Abstract
The eradication of smallpox (variola) and the subsequent cessation of routine vaccination have left modern society vulnerable to bioterrorism employing this devastating contagious disease. The existing, licensed vaccines based on live vaccinia virus (VACV) are contraindicated for a substantial number of people, and prophylactic vaccination of large populations is not reasonable when there is little risk of exposure. Consequently, there is an emerging need to develop efficient and safe therapeutics to be used shortly before or after exposure, either alone or in combination with vaccination. We have characterized the human antibody response to smallpox vaccine (VACV Lister) in immunized volunteers and isolated a large number of VACV-specific antibodies that recognize a variety of different VACV antigens. Using this broad antibody panel, we have generated a fully human, recombinant analogue to plasma-derived vaccinia immunoglobulin (VIG), which mirrors the diversity and specificity of the human antibody immune response and offers the advantage of unlimited supply and reproducible specificity and activity. The recombinant VIG was found to display a high specific binding activity toward VACV antigens, potent in vitro VACV neutralizing activity, and a highly protective efficacy against VACV challenge in the mouse tail lesion model when given either prophylactically or therapeutically. Altogether, the results suggest that this compound has the potential to be used as an effective postexposure prophylaxis or treatment of disease caused by orthopoxviruses.
Although smallpox (variola) was eradicated over 30 years ago by a worldwide vaccination campaign, the threat of bioterrorism has reintroduced this deadly and highly contagious disease as a serious hazard to public health and notably to today's unvaccinated inhabitants of crowded urban settings. Prophylactic vaccination employing the smallpox-related vaccinia virus (VACV) is associated with rare, but potentially life-threatening, adverse events (27), and unfortunately vaccination is contraindicated for people (and their household contacts) with compromised immune systems or skin conditions (such as eczema, dermatitis, and varicella), pregnant women, infants, and those receiving immunosuppressive medicines. Complications due to VACV vaccination may be treated with plasma-derived vaccinia immunoglobulin (VIG) isolated from vaccinated donors. However, since only a small fraction of the injected immunoglobulin targets the antigens of interest, large injection volumes are required, and it is therefore probably not realistic to use plasma-derived VIG in treating a generalized smallpox outbreak. Furthermore, since prophylactic vaccination of large populations is not reasonable when there is little risk of exposure, the urgent concerns over the implications of an accidental or intentional release of smallpox and also the possible outbreak of zoonotic poxvirus diseases such as monkeypox have led to a renewed interest in investigating antiviral treatment options and in understanding the humoral immune response to virus exposure.
Variola virus, which is the causative agent of smallpox, VACV, and monkeypox virus all belong to the Orthopoxvirus genus of the Poxviridae family. Characteristically, these viruses are large (approximately 200-kb genome) and have a complex mode of assembly and appearance, including multiple viral membranes and surface proteins with various functions. In addition, orthopoxviruses have two types of infectious virions: intracellular mature virions (IMV) and extracellular enveloped virions (EEV). The IMV are assembled in the cytoplasm and consist of a virally encoded membrane surrounding a core particle containing the genome. The IMV can either be released from the infected cell by cellular lysis or be further processed by wrapping of virus particles in a host-derived membrane to generate EEV. Each type of virions has distinct functions, with IMV being involved in transmission between hosts and EEV thought to be primarily involved in dissemination within the host (54). Neutralizing antibodies mainly exert their effect by recognizing surface proteins expressed on the outer virion membranes. These proteins are unique to either IMV or EEV, and the two virion types thus present different sets of targets to the humoral defense (15, 54). When the present study was initiated in 2004, animal studies had identified neutralizing antibodies against five VACV IMV-specific antigens (L1R, A27L, A17L, H3L, and D8L) and two EEV-specific antigens (B5R and A33R) (reviewed in reference 2). Although the exact biological function of these proteins remains unclear, essential functions during virion assembly and virus entry have been assigned to specific proteins (15, 54).
Early attempts to use inactivated VACV preparations composed largely of IMV for vaccination resulted in poor protection and led to the conclusion that a neutralizing antibody response to VACV needs to comprise antibodies to both viral particle types (6). More recent studies in animal models have confirmed that although some protection against virus challenge can be obtained with single-protein vaccination or antibodies directed against individual IMV or EEV surface antigens, the best protection is afforded when a combinatory approach targeting both IMV and EEV is employed (24, 30, 31, 38, 46). Lustig et al. have provided one explanation for the improved protective effect of combinations of antibodies to the two virion types as they have shown that IgG against the A33R EEV surface protein, which was not in vitro neutralizing on its own, was capable of eliciting complement-mediated lysis of the outer EEV membrane, thereby making the inner IMV particle susceptible to a neutralizing L1R-specific antibody (47). Recently, other protective mechanisms involving complement, such as direct neutralization through coating of the virion surface and complement-dependent lysis of VACV-infected cells, have been demonstrated for B5R-specific antibodies (4). The neutralization in this case did not involve virion lysis and did not require the presence of a neutralizing IMV-specific antibody (4), thus further illustrating the complex nature of antibody-mediated protection against VACV.
Altogether, given the complexity of VACV, which includes two types of infectious virions and a multitude of antigens, it seems clear that the optimal therapeutic antibody against VACV should not be limited to a single antigen but, rather, should consist of a combination of specificities. Antibody therapeutics targeting multiple epitopes, e.g., bispecific antibodies or cocktails consisting of two or more separately produced antibodies, are increasingly being developed to raise the therapeutic effectiveness when large and complex entities are targeted (3, 55). Such antibody mixtures may constitute entirely new treatment modalities but may also replace existing plasma-derived immunoglobulin products, which, as already noted, suffer certain drawbacks that make them a less attractive therapeutic option. In contrast, target-specific recombinant polyclonal antibodies offer the advantage of unlimited supply, high and reproducible specificity and activity, and an improved safety profile (8).
In this study, we have characterized the antibody response to smallpox vaccine (VACV Lister) in immunized human volunteers and employed the Symplex technology (48, 49) to isolate a large number of VACV-specific antibodies recognizing a variety of different VACV antigens. Using this broad panel of antibodies, we have generated a fully human recombinant VIG that mirrors the diversity and specificity of the human antibody immune response and has the potential to be used as an effective postexposure prophylaxis or treatment of disease caused by orthopoxviruses.
MATERIALS AND METHODS
Blood donors.
Twelve voluntary blood donors were recruited from a smallpox vaccination program of British first-responder health care workers. The participants in this study gave signed informed consent, and approval was obtained from the Salisbury Research Ethics Committee (United Kingdom) and the Regional Research Ethics Committee of Copenhagen (Denmark) prior to initiation of the study. The blood donors included both first-time vaccinees and revaccinees who received dried smallpox vaccine of the VACV Lister strain (batch 347; National Collection of Pathogenic Viruses, Health Protection Agency, Centre for Emergency Preparedness and Response, Porton Down, United Kingdom), and blood was drawn in the range of 9 to 20 days after vaccination.
Viruses, antigens, and reagents.
Live VACV of the NYCBOH and IHD-W strains was obtained from the ATCC (VR-1536 and VR-1441, respectively), and the Lister strain was provided by the National Collection of Pathogenic Viruses, United Kingdom. Purified VACV particles of the Lister and IHD-W strains inactivated by the UV-psoralen method (Advanced Biotechnologies, Columbia, MD) were used for enzyme-linked immunospot assay (ELISPOT) and binding assays. Similarly inactivated particles of the IHD-J strain were kindly provided by Grant McFadden (University of Western Ontario, London, Ontario, Canada).
Recombinant VACV proteins were produced in the baculovirus expression system. B5R and VACV complement protein (VCP or C3L; both Lister strain) were obtained from Qbiogene (Illkirch, France) and A27L, A33R, and L1R (all WR strain) were from the University of Pennsylvania (Philadelphia, PA).
In vitro translated proteins (e.g., A10L, A56R, D8L, and H3L) were obtained by PCR amplification of the individual genes from genomic VACV Lister DNA, followed by expression using a TNT T7 Quick for PCR DNA coupled transcription/translation system (Promega, Madison, WI) with Transcend biotinylated tRNA according to the manufacturer's instructions.
Vaccinia immune globulin intravenous (human) ([VIG] lot number 17304043; Cangene, Winnipeg, MB, Canada) was obtained from Statens Serum Institut (Copenhagen, Denmark).
Characterization of the donor VACV-specific immune response.
Following isolation of peripheral blood mononuclear cells (PBMC) from donor blood using Lymphoprep (Axis-Shield, Oslo, Norway), the CD19+ B cell population was purified using magnetic cell sorting (MACS) as described by Meijer et al. (48). Serum (diluted 1:2 with phosphate-buffered saline [PBS]) was also collected and stored at −20°C. The frequency of plasma cells producing VACV-specific antibodies was determined using an ELISPOT assay. Briefly, MACS-purified CD19+ cells (1 × 105 cells per well) were added to nitrocellulose-bottomed (HA filter type) plates (Millipore, Bedford, MA) coated with a solution of 20 μg/ml inactivated VACV particles. Following incubation and washing, immobilized vaccinia virus-specific human IgG and IgA were detected separately using horseradish peroxidase (HRP)-conjugated anti-human IgG (CalTag, Burlingame, CA) and HRP-conjugated anti-human IgA (AbD Serotec, Oxford, United Kingdom). The plates were developed as described previously (48), and the number of spots was counted manually using a stereomicroscope.
The serum IgG response against VACV particles and recombinant antigens was characterized by enzyme-linked immunosorbent assay (ELISA). Ninety-six-well ELISA plates (MaxiSorp; Nunc A/S, Roskilde, Denmark) were coated with antigen, blocked, and washed according to standard protocols. Following incubation with a dilution series of the serum samples and additional washing, bound IgG was detected using HRP-conjugated anti-human IgG (CalTag), and the plates were developed as described before (48). Each serum sample was assayed in duplicate against each antigen on two separate plates. Endpoint titers were determined as the reciprocal dilution giving a signal 4-fold above background by interpolation from the titration curves.
Generation of antibody repertoires.
Plasma cells (PC) were selected from the CD19+ cell population by fluorescence-activated cell sorting (FACS) based on the expression profile of CD19, CD38, and CD45 cell surface proteins as described by Meijer et al. (48). The resulting cell population was single-cell sorted into 96-well PCR plates containing PCR buffer (Phusion HF buffer; Finnzymes, Espoo, Finland), primers for reverse transcription-PCR (RT-PCR) (49), and RNase inhibitor (RNasin; Promega). Antibody repertoires were generated from the single-cell-sorted PC using the Symplex PCR (48). Cells from different blood donors were processed separately, and the resulting antibody repertoires were also kept separate. The overall Symplex PCR procedure was performed as described elsewhere (49), except for the use of a different enzyme combination in the multiplex overlap extension RT-PCR. The following was added to each well to obtain the given final concentration: deoxynucleoside triphosphate (dNTP) mix (200 μM each), RNasin (20 U/μl), Sensiscript reverse transcriptase (diluted 1:320; Qiagen, Hilden, Germany), and Phusion DNA polymerase (0.02 U/μl; Finnzymes). Following reverse transcription for 1 h at 37°C, antibody genes were amplified and linked using the following PCR cycle: initial denaturation at 98°C for 30 s; 30 cycles of 98°C for 20 s, 60°C for 30 s, and 72°C for 45 s; final extension at 72°C for 45 s. Following further amplification of the multiplex overlap extension PCR products in a nested PCR (49), the linked antibody genes were cloned into the Fab expression vector pJSK301 (48, 49). The generated repertoire of expression vectors was propagated in Escherichia coli (TOP10), and individual bacterial colonies were arrayed in 384-well master plates and stored as glycerol stocks.
Screening of cloned repertoires.
E. coli expression of Fab fragments was performed in 384 deep-well plates essentially as described previously (48). The Fab-containing supernatants were cleared by centrifugation and used for screening of individual clones in a bead-based fluorescence-linked immunosorbent assay (FLISA) (56). Briefly, inactivated virus particles of the Lister strain, the IHD-W strain, and the recombinant protein antigens B5R, VCP, and A33R were immobilized individually on polystyrene beads (6.79-μm diameter; Spherotech Inc., Lake Forest, IL) by incubating 16.5 μg of protein or 20 μg of virus particles with 100 μl of 5% (wt/vol) beads. The coated beads, goat anti-human IgG Alexa Fluor 647 conjugate (Molecular Probes/Invitrogen, Carlsbad, CA), and supernatant containing Fab fragments were mixed in assay-compatible 384-well plates (Nunc 142761), and the fluorescence at the bead surface in individual wells was recorded using an 8200 Cellular Detection System (Applied Biosystems, Foster City, CA). Cutoff was set at 50 counts (fluorescent beads) during the primary screening in order to identify as many clones reactive with viral particles or antigens as possible. The expressed Fab fragment repertoires were screened against all five populations of coated beads.
Clones that were positive in the primary screening were retrieved from the original master plates and reexpressed in 96-well plates and tested in a secondary screening against the same antigens by both FLISA and standard ELISA (48).
Sequence analysis.
Plasmids encoding clones that were identified as positive in the secondary screening were subjected to DNA sequencing (MWG Biotech, Ebersberg, Germany), and the obtained sequences were clustered based on sequence homology. Immunoglobulin variable (V), diversity (D), and joining (J) gene segment usage was determined for each sequence by comparison to germ line sequences from the ImMunoGeneTics (IMGT [www.imgt.org]) reference directory (44). Antibody clones that share V and J segment usage, complementarity determining region 3 (CDR3) lengths in the heavy chain, and consensus sequences in the CDR3 of both the heavy and light chains (CDRH3 and CDRL3, respectively) were assigned to the same clonotype.
Expression of full-length recombinant human antibodies.
Selected antibody heavy and light V gene pairs were transferred to a mammalian antibody expression vector, described in Meijer et al. (49), to allow for production of full-length IgG1 molecules. In the process of transferring the genes, mutations introduced by cross-priming during the Symplex PCR were also corrected. The genes encoding the V genes of an antibody of interest were individually PCR amplified with a primer set containing sequences corresponding to the identified variable germ line genes, thereby correcting any changes introduced by cross-priming. The resulting products were digested and sequentially inserted into the mammalian expression vector using conventional ligation procedures (57). A FastStart High Fidelity PCR system (Roche, Penzberg, Germany) was used for the amplification of the genes. All restriction enzymes and the T4 DNA ligase were obtained from New England BioLabs (Ipswich, MA).
The resulting constructs were individually transfected into a subclone of the CHO Flp-In cell line (Invitrogen), CHO019 (57). Following selection of stable cell lines in the presence of Geneticin (Invitrogen), each cell line was adapted to suspension culture in serum-free EX-CELL 302 medium (Sigma, St. Louis, MO), expanded, and cryopreserved at −150°C. Twenty-six of the individual cell lines (expressing antibody clones 029, 037, 058, 086, 147, 186, 188, 195, 197, 203, 211, 229, 235, 286, 295, 303, 339, 461, 482, 488, 526, 551, 586, 589, 607, and 633) were selected for the generation of a polyclonal cell line, and batches of recombinant polyclonal VACV-specific antibody were produced in 5-liter bioreactors (B. Braun Biotech International, Melsungen, Germany), essentially as described previously (57). Each cell line was also cultured in shaker flasks for production of the individual antibodies. The monoclonal antibodies (MAbs) and polyclonal antibodies were individually purified from the culture supernatants by protein A affinity chromatography and analyzed by SDS-PAGE and cation exchange chromatography (57).
Assessment of antibody reactivity.
Following identification of unique antibody clones, large-scale batches of Fab fragments of each clone were prepared for final confirmation of the reactivity with inactivated virus particles of the Lister, IHD-W, and IHD-J strains and the recombinant antigens A27L, A33R, B5R, L1R, and VCP by ELISA as outlined in previous sections. The subsequently produced full-length antibodies were similarly analyzed.
Antigen specificities of the individual antibody clones were further analyzed by Western blotting using acetone-precipitated VACV (Lister) particles, which subsequently were dissolved in SDS loading buffer containing 8 M urea to obtain optimal resolution of the viral proteins. The proteins were separated on NuPAGE Bis-Tris gels (4 to 12% or 10%; Invitrogen) and transferred onto polyvinylidene difluoride (PVDF) membranes (Invitrogen). After a blocking step, the Western blots were probed with purified VACV-specific MAbs and HRP-conjugated anti-human IgG (CalTag) and developed using the SuperSignal West Femto Maximum Sensitivity Chemiluminescent Substrate (Pierce, Rockford, IL) according to the manufacturer's instructions.
A number of antibodies were also tested for reactivity with in vitro translated VACV antigens. Purified antibodies were immobilized in ELISA plates at 1 μg/ml and incubated with dilutions of the different in vitro translated antigens. Bound antigen was detected by streptavidin-peroxidase polymer (Sigma), and the plates were developed as described above.
A panel of purified MAbs (029, 037, 058, 086, 147, 186, 188, 195, 197, 203, 211, 229, 235, 286, 295, 303, 339, 461, 482, 488, 526, 551, 586, 589, 607, and 633) was also tested for reactivity with VACV (WR strain) proteome arrays (Vaccinia Chip 9, versions 1,2, and 10b; Antigen Discovery, Irvine, CA). The arrays contained VACV proteins produced in a cell-free transcription and translation system, with or without disulfide bridge formation (20), and the 10b array also contained purified proteins produced in E. coli or mammalian cells. Each MAb underwent duplicate analyses on the arrays. The data were averaged for each sample, and the average negative-control values (plus 10 standard deviations) were subtracted.
Affinity measurements.
The affinities of selected antibody clones were determined by surface plasmon resonance using a Biacore 2000 (Biacore AB, Uppsala, Sweden). The antigens (B5R, VCP, A33R, and A27L) were immobilized individually on a CM5 chip surface using standard amine coupling chemistry to a level resulting in a maximum resonance unit (RUmax) value of approximately 100 RU or less. Purified antibody Fab fragments prepared by papain cleavage (ImmunoPure Fab preparation Kit; Pierce) were diluted serially in HBS-EP (10 mM HEPES [pH 7.4], 150 mM NaCl, 3 mM EDTA, 0.005% P20 surfactant) running-buffer (Biacore AB) and passed over the chip at 10 to 50 μl/min. Binding kinetics and affinity constants (KD) were determined using the BIAevaluation software (Biacore AB) by global fitting of four to six different concentrations passed over the same sensor surface.
Preparation of VACV for use in vitro and in vivo.
Infectious VACV particles were added to subconfluent B-SC-1 cells in serum-free Earle's minimal essential medium (MEM; Invitrogen), and the virus was allowed to adsorb for 45 min at 37°C. Following addition of MEM containing 2% (vol/vol) fetal calf serum (2% MEM), the cells were incubated at 37°C until they showed full cytopathic effect. The supernatant was discarded, and the cells were detached into PBS, centrifuged, resuspended in PBS, and frozen at −80°C. After samples were thawed, the cell debris was removed by centrifugation, and aliquots of the supernatant were stored at −80°C. The titer was determined by a plaque reduction assay (see below).
Plaque reduction and neutralization assay.
For the evaluation of in vitro neutralizing activity, antibodies were diluted in serum-free MEM and preincubated for 1 h at 37°C with 1 × 104 PFU of Lister strain virus particles. The mixture was applied to a monolayer of Vero cells preseeded in 24-well plates, which were incubated for 4 h at 37°C in 5% CO2. The infected cells were overlaid with 2% MEM containing 2% carboxymethylcellulose, followed by incubation for 3 days at 37°C in 5% CO2. The cells were fixed by incubation with 10% formalin in PBS and stained using 20% ethanol containing 0.1% crystal violet, and the number of plaques in each well was counted.
Tail lesion model.
The mouse tail lesion model (7) was used to evaluate the in vivo protective activity of the recombinant VIG. All experiments were performed according to the regulations of the United Kingdom Home Office Animals (Scientific Procedures) Act (1986).
Female BALB/c, 6- to 8-week-old mice (Health Protection Agency) were challenged with infectious VACV particles of either the Lister strain (2 × 106 PFU) or the NYCBOH strain (5 × 105 PFU) by injection into the tail vein. The virus challenge doses were determined in separate sighting studies as the number of PFU required to produce 30 to 40 lesions per tail in untreated mice. Twenty-four hours before or after challenge, groups of eight mice were treated intramuscularly with different amounts of recombinant VIG or plasma-derived VIG (Cangene) or with 300 μg of a negative-control antibody (anti-rhesus D recombinant polyclonal antibody [57]) in 500 μl of PBS. One group of mice was treated intramuscularly with 2.5 mg of cidofovir (Vistide; Gilead Sciences, Inc., Foster City, CA) as a positive control. Seven days after virus challenge, the lesions on each tail were counted in a blinded fashion, and the number of lesions in the groups treated with recombinant or plasma-derived VIG or with cidofovir was expressed as a percentage of the lesions in the mice treated with the negative-control antibody, which had no effect on the number of tail lesions. Statistical significance was determined using a homoscedastic Student's t test.
Nucleotide sequence accession numbers.
Complete V region sequences of a representative antibody clone from each clonotype can be found in GenBank under accession numbers HQ378325 to HQ378502.
RESULTS
Characterization of the immune response in smallpox vaccinees.
To characterize the human immune response to VACV and select the optimal starting material for isolation of VACV-specific antibodies, the blood samples obtained from the smallpox vaccinees were analyzed in terms of frequencies of plasma cells (PC) producing VACV-specific antibodies and serum antibody levels to VACV antigens.
Circulating PC were selected from the CD19+ B cell population by FACS (48), and the percentages of PC secreting VACV-specific antibodies were estimated by ELISPOT assay. Most of the vaccinees had a clearly detectable frequency of VACV-specific PC, and the revaccinees had in general the highest frequencies, reaching up to 0.6% of the total number of PC (Table 1). A detailed analysis of the kinetics of the B cell response was not possible since the majority of the blood samples were, for logistical reasons, obtained 14 or 15 days after the vaccination. However, the only sample that was obtained earlier (donor identification number [ID] 11; 9 days after vaccination) also had the highest frequency of VACV-specific IgG-secreting PC (Table 1), thus suggesting that the B cell response induced by smallpox vaccination peaked before the time point where most of the blood samples were obtained.
TABLE 1.
Overview of the vaccinated donors, their immune responses, and the cloned antibody repertoires
| Donor ID | Previous vaccination(s) (yr) | Response period (days) | % PC of CD19+ cells | % VACV-specific PC |
Estimated repertoire size (no. of PCR products) | No. of confirmed VACV-specific antibodies | |
|---|---|---|---|---|---|---|---|
| IgG | IgA | ||||||
| 01 | 1950, 1970 | 14 | 12 | 0.40 | 0.20 | 600 | 10 |
| 02 | 1966, 1979 | 14 | 2.6 | 0.32 | 0.15 | 400 | 9 |
| 03 | 20 | 1.7 | 0.21 | 0.03 | |||
| 04 | 18 | 2.5 | 0.13 | 0 | |||
| 05 | 1978-1979 | 14 | 2.4 | 0.25 | 0.06 | 1,200 | 18 |
| 07 | 1956-1958 | 15 | NDb | ND | ND | ||
| 08 | Yesa | 15 | 15 | ND | ND | 300 | 14 |
| 09 | 14 | 20 | 0.07 | 0.03 | |||
| 10 | 14 | 2.0 | 0.03 | 0 | |||
| 11 | 1966 | 9 | 7.2 | 0.47 | 0.01 | 1,050 | 38 |
Date of previous vaccination(s) unknown.
ND, not determined.
The serum antibody titers against inactivated viral particles from three virus strains (Lister, IHD-W, and IHD-J) and five recombinant antigens (B5R, VCP, A27L, A33R, and L1R) were determined by standard ELISA. The Lister strain was commonly used in Western Europe during the WHO smallpox eradication campaign (23) and was also used for vaccination in this study. Both the IHD-J and IHD-W strains are derived from the NYCBOH strain (www.atcc.org), which was used in the United States during the eradication of smallpox (23).
The serum response against the different antigens varied greatly both between and within individual donors, likely a reflection of the differences in vaccination responsiveness, response period, vaccination history, and immunogenicity of the different antigens. Four out of the six previously vaccinated donors (IDs 01, 02, 07, and 08) exhibited strong serum responses against both VACV particles and recombinant antigens, whereas the other two donors (IDs 05 and 11) had low or almost undetectable titers against all antigens (Fig. 1). As already mentioned, donor 11 also had the shortest response period, i.e., 9 days, which may explain its poor serum response. The antibody response in revaccinees has been reported to increase rapidly from day 6 to 8 until it peaks around day 12 to 15 after vaccination (26).
FIG. 1.
The natural antibody response to smallpox vaccination is highly variable. Serum reactivity with VACV particles (strains Lister, IHD-J, and IHD-W) and recombinant antigens (A27L, A33R, B5R, L1R, and VCP) was determined in 10 human smallpox vaccinees by ELISA. The endpoint titer corresponds to the reciprocal dilution giving a signal 4-fold above background. All determinations were performed in duplicate, and the results are presented as means ± standard errors of the means.
Due to the presence of multiple targets for antibodies on the VACV particles, the response was expected to be stronger against these than against the single recombinant antigens. This was indeed the case for the IHD-J strain particles but not for the other two virus strains. A part of the observed difference may naturally be attributed to differences in coating efficiency and uncertainties in the concentration determination, but there may also be differences in the recognition of dominant antigens, in particular for the IHD-W particles, which were poorly recognized by all sera. IHD-J has been shown to have an increased EEV particle production (51), whereas IHD-W is a hemagglutinin (A56R)-negative strain derived from the IHD-J strain (17). In agreement with previous studies, the serum response to L1R was very weak, or nearly undetectable, in all donors (5, 43, 52).
Generation and screening of antibody repertoires.
Based on the serum reactivity, the ELISPOT analysis, and the expectation that the recall antibody response generated by repeated vaccination will yield antibodies of higher affinity, it was decided to use cells from five of the revaccinees as the starting material for the generation of antibody repertoires. The protocol used for this study is based on the capture of immunoglobulin V genes from single B cells that have a high expression of antibody (i.e., PC). Since donors 01, 02, 05, and 11 had the highest frequencies of VACV-specific PC (Table 1), they constituted the prime candidates. Cells from donor 08 could not be analyzed by ELISPOT due to the small amount of blood obtained, but based on the high overall frequency of PC in the CD19+ cell population and the vaccination history (Table 1), this donor was also included.
The nucleic acids encoding the antibody repertoires were isolated from the single-cell-sorted PC by the Symplex PCR, which creates a physical link between the heavy V region gene fragment and the full-length light chain (48, 49). The protocol was designed to amplify antibody genes of all human immunoglobulin heavy chain and kappa light chain V region gene families by the use of two primer sets, one for heavy V region (IgG and IgA isotypes) amplification and one for kappa light-chain amplification. Using approximately 5,000 cells from each donor, repertoires of between 300 and 1,200 overlap products were generated by the multiplex overlap extension RT-PCR (Table 1). The size of the repertoires was estimated by analyzing a representative number of reactions on agarose gels (49) (data not shown). The overlap products from each donor were pooled separately and inserted into a Fab expression vector for production and screening of the individual antibody specificities.
Approximately three times as many antibody-expressing clones as the estimated number of input PCR products (Table 1) were arrayed and screened on different VACV antigens, thus giving 95% likelihood for the representation of all unique V gene pairs amplified by the Symplex PCR (14). The large number of hits obtained in the screening of the five repertoires was retested against the same VACV antigens to verify reactivities, and approximately 700 clones were subjected to sequencing and clustering according to sequence similarity. A group of clones that shared V and J segment usage in both the heavy and light V region, heavy-chain CDR3 length, and consensus sequences in both CDRH3 and CDRL3 were assigned to the same cluster or clonotype. The number of clones assigned to a clonotype varied from a single clone to more than 30 in the case of one of the A33R-specific clonotypes (Table 2 ). As shown in Fig. 2 and Table 2, the isolated repertoire of VACV-specific antibodies displays a high degree of genetic diversity. In fact, all human immunoglobulin heavy-chain variable region (IGHV) gene families, as well as the immunoglobulin kappa light-chain variable region (IGKV) gene families 1 to 4 (44; IMGT [www.imgt.org]), were found among the confirmed VACV-specific clones. Genes belonging to the IGKV5 and IGKV6 families, which were not found among the confirmed clones, are only rarely expressed (25). The heavy-chain V gene usage correlates well with what has been found previously in human antibody repertoires (9, 22, 33), with a frequent usage of the IGHV3, IGHV1, and IGHV4 families. The light-chain V gene usage was dominated by the IGKV1 family, mainly due to the frequent usage of the IGKV1-39 genes (Fig. 2 and Table 2). These genes are also among the most frequently used light-chain V genes in the human immunoglobulin repertoire (16, 22, 25). As a whole, the IGKV1 genes made up 75% of the repertoire. The only other IGKV gene of prominent usage in the isolated repertoire was IGKV3-20, which also is the single most frequently expressed light-chain gene in humans (22). The observed genetic diversity is indicative of an unbiased recovery of V genes by the Symplex PCR and suggests that the isolated antibody repertoire mirrors the diversity of the natural humoral response to VACV in humans.
TABLE 2.
Summary of the genetic origin and reactivity of the 89 confirmed VACV-specific antibodies
| Antibody clone | IGHV gene | CDRH3 sequence | IGKV gene | CDRL3 sequence | No. of clones in cluster | Specificitya |
|---|---|---|---|---|---|---|
| 029 | 1-24 | CATDVWRRTPEGGTDWF-------DPW | 3-11 | CLQRSNWP-ITF | 12 | H3L |
| 031 | 4-34 | CAGSRSFDLLTAYDLFHRKGNAM-DVW | 1-39 | CQQSYRTL-YTF | 6 | (H3L) |
| 037 | 6-1 | CARGDVLRYF--------------DYW | 1-5 | CQQYNGYSEVTF | 4 | WR148 |
| 058 | 3-30 | CAKGGLGTNEF-------------DHW | 1-5 | CQQYDTYP-ITF | 2 | WR148 |
| 086 | 5-51 | CATLPRYDAYGARIR---------DYW | 1-33 | CQQYDNLP-PTF | 16 | I1L |
| 089 | 1-2 | CARYCSSPTCS-------------IVW | 3-20 | CQQYGRSP-LTF | 2 | (WR148) |
| 112 | 2-5 | CAHSSQRVVTGL------------DFW | 1-39 | CQQSYNTP-ATF | 10 | A27L |
| 113 | 2-70 | CARIRTCYPDLYGDYNDAF-----DIW | 1-33 | CQQYENVP-YTF | 2 | VCP |
| 147 | 3-7 | CARAADYGDY--------------VRP | 2-28 | CMQALQIP-RTF | 8 | H5R |
| 156 | 3-30 | CAKHVAAGGTL-------------DYW | 2-24 | CMQTTHIP-HTF | 3 | (A14L) |
| 159 | 1-46 | CARVIRKYYTSSNSYLTEQAF---DIW | 1-39 | CQQTYGNP-LTF | 9 | A27L |
| 160 | 3-9 | CVKETVAGRRGAF-----------DYW | 1-39 | CQQSFRTP-HTF | 10 | H3L |
| 166 | 4-34 | CARGREWPSNF-------------DSW | 3-11 | CQQRAIWP-PEF | 1 | |
| 169 | 5-51 | CARRGSTYYY--------------DTW | 1-39 | CQQSFTSW-WTF | 2 | B5R |
| 172 | 3-21 | CASKPYGGDFG-------------SYW | 1-5 | CQQYSNYP-ITF | 2 | B5R |
| 183 | 5-51 | CARPPSNWDESF------------DIW | 1-17 | CLQHNSF--LTF | 1 | (H3L) |
| 186 | 1-3 | CARDPTQWLLQGDVYDM-------DVW | 2-30 | CMQGTHWP-PAF | 6 | A27L |
| 188 | 4-30-4 | CAREAWLGEPLLLGDDAF------DIW | 1-17 | CLQLHTFP-RTF | 3 | A27L |
| 189 | 3-30 | CARDGAGEWDLLMRRDF-------DYW | 1-39 | CQQGYSTP-YTF | 4 | (H3L) |
| 195 | 1-18 | CARDPARRPRSGYSVF--------EYW | 3-15 | CHQYNYWPPLAF | 1 | H3L |
| 197 | 4-4 | CARDNRQSSSWVEGFFYYYGM---DVW | 3-20 | CQQYGISP-RTF | 1 | H3L |
| 201 | 1-46 | CARLRLGATIGRD-----------DYW | 2-28 | CMQALQTP-HTF | 2 | H3L |
| 203 | 4-30-4 | CARDRASSGYDSRVWF--------DPW | 1-33 | CHQYDSLP-FTF | 2 | A27L |
| 205 | 3-30 | CARTYRVYAKFDPF----------DVW | 3-20 | CQQFGYSPRFTF | 5 | H3L |
| 211 | 3-9 | CGKDGVPGRRGYI-----------EDW | 1-39 | CQQTYITP-KSF | 4 | H3L |
| 214 | 3-9 | CVKDNIAGRRGSF-----------DSW | 1-39 | CQQSYRTP-LTF | 3 | (H3L) |
| 215 | 1-2 | CARDYIRATGATPSKYFIYYYGM-GVW | 1-27 | CQKYDSAP-YTF | 12 | (H3L) |
| 219 | 1-18 | CARARRVTNSPNNWF---------DPW | 1-39 | CQQTYTIP-LTF | 1 | (H3L) |
| 225 | 4-34 | CARAGERSGSGSFVLGRF------DFW | 1-39 | CQQSYSTL-RTF | 2 | A14L |
| 229 | 3-49 | CTNTSSLAVA--------------GNW | 4-1 | CQQYYKTP-PTF | 3 | A27L |
| 232 | 4-39 | CARIPQQRVNYF------------DYW | 1-5 | CQQYNSYP-LTF | 1 | H3L |
| 235 | 4-39 | CARLPGQRTTFF------------DYW | 1-5 | CQQYNFY--GTF | 1 | H3L |
| 237 | 6-1 | CARGRRFEDDAF------------DIW | 1-39 | CQQSYSIP-RTF | 3 | (H3L) |
| 238 | 3-9 | CVKDSVAGRRGGF-----------DHW | 1-39 | CQQSYSMSPYTF | 3 | (H3L) |
| 242 | 1-f | CATRDGDF----------------DHW | 1-16 | CQQYNSFP-LTF | 8 | |
| 243 | 5-51 | CVRHGTRYSFGRSDII--------DIW | 1-17 | CLQQNNYP-WTF | 9 | (I1L) |
| 246 | 5-51 | CTKTPARGAYGDYIS---------GSW | 1-33 | CQQYDNFP-YTF | 3 | (H3L) |
| 250 | 3-9 | CAKSTKAVRRGSF-----------DYW | 1-39 | CQQTYIST-RTF | 3 | (H3L) |
| 267 | 1-69 | CARGGKLYEGNGYYSFHYF-----DYW | 1-33 | CQQYDNLP--LF | 1 | VCP |
| 269 | 2-5 | CAHTELAF----------------DYW | 1-33 | CQQYDNLI--TF | 1 | VCP |
| 271 | 1-69 | CARVKGTQNYYGM-----------DVW | 1-5 | CQQYESDI-FTF | 2 | VCP |
| 274 | 4-34 | CHYYDSTGYYVS------------DFW | 1-16 | CQQYGRYP-LTF | 1 | VCP |
| 286 | 1-18 | CARGVVLIQTILF-----------DYW | 1-39 | CQQSHRTP-YTF | 4 | VCP |
| 290 | 1-69 | CARTVLDSGAYSYY----------DSR | 1-5 | CQQYQTY--STF | 1 | VCP |
| 291 | 1-69 | CARSRGSQDYYGM-----------DVW | 1-5 | CQQYESDS-WTF | 2 | VCP |
| 294 | 3-23 | CAKLRDSSVYSAYVFRVIF-----DCW | 1-33 | CQQYDNLP-FTF | 1 | VCP |
| 295 | 3-11 | CATLTVASTY--------------DYW | 2-30 | CMQGTHWP-YSF | 8 | B5R |
| 297 | 3-9 | CVKDTVALLTSRGGCM--------DVW | 1-12 | CQQAYSFP-WTF | 12 | VCP |
| 302 | 2-5 | CAHSPPHGG---------------DYW | 1-33 | CQQYDNL--PTF | 1 | VCP |
| 303 | 3-30 | CAKGLSQALNYYGSGS--------PFL | 3-20 | CQQYGTSP-WTF | 3 | B5R |
| 335 | 3-30 | CAKDRGVSAWYPRDAF--------DIW | 1-39 | CHQSYSLP-FTF | 1 | (H3L) |
| 339 | 4-59 | CARVPLIEAGITIFAKIGAF----DIW | 3-20 | CQQYVASP-FTF | 2 | D8L |
| 349 | 5-51 | CARGSPMIKFYF------------DYW | 3-20 | CHQYGTSP-RTF | 2 | (A14L) |
| 351 | 3-9 | CARATGAGRRNPL-----------DYW | 1-39 | CQQSYITP-YTF | 1 | (H3L) |
| 431 | 1-46 | CARPYRSYSSSPQ-----------DYW | 3-20 | CQQYGSSP-YTF | 9 | A56R |
| 437 | 4-31 | CARYYYSSGPKF------------DYW | 1-12 | CQQANSFP-FTF | 9 | |
| 446 | 1-69 | CARNNRPLGALFGM----------DVW | 1-9 | CQQLDTYP-LTF | 4 | |
| 461 | 1-18 | CARGGFNRLV--------------DPW | 3-20 | CQQYASSP-YTF | >30 | A33R |
| 482 | 4-31 | CARNTGIYLGGSPGGTRNNWF---DPW | 1-39 | CQHSSKTP-FTF | 5 | A33R |
| 488 | 5-51 | CARQQAKTLYYDSSGSKSAF----DIW | 1-5 | CQQYSSY--LSF | 2 | A27L |
| 515 | 4-31 | CARVRGNIVATTAFYYYYGL----DAW | 1-12 | CQQANGFL-WTF | 1 | |
| 516 | 3-11 | CAKFDYGEGAYHF-----------DFW | 1D-12 | CQQAHSFP-VTF | 2 | A56R |
| 517 | 4-31 | CARDPIALPGRGVF----------DYW | 3-20 | CQQYGSSP-LTF | 1 | (D8L) |
| 520 | 4-34 | CSSGYYFAGGEF------------DYW | 1-12 | CQQANSFP-RTF | 4 | |
| 526 | 3-49 | CTRDRGYSDHTGLYTRFGF-----DSW | 2-28 | CMQSLQT--VTF | 2 | B5R |
| 532 | 1-69 | CARWRGGYSGYGDYF---------DSW | 1-39 | CQHSYETPPYTF | 3 | |
| 536 | 4-4 | CARDPQKPRQHLWPNPYYYSGM--DVW | 1-5 | CQQYNFYP-WTF | 2 | |
| 551 | 1-69 | CARGRSWRGYL-------------DYW | 3-20 | CQQYGGSP-QTF | 1 | A56R |
| 559 | 1-46 | CARDYGDYCGGDCPYDAF------DIW | 1-27 | CQKYNSAP-LTF | 1 | |
| 572 | 1-69 | CARDKGESDINGWQTGAFYYGM--DVW | 1-12 | CQQANSFP-VTF | 3 | |
| 575 | 2-26 | CARIDSVGWPSSHYYGM-------DVW | 1-12 | CQQTHSFPPWTF | 2 | |
| 586 | 4-59 | CARDRITGYDSSGHAF--------DIW | 1-5 | CQQYHHYS-PTF | 1 | |
| 589 | 4-34 | CARGGKFCGSTSCFTEGRL-----DYW | 1-39 | CQQSYSTL-RTF | 1 | A14L |
| 607 | 3-30 | CAKDSGRYSSLGHYYYYGM-----DVW | 1-39 | CQQGYSTP-PTF | 1 | A10L |
| 611 | 5-51 | CARGYYYDTSGYRPGSF-------QHW | 1-5 | CQQYDSYP-YTF | 2 | |
| 612 | 3-30 | CARPLFYGAGDAF-----------DIW | 1-39 | CQQSYSTP-RTF | 2 | |
| 614 | 1-69 | CADWVVGNYNGL------------DVW | 3-20 | CQQYSDS--LTF | 1 | |
| 617 | 1-46 | CAREGKHDFWRGYFSPLGM-----DVW | 3-20 | CHQYGGAQ-GTF | 5 | |
| 621 | 1-69 | CATARNSSNWYEGHYYL-------AHW | 1-33 | CQQYDTLPPITF | 1 | |
| 626 | 4-30-4 | CANMVVVATQPKNWF---------DPW | 1-39 | CQQSHSSP-WTF | 1 | |
| 628 | 4-30-4 | CASYGSGMGSEYYF----------GHW | 1-39 | CQQSYLAP-WTF | 1 | A56R |
| 632 | 1-2 | CGRVGREAFYYYGM----------DVW | 1-9 | CQQFNNYP-YTF | 3 | |
| 633 | 1-2 | CARASRRLTTHNYF----------DGW | 1-16 | CQQYHTYP-FTF | 3 | H3L |
| 634 | 3-48 | CARVRGGRYF--------------DYW | 3-20 | CQKYGRSPTWTF | 1 | |
| 640 | 5-51 | CASGSGYDSYYNM-----------DVW | 1-39 | CQQGFTTP-ITF | 3 | |
| 643 | 7-4-1 | CARDSSTVTGLMTEYNWF------DPW | 1-39 | CQQSYSTP-YTF | 1 | |
| 649 | 4-31 | CATEKGSGGDVGKF----------DNW | 1-39 | CQQSYTIPLWTF | 1 | |
| 650 | 1-69 | CATGQQLYSL--------------HYW | 2-30 | CMQGTHWP-PTF | 1 | |
| 651 | 1-2 | CAREGPQFYYDSGDYYSAHSPGDFDHW | 1-39 | CQQSDSTP-YTF | 1 | (H3L) |
Parentheses indicate that the specificity was assigned by comparison of reactivities determined by Western blotting.
FIG. 2.
The captured repertoire of VACV-specific antibodies mirrors the natural genetic diversity of a human antibody response. The frequencies of IGHV (left panel) and IGKV (right panel) gene usage in the VACV-specific antibody repertoire were calculated based on the genetic origin of each of the 89 confirmed VACV-specific antibodies (Table 2). The highly homologous gene pair IGKV1-12/IGKV1D-12 was for clarity treated as a single entity.
In addition, the sequence analysis revealed that six clonotypes that were isolated from three different repertoires utilize highly similar VDJ and VJ rearrangements. The clonotypes represented by clones 160 and 250 were isolated from donors 05 and 08, respectively, and the four clonotypes represented by clones 211, 214, 238, and 351 were all isolated from donor 11. These clonotypes all use the IGHV3-9 and IGKV1-39 genes and have highly similar CDRH3 sequences (Table 2). The Western blot analysis also showed that these clones all recognize the same antigen, subsequently identified as H3L (see below). However, the differences in CDRL3 sequence (Table 2), the use of different D and J segment genes, and the distinct patterns of somatic mutations (data not shown) demonstrate that these clonotypes have clearly arisen through independent rearrangements. In donor 11, this type of rearrangement has apparently arisen four times.
Characterization of individual antibody specificities.
Following sequencing and identification of unique clonotypes, a representative clone from each cluster was tested as a Fab fragment or as a full-length IgG after transfer of the V genes to a mammalian expression vector. Together with the sequence analysis, this characterization revealed 89 clonotypes that were reactive with inactivated VACV particles of the Lister, IHD-W, or IHD-J strain or the recombinant antigens B5R and VCP (Table 2). All five donor repertoires contributed to different extents to this panel of VACV-specific antibodies (Table 1).
The confirmed B5R- and VCP-specific clones were mainly identified by screening on the recombinant antigens, and the reactivities were verified by ELISA using the same antigens (data not shown). However, all five B5R-specific clones also bound to inactivated VACV (Lister) particles to some extent. Some of the 11 VCP-specific clones also bound weakly to inactivated VACV particles (Lister or IHD-W strain), indicating that VCP is associated with the viral particle preparations despite being a secreted protein. The two A33R-specific clonotypes were identified by screening on the VACV Lister particles, and screening on recombinant antigen did not yield any additional clones. The reactivity of both clones was determined by ELISA using the recombinant antigen. The seven A27L-specific clonotypes were also identified by screening on virus particles, and the specificity was subsequently identified by ELISA using recombinant A27L.
In order to assign antigenic specificities to the clonotypes that were not reactive with any of the recombinant antigens, an extensive analysis was performed using Western blotting, capture ELISA with in vitro translated VACV antigens, and VACV protein array analysis. The Western blotting using acetone-precipitated VACV Lister particles revealed seven distinct reactivities, arbitrarily labeled B, C, D, E, G, HI, and L, and also confirmed the already identified reactivities with A27L, A33R, B5R, and VCP. Figure 3 shows representative results from the analysis. The VCP-specific clones reacted strongly with the recombinant protein in ELISA, but only a few of them recognized the protein in Western blotting with VACV particles. As also noted by other investigators (37), a cluster of proteins with molecular masses between 30 and 40 kDa was recognized by the VACV-specific antibodies (C, E, G, and HI reactivities and B5R and VCP) (Fig. 3). The HI reactivity was found to be the predominant, with a total of 22 clonotypes, including the six that use similar rearrangements of IGHV3-9 and IGKV1-39 (160, 211, 214, 238, 250, and 351), displaying this profile. The other reactivities were represented by only a few clonotypes each. Approximately one-third of the clones that were reactive with VACV particles when tested by ELISA and FLISA did not recognize a specific antigen in the Western blot analysis.
FIG. 3.
The confirmed VACV-specific antibodies display a number of distinct reactivities with VACV proteins. Representative Western blots of the identified reactivities B, C, HI, E, D, G, and L and recombinant A33R, B5R, VCP, and A27L, as indicated above each panel, are shown. Virus particles or recombinant antigens were used as target antigen and probed with different VACV-specific antibodies (clone numbers are given above the right lane in each panel). M, molecular size marker (kDa).
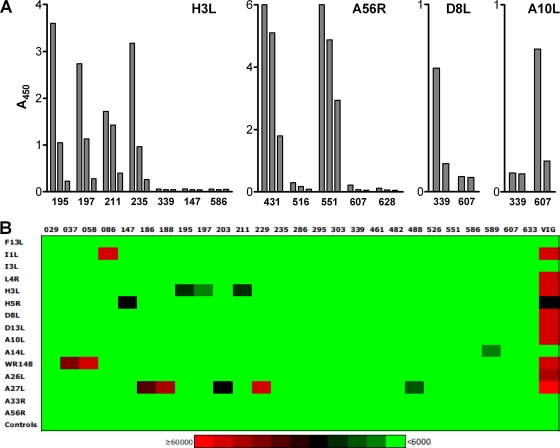
Results from the capture ELISA using in vitro translated VACV antigens demonstrated that the clones displaying the HI reactivity profile recognized the H3L protein of the Lister strain (Fig. 4 A). Similarly, it was shown that the antibody clones displaying the E and D reactivities recognized D8L and A56R, respectively (Fig. 4A). These results correlate well with the observed Western blotting reactivity profiles (Fig. 3) as H3L has been shown to migrate as a single band of 35 kDa (59), D8L as a single band of about 34 kDa (50), and A56R as two bands with apparent sizes of 85 and 68 kDa (10). Clone 607, which did not recognize a specific antigen in the Western blot analysis, was reactive with the in vitro translated A10L (major core protein) (Fig. 4A).
FIG. 4.
The confirmed anti-VACV antibodies recognize a wide range of VACV proteins. (A) Reactivity of selected antibody clones with in vitro translated VACV proteins as determined by capture ELISA. Each protein was incubated in serial dilutions (approximately 1:10 to 1:100 dilution of the crude in vitro translation reaction) with the different immobilized anti-VACV antibodies, and the bound antigen was detected by streptavidin-peroxidase polymer. (B) Representative heat map showing the interaction of monoclonal antibodies at 50 ng/ml and commercial, plasma-derived VIG at 5 μg/ml with VACV antigens on Vaccinia Chip 9, version 1 (Antigen Discovery). Each interaction was assigned a color shade based on the signal intensity (see scale at the bottom of figure) after subtraction of the average control signal intensity plus 10 standard deviations from the average raw signal intensity.
The VACV protein array analysis (20), which was performed with a set of 26 antibody clones produced as full-length IgG, verified several of the already identified specificities and also revealed a few additional specificities (Fig. 4B and data not shown). All five A27L-specific antibodies that were tested, 186, 188, 203, 229, and 488, were reactive with the in vitro translated protein on the VACV array. Three of the previously identified H3L-specific clones, 195, 197, and 211, also reacted with the protein expressed in an E. coli-based cell-free system, and two others, 029 and 235, recognized the protein when it was expressed in a system allowing for disulfide bond formation. Clone 633, which displayed weak reactivity in Western blotting, was also found to recognize H3L on the protein arrays (data not shown). Clone 607 was also verified as being A10L specific, in accordance with the capture ELISA using in vitro translated protein (Fig. 4A).
Two clones that displayed the B reactivity profile in the Western blot analysis (Fig. 3), 037 and 058, reacted strongly with WR148, the VACV A-type inclusion (ATI) protein homologue (Fig. 4B). This protein has no direct VACV Copenhagen homologue, but is usually referred to as A25L (www.poxvirus.org). The B reactivity clones recognized an ∼82-kDa protein in the Western blotting with Lister strain viral particles (Fig. 3), which is also the size reported for the ATI protein (37). Three additional reactivities were also discovered as clones 086, 147, and 589 were found to recognize I1L (DNA-binding core protein), H5R (viral late gene transcription factor) and A14L (major IMV membrane component), respectively (Fig. 4B). The reactivities in Western blotting (clone 086, C; clone 147, G; and clone 589, L) (Fig. 3) correlate well with the reported sizes of 36 kDa for both I1L and H5R (39, 40) and 14 to 16 kDa for A14L (28).
A number of antibody clones, including three B5R-specific clones (295, 303, and 526), the two A33R-specific clones (461 and 482), and one A56R-specific clone (551), recognized only their respective targets as purified proteins expressed in mammalian cells. The D8L-specific clone 339 recognized the target gene product only when it was expressed in a cell-free system which allowed for disulfide bond formation (data not shown). Not surprisingly, correct folding, disulfide bond formation, and/or posttranslational modifications appear to be required for specific recognition by some antibodies.
The VACV protein array analysis failed to identify the target antigen for only two antibody clones, 286 and 586. Neither of these reacted specifically with any particular in vitro expressed VACV protein, regardless of the expression system used (Fig. 4B and data not shown). Clone 286 had already been identified as VCP specific and, as discussed further below, recognizes recombinant VCP expressed in insect cells with nanomolar affinity (Fig. 5). The lack of reactivity with the protein arrays may be due to an improper conformation of the in vitro expressed protein. In fact, protein array analysis has failed to detect anti-VCP antibodies in both commercial, plasma-derived VIG, and hyperimmune serum (18, 20; this study). Clone 586 did not react specifically in any of the confirmatory assays used, and the specificity of this clone remains unknown.
FIG. 5.
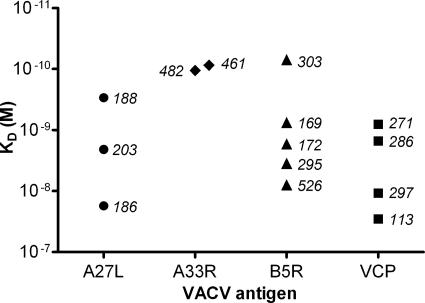
Affinities of human VACV-specific antibodies isolated by the Symplex technology. The affinity distributions among antibody clones reactive with VACV antigens A27L, A33R, B5R, and VCP were determined by surface plasmon resonance. Each symbol represents a unique antibody clone, identified by number.
In summary, using a variety of assays with both natural and recombinant VACV proteins, antigenic specificities have been assigned to more than two-thirds of the 89 confirmed VACV-specific antibodies. The analysis showed that at least 12 different VACV proteins, including A10L, A14L, A27L, A33R, A56R, B5R, D8L, H3L, H5R, I1L, VCP, and the ATI protein, are recognized by the isolated human VACV-specific antibodies, thus further demonstrating the diversity of the captured antibody repertoire. For a comparison, commercial, plasma-derived VIG reacted with A10L, A14L, A27L, A33R, A56R, D8L, D13L, F13L, H3L, H5R, I1L, I3L, L4R, and WR148 (ATI protein) on the VACV proteome arrays (Fig. 4B). In addition, plasma-derived VIG also recognized recombinant A33R, B5R, L1R, and VCP in ELISA (Fig. 6 and data not shown), whereas the anti-rhesus D recombinant polyclonal antibody, which was used as a negative control, did not display any reactivity toward the tested antigens (Fig. 6). Antigenic specificities could not be assigned to the remaining antibody clones as these did not recognize any particular antigen in any of the applied assays. The cause for the absence of reactivity may be that the epitopes recognized by these antibodies are poorly represented in the antigenic preparations, either due to a lack of proper conformation or posttranslational modifications or to the absence of complex formation in the case of epitopes made up by more than one protein. For VACV, such complexes have been shown to be formed by, e.g., A33R, A34R, and A36R; A34R and B5R; and A17L, A26L, and A27L (32, 53, 58). Judging from the binding characteristics, some of the unassigned antibodies appear to recognize antigens of low abundance in the VACV particle preparations (data not shown). It should be noted that none of the confirmed VACV-specific antibody clones bound to Vero cells, i.e., the cells used to produce the VACV particles, when tested at 10 μg/ml in a FACS-based assay (data not shown). All in all, the reactivity of the isolated antibody repertoire appears to be essentially the same as that demonstrated for plasma-derived VIG.
FIG. 6.
Recombinant VIG displays a high specific binding activity toward VACV antigens. The reactivity of purified recombinant VIG (orange circles) against VACV particles (Lister and IHD-J) and recombinant antigens (A27L, A33R, B5R, and VCP) was evaluated by ELISA. Commercial, plasma-derived VIG (gray diamonds) and a negative-control polyclonal antibody (anti-rhesus D [57]; black squares) were similarly tested for comparison. All determinations were performed in duplicate, and the results are presented as means ± standard errors of the means.
Furthermore, in order to determine the quality of the isolated antibody repertoire, the binding kinetics of the interaction with the target antigen was determined for a selection of antibodies. This analysis was for practical reasons restricted to antibodies specific for antigens that were available as purified, recombinant proteins (A27L, A33R, B5R, and VCP). The kinetic measurements revealed affinity constants (KD) between 10−8 to 10−10 M for the majority of the investigated antibodies (Fig. 5), thus showing that the isolated antibodies reach into the high-affinity range. In fact, the observed affinities are well within the range of those of currently used therapeutic monoclonal antibodies (11), suggesting that the captured antibodies have therapeutic potential without further improvements in terms of affinity. A number of antibodies reactive with A27L and VCP did not recognize the antigens immobilized to the Biacore chip and were for this reason excluded from the analysis.
Generation of a recombinant VIG.
Having identified a large number of antibodies reactive with different VACV proteins, we selected 26 antibody clones for the creation of a recombinant VIG with the broadest possible reactivity against VACV strains and related orthopoxviruses. To allow for consistent production and easy characterization, the selected clones were combined and expressed as a recombinant polyclonal antibody mixture using the Sympress technology. This technology has also been used to generate an anti-rhesus D recombinant polyclonal antibody comprised of 25 unique antibodies (57), which is currently in clinical trials (identifier NCT00718692 [clinical trials.gov]). Due to the complexity of antibody-mediated protection against VACV infection, which makes it difficult to assess the full impact of individual antibodies on the basis of in vitro assays, antibody clones reactive with all the identified antigens were included in the recombinant VIG. Where possible, more than one antibody clone per target was included to obtain optimal epitope coverage, and the numbers of antibodies against each antigen was also selected as to reflect the frequency in the captured repertoire (Table 2) and, thus, the natural antibody response toward each antigen (5, 20, 37, 52). Multiple antibodies against the dominating antigens, A27L and H3L, were for this reason included in the recombinant VIG. As described in the previous section, VCP also yielded numerous antibodies, but due to the secreted nature of the protein, only a single, high-affinity antibody (Fig. 5) specific for this antigen was included.
The recombinant VIG thus contains the following reactivities: A10L (clone 607), A14L (clone 589), A27L (clones 186, 188, 203, 229, and 488), A33R (clones 461 and 482), A56R (clone 551), B5R (clones 295, 303, and 526), D8L (clone 339), H3L (clones 029, 195, 197, 211, 235, and 633), H5R (clone 147), I1L (clone 086), VCP (clone 286), and the ATI protein (clones 037 and 058). In addition, a single antibody clone (586) for which no specificity could be assigned was also included as a representative of the group of antibody clones that react with unknown antigens of low abundance in the VACV particle preparations (see above).
The resulting recombinant VIG was tested for reactivity with different VACV strains and recombinant VACV antigens by ELISA, and the in vitro neutralizing activity was determined in a plaque reduction neutralization assay against the Lister strain. As shown in Fig. 6, the recombinant VIG displayed a high specific binding activity, approximately 250-fold higher than the commercial, plasma-derived VIG, more or less irrespective of the target antigen tested. Furthermore, the recombinant VIG was found to exhibit an 800-fold higher virus neutralizing activity against the Lister VACV strain than the plasma-derived VIG (50% effective concentration [EC50], 0.125 versus 100 μg/ml).
Once it was established that the recombinant VIG harbors both high VACV-specific binding activity and in vitro neutralizing activity, the in vivo antiviral activity was assessed using the mouse tail lesion model against the Lister and NYCBOH VACV strains. Inoculation of VACV into the tail vein of mice has been shown to produce a nonlethal and self-limiting infection that results in discrete dermal lesions along the entire surface of the tail (7), in close resemblance to the reaction at the vaccination site following successful smallpox vaccination in humans. The mouse tail lesion model represents a simple and reliable way of assessing protective efficacy and is generally more ethically acceptable than lethal animal models. The antibodies were administered intramuscularly 1 day before or 1 day after virus challenge to evaluate both prophylactic and therapeutic efficacies in early infection. The effect of antibody treatment on tail lesion formation was determined 7 days after virus challenge by manual counting of the lesions on each tail. A challenge dose that produced 30 to 40 lesions per tail in the groups receiving the negative-control antibody was used in both settings. As shown in Fig. 7, both the recombinant and the plasma-derived VIG were capable of inhibiting lesion formation in both the prophylactic and therapeutic settings. However, the recombinant VIG was found to be at least 200-fold more potent than the commercial, plasma-derived VIG against both virus strains in both settings. In addition, at the highest dose tested (250 μg/mouse), the recombinant VIG almost completely abolished the formation of VACV lesions (Fig. 7). This was in stark contrast to the plasma-derived VIG, which was capable of inhibiting lesion formation only to 30 to 40% of the negative control at a 20 times higher dose (5 mg/mouse). For a comparison, prophylaxis and treatment with a standard dose of 2.5 mg/mouse of the small-molecule antiviral drug cidofovir (21) against both virus strains reduced lesion formation to 25 to 28% of that in the control group (data not shown). The highest dose of recombinant VIG was significantly more effective at inhibiting lesion formation than both the commercial, plasma-derived VIG at 5 mg/mouse and cidofovir at 2.5 mg/mouse in both settings (P < 0.02).
FIG. 7.
Recombinant VIG protects mice against VACV challenge in vivo. Groups of eight mice were treated with recombinant VIG (orange circles) or commercial, plasma-derived VIG (gray diamonds) at the indicated doses 24 h before (solid lines) or after (broken lines) challenge with VACV (Lister strain or NYCBOH strain). Tail lesions were enumerated in a blinded fashion 7 days after virus challenge, and the results are expressed relative to the negative control (anti-rhesus D polyclonal antibody [57]). Data are shown as means ± standard errors of the means.
DISCUSSION
The concerns over the potential use of smallpox virus in biological warfare or terrorism and the increasing risk of zoonotic poxvirus outbreaks have revived the need for treatment options to be used alone or in combination with vaccination. For this reason, we set out to characterize and capture the natural human antibody response toward VACV in vaccinated donors with the aim of creating a recombinant VIG that would mimic the natural antibody response but have a higher degree of specificity and therapeutic efficacy and improved safety profile than plasma-derived products. Using the Symplex technology, a diverse repertoire consisting of 89 unique human VACV-specific antibodies recognizing at least 12 different VACV antigens with affinities similar to those of licensed monoclonal antibody therapeutics was isolated. A thorough analysis of the sequences of the isolated VACV-specific antibodies showed that these are of a highly diverse genetic origin (Fig. 2), which corresponds well to that of normal human antibody responses (9, 16, 22, 25, 33). Furthermore, the antigen recognition of the captured antibody repertoire also corresponds to the specificity of the serum response induced by vaccination with VACV (5, 20, 37, 52), thus demonstrating an effective and unbiased capture of VACV-specific antibodies using the Symplex technology.
The antigenic specificities identified in the captured VACV-specific repertoire and also included in the recombinant VIG include both IMV- and EEV-associated antigens and one secreted antigen (VCP). Five of the recognized proteins are surface proteins of the IMV (A27L, D8L, and H3L) or the EEV (A33R and B5R) that have been shown to induce neutralizing and/or in vivo protective antibodies in animal models or humans (for a review, see reference 2) and constitute thus obvious targets for a therapeutic antibody composition. In agreement with the findings by other investigators (19), H3L was found to be the most frequently recognized antigen within the captured repertoire. Besides confirming the immunodominance of H3L in humans, the results from this study also show that H3L seems to select for a common antibody sequence motif in different individuals, possibly indicating the presence of an imprint in the germ line-encoded antibody repertoire required for recognition of this antigen. A similar bias in V gene usage has been observed for a number of other antigens, including viral proteins, bacterial polysaccharides, and allergens, and may in part explain the immunodominance of certain antigens or epitopes (reviewed in reference 42). Two additional surface proteins, A14L and A56R, were also recognized by the isolated antibodies. A14L, which is an IMV membrane protein, is a known target for antibodies, and it has been shown to evoke an antibody response that correlates with virus neutralization in smallpox vaccinees (5). A56R is the VACV hemagglutinin protein, and it is considered a constituent of the EEV envelope (54). However, the role of A56R-specific antibodies in the protection against VACV infection is unclear. Depletion of A56R-reactive antibodies from hyperimmune human serum does not affect the neutralizing activity (52), but, as discussed further below, this may be an effect of the redundancy in the neutralizing response toward VACV. It has also been debated whether A56R is a true EEV envelope protein since it does not seem to accumulate intracellularly in virion-like structures, and approximately one-third of EEV particles also lack A56R (41, 45). Furthermore, not all VACV strains express A56R, as in the case of IHD-W (17). The fact that A56R is expressed on the surface of infected cells (45) suggests that it still may be of importance, e.g., for opsonization of infected cells.
In addition, a number of nonenvelope IMV proteins, including A10L (major core protein), I1L (DNA-binding core protein), H5R (viral late gene transcription factor), and the ATI protein, were recognized. It was not unexpected to retrieve binders against these proteins as it has been shown that a significant part of the antibody response following smallpox vaccination is directed against these antigens (20, 37). With the exception of H5R, they are also major components of IMV or are abundantly expressed in VACV-infected cells (13, 20). Recently, it was shown that polyclonal antibody raised against the ATI protein was capable of blocking VACV plaque formation in vitro, thus demonstrating that this protein is actually a target of neutralizing antibodies (12). The ATI protein is not an integral envelope protein, but it appears to be associated with the surface of IMV through complex formation with other proteins. Whereas it seems unlikely that the other three proteins (A10L, I1L, and H5R) also are targets for neutralizing antibodies, they are clearly being targeted by the natural human antibody response, and these specificities were for that reason included in the recombinant VIG.
VCP (C3L) is a secretory protein of VACV that is capable of inhibiting complement activation, thereby preventing antibody-mediated virus neutralization and allowing the virus to evade the host immune response (35). Due to its homology to human proteins, it has been suggested that VCP is poorly immunogenic and will not elicit an antibody response (36). As shown in this study, this is clearly not the case since a majority of the tested donor sera contained high titers against the recombinant VCP (Fig. 1), and multiple VCP-specific antibodies were isolated from the cloned repertoires (Table 2). Other investigators have also shown that VCP is the major secretory VACV protein that is recognized by VIG (1). Being a secreted protein, VCP is not a target for neutralizing antibodies, and it has also been shown that the humoral response induced by vaccination with recombinant VCP fails to protect against lethal VACV challenge in a mouse model (1). However, antibodies raised against VCP have been shown to block its activity in vitro (1, 34), and it is possible that anti-VCP antibodies have a role in ameliorating disease or enhancing the effect of neutralizing antibodies. VCP is thus a natural and potentially important reactivity to include in a recombinant VIG.
As indicated by the size of the VACV genome and number of reactivities against VACV in VIG and hyperimmune serum observed in this study (Fig. 4) and elsewhere (18, 20), the antigens recognized by the captured antibody repertoire do not represent all the VACV antigens that the humoral immune response is potentially capable of recognizing. However, protein array profiling studies have shown that only a limited number of VACV proteins are consistently recognized by VIG and/or human hyperimmune serum. The list of recognized proteins includes A10L, A13L, A17L, A27L, A33R, A56R, B5R, D8L, D13L, E3L, F13L, H3L, H5R, L4R, and WR148 (ATI protein), and, when expressed under the proper conditions, L1R (18, 20). The majority of these antigens were also recognized by the members of the isolated VACV-specific repertoire, including most of the surface antigens shown to induce neutralizing or protective antibodies (2). One significant exception that was not found in the isolated repertoire is L1R. This is an IMV envelope protein that has been shown to be the target of both neutralizing and in vivo protective antibodies (reviewed in reference 2). However, as demonstrated both here (Fig. 1) and elsewhere (5, 43, 52), L1R appears to be poorly immunogenic in humans, and the serum response to L1R is generally weak, or nearly undetectable, in vaccinated humans. The failure to identify any L1R-specific antibodies in this study is therefore likely due to an absence or a very low frequency of L1R-specific B cells in the starting material for the Symplex PCR. Dedicated screening using recombinant or purified proteins might have yielded additional hits against individual proteins, including L1R. However, due to the abundance of antibodies against other IMV envelope proteins, this was not implemented for L1R. A number of studies have suggested that there is a significant degree of redundancy in the neutralizing antibody response toward IMV; i.e., in the absence of one or more neutralizing specificities, other specificities are able to compensate for the loss. For instance, Benhnia et al. have demonstrated that although purified anti-H3L IgG is sufficient to neutralize VACV, depletion of H3L antibodies from serum does not significantly reduce the neutralizing activity (5). Comparable results have also been obtained for anti-L1R and also for a combination of L1R and H3L antibodies, thus leading to the proposal that the natural immune response to the IMV form of VACV is not directed toward a single key antigen but is instead aimed at the development of a highly functionally redundant response of neutralizing antibodies to multiple different viral proteins (5). Pütz and colleagues have also showed that depletion of hyperimmune sera of antibodies against A27L, H3R, or L1R only had limited effect on the residual neutralizing activity (52), and He et al. have presented similar results for A27L (29). Thus, the collective IMV reactivity, which includes antibodies to A14L, A27L, D8L, and H3L, in the recombinant VIG is likely more than able to substitute for the missing anti-L1R reactivity. On the other hand, similar depletion experiments with EEV antigens have suggested that B5R is the only target of importance for EEV neutralization as depletion of A33R- and A56R-specific antibodies had no impact on the residual neutralizing activity, whereas depletion of antibodies against B5R fully abrogated the neutralizing activity (52).
Since the recombinant VIG was designed to consist exclusively of VACV-specific antibodies, it did not come as a surprise that it displayed a very high specific binding activity toward VACV particles and proteins. The binding activity was also reflected in the in vitro and in vivo potency as the recombinant VIG displayed an 800-fold higher in vitro neutralizing activity and at least 200-fold higher in vivo protective capacity than the commercial, plasma-derived VIG. Based on the binding activity and the in vitro neutralizing activity, it was expected that prophylactically administered recombinant VIG would elicit a profound antiviral effect. However, it was not anticipated that the effect of postchallenge treatment would be nearly identical to the effect of prophylaxis for all of the administered therapeutics, including cidofovir. This result probably reflects a limitation of the mouse model used, in which a large number of infectious virus particles (>105 PFU) are injected into the tail vein, thereby creating a temporary high local virus concentration, which gives rise to 30 to 40 lesions in untreated mice. The temporary high concentration of virus will likely exceed the concentration of the systemically administered VACV-specific antibody, which thereby becomes more likely to influence continued virus dissemination rather than blocking of the primary infection. If this is the dynamic of the in vivo model used in this study, no profound differentiation between prophylactic and therapeutic administration of VACV-specific antibody can be expected, in correlation with the observed result. However, potent prophylactic and therapeutic efficacy of the recombinant VIG has also been demonstrated in a lethal mouse model based on intranasal challenge, thus corroborating the observed protective efficacy (H. S. Nielsen et al., unpublished data).
Taken together, the in vitro and in vivo data clearly demonstrate that a high degree of relevant antigenic specificities is included in the recombinant VIG and that it contains a high titer of protective antibodies. However, as suggested by the studies demonstrating a redundancy in the neutralizing antibody response toward VACV (5, 29, 52) and the fact that a number of antigenic specificities with no clear role in the protection against VACV were included in the antibody composition, it is possible that a less complex antibody composition with equal or even higher efficacy could be created by careful selection of the appropriate specificities. It should be kept in mind, though, that several studies have suggested that multiple specificities targeting antigens of both the IMV and EEV are required for optimal protection against the virus (24, 30, 31, 38, 46). In addition, the requirements for effective protection against other orthopoxviruses such as variola virus or monkeypox virus have not been investigated in detail, and it seems reasonable to believe that multiple specificities are needed in order to achieve broad and efficacious protection against the various viruses of the genus.
In conclusion, we have demonstrated the generation of a recombinant VIG that mirrors the diversity and specificity of the natural human antibody immune response to VACV and displays a high protective efficacy against VACV. We believe that this recombinant VIG has the potential to be used against orthopoxvirus infections either as postexposure prophylaxis or treatment. A detailed characterization of the biological activity of the recombinant VIG against different viruses both in vitro and in animal models will be presented elsewhere (Nielsen et al., unpublished).
Acknowledgments
We thank Barbara T. Andersen, Berit Mikkelsen, Bettina C. Overgaard, Camilla Holst Dahl, Elisabeth V. Andersen, Finn C. Wiberg, Hanne Wagner, Maria Schmidt, Vincent W. Coljee, and Yvonne Berger Larsen for excellent technical assistance and Ulla Jessen for editorial assistance.
The study was supported by award U01AI070378 from the National Institute of Allergy and Infectious Diseases.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
A.J., J.L., M.H.H., S.K.R., and L.S. are inventors on a patent application on the recombinant VIG described here.
Footnotes
Published ahead of print on 8 December 2010.
REFERENCES
- 1.Adamo, J. E., C. A. Meseda, J. P. Weir, and M. J. Merchlinsky. 2009. Smallpox vaccines induce antibodies to the immunomodulatory, secreted vaccinia virus complement control protein. J. Gen. Virol. 90:2604-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanna, I. J., M. K. Slifka, and S. Crotty. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211:320-337. [DOI] [PubMed] [Google Scholar]
- 3.Beck, A., T. Wurch, C. Bailly, and N. Corvaia. 2010. Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 10:345-352. [DOI] [PubMed] [Google Scholar]
- 4.Benhnia, M. R., et al. 2009. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J. Virol. 83:1201-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benhnia, M. R., et al. 2008. Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J. Virol. 82:3751-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulter, E. A., H. T. Zwartouw, D. H. Titmuss, and H. B. Maber. 1971. The nature of the immune state produced by inactivated vaccinia virus in rabbits. Am. J. Epidemiol. 94:612-620. [DOI] [PubMed] [Google Scholar]
- 7.Boyle, J. J., R. F. Haff, and R. C. Stewart. 1966. Evaluation of antiviral compounds by suppression of tail lesions in vaccinia-infected mice. Antimicrob. Agents Chemother. 6:536-539. [PubMed] [Google Scholar]
- 8.Bregenholt, S., A. Jensen, J. Lantto, S. Hyldig, and J. S. Haurum. 2006. Recombinant human polyclonal antibodies: a new class of therapeutic antibodies against viral infections. Curr. Pharm. Des. 12:2007-2015. [DOI] [PubMed] [Google Scholar]
- 9.Brezinschek, H. P., et al. 1997. Analysis of the human VH gene repertoire. Differential effects of selection and somatic hypermutation on human peripheral CD5+/IgM+ and CD5−/IgM+ B cells. J. Clin. Invest. 99:2488-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, C. K., P. C. Turner, and R. W. Moyer. 1991. Molecular characterization of the vaccinia virus hemagglutinin gene. J. Virol. 65:3598-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter, P. J. 2006. Potent antibody therapeutics by design. Nat. Rev. Immunol. 6:343-357. [DOI] [PubMed] [Google Scholar]
- 12.Chang, S. J., Y. X. Chang, R. Izmailyan, Y. L. Tang, and W. Chang. 2010. Vaccinia virus A25 and A26 proteins are fusion suppressors for mature virions and determine strain-specific virus entry pathways into HeLa, CHO-K1, and L cells. J. Virol. 84:8422-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung, C. S., et al. 2006. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J. Virol. 80:2127-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke, L., and J. Carbon. 1976. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell 9:91-99. [DOI] [PubMed] [Google Scholar]
- 15.Condit, R. C., N. Moussatche, and P. Traktman. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 66:31-124. [DOI] [PubMed] [Google Scholar]
- 16.Cox, J. P., I. M. Tomlinson, and G. Winter. 1994. A directory of human germ-line V kappa segments reveals a strong bias in their usage. Eur. J. Immunol. 24:827-836. [DOI] [PubMed] [Google Scholar]
- 17.Dales, S., et al. 1978. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology 84:403-428. [DOI] [PubMed] [Google Scholar]
- 18.Davies, D. H., et al. 2005. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. U. S. A. 102:547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies, D. H., et al. 2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 79:11724-11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies, D. H., et al. 2007. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics 7:1678-1686. [DOI] [PubMed] [Google Scholar]
- 21.De Clercq, E. 2002. Cidofovir in the treatment of poxvirus infections. Antiviral Res. 55:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wildt, R. M., R. M. Hoet, W. J. van Venrooij, I. M. Tomlinson, and G. Winter. 1999. Analysis of heavy and light chain pairings indicates that receptor editing shapes the human antibody repertoire. J. Mol. Biol. 285:895-901. [DOI] [PubMed] [Google Scholar]
- 23.Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 24.Fogg, C., et al. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster, S. J., H. P. Brezinschek, R. I. Brezinschek, and P. E. Lipsky. 1997. Molecular mechanisms and selective influences that shape the kappa gene repertoire of IgM+ B cells. J. Clin. Invest. 99:1614-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey, S. E., F. K. Newman, L. Yan, K. R. Lottenbach, and R. B. Belshe. 2003. Response to smallpox vaccine in persons immunized in the distant past. JAMA 289:3295-3299. [DOI] [PubMed] [Google Scholar]
- 27.Fulginiti, V. A., A. Papier, J. M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251-271. [DOI] [PubMed] [Google Scholar]
- 28.Grosenbach, D. W., S. G. Hansen, and D. E. Hruby. 2000. Identification and analysis of vaccinia virus palmitylproteins. Virology 275:193-206. [DOI] [PubMed] [Google Scholar]
- 29.He, Y., et al. 2007. Antibodies to the A27 protein of vaccinia virus neutralize and protect against infection but represent a minor component of Dryvax vaccine-induced immunity. J. Infect. Dis. 196:1026-1032. [DOI] [PubMed] [Google Scholar]
- 30.Hooper, J. W., D. M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooper, J. W., et al. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howard, A. R., T. G. Senkevich, and B. Moss. 2008. Vaccinia virus A26 and A27 proteins form a stable complex tethered to mature virions by association with the A17 transmembrane protein. J. Virol. 82:12384-12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang, S. C., R. Jiang, A. M. Glas, and E. C. Milner. 1996. Non-stochastic utilization of Ig V region genes in unselected human peripheral B cells. Mol. Immunol. 33:553-560. [DOI] [PubMed] [Google Scholar]
- 34.Isaacs, S. N., E. Argyropoulos, G. Sfyroera, S. Mohammad, and J. D. Lambris. 2003. Restoration of complement-enhanced neutralization of vaccinia virus virions by novel monoclonal antibodies raised against the vaccinia virus complement control protein. J. Virol. 77:8256-8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaacs, S. N., G. J. Kotwal, and B. Moss. 1992. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. U. S. A. 89:628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jha, P., and G. J. Kotwal. 2003. Vaccinia complement control protein: multi-functional protein and a potential wonder drug. J. Biosci. 28:265-271. [DOI] [PubMed] [Google Scholar]
- 37.Jones-Trower, A., et al. 2005. Identification and preliminary characterization of vaccinia virus (Dryvax) antigens recognized by vaccinia immune globulin. Virology 343:128-140. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman, D. R., et al. 2008. Differential antigen requirements for protection against systemic and intranasal vaccinia virus challenges in mice. J. Virol. 82:6829-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klemperer, N., J. Ward, E. Evans, and P. Traktman. 1997. The vaccinia virus I1 protein is essential for the assembly of mature virions. J. Virol. 71:9285-9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovacs, G. R., and B. Moss. 1996. The vaccinia virus H5R gene encodes late gene transcription factor 4: purification, cloning, and overexpression. J. Virol. 70:6796-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krauss, O., R. Hollinshead, M. Hollinshead, and G. L. Smith. 2002. An investigation of incorporation of cellular antigens into vaccinia virus particles. J. Gen. Virol. 83:2347-2359. [DOI] [PubMed] [Google Scholar]
- 42.Lantto, J. 2002. Antibody evolution and repertoire development. Ph.D. thesis. Lund University, Lund, Sweden.
- 43.Lawrence, S. J., et al. 2007. Antibody responses to vaccinia membrane proteins after smallpox vaccination. J. Infect. Dis. 196:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefranc, M.-P., and G. Lefranc. 2001. The immunoglobulin factsbook. Academic Press, San Diego, CA.
- 45.Lorenzo, M. M., I. Galindo, G. Griffiths, and R. Blasco. 2000. Intracellular localization of vaccinia virus extracellular enveloped virus envelope proteins individually expressed using a Semliki Forest virus replicon. J. Virol. 74:10535-10550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lustig, S., et al. 2005. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 79:13454-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lustig, S., C. Fogg, J. C. Whitbeck, and B. Moss. 2004. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology 328:30-35. [DOI] [PubMed] [Google Scholar]
- 48.Meijer, P. J., et al. 2006. Isolation of human antibody repertoires with preservation of the natural heavy and light chain pairing. J. Mol. Biol. 358:764-772. [DOI] [PubMed] [Google Scholar]
- 49.Meijer, P. J., L. S. Nielsen, J. Lantto, and A. Jensen. 2009. Human antibody repertoires. Methods Mol. Biol. 525:261-277. [DOI] [PubMed] [Google Scholar]
- 50.Niles, E. G., and J. Seto. 1988. Vaccinia virus gene D8 encodes a virion transmembrane protein. J. Virol. 62:3772-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Payne, L. G. 1979. Identification of the vaccinia hemagglutinin polypeptide from a cell system yielding large amounts of extracellular enveloped virus. J. Virol. 31:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pütz, M. M., C. M. Midgley, M. Law, and G. L. Smith. 2006. Quantification of antibody responses against multiple antigens of the two infectious forms of vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 12:1310-1315. [DOI] [PubMed] [Google Scholar]
- 53.Röttger, S., F. Frischknecht, I. Reckmann, G. L. Smith, and M. Way. 1999. Interactions between vaccinia virus IEV membrane proteins and their roles in IEV assembly and actin tail formation. J. Virol. 73:2863-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 55.Swann, P. G., et al. 2008. Considerations for the development of therapeutic monoclonal antibodies. Curr. Opin. Immunol. 20:493-499. [DOI] [PubMed] [Google Scholar]
- 56.Swartzman, E. E., S. J. Miraglia, J. Mellentin-Michelotti, L. Evangelista, and P. M. Yuan. 1999. A homogeneous and multiplexed immunoassay for high-throughput screening using fluorometric microvolume assay technology. Anal. Biochem. 271:143-151. [DOI] [PubMed] [Google Scholar]
- 57.Wiberg, F. C., et al. 2006. Production of target-specific recombinant human polyclonal antibodies in mammalian cells. Biotechnol. Bioeng. 94:396-405. [DOI] [PubMed] [Google Scholar]
- 58.Wolffe, E. J., A. S. Weisberg, and B. Moss. 2001. The vaccinia virus A33R protein provides a chaperone function for viral membrane localization and tyrosine phosphorylation of the A36R protein. J. Virol. 75:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zinoviev, V. V., N. A. Tchikaev, O. Y. Chertov, and E. G. Malygin. 1994. Identification of the gene encoding vaccinia virus immunodominant protein p35. Gene 147:209-214. [DOI] [PubMed] [Google Scholar]