Abstract
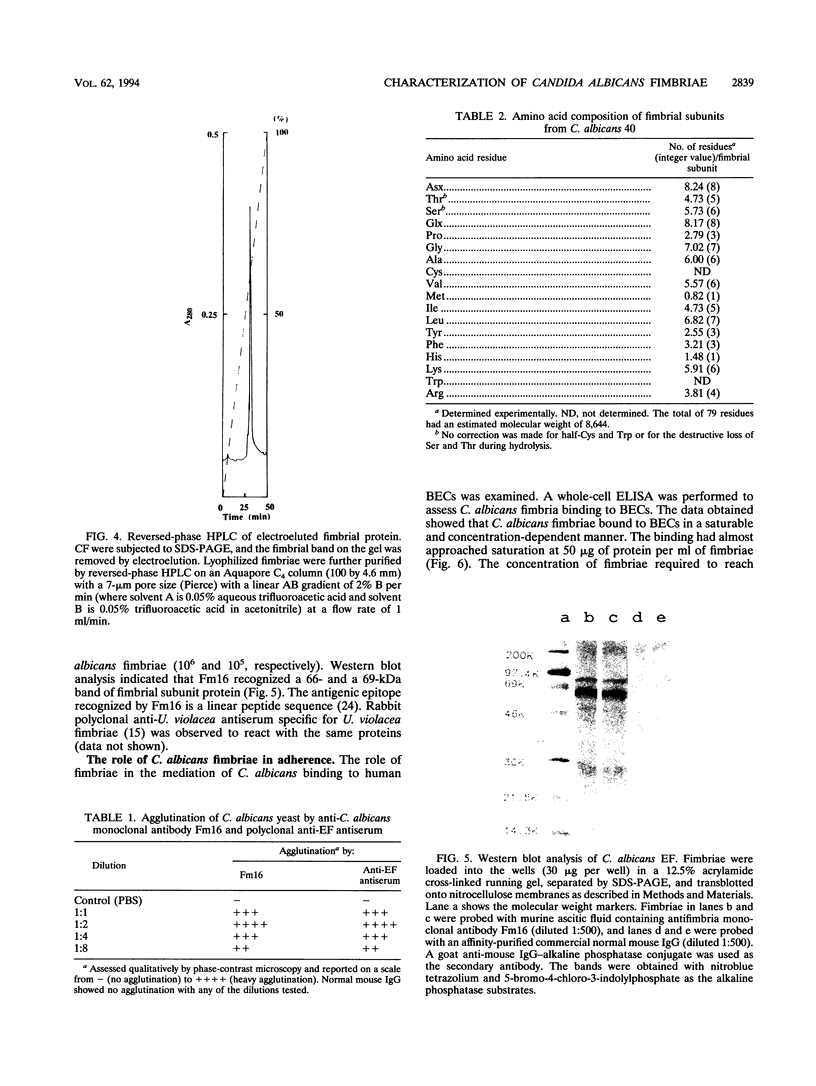
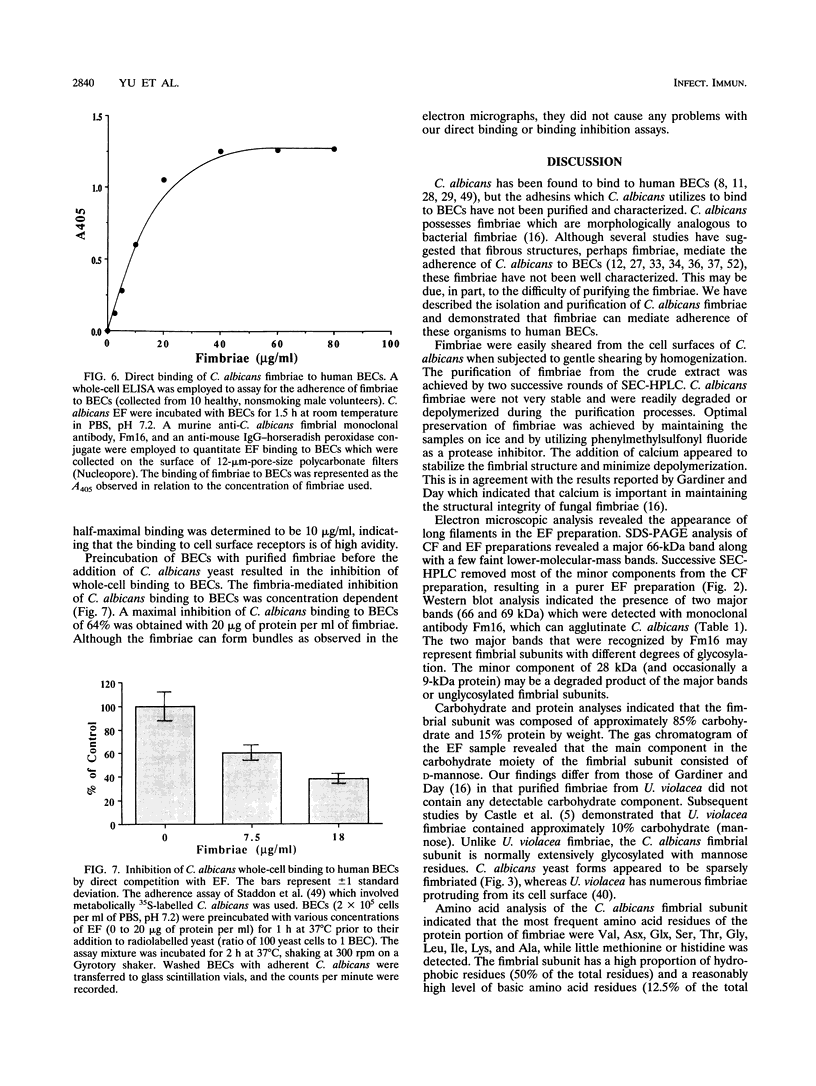
Candida albicans is the primary etiologic agent of candidiasis, a disease that can vary from superficial mucosal lesions to life-threatening systemic or disseminated diseases. Strains of C. albicans have been reported to possess long, thin filamentous protein cell surface appendages termed fimbriae (R.B. Gardiner, M. Canton, and A. W. Day, Bot. Gaz. 143:534-541, 1982). These fimbriae were isolated, purified, and partially characterized. The major structural subunit of the fimbriae is a glycoprotein which consists of 80 to 85% carbohydrate (consisting primarily of D-mannose) and 10 to 15% protein. The molecular weight of the glycosylated fimbrial subunit is approximately 66,000, while unglycosylated protein has an approximate molecular weight of 8,644. The fimbriae function as adhesins mediating C. albicans binding to human buccal epithelial cells. Amino acid analysis of the purified fimbrial subunit indicates that the fimbrial subunit is composed of 50% hydrophobic amino acid residues. The N terminus of the fimbrial subunit is blocked to N-terminal sequencing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borg M., Rüchel R. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect Immun. 1988 Mar;56(3):626–631. doi: 10.1128/iai.56.3.626-631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryn K., Jantzen E. Quantification of 2-keto-3-deoxyoctonate in (lipo)polysaccharides by methanolytic release, trifluoroacetylation and capillary gas chromatography. J Chromatogr. 1986 Nov 26;370(1):103–112. doi: 10.1016/s0021-9673(00)94678-8. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Lehrer N., Segal E. Adherence of Candida albicans to buccal and vaginal epithelial cells: ultrastructural observations. Can J Microbiol. 1984 Aug;30(8):1001–1007. doi: 10.1139/m84-156. [DOI] [PubMed] [Google Scholar]
- Castle N. A. Differential inhibition of potassium currents in rat ventricular myocytes by capsaicin. Cardiovasc Res. 1992 Nov;26(11):1137–1144. doi: 10.1093/cvr/26.11.1137. [DOI] [PubMed] [Google Scholar]
- Cox F. Adherence of Candida albicans to buccal epithelial cells in children and adults. J Lab Clin Med. 1983 Dec;102(6):960–972. [PubMed] [Google Scholar]
- Critchley I. A., Douglas L. J. Role of glycosides as epithelial cell receptors for Candida albicans. J Gen Microbiol. 1987 Mar;133(3):637–643. doi: 10.1099/00221287-133-3-637. [DOI] [PubMed] [Google Scholar]
- Cutler J. E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- Day A. W., Poon N. H. Fungal fimbriae. II. Their role in conjugation in Ustilago violacea. Can J Microbiol. 1975 Apr;21(4):547–557. doi: 10.1139/m75-077. [DOI] [PubMed] [Google Scholar]
- Doig P., Sastry P. A., Hodges R. S., Lee K. K., Paranchych W., Irvin R. T. Inhibition of pilus-mediated adhesion of Pseudomonas aeruginosa to human buccal epithelial cells by monoclonal antibodies directed against pili. Infect Immun. 1990 Jan;58(1):124–130. doi: 10.1128/iai.58.1.124-130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas L. J. Adhesion of Candida species to epithelial surfaces. Crit Rev Microbiol. 1987;15(1):27–43. doi: 10.3109/10408418709104446. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Brawner D. L., Riesselman M. H., Jutila M. A., Cutler J. E. Differential adherence of hydrophobic and hydrophilic Candida albicans yeast cells to mouse tissues. Infect Immun. 1991 Mar;59(3):907–912. doi: 10.1128/iai.59.3.907-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Lay J. G., Hazen B. W., Fu R. C., Murthy S. Partial biochemical characterization of cell surface hydrophobicity and hydrophilicity of Candida albicans. Infect Immun. 1990 Nov;58(11):3469–3476. doi: 10.1128/iai.58.11.3469-3476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C. Participation of yeast cell surface hydrophobicity in adherence of Candida albicans to human epithelial cells. Infect Immun. 1989 Jul;57(7):1894–1900. doi: 10.1128/iai.57.7.1894-1900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett J. A., Squier C. A. Candida albicans ultrastructure: colonization and invasion of oral epithelium. Infect Immun. 1980 Jul;29(1):252–260. doi: 10.1128/iai.29.1.252-260.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Lee J. C., Morris A. L. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun. 1980 Feb;27(2):667–674. doi: 10.1128/iai.27.2.667-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Gerhard W., Croce C. M. Production of antibodies against influenza virus by somatic cell hybrids between mouse myeloma and primed spleen cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2985–2988. doi: 10.1073/pnas.74.7.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcantonio E. E., Hynes R. O. Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988 May;106(5):1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. The ultrastructure of Candida albicans infections. Can J Microbiol. 1981 Nov;27(11):1156–1164. doi: 10.1139/m81-181. [DOI] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition, adherence, and virulence of Candida albicans. Infect Immun. 1984 Jul;45(1):6–12. doi: 10.1128/iai.45.1.6-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachran D. W., Irvin R. T. Adhesion of Pseudomonas aeruginosa to human buccal epithelial cells: evidence for two classes of receptors. Can J Microbiol. 1985 Jun;31(6):563–569. doi: 10.1139/m85-105. [DOI] [PubMed] [Google Scholar]
- Mohamed A. M. Ultrastructural aspects of chronic oral candidosis. J Oral Pathol. 1975 Oct-Nov;4(4):180–194. doi: 10.1111/j.1600-0714.1975.tb01741.x. [DOI] [PubMed] [Google Scholar]
- Montes L. F., Wilborn W. H. Ultrastructural features of host-parasite relationship in oral candidiasis. J Bacteriol. 1968 Oct;96(4):1349–1356. doi: 10.1128/jb.96.4.1349-1356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T., Song Z. L., Hinenoya H., Ohtsuka A., Taguchi T., Liu J. J., Sano T. Lysine-mediated tissue osmication in combination with a tannin-osmium conductive staining method for non-coated scanning electron microscopy of biological specimens. Arch Histol Jpn. 1987 Dec;50(5):485–493. doi: 10.1679/aohc.50.485. [DOI] [PubMed] [Google Scholar]
- Poon N. H., Day A. W. Fungal fimbriae. I. Structure, origin, and synthesis. Can J Microbiol. 1975 Apr;21(4):537–546. doi: 10.1139/m75-076. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Digre K. B., Payne C. D. Adherence of Candida species to human epidermal corneocytes and buccal mucosal cells: correlation with cutaneous pathogenicity. J Invest Dermatol. 1984 Jul;83(1):37–41. doi: 10.1111/1523-1747.ep12261661. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Payne C. D. Scanning electron microscopy of epidermal adherence and cavitation in murine candidiasis: a role for Candida acid proteinase. Infect Immun. 1988 Aug;56(8):1942–1949. doi: 10.1128/iai.56.8.1942-1949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotrosen D., Calderone R. A., Edwards J. E., Jr Adherence of Candida species to host tissues and plastic surfaces. Rev Infect Dis. 1986 Jan-Feb;8(1):73–85. doi: 10.1093/clinids/8.1.73. [DOI] [PubMed] [Google Scholar]
- Shepherd M. G., Poulter R. T., Sullivan P. A. Candida albicans: biology, genetics, and pathogenicity. Annu Rev Microbiol. 1985;39:579–614. doi: 10.1146/annurev.mi.39.100185.003051. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Staddon W., Todd T., Irvin R. T. Equilibrium analysis of binding of Candida albicans to human buccal epithelial cells. Can J Microbiol. 1990 May;36(5):336–340. doi: 10.1139/m90-058. [DOI] [PubMed] [Google Scholar]
- Tosh F. D., Douglas L. J. Characterization of a fucoside-binding adhesin of Candida albicans. Infect Immun. 1992 Nov;60(11):4734–4739. doi: 10.1128/iai.60.11.4734-4739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Poulain D., Vernes A. Cytochemical and ultrastructural studies of Candida albicans. III. Evidence for modifications of the cell wall coat during adherence to human buccal epithelial cells. Arch Microbiol. 1984 Oct;139(2-3):221–224. doi: 10.1007/BF00402004. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]