots were developed for 15 min at room temperature. Plates were dried and analyzed by using an automated CTL ELISPOT reader system (Cellular Technology Ltd., Cleveland, OH).
Pneumococcal challenge.
At week 10, mice were challenged by intraperitoneal injection with 2 × 104 CFU of S. pneumoniae WU2 in 100 μl of BSG buffer (33), equivalent to 100 times the 50% lethal dose (LD50). Challenged mice were monitored daily for 30 days.
Statistical analysis.
Statistical analyses were performed by using the GraphPad Prism 5 software package (Graph Software, San Diego, CA). Antibody titers were expressed as means ± standard deviations. The methods for comparison are shown in the figure legends. Differences were considered significant at P values of <0.05. The survival data were analyzed using the Kaplan-Meier method, and survival comparisons were made using the Mantel-Cox test method.
RESULTS
Systems for comparison of IVIPs and RDAS.
Different combinations of strains and plasmids were set up to compare the IVIP and RDAS systems. For PpagC and PssaG plasmids, we cloned long promoter sequences to ensure inclusion of all the elements required for regulation (67, 74, 89). Two empty vector plasmids, pYA4569 (PssaG) and pYA4570 (PpagC), were generated to include the same β-lactamase N-terminal secretion signal as that present in Ptrc plasmid pYA3493 (46) (Fig. 1A). The pspA gene encoding amino acids 3 to 285 was cloned into pYA4569 and pYA4570 to generate IVIP expression plasmids pYA4571 (PssaG pspA) and pYA4572 (PpagC pspA) (Fig. 1). Plasmid pYA4088, a derivative of plasmid pYA3493, carries the same pspA fragment under transcriptional control of the Ptrc promoter (91) (Fig. 1A). Plasmid pYA4571 carries a 467-bp fragment encoding PssaG, plasmid pYA4572 carries a 726-bp fragment encoding PpagC (67), and plasmid pYA4088 carries an 82-bp fragment encoding Ptrc (Fig. 1B). The only differences among plasmids pYA4571, pYA4572, and pYA4088 are the promoters driving transcription of pspA (Fig. 1B). Strain χ9555, which does not express lacI, was used as the vector for IVIP plasmids pYA4571 and pYA4572. RDAS strain χ9241, isogenic to χ9555 except for the presence of the ΔrelA198::araC PBAD lacI TT cassette that directs arabinose-regulated LacI synthesis, was used as the bacterial host for plasmid pYA4088 (91). Thus, we have the direct comparison of RDAS vaccine strain χ9241(pYA4088) to IVIP strains χ9555(pYA4071) and χ9555(pYA4072). We also included strains χ9241(pYA4071) and χ9241(pYA4072) in our analysis to determine the effect of LacI on the efficacy of the IVIP strains.
Synthesis of rPspA in Salmonella.
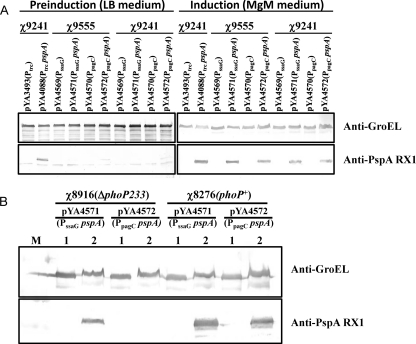
We evaluated PspA synthesis in RDAS vaccine strain χ9241(pYA4088) and IVIP strains χ9555(pYA4071), χ9555(pYA4072), χ9241(pYA4071), and χ9241(pYA4072), grown in LB broth and in MgM medium, to mimic in vivo conditions for induction of PpagC and PssaG. Although strain χ9241(pYA4088) was grown in LB with arabinose, some PspA synthesis was observed in the culture (Fig. 2A). When the χ9241 (lacI) and χ9555 (no lacI) strains were grown in LB, there was detectable PspA synthesis in the PpagC constructs (pYA4572) but not in the PssaG constructs (pYA4571), consistent with previous reports (62) (Fig. 2A). However, after induction in MgM medium, which lacks arabinose, strain χ9241(pYA4088) produced levels of PspA similar to the two IVIP constructs in both χ9241 and χ9555 backgrounds (Fig. 2A). These results demonstrate that all three promoters can drive similar levels of pspA expression under MgM-inducing conditions. The presence of lacI in the chromosome of χ9241 had no effect on PspA synthesis directed by the IVIPs.
FIG. 2.
PspA synthesis in S. Typhimurium vaccine strains. Sample preparation and the Western blot procedure are described in Materials and Methods. Nitrocellulose membranes were probed with polyclonal antibodies specific for either PspA or GroEL. GroEL was used as a standardization marker. Relevant portions of each blot are shown. (A) Western blots showing PspA and GroEL synthesis in strains χ9241 and χ9555 harboring the indicated plasmids. (B) PspA synthesis in S. Typhimurium vaccine strains with or without phoP mutation. The Western blot shows PspA and GroEL synthesis in ΔphoP strains χ8916(pYA4571) (PssaG pspA) and χ8916(pYA4572) (PpagC pspA) and PhoP+ strains χ8276(pYA4571) (PssaG pspA) and χ8276(pYA4572) (PpagC pspA). M, protein marker. Lane 1, cells grown in LB medium (preinduction sample); lane 2, cells grown in MgM medium (induction sample).
PssaG and PpagC are affected by phoP.
To confirm that expression from PssaG and PpagC in our constructs is regulated by phoP (4, 7, 74), we evaluated PspA synthesis in isogenic strains χ8276 and χ8916, differing only at the phoP locus (Table 1). Strain χ8916 is a ΔphoP mutant. Before induction, there was no detectable PspA synthesis in either strain carrying plasmids pYA4571 (PssaG pspA) or pYA4572 (PpagC pspA) (Fig. 2B, lane 1). After induction, high levels of PspA synthesis were detected in phoP+ strains χ8276(pYA4571) and χ8276(pYA4572). Low-level PspA synthesis was detected in ΔphoP233 strain χ8916(pYA4571) (PssaG pspA) but not in strain χ8916(pYA4572) (PpagC pspA) (Fig. 2B). Our results indicate that phoP regulates expression from both promoters but has a more profound influence on PpagC than it does on PssaG.
Colonization of mouse tissues after oral immunization with recombinant attenuated Salmonella vaccines (RASVs) expressing pspA.
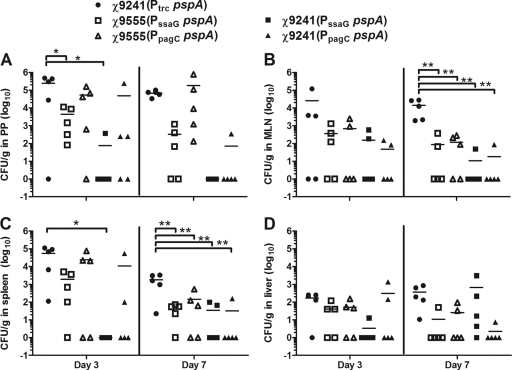
The abilities of the vaccine strains to colonize mouse PPs, MLNs, spleens, and livers were analyzed on days 3 and 7 after oral inoculation. All strains colonized PPs equally well on day 3 except the strains carrying the PssaG pspA promoter, which colonized less well (Fig. 3A). In particular, we were unable to isolate the strain expressing lacI, χ9241(pYA4571) (PssaG pspA), from four of the five mice examined on day 3. On day 7, we were unable to recover χ9241(pYA4571) from any of the five mice examined, although this did not reach statistical significance due to the wide variations in numbers of bacteria recovered from the tissues of mice inoculated with other strains expressing pspA from IVIPs. The poor colonization of this strain was also reflected in the MLN (Fig. 3B), spleen (Fig. 3C), and liver (Fig. 3D). By day 7, the numbers of the RDAS strain χ9241(pYA4088) (Ptrc pspA) CFU recovered from MLNs (Fig. 3B) and spleens (Fig. 3C) were significantly greater than those of all other strains (P < 0.01), although no differences were observed between strains for liver colonization (Fig. 3D). Of note is the fact that, even in the absence of statistical significance, the two strains expressing lacI (χ9241 derivatives) and carrying IVIP-driven pspA appeared to be at a disadvantage with regard to tissue colonization based on the fact that the strain was not recovered from a majority of the mice inoculated with those strains. The only exception was for day 7 recovery from the liver for strain χ9241(pYA4572) (PpagC pspA) (Fig. 3D).
FIG. 3.
Colonization of BALB/c mice by attenuated S. Typhimurium vaccine strains. Bacterial numbers shown are recovered from PPs (A), MLNs (B), spleens (C), and livers (D) at 3 and 7 days postinoculation. For representation in graphic and statistical analysis, log10 was applied to the values. For graphic and statistical analysis, an entry of 1 CFU/g was listed in cases where no bacteria were recovered from a given tissue. The horizontal lines represent the means of each data set. Significant differences between groups are indicated and were determined using one-way analysis of variance (ANOVA) and Tukey's tests (*, P < 0.05; **, P < 0.01).
We also evaluated the in vivo stability of the RDAS and IVIP strains using the bacteria recovered from spleens on day 7. Six colonies from each vaccine strain were randomly chosen and grown overnight in MgM medium. Each culture was analyzed by Western blot analysis using antiserum against PspA. In each case, all six isolates synthesized PspA at levels identical to those seen in the starting cultures (data not shown). These results demonstrate that pspA expression in both the RDAS and IVIP strains is stable in vivo.
Antibody responses in mice immunized with χ9555 and χ9241 harboring pspA expression plasmids.
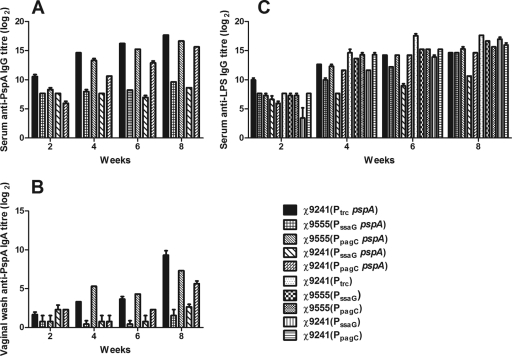
We compared the antibody responses induced by three plasmids delivered by strain χ9241 (ΔrelA198::araC PBAD lacI TT) and the two IVIP plasmids delivered by strain χ9555 (no lacI). The two strains are isogenic, differing only in the presence of an arabinose-regulated lacI gene. The lacI gene in χ9241 was codon optimized to increase LacI synthesis and provide tighter control over the Ptrc promoter present in pYA4088 (91). All mice immunized with RASVs expressing pspA developed titers of anti-rPspA serum IgG (Fig. 4A). The anti-PspA serum IgG titers in mice immunized with RDAS strain χ9241(pYA4088) were higher than titers in mice immunized with either strain carrying the PssaG plasmid pYA4571. The difference in titers was greater than 100-fold by week 4 (Fig. 4A). The anti-PspA titers were about 2-fold higher in the serum of RDAS-immunized mice than in the serum of mice immunized with χ9555(pYA4572) (PpagC pspA). The presence of lacI had a negative impact on antibody responses generated by χ9241(pYA4572) compared to χ9555(pYA4572) at all times and χ9241(pYA4571) compared to χ9555(pYA4571) at 6 and 8 weeks, although the differences were less than 4-fold in most cases. By week 8, identical strains carrying PpagC plasmid pYA4572 elicited anti-PspA serum IgG titers 100-fold greater than those of strains carrying PssaG plasmid pYA4571, regardless of whether the strains expressed lacI. No anti-PspA IgG was detected in mice immunized with strains carrying control plasmid pYA4569, pYA4570, or pYA3493 (data not shown).
FIG. 4.
Reciprocal antibody titers in immunized mice. (A) Serum IgG against rPspA; (B) mucosal IgA against rPspA; (C) serum IgG against S. Typhimurium LPS. Mice were orally immunized with approximately 1 × 109 CFU of the indicated strains. Serum and mucosal antibody titers in pooled samples were determined by ELISA. The data represent antibody in pooled sera from mice orally immunized with attenuated Salmonella harboring either control vector plasmids or pspA expression plasmids. The error bars represent the standard deviations.
The common mucosal system in mice (61) facilitates the production of antigen-specific antibody responses at mucosal sites distant from the site of mucosal immunization, including both the upper respiratory and genital tract (42). Therefore, vaginal washes can be used as a surrogate for nasal or lung secretions. This approach also provided a convenient way to obtain multiple mucosal samples from the same animal and allowed us to keep the animals alive for challenge studies. There were no differences in mucosal anti-PspA IgA responses at 2 weeks, but there were clear differences by 4 weeks. RDAS strain χ9241(pYA4088) and strain χ9555(pYA4571) (PpagC pspA) induced detectable anti-PspA IgA titers, while titers of mucosal samples from mice immunized with the other three strains were below the limits of detection (Fig. 4B). By week 8, mice immunized with either the RDAS strain or χ9555 carrying the PpagC pspA plasmid had developed mucosal IgA titers >100-fold or >50-fold greater than the titers in mice immunized with χ9555(pYA4572) (PssaG pspA), respectively. The negative effect of lacI on the immunogenicity of the PpagC strains was similar to the effect observed for the serum IgG responses (Fig. 4A and B). The absence of any detectable effect of lacI on the IgA titers elicited by the PssaG strains was probably due to the low magnitude of the overall response.
The anti-LPS serum IgG responses were similar for RDAS strain χ9241(pYA4088), IVIP strains χ9555(pYA4571) (PssaG pspA) and χ9555(pYA4572) (PpagC pspA), and their matched control strains carrying empty plasmid vectors (Fig. 4C). In fact, all strains induced similar responses except strain χ9241(pYA4571) (PssaG pspA), which elicited anti-LPS titers 80-fold lower than those of its matched control strain χ9241(pYA4569) (PssaG) by 8 weeks. The LPS response for χ9241(pYA4569) (PssaG) at 2 weeks lagged behind the rest, and the titers increased to levels comparable with those of the other strains by 4 weeks. These results indicate that, with the exception of χ9241(pYA4571) (PssaG pspA), all the strains were able to interact with the mouse immune system in similar fashions.
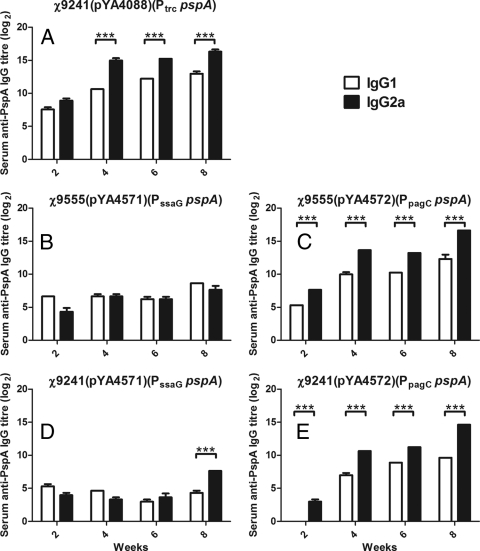
We then evaluated the anti-PspA IgG isotype subclasses IgG1 and IgG2a in these five strains (Fig. 5). Immunization with lacI strains χ9241(pYA4088) (Ptrc pspA) or χ9241(pYA4572) (PpagC pspA) or the non-LacI-producing strain χ9555(pYA4572) (PpagC pspA) induced a Th1-biased response, based on the higher titers of IgG2a than of IgG1 in these mice. Immunization with either strain harboring plasmid pYA4571 (PssaG pspA) resulted in a mixed Th1-Th2 response, except the week 8 samples from mice immunized with strain χ9241(pYA4571), which indicated a Th1-biased response.
FIG. 5.
Serum IgG1 and IgG2a responses to rPspA in mice orally immunized with χ9241(pYA4088) (A), χ9555(pYA4571) (B), χ9555(pYA4572) (C), χ9241(pYA4571) (D), or χ9241(pYA4572) (E). Levels of IgG1 and IgG2a were determined in pooled serum samples by ELISA. The error bars represent the standard deviations. ***, P < 0.001.
IFN-γ and IL-4 responses in immunized mice.
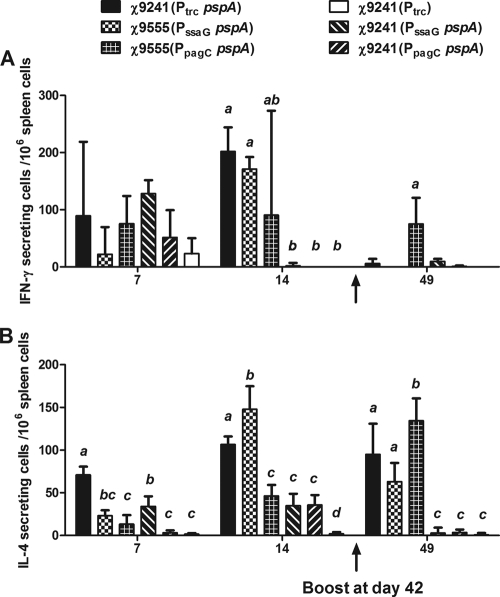
The ELISPOT assay is widely used to detect the function and frequency of antigen-specific T cell responses. Using the ELISPOT assay, we evaluated PspA-specific IFN-γ and IL-4 stimulation in splenocytes and MLN-derived cells harvested from immunized mice on days 7, 14, and 49 postimmunization (Fig. 6). IFN-γ production reached its peak by day 14 and was greatly reduced on day 49, 1 week after boosting (Fig. 6A). IL-4 responses peaked by day 14 and were still strong for some strains on day 49 (Fig. 6B). In mice immunized with χ9241(pYA4088) (Ptrc pspA), χ9555(pYA4571) (PssaG pspA), and χ9555(pYA4572) (PpagC pspA), IL-4-secreting cells were detected at all time points, and IFN-γ-secreting cells were detected at days 7 and 14. On days 7 and 14, we detected significantly greater numbers of IL-4-secreting cells in the spleens of mice immunized with RDAS strain χ9241(pYA4088) than in those of mice immunized with other strains except χ9555(pYA4571) at day 14 (P < 0.05). The RDAS vaccine strain also generated significantly greater numbers of IFN-γ-secreting cells than χ9555(pYA4572) (PpagC pspA), χ9241(pYA4571) (PssaG pspA), and χ9241(pYA4572) (PpagC pspA) on day 14 (Fig. 6A) (P < 0.01). At day 49, 1 week after boosting, we detected greater numbers of IFN-γ-secreting cells in mice immunized with strain χ9555(pYA4572) (PpagC pspA) than in any of the other strains (Fig. 6A). The numbers of IL-4-secreting cells detected in mice immunized with strains χ9241(pYA4088) (RDAS, Ptrc pspA), χ9555(pYA4571) (PssaG pspA), and χ9555(pYA4572) (PpagC pspA) were significantly greater than those of control groups (P < 0.01). After the boost, the IL-4 responses were significantly higher in mice immunized with χ9555(pYA4572) (PpagC pspA) than in mice immunized with χ9555(pYA4571) (PssaG pspA) (P < 0.01) and χ9241(pYA4088) (Ptrc pspA) (P < 0.01). We detected background levels of IFN-γ- and IL-4-secreting cells only from MLNs at day 7 and day 14 (data not shown).
FIG. 6.
PspA-specific cytokine stimulation in mice immunized with χ9241(pYA3493) (Ptrc), χ9241(pYA4088) (Ptrc pspA), χ9555(pYA4571) (PssaG pspA), χ9555(pYA4572) (PpagC pspA), χ9241(pYA4571), and χ9241(pYA4572). Numbers of IFN-γ-producing (A) and IL-4-producing (B) cells were determined by ELISPOT assay. Splenectomies were performed on euthanized mice 7, 14, and 49 days after the first immunization. Splenocytes were harvested from three mice per group, and cells from each spleen were assayed in duplicate. The results from each well are expressed as ELISPOTs per million splenocytes minus the background (typically approximately 15 spots) from unpulsed mock controls. Significant differences between groups are indicated and were determined using one-way ANOVA and Tukey's tests. Groups with different letters are statistically different (P < 0.05). Groups with no letters or the same letters were not different.
Protection of mice immunized with RDAS and IVIP vaccine strains against S. pneumoniae challenge.
When immunized mice were challenged intraperitoneally with 100 times the LD50 of S. pneumoniae WU2, all groups that received PspA-producing strains were significantly protected compared with control strains that did not express pspA (P < 0.05) (Table 2), with the exception of the groups immunized with χ9241(pYA4571) (PssaG pspA). There was no significant difference in protection between mice immunized with χ9241(pYA4088) (Ptrc pspA), χ9555(pYA4571) (PssaG pspA), χ9555(pYA4572) (PpagC pspA), or χ9241(pYA4572) (PpagC pspA). Although 15% of mice immunized with χ9241(pYA4571) (PssaG pspA) survived challenge, the level of protection was not significant compared to that for controls.
TABLE 2.
Oral immunization with PspA-expressing Salmonella strains protects BALB/c mice against intraperitoneal challenge with capsular type 3 S. pneumoniae WU2
| Vaccine straina | Promoter | Presence of lacI/pspAb | No. of challenged mice | No. of days to death (no. of mice)c | % survivald |
|---|---|---|---|---|---|
| χ9241(pYA4088) | Ptrc | +/+ | 22 | 3 (4), 4 (8), >15 (10) | 45.5e |
| χ9555(pYA4571) | PssaG | −/+ | 23 | 2 (2), 3 (8), 4 (3), 5 (1), >15 (9) | 39.1f |
| χ9555(pYA4572) | PpagC | −/+ | 22 | 3 (7), 4 (7), >15 (8) | 36.4g |
| χ9241(pYA4571) | PssaG | +/+ | 20 | 2 (4), 3 (11), 4 (1), 5 (1), >15 (3) | 15 |
| χ9241(pYA4572) | PpagC | +/+ | 20 | 2 (2), 3 (6), 4 (2), 5 (1), >15 (9) | 45h |
| χ9241(pYA3493) | Ptrc | +/− | 8 | 2 (5), 3 (3) | 0 |
| χ9555(pYA4569) | PssaG | −/− | 8 | 2 (5), 3 (3) | 0 |
| χ9555(pYA4570) | PpagC | −/− | 8 | 2 (4), 3 (4) | 0 |
| χ9241(pYA4569) | PssaG | +/− | 3 | 2 (2), 3 (1) | 0 |
| χ9241(pYA4570) | PpagC | +/− | 3 | 3 (3) | 0 |
Mice were orally immunized with two doses of the indicated vaccine strains at 0 and 6 weeks.
+, presence; −, absence.
Ten weeks after the primary oral immunization, mice were challenged with approximately 2 × 104 CFU of S. pneumoniae WU2. The LD50 of WU2 in nonimmunized BALB/c mice is 2 × 102 CFU (53).
The survival data were analyzed using the Kaplan-Meier method, and survival comparisons were done by the Mantel-Cox test method.
Compared to χ9241(pYA4571)-immunized mice, P = 0.002. Compared to χ9241(pYA3493)-immunized mice, P < 0.001.
Compared to χ9241(pYA4571)-immunized mice, P = 0.046. Compared to χ9555(pYA4569)-immunized mice, P < 0.001.
Compared to χ9555(pYA4570)-immunized mice, P < 0.001.
Compared to χ9241(pYA4571)-immunized mice, P = 0.03.
DISCUSSION
Live attenuated Salmonella vaccines hold great potential for delivering heterologous antigens or DNAs to a variety of animal hosts to elicit protection against a number of viral, bacterial, and parasite pathogens (18, 20, 33, 52, 80). While there appears to be a need for the vaccine strain to produce high levels of antigen to elicit protection, there is a metabolic flux readjustment and redirection imposed by the diversion of the cell's resources to antigen synthesis (31, 32, 37, 52). This can lead to a reduction in plasmid and/or antigen stability and the ability of the vaccine strain to interact with the host immune system. In this work, we evaluated two strategies for reducing the metabolic burden on the cell, the use of IVIPs and the use of our recently developed RDAS system (91). Our results show that in many ways, the two systems, in particular RDAS and PpagC, are comparable.
Both systems tested were capable of directing similar amounts of the test antigen PspA under in vitro induction conditions (Fig. 2). The RDAS system, which relies on the Ptrc promoter, was induced in MgM medium. The basis for this induction is probably related to the absence of arabinose in MgM medium, as we have previously shown that RDAS strains are induced in LB broth without arabinose (91). Vaccine isolates recovered from mouse tissues could synthesize PspA, indicating that PspA production in both RDAS and IVIP strains was stable in vivo. Although all strains effectively colonized gut-associated lymphatic tissue (Peyer's patches) and systemic sites (spleens, livers, and MLNs), the RDAS strain χ9241(pYA4088) colonized mouse tissues as well or better than the IVIP strains on days 3 and 7 (Fig. 3). Three strains, χ9241(pYA4088), χ9555(pYA4571), and χ9555(pYA4572), were able to induce serum IgG responses against PspA in immunized mice, although the titers induced by the PssaG strain were much lower than those of the other two strains (Fig. 4A). The deficiency in the PssaG strain also extended to the titers of mucosal IgA (Fig. 4B). The RDAS strain χ9241(pYA4088) and the PpagC strain χ9555(pYA4572) induced similar levels of mucosal IgA, a result that correlated well with their comparable abilities to colonize host tissues (Fig. 3).
Examination of the serum IgG isotypes revealed that both the RDAS strain and the PpagC strain elicited a Th1-biased response typical of those induced by Salmonella vaccines (Fig. 5) (46, 71, 72, 75, 91). The PssaG strain elicited a more balanced Th1-Th2 response, although the overall anti-PspA serum IgG titers were very low. Despite the results suggested by analysis of IgG1/IgG2a, the RDAS strain induced a strong IFN-γ response on days 7 and 14 but not on day 49 (Fig. 6A). The PssaG strain induced strong IFN-γ and IL-4 responses on day 14, consistent with the mixed Th1-Th2 response we observed based on the IgG1/IgG2a ratios (Fig. 5). Conversely, the PpagC strain χ9555(pYA4572) elicited a significant number of IFN-γ-secreting spleen cells at all time points examined, suggesting that the PpagC promoter system may have an advantage for inducing strong cellular immunity compared to either the RDAS system or the PssaG promoter (Fig. 6B). Interestingly, all three strains elicited PspA-responsive IL-4-secreting cells. Induction of IL-4 by the PpagC strain was low on day 14 but significantly increased after the boost.
Ultimately, the most important test of a vaccine antigen delivery system is the ability to elicit protective immunity. Surprisingly, despite the differences in immune responses among strains, all three strains, χ9241(pYA4088), χ9555(pYA5471), and χ9555(pYA4572), protected immunized mice equally well from challenge with virulent S. pneumoniae (Table 2). Although the antibody responses against S. pneumoniae are important for protection, other factors, including T cell responses and production of cytokines such as IL-17, are also important (59). We found that spleen cells harvested from mice 14 days postimmunization with χ9241(pYA4088) produced less IL-17 than those from mice immunized with χ9555(pYA4571) and χ9555(pYA4572) when restimulated with PspA (data not shown). It was also reported that PssaG can induce high levels of antigen-specific T cell responses (95). Thus, the RDAS and IVIPs may stimulate different aspects of the immune system; the former can induce a stronger antibody response, while the latter provides for better induction of cellular immunity.
Our findings show that each system works in our test scenario. The concept of regulated antigen synthesis to reduce the effect of antigen production on the carrier is especially helpful for toxic antigens. In a previous study, we found that an RDAS-synthesizing LacI strain, but not a strain lacking LacI, could support the growth of a high-copy-number pUC-based plasmid expressing pspA from the Ptrc promoter (91). The RDAS system may have certain advantages over the IVIP system. Expression from IVIPs can be influenced by the genetic background of the host. IVIPs are regulated by native Salmonella proteins that are themselves virulence genes that can be targeted for their ability to attenuate virulence. For example, the PpagC and PssaG promoters are regulated by phoP (Fig. 2B) (4, 7, 74). Thus, while phoP Salmonella mutants are attenuated and immunogenic (3, 38, 40), these two promoters cannot be used in phoP strains. Therefore, selection of an IVIP is limited by the mode of attenuation used for the Salmonella delivery strain. The PssaG promoter is also regulated by the master sensor-kinase system SsrA-SsrB from the Salmonella pathogenicity island 2 (SPI-2) (51, 87). Expression of SPI-2 type III secretion system apparatus genes, including ssaG, is also controlled by a regulatory hierarchy that includes the two-component systems OmpR-EnvZ and PhoP-PhoQ, the MarR homologue SlyA, and nucleoid-associated proteins Fis, H-NS, and YdgT in response to several environmental signals, such as low osmolarity, acidic pH, and cation concentration (7, 17, 26, 35, 47, 49, 51, 55, 60). The regulatory proteins Fis, SsrB, and H-NS bind directly to PssaG (27, 47, 54, 89). Other IVIPs evaluated for antigen delivery may also be controlled by different environment factors and/or global regulators, a factor which needs to be considered when selecting an IVIP. Even a group of promoters regulated by the same regulator may show differences in strength of expression, efficiency, and genetic background (95). Another disadvantage of IVIPs may be that if they are used to direct antigen expression from multicopy plasmids, as we have done here, the presence of multiple copies of the promoter may interfere with its regulation or the stability of the plasmid due to titration of effector proteins. The strain harboring the PpagC plasmid was not as stable as Ptrc in vivo in MLNs and PPs (Fig. 3) (22). Inserting the IVIP into the bacterial chromosome, as has been done with PssaG driving eltB (41, 82), may be a better choice to ensure proper regulation. Previous work that directly compares different IVIPs showed that the most effective promoter to achieve the desired immune response varied for different antigens (9, 14, 22, 29, 63, 77); in some cases, a constitutive promoter may be optimal (30, 45, 83). In addition, for IVIPs, measures of in vitro expression may have limited correlation with their performance in vivo (95). Thus, selection of the IVIPs may require an empirical analysis for each antigen, strain, and bacterial species. Modular design of expression cassettes and systematic comparisons are needed to select optimal IVIPs (95). Currently, the RDAS system relies on the Ptrc promoter and the lacI gene, neither of which are naturally occurring in most Salmonella species. Therefore, theoretically, this system can be used with any attenuation strategy and should provide a greater degree of flexibility with respect to various antigens and attenuation strategies. In our case, the regulatable repression element is arabinose-regulated lacI, and the regulatable promoter is LacI-regulated Ptrc. Other repressor/promoter pairs, such as C2 from phage P22, which can repress promoter PL or PR from phage P22, can also be applied in this system and are currently being evaluated in our laboratory. In other cases, the phage λ cI repressor and λ promoters PL or PR can be used. Adopting these combinations will provide more choices for optimizing RDAS in different bacterial species.
One potential disadvantage of the RDAS system is its reliance on lacI. It was recently shown that LacI is an antivirulence factor and that overexpression of lacI in S. Typhimurium can impair the organism's ability to invade host tissues (24). However, in that study, lacI was expressed from a high-copy-number plasmid, while in our system, lacI is expressed from a single copy in the chromosome. Our data show that the level of LacI synthesized in our system had little effect on the ability of χ9241(pYA4572) (PpagC pspA) to elicit an anti-PspA serum IgG or mucosal IgA response (Fig. 4A), although the IFN-γ and IL-4 responses were impaired compared to that of χ9555(pYA4572) at day 49 (Fig. 6). On the other hand, while there was no difference in anti-PspA serum IgG titers, we did observe a reduction in anti-LPS IgG titers when comparing the lacI strain χ9241(pYA4571) (PssaG pspA) with strain χ9555(pYA4571), which does not express lacI (Fig. 4C). Strain χ9555(pYA4571) induced higher numbers of IL-4-secreting cells than χ9241(pYA4571), indicating that the presence of lacI had a negative influence on the strain's ability to induce cellular immunity. Finally, there was a significant reduction in protection from challenge in mice immunized with χ9241(pYA4571) (PssaG pspA) compared to that in mice immunized with χ9555(pYA4571). One explanation for why the impact of LacI was greater for the PssaG strain than the PpagC strain is that LacI interfered with the regulation of pspA expression from PssaG in vivo. This hypothesis is supported by our observation that there are comparable levels of PspA synthesis in strains with or without LacI carrying the PssaG pspA and PpagC pspA plasmids grown under inducing conditions in MgM medium (Fig. 2). Further support for this explanation is that ssrA expression is downregulated about 2-fold in strains overexpressing lacI from a multicopy plasmid, as determined by microarray analysis and reverse transcription (RT)-PCR (24). Since SsrA-SsrB constitute the primary regulators of expression from PssaG, downregulation of ssrA may interfere with the regulation of pspA expression in strain χ9241(pYA4571). While phoP was also shown to be downregulated in minimal medium in the same study, there was no reduction of phoP expression in macrophages as determined by RT-PCR. Taken together, the results indicate that LacI can have a negative impact on vaccine strains that rely on the two IVIPs examined in this study. This impact was greater in the PssaG promoter strain, which did not perform as well even in the absence of lacI, than in the PpagC promoter strain.
LacI was not necessary for expression from IVIPs, and its presence reduced colonization and cytokine induction in the strain carrying the PssaG plasmid (Fig. 3 and 6). In contrast, we have shown previously that when the Ptrc promoter was used to drive antigen gene expression, inclusion of the ΔrelA198::araC PBAD lacI TT cassette improved the performance of the vaccine, including a significant increase in protection from challenge (91). In addition, the RDAS strain induced higher anti-PspA IgG and IgA responses than the PssaG strain regardless of whether the PssaG host expressed lacI (Fig. 4). Our system also provides suitable flexibility to optimize the synthesis of a given antigen. We have constructed two other relA::araC PBAD lacI TT alleles, relA196 and relA197, which were engineered to direct the synthesis of less LacI than the relA198 allele (91). These may be useful in situations where antigen genes are expressed from low-copy-number plasmids or from the chromosome. The choice of arabinose concentration can be adjusted to reduce or increase the level of LacI synthesis, which will reciprocally control the levels of antigen synthesis in vitro and the time course of antigen gene derepression in vivo (91). Thus, there are a number of ways to optimize the RDAS system that require less work and expense than when choosing the optimal IVIP.
The RDAS strain elicited antibody titers and IL-4 responses equal to or greater than those of any of the IVIP strains, suggesting that the levels of LacI in our system did not impair the vaccine's ability to induce a robust immune response. This may be due to the fact that the level of LacI synthesized did not compromise the ability of the strain to invade host tissues. This notion is supported by the fact that the plasmid carrying the PpagC promoter driving expression of pspA stimulated similar levels of protective immunity regardless of whether lacI was present (Table 2).
In summary, we have made a direct comparison of the new RDAS system and two IVIPs, each driving the expression of pspA. Overall, both systems worked well to provide protection against challenge with virulent S. pneumoniae. This work, combined with previous work (91), establishes the RDAS system as a practical alternative to IVIPs to overcome the metabolic burden imposed by constitutive antigen gene expression.
Acknowledgments
The work was supported by NIH R01 grants AI24533, AI057885, and AI056289 and the Bill and Melinda Gates Foundation grant 37863.
We thank David E. Briles and Susan K. Hollingshead (University of Alabama at Birmingham) for providing S. pneumoniae strain W42 and Qing Liu for purifying OMPs and PspA protein.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Altboum, Z., E. M. Barry, G. Losonsky, J. E. Galen, and M. M. Levine. 2001. Attenuated Shigella flexneri 2a ΔguaBA strain CVD 1204 expressing enterotoxigenic Escherichia coli (ETEC) CS2 and CS3 fimbriae as a live mucosal vaccine against Shigella and ETEC infection. Infect. Immun. 69:3150-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altboum, Z., M. M. Levine, J. E. Galen, and E. M. Barry. 2003. Genetic characterization and immunogenicity of coli surface antigen 4 from enterotoxigenic Escherichia coli when it is expressed in a Shigella live-vector strain. Infect. Immun. 71:1352-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelakopoulos, H., and E. L. Hohmann. 2000. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar Typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect. Immun. 68:2135-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belden, W. J., and S. I. Miller. 1994. Further characterization of the PhoP regulon: identification of new PhoP-activated virulence loci. Infect. Immun. 62:5095-5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beuzon, C. R., G. Banks, J. Deiwick, M. Hensel, and D. W. Holden. 1999. pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol. Microbiol. 33:806-816. [DOI] [PubMed] [Google Scholar]
- 7.Bijlsma, J. J., and E. A. Groisman. 2005. The PhoP/PhoQ system controls the intramacrophage type three secretion system of Salmonella enterica. Mol. Microbiol. 57:85-96. [DOI] [PubMed] [Google Scholar]
- 8.Briles, D. E., et al. 1996. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine 14:858-867. [DOI] [PubMed] [Google Scholar]
- 9.Bullifent, H. L., et al. 2000. Antibody responses to Yersinia pestis F1-antigen expressed in Salmonella typhimurium aroA from in vivo-inducible promoters. Vaccine 18:2668-2676. [DOI] [PubMed] [Google Scholar]
- 10.Bumann, D. 2002. Examination of Salmonella gene expression in an infected mammalian host using the green fluorescent protein and two-colour flow cytometry. Mol. Microbiol. 43:1269-1283. [DOI] [PubMed] [Google Scholar]
- 11.Bumann, D. 2001. Regulated antigen expression in live recombinant Salmonella enterica serovar Typhimurium strongly affects colonization capabilities and specific CD4+-T-cell responses. Infect. Immun. 69:7493-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakravortty, D., M. Rohde, L. Jager, J. Deiwick, and M. Hensel. 2005. Formation of a novel surface structure encoded by Salmonella pathogenicity island 2. EMBO J. 24:2043-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatfield, S. N., et al. 1992. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Biotechnology 10:888-892. [DOI] [PubMed] [Google Scholar]
- 14.Chen, H., and D. M. Schifferli. 2001. Enhanced immune responses to viral epitopes by combining macrophage-inducible expression with multimeric display on a Salmonella vector. Vaccine 19:3009-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, I., et al. 1998. A recombinant live attenuated strain of Vibrio cholerae induces immunity against tetanus toxin and Bordetella pertussis tracheal colonization factor. Infect. Immun. 66:1648-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coombes, B. K., N. F. Brown, Y. Valdez, J. H. Brumell, and B. B. Finlay. 2004. Expression and secretion of Salmonella pathogenicity island-2 virulence genes in response to acidification exhibit differential requirements of a functional type III secretion apparatus and SsaL. J. Biol. Chem. 279:49804-49815. [DOI] [PubMed] [Google Scholar]
- 17.Coombes, B. K., M. E. Wickham, M. J. Lowden, N. F. Brown, and B. B. Finlay. 2005. Negative regulation of Salmonella pathogenicity island 2 is required for contextual control of virulence during typhoid. Proc. Natl. Acad. Sci. U. S. A. 102:17460-17465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtiss, R., III., et al. 2005. Antigen delivery systems II: development of live recombinant attenuated bacterial antigen and DNA vaccine delivery vector vaccines, p. 1009-1037. In J. Mestecky, M. E. Lamm, J. R. McGhee, J. Bienenstock, L. Mayer, and W. Strober (ed.), Mucosal immunology, 3rd ed. Academic Press, Burlington, MA.
- 19.Curtiss, R., III., et al. 1991. Nonrecombinant and recombinant avirulent Salmonella live vaccines for poultry, p. 169-198. In L. C. Blankenship, J. H. S. Bailey, N. A. Cox, N. J. Stern, and R. J. Meinersmann (ed.), Colonization control of human bacterial enteropathogens in poultry. Academic Press, New York, NY.
- 20.Curtiss, R., III., et al. 2007. Induction of host immune responses using Salmonella-vectored vaccines, p. 297-313. In K. A. Brogden, N. Cornick, T. B. Stanton, Q. Zhang, L. K. Nolan, and M. J. Wannemuehler (ed.), Virulence mechanisms of bacterial pathogens, 4th ed. ASM Press, Washington, DC.
- 21.Deiwick, J., T. Nikolaus, S. Erdogan, and M. Hensel. 1999. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol. Microbiol. 31:1759-1773. [DOI] [PubMed] [Google Scholar]
- 22.Dunstan, S. J., C. P. Simmons, and R. A. Strugnell. 1999. Use of in vivo-regulated promoters to deliver antigens from attenuated Salmonella enterica var. Typhimurium. Infect. Immun. 67:5133-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 24.Eswarappa, S. M., G. Karnam, A. G. Nagarajan, S. Chakraborty, and D. Chakravortty. 2009. lac repressor is an antivirulence factor of Salmonella enterica: its role in the evolution of virulence in Salmonella. PLoS One 4:e5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faucher, S. P., S. Porwollik, C. M. Dozois, M. McClelland, and F. Daigle. 2006. Transcriptome of Salmonella enterica serovar Typhi within macrophages revealed through the selective capture of transcribed sequences. Proc. Natl. Acad. Sci. U. S. A. 103:1906-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng, X., R. Oropeza, and L. J. Kenney. 2003. Dual regulation by phospho-OmpR of ssrA/B gene expression in Salmonella pathogenicity island 2. Mol. Microbiol. 48:1131-1143. [DOI] [PubMed] [Google Scholar]
- 27.Feng, X., D. Walthers, R. Oropeza, and L. J. Kenney. 2004. The response regulator SsrB activates transcription and binds to a region overlapping OmpR binding sites at Salmonella pathogenicity island 2. Mol. Microbiol. 54:823-835. [DOI] [PubMed] [Google Scholar]
- 28.Fontana, M. R., et al. 2000. IEM101, a naturally attenuated Vibrio cholerae strain as carrier for genetically detoxified derivatives of cholera toxin. Vaccine 19:75-85. [DOI] [PubMed] [Google Scholar]
- 29.Foynes, S., J. L. Holley, H. S. Garmory, R. W. Titball, and N. F. Fairweather. 2003. Vaccination against type F botulinum toxin using attenuated Salmonella enterica var. Typhimurium strains expressing the BoNT/F HC fragment. Vaccine 21:1052-1059. [DOI] [PubMed] [Google Scholar]
- 30.Galen, J. E., et al. 1997. A murine model of intranasal immunization to assess the immunogenicity of attenuated Salmonella typhi live vector vaccines in stimulating serum antibody responses to expressed foreign antigens. Vaccine 15:700-708. [DOI] [PubMed] [Google Scholar]
- 31.Galen, J. E., and M. M. Levine. 2001. Can a ‘flawless’ live vector vaccine strain be engineered? Trends Microbiol. 9:372-376. [DOI] [PubMed] [Google Scholar]
- 32.Galen, J. E., et al. 1999. Optimization of plasmid maintenance in the attenuated live vector vaccine strain Salmonella typhi CVD 908-htrA. Infect. Immun. 67:6424-6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galen, J. E., et al. 2009. Salmonella enterica serovar Typhi live vector vaccines finally come of age. Immunol. Cell Biol. 87:400-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galen, J. E., et al. 2010. A new generation of stable, nonantibiotic, low-copy-number plasmids improves immune responses to foreign antigens in Salmonella enterica serovar Typhi live vectors. Infect. Immun. 78:337-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garmendia, J., C. R. Beuzon, J. Ruiz-Albert, and D. W. Holden. 2003. The roles of SsrA-SsrB and OmpR-EnvZ in the regulation of genes encoding the Salmonella typhimurium SPI-2 type III secretion system. Microbiology 149:2385-2396. [DOI] [PubMed] [Google Scholar]
- 36.Heithoff, D. M., et al. 1997. Bacterial infection as assessed by in vivo gene expression. Proc. Natl. Acad. Sci. U. S. A. 94:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann, F., J. Weber, and U. Rinas. 2002. Metabolic adaptation of Escherichia coli during temperature-induced recombinant protein production. 1. Readjustment of metabolic enzyme synthesis. Biotechnol. Bioeng. 80:313-319. [DOI] [PubMed] [Google Scholar]
- 38.Hohmann, E. L., C. A. Oletta, K. P. Killeen, and S. I. Miller. 1996. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J. Infect. Dis. 173:1408-1414. [DOI] [PubMed] [Google Scholar]
- 39.Hohmann, E. L., C. A. Oletta, W. P. Loomis, and S. I. Miller. 1995. Macrophage-inducible expression of a model antigen in Salmonella typhimurium enhances immunogenicity. Proc. Natl. Acad. Sci. U. S. A. 92:2904-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hohmann, E. L., C. A. Oletta, and S. I. Miller. 1996. Evaluation of a phoP/phoQ-deleted, aroA-deleted live oral Salmonella typhi vaccine strain in human volunteers. Vaccine 14:19-24. [DOI] [PubMed] [Google Scholar]
- 41.Hone, D., S. Attridge, L. van den Bosch, and J. Hackett. 1988. A chromosomal integration system for stabilization of heterologous genes in Salmonella based vaccine strains. Microb. Pathog. 5:407-418. [DOI] [PubMed] [Google Scholar]
- 42.Hu, K. F., J. Ekstrom, M. Merza, K. Lovgren-Bengtsson, and B. Morein. 1999. Induction of antibody responses in the common mucosal immune system by respiratory syncytical virus immunostimulating complexes. Med. Microbiol. Immunol. 187:191-198. [DOI] [PubMed] [Google Scholar]
- 43.Huang, Y., G. Hajishengallis, and S. M. Michalek. 2000. Construction and characterization of a Salmonella enterica serovar Typhimurium clone expressing a salivary adhesin of Streptococcus mutans under control of the anaerobically inducible nirB promoter. Infect. Immun. 68:1549-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Husseiny, M. I., and M. Hensel. 2005. Evaluation of an intracellular-activated promoter for the generation of live Salmonella recombinant vaccines. Vaccine 23:2580-2590. [DOI] [PubMed] [Google Scholar]
- 45.John, M., T. I. Crean, S. B. Calderwood, and E. T. Ryan. 2000. In vitro and in vivo analyses of constitutive and in vivo-induced promoters in attenuated vaccine and vector strains of Vibrio cholerae. Infect. Immun. 68:1171-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang, H. Y., J. Srinivasan, and R. Curtiss III. 2002. Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect. Immun. 70:1739-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly, A., et al. 2004. A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology 150:2037-2053. [DOI] [PubMed] [Google Scholar]
- 48.Khan, S., et al. 2007. Ability of SPI2 mutant of S. typhi to effectively induce antibody responses to the mucosal antigen enterotoxigenic E. coli heat labile toxin B subunit after oral delivery to humans. Vaccine 25:4175-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim, C. C., and S. Falkow. 2004. Delineation of upstream signaling events in the Salmonella pathogenicity island 2 transcriptional activation pathway. J. Bacteriol. 186:4694-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konjufca, V., S. Y. Wanda, M. C. Jenkins, and R. Curtiss III. 2006. A recombinant attenuated Salmonella enterica serovar Typhimurium vaccine encoding Eimeria acervulina antigen offers protection against E. acervulina challenge. Infect. Immun. 74:6785-6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee, A. K., C. S. Detweiler, and S. Falkow. 2000. OmpR regulates the two-component system SsrA-ssrB in Salmonella pathogenicity island 2. J. Bacteriol. 182:771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis, G. K. 2007. Live-attenuated Salmonella as a prototype vaccine vector for passenger immunogens in humans: are we there yet? Expert Rev. Vaccines 6:431-440. [DOI] [PubMed] [Google Scholar]
- 53.Li, Y., et al. 2009. Evaluation of new generation Salmonella enterica serovar Typhimurium vaccines with regulated delayed attenuation to induce immune responses against PspA. Proc. Natl. Acad. Sci. U. S. A. 106:593-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim, S., B. Kim, H. S. Choi, Y. Lee, and S. Ryu. 2006. Fis is required for proper regulation of ssaG expression in Salmonella enterica serovar Typhimurium. Microb. Pathog. 41:33-42. [DOI] [PubMed] [Google Scholar]
- 55.Linehan, S. A., A. Rytkonen, X. J. Yu, M. Liu, and D. W. Holden. 2005. SlyA regulates function of Salmonella pathogenicity island 2 (SPI-2) and expression of SPI-2-associated genes. Infect. Immun. 73:4354-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loessner, H., et al. 2007. Remote control of tumour-targeted Salmonella enterica serovar Typhimurium by the use of l-arabinose as inducer of bacterial gene expression in vivo. Cell. Microbiol. 9:1529-1537. [DOI] [PubMed] [Google Scholar]
- 57.Londono, L. P., et al. 1996. Immunisation of mice using Salmonella typhimurium expressing human papillomavirus type 16 E7 epitopes inserted into hepatitis B virus core antigen. Vaccine 14:545-552. [DOI] [PubMed] [Google Scholar]
- 58.Londono-Arcila, P., et al. 2002. Attenuated Salmonella enterica serovar Typhi expressing urease effectively immunizes mice against Helicobacter pylori challenge as part of a heterologous mucosal priming-parenteral boosting vaccination regimen. Infect. Immun. 70:5096-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu, Y. J., et al. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lucchini, S., et al. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDermott, M. R., and J. Bienenstock. 1979. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 122:1892-1898. [PubMed] [Google Scholar]
- 62.McKelvie, N. D., et al. 2004. Expression of heterologous antigens in Salmonella typhimurium vaccine vectors using the in vivo-inducible, SPI-2 promoter, ssaG. Vaccine 22:3243-3255. [DOI] [PubMed] [Google Scholar]
- 63.McSorley, S. J., D. Xu, and F. Y. Liew. 1997. Vaccine efficacy of Salmonella strains expressing glycoprotein 63 with different promoters. Infect. Immun. 65:171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller, V. L., K. B. Beer, W. P. Loomis, J. A. Olson, and S. I. Miller. 1992. An unusual pagC::TnphoA mutation leads to an invasion- and virulence-defective phenotype in salmonellae. Infect. Immun. 60:3763-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mollenkopf, H., G. Dietrich, and S. H. Kaufmann. 2001. Intracellular bacteria as targets and carriers for vaccination. Biol. Chem. 382:521-532. [DOI] [PubMed] [Google Scholar]
- 66.Nakayama, K., S. M. Kelly, and R. Curtiss III. 1988. Construction of an Asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Nat. Biotechnol. 6:693-697. [Google Scholar]
- 67.Navarre, W. W., et al. 2005. Co-regulation of Salmonella enterica genes required for virulence and resistance to antimicrobial peptides by SlyA and PhoP/PhoQ. Mol. Microbiol. 56:492-508. [DOI] [PubMed] [Google Scholar]
- 68.Nordstrom, K., and B. E. Uhlin. 1992. Runaway-replication plasmids as tools to produce large quantities of proteins from cloned genes in bacteria. Nat. Biotechnol. 10:661-666. [DOI] [PubMed] [Google Scholar]
- 69.Orr, N., J. E. Galen, and M. M. Levine. 2001. Novel use of anaerobically induced promoter, dmsA, for controlled expression of fragment C of tetanus toxin in live attenuated Salmonella enterica serovar Typhi strain CVD 908-htrA. Vaccine 19:1694-1700. [DOI] [PubMed] [Google Scholar]
- 70.Paccez, J. D., et al. 2006. Stable episomal expression system under control of a stress inducible promoter enhances the immunogenicity of Bacillus subtilis as a vector for antigen delivery. Vaccine 24:2935-2943. [DOI] [PubMed] [Google Scholar]
- 71.Pascual, D. W., et al. 1999. Expression of recombinant enterotoxigenic Escherichia coli colonization factor antigen I by Salmonella typhimurium elicits a biphasic T helper cell response. Infect. Immun. 67:6249-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pashine, A., B. John, S. Rath, A. George, and V. Bal. 1999. Th1 dominance in the immune response to live Salmonella typhimurium requires bacterial invasiveness but not persistence. Int. Immunol. 11:481-489. [DOI] [PubMed] [Google Scholar]
- 73.Poirier, K., et al. 2008. Escherichia coli O157:H7 survives within human macrophages: global gene expression profile and involvement of the Shiga toxins. Infect. Immun. 76:4814-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pulkkinen, W. S., and S. I. Miller. 1991. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J. Bacteriol. 173:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramarathinam, L., D. W. Niesel, and G. R. Klimpel. 1993. Salmonella typhimurium induces IFN-γ production in murine splenocytes. Role of natural killer cells and macrophages. J. Immunol. 150:3973-3981. [PubMed] [Google Scholar]
- 76.Rathman, M., M. D. Sjaastad, and S. Falkow. 1996. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect. Immun. 64:2765-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roberts, M., J. Li, A. Bacon, and S. Chatfield. 1998. Oral vaccination against tetanus: comparison of the immunogenicities of Salmonella strains expressing fragment C from the nirB and htrA promoters. Infect. Immun. 66:3080-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schödel, F., S. M. Kelly, D. L. Peterson, D. R. Milich, and R. Curtiss III. 1994. Hybrid hepatitis B virus core-pre-S proteins synthesized in avirulent Salmonella typhimurium and Salmonella typhi for oral vaccination. Infect. Immun. 62:1669-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sirard, J. C., M. Weber, E. Duflot, M. R. Popoff, and M. Mock. 1997. A recombinant Bacillus anthracis strain producing the Clostridium perfringens Ib component induces protection against iota toxins. Infect. Immun. 65:2029-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spreng, S., G. Dietrich, and G. Weidinger. 2006. Rational design of Salmonella-based vaccination strategies. Methods 38:133-143. [DOI] [PubMed] [Google Scholar]
- 81.Stocker, B. A. 2000. Aromatic-dependent salmonella as anti-bacterial vaccines and as presenters of heterologous antigens or of DNA encoding them. J. Biotechnol. 83:45-50. [DOI] [PubMed] [Google Scholar]
- 82.Stratford, R., et al. 2005. Optimization of Salmonella enterica serovar Typhi ΔaroC ΔssaV derivatives as vehicles for delivering heterologous antigens by chromosomal integration and in vivo inducible promoters. Infect. Immun. 73:362-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tacket, C. O., et al. 2000. Safety and immune responses to attenuated Salmonella enterica serovar Typhi oral live vector vaccines expressing tetanus toxin fragment C. Clin. Immunol. 97:146-153. [DOI] [PubMed] [Google Scholar]
- 84.Terry, T. D., J. E. Downes, S. J. Dowideit, A. N. Mabbett, and M. P. Jennings. 2005. Investigation of ansB and sspA derived promoters for multi- and single-copy antigen expression in attenuated Salmonella enterica var. Typhimurium. Vaccine 23:4521-4531. [DOI] [PubMed] [Google Scholar]
- 85.Torres-Escobar, A., et al. 2010. Fine-tuning synthesis of Yersinia pestis LcrV from runaway-like replication balanced-lethal plasmid in a Salmonella enterica serovar Typhimurium vaccine induces protection against a lethal Y. pestis challenge in mice. Infect. Immun. 78:2529-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tzschaschel, B. D., C. A. Guzmán, K. N. Timmis, and V. de Lorenzo. 1996. An Escherichia coli hemolysin transport system-based vector for the export of polypeptides: export of Shiga-like toxin IIeB subunit by Salmonella typhimurium aroA. Nat. Biotechnol. 14:765-769. [DOI] [PubMed] [Google Scholar]
- 87.Valdivia, R. H., and S. Falkow. 1997. Fluorescence-based isolation of bacterial genes expressed within host cells. Science 277:2007-2011. [DOI] [PubMed] [Google Scholar]
- 88.Van Gerven, N., V. Derous, and J. P. Hernalsteens. 2008. Expression of in vivo-inducible Salmonella enterica promoters during infection of Caenorhabditis elegans. FEMS Microbiol. Lett. 278:236-241. [DOI] [PubMed] [Google Scholar]
- 89.Walthers, D., et al. 2007. The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol. Microbiol. 65:477-493. [DOI] [PubMed] [Google Scholar]
- 90.Wang, J., V. Michel, C. Leclerc, M. Hofnung, and A. Charbit. 1999. Immunogenicity of viral B-cell epitopes inserted into two surface loops of the Escherichia coli K-12 LamB protein and expressed in an attenuated aroA strain of Salmonella typhimurium. Vaccine 17:1-12. [DOI] [PubMed] [Google Scholar]
- 91.Wang, S., et al. 2010. Salmonella vaccine vectors displaying delayed antigen synthesis in vivo to enhance immunogenicity. Infect. Immun. 78:3969-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang, S., et al. 2010. Immune responses to recombinant pneumococcal PsaA antigen delivered by a live attenuated Salmonella vaccine. Infect. Immun. 78:3258-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weiss, S., and T. Chakraborty. 2001. Transfer of eukaryotic expression plasmids to mammalian host cells by bacterial carriers. Curr. Opin. Biotechnol. 12:467-472. [DOI] [PubMed] [Google Scholar]
- 94.Xu, D., et al. 1998. Protective effect on Leishmania major infection of migration inhibitory factor, TNF-α, and IFN-γ administered orally via attenuated Salmonella typhimurium. J. Immunol. 160:1285-1289. [PubMed] [Google Scholar]
- 95.Xu, X., M. I. Husseiny, A. Goldwich, and M. Hensel. 2010. Efficacy of intracellular activated promoters for generation of Salmonella-based vaccines. Infect. Immun. 78:4828-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang, X., et al. 2007. Oral vaccination with Salmonella simultaneously expressing Yersinia pestis F1 and V antigens protects against bubonic and pneumonic plague. J. Immunol. 178:1059-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]