Abstract
Stress granules (SGs) are dynamic cytosolic aggregates composed of ribonucleoproteins that are induced during cellular stress when protein synthesis is inhibited. The function of SGs is poorly understood, but they are thought to be sites for reorganizing mRNA and protein. Several viruses can modulate SG formation, suggesting that SGs have an impact on virus infection. In this study, we have investigated the relationship of SG formation in Drosophila S2 cells infected by cricket paralysis virus (CrPV), a member of the Dicistroviridae family. Despite a rapid shutoff of host translation during CrPV infection, several hallmark SG markers such as the Drosophila TIA-1 and G3BP (RasGAP-SH3-binding protein) homologs, Rox8 and Rin, respectively, do not aggregate in CrPV-infected cells, even when challenged with potent SG inducers such as heat shock, oxidative stress, and pateamine A treatment. Furthermore, we demonstrate that a subset of P body markers become moderately dispersed at late times of infection. In contrast, as shown by fluorescent in situ hybridization, poly(A)+ RNA granules still form at late times of infection. These poly(A)+ RNA granules do not contain viral RNA nor do they colocalize with P body markers. Finally, our results demonstrate that the CrPV viral 3C protease is sequestered to SGs under cellular stress but not during virus infection. In summary, we propose that dicistrovirus infection leads to the selective inhibition of distinct SGs so that viral proteins are available for viral processing.
In response to environmental stress such as oxidative stress or heat shock, cells respond by shutting down overall protein synthesis. This results in the disassembly of polyribosomes, leading to stalled initiation complexes that are dynamically recruited to cytoplasmic foci called stress granules (SGs) (reviewed in references 2, 4, and 9). SGs are not required for global translation repression (8, 36, 41, 44, 49) or global mRNA stability (8, 26). Instead, it has been proposed that SGs are sites where the increased local concentration of proteins and mRNAs allows for remodeling and redistribution of mRNPs (9). Alternatively, it has been shown that specific proteins can be selectively sequestered to or away from SGs, thus affecting biochemical processes in the cell. For instance, recruitment to SGs of specific proteins such as RACK1, which is required to activate the apoptosis-inducing MTK1 kinase during mild stress, can inhibit apoptosis (3). Interestingly, it has become apparent that viruses can affect SG formation, suggesting that SGs influence the virus life cycle (reviewed in reference 4). Because the function of SGs is still poorly understood, further examination into the interplay between SGs and virus infection may shed light on this process.
The primary trigger for SG assembly is the inhibition of protein synthesis, where stalled initiation complexes are shuttled to and aggregate into SG foci. SG formation can occur in cells that respond to environmental stresses or through the addition of chemicals that block the activity of specific translation initiation factors (reviewed in references 2 and 9). One of the best-described pathways is through eIF2α (eukaryotic initiation translation factor 2α) phosphorylation (30, 33). In response to distinct stresses, eIF2α kinases are activated to target and phosphorylate eIF2α, which inhibits a key step in translation initiation, leading to stalled initiation complexes on mRNA and subsequent movement to SGs (33). SGs can also be induced in an eIF2α-independent manner. For example, treatment of cells with hippuristanol or pateamine A (PatA), which alters the activity of the helicase, eIF4A, also induces SG formation (12, 42). In general, inhibition of translation that results in the release of translating ribosomes will trigger SG assembly. However, there are exceptions. In one study, preventing 60S subunit joining with the 40S subunit does not lead to SG assembly, suggesting that translational repression can be uncoupled from SG induction (44). Thus, SGs may form only through the inhibition of specific translation factors or within a defined window during translation initiation (9).
SGs are nonmembranous dense complexes composed of several proteins and RNA. In addition to stalled initiation complexes composed of translation initiation factors such as eIF4E, eIF2, eIF3, poly(A)-binding protein (PABP), and the small 40S ribosomal subunit, SGs contain hallmark protein markers such as T-cell intracellular antigen 1 (TIA-1), TIA-1-related protein (TIAR), and GTPase (Src homology 3 [SH3] domain) binding protein G3BP (31, 60). TIA-1 and TIAR are closely related proteins that contain RNA recognition motifs and are implicated in RNA metabolism (59). G3BP, a member of the Ras signaling pathway, was discovered by its ability to bind to the SH3 domain of RasGAP and has since been implicated in a number of biological processes including RNA metabolism (28). All three proteins contain domains that are important for the aggregation and formation of SGs. For example, TIA-1 and TIAR lacking the QN-rich prion-like domain, which allows for self-aggregation, can no longer form SGs (23). There are currently more than 50 proteins that are associated with SGs (reviewed in references 2 and 9). However, not all SGs are compositionally homogeneous. For example, tristetraprolin (TTP) is recruited to SGs when cells are treated with the mitochondrial inhibitor FCCP [carbonyl cyanide-p-(trifluoromethoxy)phenylhydrazone] but not with arsenite (56). Thus, compositionally distinct SGs may form depending on the type of cellular stress. Furthermore, a recent report using a small interfering RNA (siRNA) screen identified more than 100 genes involved in the assembly and disassembly of SGs (49). Which of these genes are specifically regulating the assembly of distinct SGs remains to be determined. Another key component of SGs is mRNA. Currently, it is not clear if all mRNAs or a distinct subset of mRNAs are recruited to SGs. Interestingly, a recent report described the recruitment of cleaved tRNAs to SGs under angiotensin-induced stress, suggesting that distinct RNA populations may exist in SGs (15).
SG assembly is dynamic. Under stress, stalled initiation complexes are shuttled to SGs (30, 33, 34). Upon recovery from the stress, SGs disassemble, and stalled initiation complexes can now reenter the translation cycle and assemble onto polyribosomes. Inhibiting mRNA release from translating ribosomes by treating cells with translation elongation inhibitors such as emetine or cycloheximide can inhibit SG formation (31). Moreover, fluorescence recovery after photobleaching (FRAP) studies have shown that proteins shuttle in and out of SGs rapidly (20, 24, 31, 32, 45). Thus, SG assembly and disassembly are in equilibrium with translating polysomes, and this process is likely highly regulated. A recent study reported that dynein and kinesin motors are required for SG assembly and disassembly, indicating that RNPs are delivered to SGs on microtubule tracks (41).
P bodies (PBs) are another type of cytoplasmic RNA granule distinct from SGs (reviewed in references 16 and 50). Normally present under basal conditions, PBs become larger and more abundant under cellular stress such as oxidative stress and glucose deprivation (57). Like SG formation, PB formation is dependent on the pool of nontranslating mRNAs, and PBs are composed of proteins involved in the mRNA degradation pathway including DCP1, DCP2, LSM1 to LSM7, XRN1, and EDC3 (50). In addition, PBs are sometimes composed of factors involved in the microRNA (miRNA) repression machinery and nonsense-mediated decay (50). PBs often associate with and are adjacent to SGs and share a subset of proteins with SGs such as the decapping enzyme eIF4E and Rck/p54 (32). These observations have led to the idea that mRNPs cycle between cytosol, PBs, and SGs (9, 32). Finally, although inhibition of protein synthesis can lead to an increase in the number and size of SGs and PBs, the study by Ohn et al. has shown that SGs and PBs can assemble independently, suggesting that formation of these RNA granules may be specifically regulated (49).
The importance of SGs and PBs has been highlighted by observations that several viruses can modulate SG assembly (reviewed in reference 4). Viruses including the alphavirus Semliki Forest virus, rotavirus, dengue virus, and West Nile virus (WNV) inhibit SG formation (14, 43, 46). Moreover, it appears that these viruses may inhibit SG formation via distinct strategies. In poliovirus-infected cells, translation arrest early in infection leads to induction of G3BP-containing SGs that disassemble at later times of infection (63). This study showed that SG disassembly is due to cleavage of G3BP by the poliovirus 3C protease and that expression of a noncleavable G3BP in poliovirus-infected cells attenuates virus replication (63). However, a recent study reported that SGs containing poly(A)+ RNA and TIA-1 are still induced in poliovirus-infected cells, suggesting that compositionally distinct SGs may be modulated during infection (51). In WNV-infected cells, the recruitment of TIA-1 and TIAR to the minus-strand viral RNA for viral replication may play a part in inhibiting SG assembly (14, 39). These studies suggest that viruses disassemble or modulate SGs to evade being sequestered to nontranslating cytoplasmic foci and that SG induction may act as a host antiviral response. Further investigation into the antiviral role of SGs is needed. Like SGs, PBs also play important roles during virus infection. For example, depletion of PBs increases translation of viral RNAs in HIV-infected cells (47). Moreover, it was demonstrated that miR-29A interactions with the HIV RNA are sequestered to PBs, suggesting that PBs play an antiviral role (47). Clearly, understanding how viruses modulate SGs and PBs may provide insight into the significance of these mRNPs during the viral life cycle.
Dicistroviruses are positive-sense, single-stranded RNA viruses that primarily infect insects and arthropods. Members of the Dicistroviridae family include the cricket paralysis virus (CrPV), Drosophila C virus (DCV), Taura syndrome virus (TSV), and Israeli acute paralysis virus (IAPV) (reviewed in reference 6). Several of these viruses have had a negative impact economically and agriculturally. TSV infection led to the collapse of shrimp farms on the American continent in the 1990s (7), whereas IAPV has been recently linked to colony collapse disorder in honeybees (11). Dicistroviruses encode an 8- to 10-kb-long RNA genome which contains two open reading frames, encoding the nonstructural and structural proteins (reviewed in reference 6). Both open reading frames are translated by distinct internal ribosome entry sites (21, 29, 64). CrPV infection of Drosophila cells leads to a rapid shutoff of host protein synthesis concomitant with phosphorylation of eIF2α (21, 64). Given that these properties are cellular signatures for SG induction, we investigated whether SG assembly is affected during dicistrovirus infection. We demonstrate that SGs and also PBs are modulated in both CrPV- and DCV-infected cells, suggesting that disruption of these RNA granules is important for virus infection. Our results also show that the CrPV 3C protease is sequestered in SGs under cellular stress but becomes dispersed during viral infection. We propose that CrPV inhibits SG formation to keep viral proteins and RNA available for viral replication and translation. We discuss the potential role that SGs and PBs may have in the Drosophila antiviral response.
MATERIALS AND METHODS
Cell culture and virus.
S2 cells (Drosophila Schneider 2) were maintained and passaged in M3-BPYE (M3 insect medium supplemented with bactopeptone-yeast extract) medium containing 10% fetal bovine serum. CrPV and DCV production in Drosophila S2 cells has been previously described (21). A total of 3 × 109 cells were infected with 1 ml of virus suspension for 30 min. S2 cells were subsequently incubated in 60 ml of conditioned medium for 14 h at 25°C. The cells were pelleted, resuspended in phosphate-buffered saline (PBS), and subjected to four freeze-thaw cycles.
Viral titers and yields were determined as described previously (21). UV-irradiated CrPV was produced as described previously (21). The lack of replication of the UV-irradiated CrPV was confirmed by infecting S2 cells and probing for the viral RNA by Northern blot analysis.
Plasmids and transfection.
pAc5.1 GFP-PABP (where GFP is green fluorescent protein) and pAc5.1 V5-eIF3 S9 were generously provided by Pam Silver (Harvard). GFP-DCP1a and GFP-GW182 were gifts from Elisa Izaurralde (Max Planck). Red fluorescent protein (RFP)-AGO2 and GFP-AGO1 were generously provided by Andrew Simmonds (University of Alberta). Full-length Rox8, FMR1, and CrPV viral proteins were amplified by reverse transcription-PCR (RT-PCR) from RNA extracted from S2 cells infected with CrPV. Full-length Rin (Rasputin) cDNA was PCR amplified from a clone obtained from the Drosophila Genomics Resource Center. Rox8 and Rin were fused with GFP by PCR amplification and ligated into pAc5.1 to produce Rox8-GFP and Rin-GFP. FMR1 and the viral 3C protein were PCR amplified and ligated into pAc5.1 fused in frame with the V5 tag to produce FMR1-V5 and 3C-V5.
For transient transfections, 1 × 107 cells were transfected with 20 μg of plasmid DNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Cells were incubated for 18 h. S2 cells were infected with CrPV or DCV at a multiplicity of infection (MOI) of 10. In detail, S2 cells were centrifuged and washed once with PBS. Cells were infected with virus for 30 min, and subsequently conditioned medium was added. Cells were treated with 50 nM pateamine A (generous gift of Jerry Pelletier, McGill University) or 500 μM arsenite or incubated at 42°C to induce SGs. In preparation for microscopy, a total of 1 × 106 S2 cells were plated on coverslips precoated with 0.5 mg/ml concanavalin A (Calbiochem) for 1 h. Cells were subsequently fixed with 3% paraformaldehyde in PBS and permeabilized in 0.5% Tween in PBS.
In situ hybridization.
In preparation for in situ hybridization, S2 cells were incubated in hybridization buffer (2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 20% formamide, 0.2% bovine serum albumin [BSA], 1 μg/μl Saccharomyces cerevisiae tRNA) for 15 min at 37°C. Subsequently, S2 cells were hybridized with 1 mg/ml DNA probes, oligo(dT) (40) conjugated to Cy-3 or Cy-5, antisense CrPV RNA conjugated to Cy-5, or antisense 18S rRNA conjugated to Cy-3 overnight at 46°C in hybridization buffer (IDT). The next day, S2 cells were washed with 2× SSC with 20% formamide twice for 5 min each at 37°C, 2× SSC twice for 5 min each at 37°C, and 1× SSC once for 5 min. If needed, cells were costained by indirect immunofluorescence. The 18S rRNA probe sequence was GTCCTAGATACTACCACAAAAGTTGATAG. The antisense CrPV RNA probe sequence was ACTAAATAAAATATACAATAAAT.
Indirect immunofluorescence assays.
After in situ hybridization, S2 cells were washed with PBS and incubated with antibodies in PBS containing 5% horse serum for 1 h at 25°C. The antibodies include 1:200 rabbit-anti-deIF4E (generous gift from Paul Lasko, McGill University), 1:500 rabbit anti-V5 IgG (Sigma), 1:200 anti-double-stranded RNA ([dsRNA] English & Scientific Consulting Bt), and 1:500 mouse anti-O-GlcNAc IgG (Covance). Cells were subsequently washed with PBS three times and incubated for 1 h at 25°C with the secondary antibodies goat anti-rabbit Texas Red IgG (1:300; Invitrogen) and goat anti-mouse Alexa Fluor 568 IgG (1:500; Invitrogen). Cells were then washed three times with PBS. Coverslips were mounted on slides with Prolong Gold Antifade Reagent (Invitrogen). Images were acquired by a confocal microscope (Olympus FV1000 using Olympus Fluoview, version 2.0a) with a 60× oil immersion lens. Images shown are representative of a single Z-section. Photoshop CS2 software was used to process the images. Quantitation of the percentage of cells with SGs or PBs was measured by manually counting the number of cells with ≥3 or ≥10 visible foci per cell.
Immunoblotting.
S2 cells were lysed in lysis buffer (20 mM HEPES, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 1 mM EDTA, 10 mM tetrapyrophosphate, 100 mM NaF, 17.5 mM β-glycerophosphate, and a protease inhibitor cocktail [Roche]). Equal amounts of lysates were loaded and separated on an SDS-PAGE gel and then transferred to a polyvinylidene difluoride Immobilon-FL membrane (Millipore). The membrane was blocked for 30 min in 5% skim milk in TBST (20 mM Tris, 150 mM NaCl, 0.1% Tween) and incubated at 4°C overnight with the following antibodies: 1:1,000 mouse anti-GFP (Roche), 1:5,000 rabbit anti-V5 (Sigma), and 1:1,000 rabbit-anti-deIF4E (generous gift from Paul Lasko, McGill University). Membranes were washed with TBST three times and incubated with IRDye 800CW goat-anti-rabbit IgG (Li-Cor Biosciences) or IRDye 680CW goat anti-mouse 1:10,000 for 1 h at room temperature in 5% skim milk in TBST. The membranes were washed three times in TBST and scanned on an Odyssey imager (Li-Cor Biosciences). In some experiments, a 1:30,000 dilution of donkey anti-rabbit IgG-horseradish peroxidase (Amersham) or a 1:10,000 dilution of goat anti-mouse IgG-horseradish peroxidase (Santa Cruz Biotechnology) was also used to detect proteins by chemiluminescence (Millipore).
RESULTS
Induction of stress granules in Drosophila S2 cells.
Previous reports demonstrated that SGs can be induced in Drosophila S2 cells under oxidative and heat shock stress, stresses that inhibit translation initiation in an eIF2α phosphorylation-dependent and -independent manner (19, 41). To further provide a comprehensive analysis of SG formation in Drosophila cells, we monitored the formation of mRNA granules by in situ hybridization using an oligo(dT)-Cy3 in S2 cells treated with arsenite (oxidative stress), heat shock (42°C), or pateamine A (PatA). PatA inhibits translation initiation by sequestering eIF4A from the cap-binding complex, which results in the assembly of SGs in mammalian cells (12, 42). In untreated S2 cells, the poly(A)+ RNA was primarily diffuse in the cytoplasm. Treatment of cells with arsenite or heat shock for 1 h induced poly(A)+ RNA granules (∼100%) (Fig. 1). Similarly, treatment of cells with PatA also induced poly(A)+ RNA granules (∼100%), demonstrating that the induction of RNA granules when eIF4A activity is inhibited is a conserved process from Drosophila to mammals (Fig. 1). To determine whether these poly(A)+ RNA granules are SGs, we monitored the subcellular localization of a panel of SG protein markers. Because antibodies to several Drosophila SG markers are not yet available, we transiently transfected expression vectors containing SG markers fused with either GFP or V5. As observed previously with some of the markers (19), expression of the fusion SG markers was cytoplasmically diffuse in untreated cells (Fig. 1). In contrast, in all stressed cells tested, exogenously expressed Rox8-GFP, GFP-PABP, FMR1-V5, and Rin-GFP were recruited to granules that colocalized with poly(A)+ RNA granules (Fig. 1A to C and E). Rox8 and Rin are the Drosophila homologues of the mammalian TIA-1 and G3BP, respectively, which are classic markers of SGs (31, 60). Other signature SG markers include translation initiation factors and the small ribosomal subunit (30, 33, 34). We monitored the subcellular localization of a subunit of the translation factor eIF3 (fusion V5-eIF3 S9) and the endogenous cap-binding protein, eIF4E, by indirect immunofluorescence. We also detected the small ribosomal subunit by fluorescent in situ hybridization using probes that hybridize to the 18S rRNA. Under all stress conditions, a fraction of V5-eIF3 S9, endogenous eIF4E, and the 18S rRNA partially colocalized with poly(A)+ RNA granules, consistent with previous reports that stalled translation complexes are recruited to SGs (Fig. 1D, F, and G). Recently, it has been shown that a subset of O-GlcNAc-modified proteins colocalizes with SG markers (49). In untreated cells, O-GlcNAc staining by immunofluorescence showed punctate foci that did not colocalize with poly(A)+ granules. In PatA-treated cells, a small fraction of O-GlcNAc staining colocalized with the poly(A)+ RNA granules, suggesting that, as in the mammalian cells, the GlcNAc modification may be important for assembly of SGs in Drosophila cells (Fig. 1H). In summary, we have established that SGs can be induced in Drosophila cells under a number of stresses using a comprehensive panel of SG protein markers.
FIG. 1.
SG markers colocalize with poly(A)+ RNA granules in stressed Drosophila S2 cells. S2 cells were transiently transfected with expression vectors containing Rox8-GFP (A), Rin-GFP (B), GFP-PABP (C), V5-eIF3 S9 (D), or FMR1-V5 (E). A day later, S2 cells were either left untreated (control) or were treated with 50 nM PatA or 500 μM arsenite or heat-shocked at 42°C for 1 h. Poly(A)+ RNA was detected by fluorescence in situ hybridization using Cy3- or Cy5-oligo(dT) (40) (red fluorescence). S2 cells were subsequently costained with antibodies to V5 (D and E), eIF4E (F), or O-GlcNAc (H) or cohybridized with Cy3-antisense 18S rRNA (G). All SG markers except for poly(A)[supb]+[r] RNA are shown in the green channel. Enlarged views of the boxed areas highlight different channels and merged views.
Modulation of SGs during cricket paralysis virus infection.
In CrPV-infected S2 cells, complete shutoff of host translation occurs by 3 to 4 h of infection, concomitant with phosphorylation of eIF2α; thus, we would predict that SGs would form during this time (21). For this analysis, cells with ≥3 visible foci per cell were counted as SG-containing cells. We found that this approach provided a reproducible and stringent assessment of a cell containing SGs. In uninfected cells, only ∼3 to 11% of transfected cells exhibited punctate foci containing Rox8-GFP, Rin-GFP, or PABP-GFP (Fig. 2 A to C). Although host translation shutoff occurs by 4 h postinfection (hpi), only ∼15 to 20% of transfected cells at 4 and 6 hpi showed recruitment of Rox8-GFP and Rin-GFP to SGs, compared to ∼9 to 11% of mock-infected cells, indicating that the majority of infected cells displayed cytoplasmically diffuse Rox8-GFP and Rin-GFP (Fig. 2A and B). Similarly, staining of V5-eIF3 S9 and FMR1-V5 by indirect immunofluorescence was primarily diffuse in both mock-infected cells and at 6 hpi (Fig. 2D and F). Also, no change in O-GlcNAc staining was observed in mock- and CrPV-infected cells, and there was a lack of colocalization between O-GlcNAc staining and poly(A)+ granules at 6 hpi (Fig. 2E). In summary, these results indicate that SG assembly as defined by these SG protein markers is inhibited during CrPV infection. However, unlike results for the SG markers above, the number of transfected cells exhibiting foci that contain GFP-PABP increased to 21% and 43% by 4 and 6 hpi, respectively, compared to ∼3% in mock-infected and at 2 hpi (Fig. 2C). This suggests that the assembly of SGs composed of GFP-PABP during CrPV infection is distinct from that of other SG markers. Interestingly, the percentage of cells containing ≥3 poly(A)+ RNA granules increased during CrPV infection from ∼20% of mock-infected cells to >90% of cells by 8 hpi (Fig. 2G). By using a more stringent analysis, the number of cells exhibiting ≥10 foci containing poly(A)+ RNA granules also increased from ∼20% in mock-infected cells to >70% in CrPV-infected cells by 8 hpi (Fig. 2G). In general, we found that the poly(A)+ RNA granules appeared smaller and more numerous than those observed in cells treated with PatA, heat shock, or arsenite. In every case, all SGs markers (Rox8-GFP, Rin-GFP, V5-eIF3, FMR1-V5, and O-GlcNAc staining) except for GFP-PABP failed to colocalize with poly(A)+ RNA granules (Fig. 2A, B, and D to F). Although primarily diffuse, a fraction of GFP-PABP colocalized with the poly(A)+ RNA granules during infection (Fig. 2C). Thus, CrPV infection inhibits SG formation but not GFP-PABP-containing SGs or poly(A)+ RNA granules in S2 cells.
FIG. 2.
Granules containing PABP and poly(A)+ RNA but not Rox8-GFP or Rin-GFP are formed in CrPV-infected cells. S2 cells transiently transfected with expression vectors containing Rox8-GFP (A), Rin-GFP (B), GFP-PABP (C), FMR1-V5 (D), or V5-eIF3 S9 (F) were mock infected or infected for 2, 4, or 6 h with CrPV at an MOI of 10. The V5 fusion proteins and O-GlcNAc-modified proteins (E) were detected by staining using anti-V5 and anti-GlcNAc antibodies, respectively. (G) Quantitation of the percentage of cells containing poly(A)+ granules. (H) Western blot analysis of the indicated SG protein markers in mock- and CrPV-infected cells at 6 hpi. (I) Inhibition of SGs in Drosophila C virus-infected cells. S2 cells transiently transfected with the expression vector containing Rox8-GFP were mock-infected or infected for 12 h with DCV at an MOI of 10. Poly(A)+ RNA was detected by in situ hybridization using Cy3-oligo(dT) (40) (red fluorescence). Percentages represent the proportion of transfected cells containing at least three granules expressing GFP or staining with V5 or O-GlcNAc. Enlarged views of the boxed areas highlight different channels and merged views. More than 100 cells were analyzed in multiple fields for each experiment. The average of three independent experiments is shown.
In poliovirus-infected cells, the inhibition of G3BP-containing SGs late in infection is due to cleavage of G3BP by the viral 3C protease (63). To rule out the possibility that the diffuse localization of SG proteins in CrPV-infected cells may be due to degradation or cleavage, we monitored the status of these proteins by Western blot analysis. The SG markers, Rin-GFP, eIF4E, Rox8-GFP, FMR1-V5 and V5-eIF3 S9, remained intact and stable in mock- and CrPV-infected cells at 6 hpi (Fig. 2H). Similarly, it has been shown that PABP is not cleaved or degraded during CrPV infection (21). Thus, the lack of SG induction in CrPV-infected cells is not due to loss of these SG markers.
To determine whether SG assembly is actively inhibited during CrPV infection, cells were challenged with PatA treatment for 1 h prior to fixation at different times of infection. PatA treatment of mock-infected cells or cells infected for 2 h induced SGs, as demonstrated by colocalization of poly(A)+ RNA granules with Rox8-GFP, Rin-GFP, and GFP-PABP (Fig. 3 A to C), indicating that the pathway leading to SG assembly is intact during the first 2 h of infection (83 to 100% of cells contained SGs). By 4 and 6 hpi, only 9 to 15% of PatA-treated cells showed foci containing Rox8-GFP and Rin-GFP while the rest of the cells exhibited diffuse GFP expression (Fig. 3A and B). In contrast, close to ∼100% of infected cells at 4 and 6 hpi exhibited ≥3 poly(A)+ RNA granules per cell (Fig. 3). Moreover, similar to observations made during CrPV infection without PatA treatment, these poly(A)+ RNA granules did not colocalize with Rox8-GFP and Rin-GFP (Fig. 3A and B). In the case of GFP-PABP, only a fraction of GFP-PABP colocalized with poly(A)+ RNA granules in PatA-treated cells at 4 and 6 hpi, which is also similar to observations in CrPV-infected cells (Fig. 2C and 3C). To determine whether CrPV infection can inhibit other SG assembly pathways, we challenged infected cells with arsenite and heat shock. Similar to results in PatA-treated cells, Rox8-GFP remained diffuse and did not colocalize with poly(A)+ RNA granules late in infection (Fig. 3D and E). In summary, these results demonstrate that the assembly of Rox8-GFP and Rin-GFP SGs but not poly(A)+ RNA granules is inhibited in CrPV-infected cells.
FIG. 3.
CrPV infection inhibits SG formation in response to PatA treatment. S2 cells transiently transfected with expression vectors containing Rox8-GFP (A), Rin-GFP (B), or GFP-PABP (C) were either mock infected or CrPV infected (MOI of 10) for 2, 4, or 6 h and treated with 50 nM PatA (A to C) or 500 μM arsenite (D) or heat shocked at 42°C (E) for 1 h prior to fixation. Poly(A)+ RNA was detected by in situ hybridization using Cy3-oligo(dT) (40) (red fluorescence). Enlarged views of boxed areas highlight different channels and merged views. Percentages of transfected cells containing at least three GFP-containing granules are shown. More than 100 cells were analyzed in multiple fields for each experiment. The average of three independent experiments is shown.
DCV infection inhibits SG formation.
We next asked whether the related dicistrovirus, Drosophila C virus (DCV), also inhibits SG formation. Like CrPV infection, DCV infection also leads to host translation shutoff but at a later time point than that in CrPV-infected cells (10). At 12 hpi in DCV-infected cells, poly(A)+ RNA granules accumulated visible foci which did not contain Rox8-GFP (Fig. 2I). The Rox8-GFP remained cytoplasmically diffuse. When challenged with PatA for 1 h at 12 hpi, DCV-infected cells still displayed diffuse Rox8-GFP which did not colocalize with poly(A)+ RNA granules (Fig. 2I). Given that both CrPV and DCV infection leads to inhibition of Rox8-containing SGs and induction of poly(A)+ RNA granules, this may suggest a general property of dicistrovirus infections in general.
CrPV replication is required for modulation of SGs.
To determine whether CrPV replication is required for the inhibition of SGs, S2 cells were infected with UV-inactivated CrPV. UV-inactivated CrPV was replication defective and did not produce viral proteins in S2 cells (data not shown). UV-inactivated CrPV failed to induce foci containing Rox8-GFP or poly(A)+ granules at 4 hpi, a time when poly(A)+ granules are normally detected in CrPV-infected cells (Fig. 4). When challenged with PatA for 1 h at 4 hpi, cells infected with UV-inactivated CrPV exhibited foci that contained both Rox8-GFP and poly(A)+ granules (Fig. 4), thus demonstrating that viral replication is necessary for inhibition of SG formation during CrPV infection.
FIG. 4.
UV-inactivated CrPV does not inhibit SGs. Transiently transfected cells expressing Rox8-GFP were mock infected or infected with UV-inactivated CrPV. Where indicated, cells were treated with PatA for 1 h prior to fixation. Poly(A)+ RNA was detected by in situ hybridization using a Cy3-oligo(dT) (40) (red fluorescence). Enlarged views of boxed areas highlight different channels and merged views.
SGs are not maintained in CrPV-infected cells.
Our experiments indicate that the formation of SGs containing markers such as Rox8 and Rin is inhibited after 4 hpi in CrPV-infected cells (Fig. 2 and 3). It is possible that CrPV infection may not only inhibit the assembly of but also promote the disassembly of SGs. To address this, after SGs were induced by the addition of PatA to infected cells at 1 hpi, Rox8-GFP-containing SGs were monitored at 2 or 4 hpi. The 4-hpi time point was chosen because this is when Rox8-containing SGs are inhibited during CrPV infection (Fig. 3). Whereas PatA treatment for 1 h at 2 hpi led to punctate Rox8-GFP granules that colocalized with poly(A)+ RNA, PatA treatment for 3 h at 4 hpi resulted in diffuse Rox8-GFP and in poly(A)+ RNA granules (Fig. 5). The lack of Rox8-GFP-containing SGs is not due to the dynamic property of SGs as cells treated with PatA for 1 or 3 h still displayed foci containing Rox8-GFP that colocalized with poly(A)+ RNA granules (Fig. 5). These results indicate that Rox8-GFP-containing SGs cannot be maintained during the course of infection.
FIG. 5.
CrPV infection leads to disassembly of SGs. Transiently transfected cells expressing Rox8-GFP were infected with CrPV for 2 h or 4 h. At 1 hpi, 50 nM Pat A was added. As controls, uninfected cells were treated with 50 nM PatA for 1 h or 3 h. Cells were subsequently fixed, and poly(A)+ RNA was detected by in situ hybridization using a Cy3-oligo(dT) (40) (red fluorescence). Enlarged views of boxed areas highlight different channels and merged views.
Characterization of poly(A)+ RNA granules in CrPV-infected cells.
The lack of colocalization of several SG markers with poly(A)+ RNA granules in CrPV-infected cells suggests that the poly(A)+ RNA granules may represent distinct RNA granules. One possibility is that these poly(A)+ RNA granules are PBs. To monitor PBs, cells were transfected with plasmids expressing GFP-GW182, GFP-DCP1, GFP-AGO1, or RFP-AGO2. Expression of these reporter proteins resulted in distinct punctate GFP or RFP expression, indicative of basal PB formation (Fig. 6A). PBs containing GFP-DCP1 or GFP-GW182 were present in 100% of transfected cells, whereas those containing GFP-AGO1 or RFP-AGO2 were present in ∼50% of cells (cells with ≥3 foci per cell were counted as PB-containing cells). As shown in this study, at 4 and 6 hpi, poly(A)+ RNA granules were induced in CrPV-infected cells. However, these granules did not colocalize with the GFP-GW182, GFP-DCP1, GFP-AGO1, or RFP-AGO2 foci, indicating that these poly(A)+ RNA granules are not PBs. Interestingly, the number of cells that contained GFP-GW182 and GFP-DCP1 PBs moderately decreased at late times of infection (Fig. 6A). Compared to mock-infected cells (100% of transfected cells), only ∼60% of CrPV-infected cells at 6 hpi displayed ≥3 foci containing GFP-GW182 or GFP-DCP1 (Fig. 6A). This decrease was not due to degradation or cleavage of the PB markers (Fig. 6B). In contrast, the percentage of cells with PBs containing GFP-AGO1 or RFP-AGO2 remained constant throughout infection (Fig. 6A). Thus, these results demonstrate that formation of PBs containing GFP-GW182 or GFP-DCP1 but not GFP-AGO1 or RFP-AGO2 is inhibited in CrPV-infected cells.
FIG. 6.
Virus-induced poly(A)+ RNA granules are not P bodies. (A) S2 cells transiently transfected with expression vectors containing GFP-AGO1, RFP-AGO2, GFP-DCP1, or GFP-GW182 were either mock or CrPV infected for 2, 4, or 6 h with CrPV at an MOI of 10. PB markers are shown in green. Poly(A)+ RNA was detected by in situ hybridization using Cy3-oligo(dT) (40) (red fluorescence). Percentages represent the proportion of transfected cells containing at least three GFP- or RFP-containing granules. Enlarged views of boxed areas highlight different channels and merged views. (B) Western blot analysis of the indicated PB protein markers in mock- and CrPV-infected cells at 6 hpi.
Since the CrPV viral RNA genome has a poly(A) tail (13), we next investigated the possibility that the poly(A)+ RNA granules contain CrPV RNA or viral replicative RNA intermediates. By in situ hybridization using a probe specific to the sense strand of CrPV RNA, fluorescence increased during the course of CrPV infection, strongly indicating that the increase in viral RNAs was specifically detected (Fig. 7). As a control, a probe containing a randomized sequence did not result in fluorescence signal over background (data not shown). Despite the increase in viral RNA, the detectable fluorescence signal did not colocalize with the poly(A)+ RNA granules (Fig. 7). To determine whether the poly(A)+ RNA granules may be sites of replicative intermediates, we used an antibody that specifically recognizes dsRNA (62). Whereas mock-infected cells displayed basal immunofluorescence, the dsRNA staining increased over time in CrPV-infected cells, consistent with an increase in replicative viral intermediates (Fig. 7). The dsRNA staining was punctate, indicating that CrPV replication is localized. However, the dsRNA staining did not colocalize with the poly(A)+ RNA granules in CrPV-infected cells (Fig. 7, compare green and red channels). From these results, we conclude that the virally induced poly(A)+ RNA granules are not PBs and do not contain viral RNA.
FIG. 7.
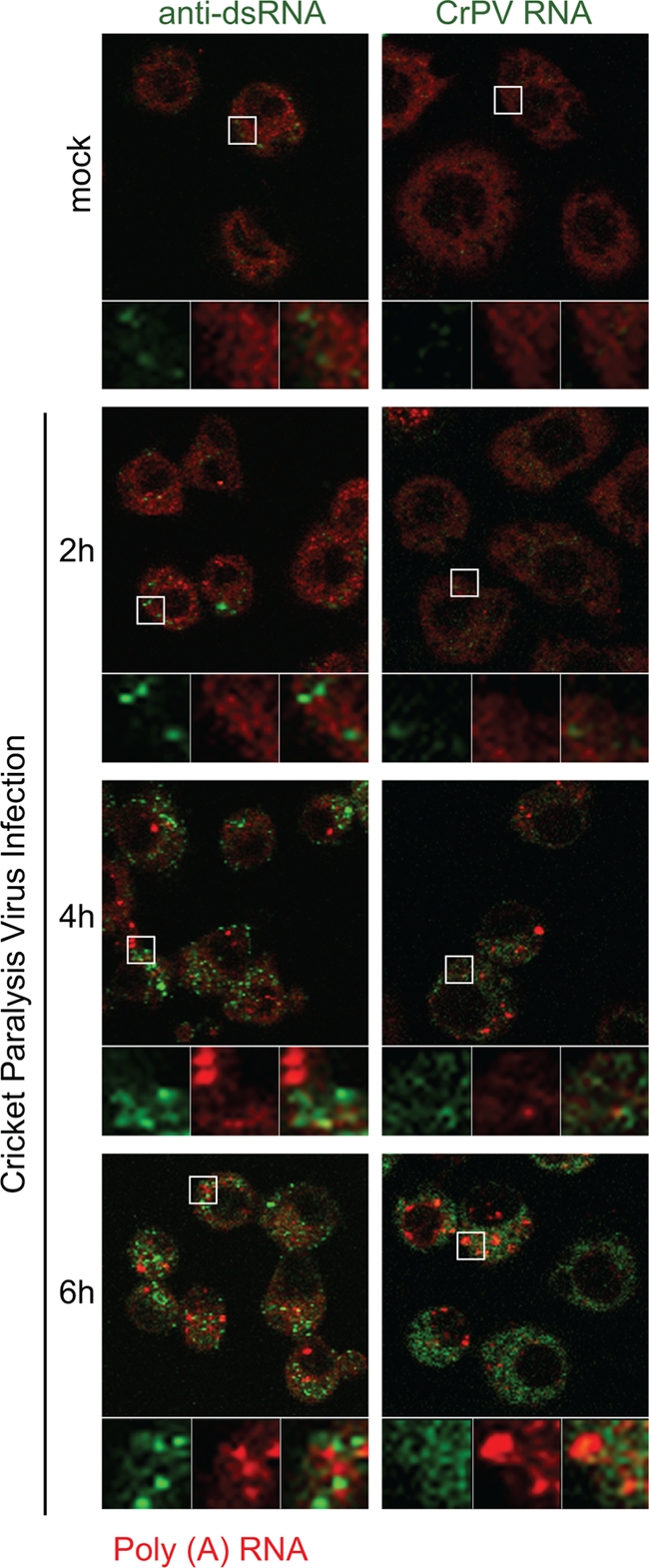
Virus-induced poly(A)+ RNA granules do not contain CrPV RNA. S2 cells were mock or CrPV infected (MOI of 10) for 2, 4, or 6 h and then processed for in situ hybridization. Poly(A)+ RNA was detected by in situ hybridization using a Cy5- or Cy3-oligo(dT) (40) (red). Viral dsRNA intermediates were detected by indirect immunofluorescence using an anti-dsRNA antibody (green). CrPV RNA was detected by using a Cy5-conjugated antisense probe that hybridized to the sense strand of CrPV RNA (green). Enlarged views of boxed areas highlight different channels and merged views.
The CrPV 3C protease is sequestered to SGs.
Our results suggest that CrPV replication is required for the inhibition of SG formation (Fig. 4). One possibility is that a CrPV protein may be mediating this effect. To begin testing this hypothesis, we subcloned individual CrPV nonstructural and structural proteins. Of the CrPV proteins tested so far, none can inhibit the formation of Rox8-GFP-containing SGs in cells treated with PatA. However, through our analysis, we noticed that in cells expressing the V5-tagged 3C protease, the staining of V5 by indirect immunofluorescence colocalized with SGs containing Rox8-GFP and poly(A)+ RNA under PatA treatment (Fig. 8, middle panel). We next tested whether the tagged 3C protease may be colocalizing with the poly(A)+ RNA granules that are induced in CrPV-infected cells. As shown previously in CrPV-infected cells, the Rox8-GFP remained diffuse, and poly(A)+ RNA granules assembled in CrPV-infected cells (Fig. 8, right panel). However, the tagged 3C protease was not recruited to the poly(A)+ granules during CrPV infection and remained cytoplasmically diffuse. Because the viral 3C protease may be susceptible to being sequestered to SGs, one possible outcome of inhibiting SG assembly during CrPV infection is that the 3C protease remains available for viral processing.
FIG. 8.
The CrPV 3C-like protease is sequestered to SGs under cellular stress. S2 cells transiently cotransfected with expression vectors containing Rox8-GFP and CrPV 3C protease-V5 were left untreated (control), treated with 50 nM PatA for 1 h, or infected with CrPV for 6 h. Fusion V5 viral proteins were detected by costaining with anti-V5 antibody (gray), and poly(A)+ RNA was detected by in situ hybridization using Cy5-oligo(dT) (40) (red). Expression of Rox8-GFP is shown in green. Enlarged views of boxed areas highlight different channels and merged views.
DISCUSSION
Viruses have evolved unique mechanisms to evade the host antiviral response and to usurp host factors for viral translation and replication. There is increasing evidence that viruses can modulate SG and PB formation, suggesting that they can impact virus infection and yield (1, 14, 25, 35, 43, 46, 51, 52, 63). Here, we have demonstrated that infection by the dicistroviruses CrPV and DCV inhibits the assembly of SGs, as defined by a set of SG protein markers, but not of granules containing poly(A)+ RNA. Our results suggest that the inhibition of SG formation may keep viral proteins available for CrPV processing, translation, and replication.
SGs are defined as aggregations of nontranslating mRNAs that colocalize with SG protein markers such as TIA-1, G3BP, and several translation initiation factors (reviewed in references 2 and 9). This study expands on previous reports on SGs in Drosophila cells, and we have now demonstrated several SG markers that aggregate and colocalize with poly(A)+ RNA under a number of cellular stress conditions (19, 41). SGs are induced in cells treated with heat shock, arsenite, and PatA, indicating that SG assembly is conserved in Drosophila and that these SGs are compositionally similar to those in mammalian cells (Fig. 1). Using this panel of SG markers, we explored the relationship of SG formation during dicistrovirus infection. In CrPV- and DCV-infected cells, despite a rapid shutoff of host protein synthesis, the majority of the classic SG markers do not aggregate and remain dispersed (Fig. 2). This was surprising as we monitored signature SG markers such as Rox8, Rin, and eIF3. Only the SG marker PABP partially aggregated and colocalized with poly(A)+ RNA granules. Our results showed that infected cells challenged with potent SG inducers such as heat shock, arsenite, or PatA could not induce aggregation of SG markers after 4 hpi but could at earlier times of infection (Fig. 3). This suggests that the inhibition of SG formation in CrPV-infected cells likely targets a general pathway of SG formation at late times of infection. Our results also indicate that Rox8-containing SGs are not maintained during CrPV infection when SGs are induced early in infection (Fig. 5). Taken together, these results indicate that CrPV infection may lead to the assembly and/or promote the disassembly of SGs at late times of infection.
Despite the lack of aggregation of most SG markers, poly(A)+ RNA granules were still induced in CrPV-infected cells (Fig. 2). This observation highlights the importance of monitoring both SG protein markers and poly(A)+ RNA and serves as an example that distinct SGs and RNA granules may be differentially regulated under specific cellular conditions. Moreover, given that there is more evidence that compositionally distinct SGs exist and may be differentially regulated, as we have observed in our studies, it is imperative to monitor as many SG markers as possible during a given cellular stress (51, 56). In our studies, the percentage of cells containing poly(A)+ RNA granules increased during infection, which correlated with viral replication (Fig. 2). To our surprise, using two approaches to detect viral RNA by in situ hybridization and by indirect immunofluorescence using an anti-dsRNA antibody, we found that these poly(A)+ RNA granules do not contain viral RNA (Fig. 7). We also showed that these poly(A)+ RNA granules do not colocalize with PB markers (Fig. 6). Currently, we do not know if these poly(A)+ RNA granules represent novel RNA granules or are distinct SGs that do not colocalize with known classic SG markers but, rather, with a yet to be discovered aggregating protein complex. A recent report showed that in poliovirus-infected cells, the induction of poly(A)+ RNA granules requires ongoing host transcription (51). In CrPV-infected cells, addition of actinomycin D did not affect the induction of poly(A)+ RNA granules or lead to the inhibition of SG marker aggregation (our unpublished results). In CrPV-infected cells, overall translation is shut off by 4 hpi, which is approximately the same time when poly(A)+ RNA granules are induced (21). It is likely that these poly(A)+ RNA granules represent nontranslating mRNAs that are released from polysomes during infection. An outstanding question is whether a subset of mRNAs or all nontranslating mRNAs are localized to these poly(A)+ RNA granules in infected cells.
What is inhibiting SGs during CrPV infection? UV-inactivated CrPV does not mediate the inhibition of SG assembly, indicating that CrPV replication is required (Fig. 4). Furthermore, the inhibition of SG formation occurred after ∼4 hpi, suggesting that a viral protein may be mediating this effect (Fig. 2 and 3). We are currently investigating whether specific CrPV structural and nonstructural proteins inhibit SG formation. SGs can assemble through the action of aggregating proteins such as TIA-1 and G3BP (31, 60). However, during CrPV and DCV infection, these and other SG proteins tested in this study do not aggregate and are not cleaved or degraded (Fig. 2). Besides protein aggregation, posttranslational modifications of specific proteins appear to be important for SG assembly. It has been reported that the deacetylase activity of histone deacetylase (HDAC) is required for SG assembly (36). Moreover, a recent siRNA screen identified several genes (>100) involved in the assembly and disassembly of SGs, suggesting that multiple factors may mediate SG assembly (49). One interesting outcome from this previous study was the discovery that the O-GlcNAc modification of proteins may be important for the assembly of SGs (49). In our studies, the staining of O-GlcNAc by immunofluorescence was localized to SGs under cellular stress but not during CrPV infection, suggesting that CrPV infection does not affect this pathway of SG assembly. Our data suggest that SGs may be actively disrupted during CrPV infection (Fig. 5). The factors Staufen 1 and eIF5A have been shown to disrupt SGs by maintaining ribosomes bound to mRNAs, thus preventing initiation complexes from shuttling to SGs (38, 58). It is unlikely that these factors are responsible for the inhibition of SGs during CrPV infection since host translation is shut off, and the majority of polysomes are disassembled (21). Clearly, more studies are required to elucidate the pathways leading to SG assembly and disassembly.
Our results demonstrated that PBs containing GW182 and DCP1 disassemble late during CrPV infection (Fig. 6). It has been proposed that PBs are sites where translationally stalled mRNPs are shuttled to and destined for degradation or for reorganization of mRNPs. Moreover, PBs are normally found adjacent to SGs during cellular stress, and proteins and mRNAs may be exchanged between PBs and SGs (32). In general, GW182, DCP1, AGO1, and AGO2 interact with each other to form cytoplasmic PB foci containing mRNA and miRNAs (5, 18, 27, 55). However, not all PBs contain the full complement of PB markers, and they can be compositionally distinct. For instance, PBs containing DCP1 and AGO2 do not colocalize under certain cellular stress conditions (37), and a recent study revealed that GW182- and AGO2-containing bodies are distinct from DCP1-containing P bodies (22). Supporting this, CrPV infection affects only granules containing DCP1 and GW182 but not AGO1 and AGO2 (Fig. 6). During CrPV infection, the virus may inhibit DCP1- and GW182-containing PBs specifically in order to keep viral RNAs from degrading. Alternatively, given that GW182 is a key component in miRNA-dependent translational control, it is possible that a miRNA that is normally shuttled to PBs may be required for CrPV infection (17, 40, 54). It has been shown that Ago2 −/− flies are hypersensitive to CrPV and DCV infection, indicating that Ago2 is an antiviral factor in Drosophila (61). Moreover, the CrPV silencing suppressor 1A protein targets and inhibits AGO2 activity, thereby antagonizing RNA interference (48). Because AGO2 remains punctate during CrPV infection, it remains to be seen whether this process may contribute to the suppression of AGO2 activity and affect viral yield.
We observed that the V5-tagged 3C protease colocalized with Rox8-GFP and poly(A)+ RNA granules under PatA-induced stress, suggesting that this viral protein has a tendency to be sequestered to SGs (Fig. 7). Under viral infection, the 3C protease remained disperse and did not colocalize with the poly(A)+ RNA granules. Thus, we propose that one purpose of inhibiting SG formation during infection is to keep the CrPV 3C protease available and free for viral protein processing. Alternatively, given that SGs may sequester specific proteins to regulate apoptosis (3), it is possible that the inhibition of SGs in CrPV-infected cells affects other cellular processes.
The significance and function of SGs are not well understood. However, modulation of SGs appears to be a common host response during viral infection, as several viruses have been shown to modulate SGs and PBs. Infection with viruses such as alphaviruses (43, 63) leads to a transient induction of SGs at early times postinfection, but at later times SGs become dispersed. Alternatively, the flaviviruses West Nile virus and dengue virus completely inhibit SG induction even when challenged with strong SG inducers, similar to results in this study with dicistrovirus infections (14). There is some indication that SGs act to limit viral infections. For instance, mouse embryonic fibroblasts lacking TIA-1 show increased coronavirus production (53). In poliovirus-infected cells, expression of a cleavage-resistant G3BP attenuates virus replication (63). Finally, viruses may not just modulate SG assembly but may also utilize key SG components. For instance, the SG marker TIAR binds to the 3′ untranslated region (UTR) of the WNV RNA and augments viral replication (14, 39). In this present study, we have demonstrated that CrPV infection may limit SG assembly to maintain viral proteins available for virus infection. Whether certain SG proteins, as in the case of TIAR and WNV (14, 39), are utilized for dicistrovirus infection will be an interesting aspect to examine in future studies. In summary, our studies have expanded on the number of viruses that modulate SGs and further highlight the significance of this process during virus infection.
Acknowledgments
We thank Kurt Gustin for critical reading of the manuscript and for insightful discussions. We are grateful to Natalie Farny, Andrew Simmonds, Pamela Silver, and Elisa Izaurralde for providing SG and PB expression vectors. We also thank Paul Lasko for providing the eIF4E antibody and Jerry Pelletier for pateamine A. A special thanks goes to the laboratory of Doug Allan for assistance on the confocal microscope.
This study was supported by a Canadian Institute of Health Research Grant (CIHR) (MOP-81244). E.J. is supported by career development awards by Michael Smith Foundation for Health Research and CIHR.
Footnotes
Published ahead of print on 24 November 2010.
REFERENCES
- 1.Abrahamyan, L. G., et al. 2010. Novel Staufen1 ribonucleoproteins prevent formation of stress granules but favour encapsidation of HIV-1 genomic RNA. J. Cell Sci. 123:369-383. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P., and N. Kedersha. 2009. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 10:430-436. [DOI] [PubMed] [Google Scholar]
- 3.Arimoto, K., H. Fukuda, S. Imajoh-Ohmi, H. Saito, and M. Takekawa. 2008. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 10:1324-1332. [DOI] [PubMed] [Google Scholar]
- 4.Beckham, C. J., and R. Parker. 2008. P bodies, stress granules, and viral life cycles. Cell Host Microbe 3:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behm-Ansmant, I., et al. 2006. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 20:1885-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonning, B. C., and W. A. Miller. 2010. Dicistroviruses. Annu. Rev. Entomol. 55:129-150. [DOI] [PubMed] [Google Scholar]
- 7.Brock, J. A. 1997. Taura syndrome, a disease important to shrimp farms in the Americas. World J. Microbiol. Biotechnol. 13:415-418. [Google Scholar]
- 8.Buchan, J. R., D. Muhlrad, and R. Parker. 2008. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J. Cell Biol. 183:441-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchan, J. R., and R. Parker. 2009. Eukaryotic stress granules: the ins and outs of translation. Mol. Cell 36:932-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherry, S., et al. 2005. Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes Dev. 19:445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox-Foster, D. L., et al. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318:283-287. [DOI] [PubMed] [Google Scholar]
- 12.Dang, Y., et al. 2006. Eukaryotic initiation factor 2α-independent pathway of stress granule induction by the natural product pateamine A. J. Biol. Chem. 281:32870-32878. [DOI] [PubMed] [Google Scholar]
- 13.Eaton, B. T., and A. D. Steacie. 1980. Cricket paralysis virus RNA has a 3′ terminal poly(A). J. Gen. Virol. 50:167-171. [Google Scholar]
- 14.Emara, M. M., and M. A. Brinton. 2007. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc. Natl. Acad. Sci. U. S. A. 104:9041-9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emara, M. M., et al. 2010. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J. Biol. Chem. 285:10959-10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eulalio, A., I. Behm-Ansmant, and E. Izaurralde. 2007. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8:9-22. [DOI] [PubMed] [Google Scholar]
- 17.Eulalio, A., E. Huntzinger, and E. Izaurralde. 2008. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat. Struct. Mol. Biol. 15:346-353. [DOI] [PubMed] [Google Scholar]
- 18.Eystathioy, T., et al. 2002. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell 13:1338-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farny, N. G., N. L. Kedersha, and P. A. Silver. 2009. Metazoan stress granule assembly is mediated by P-eIF2α-dependent and -independent mechanisms. RNA 15:1814-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujimura, K., F. Kano, and M. Murata. 2008. Identification of PCBP2, a facilitator of IRES-mediated translation, as a novel constituent of stress granules and processing bodies. RNA. 14:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrey, J. L., Y. Y. Lee, H. H. Au, M. Bushell, and E. Jan. 2010. Host and viral translational mechanisms during cricket paralysis virus infection. J. Virol. 84:1124-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbings, D. J., C. Ciaudo, M. Erhardt, and O. Voinnet. 2009. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 11:1143-1149. [DOI] [PubMed] [Google Scholar]
- 23.Gilks, N., et al. 2004. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15:5383-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guil, S., J. C. Long, and J. F. Caceres. 2006. hnRNP A1 relocalization to the stress granules reflects a role in the stress response. Mol. Cell Biol. 26:5744-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henao-Mejia, J., et al. 2009. Suppression of HIV-1 Nef translation by Sam68 mutant-induced stress granules and nef mRNA sequestration. Mol. Cell 33:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilgers, V., D. Teixeira, and R. Parker. 2006. Translation-independent inhibition of mRNA deadenylation during stress in Saccharomyces cerevisiae. RNA 12:1835-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingelfinger, D., D. J. Arndt-Jovin, R. Luhrmann, and T. Achsel. 2002. The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA 8:1489-1501. [PMC free article] [PubMed] [Google Scholar]
- 28.Irvine, K., R. Stirling, D. Hume, and D. Kennedy. 2004. Rasputin, more promiscuous than ever: a review of G3BP. Int. J. Dev. Biol. 48:1065-1077. [DOI] [PubMed] [Google Scholar]
- 29.Jan, E. 2006. Divergent IRES elements in invertebrates. Virus Res. 119:16-28. [DOI] [PubMed] [Google Scholar]
- 30.Kedersha, N., et al. 2002. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 13:195-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kedersha, N., et al. 2000. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151:1257-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kedersha, N., et al. 2005. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169:871-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedersha, N. L., M. Gupta, W. Li, I. Miller, and P. Anderson. 1999. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2α to the assembly of mammalian stress granules. J. Cell Biol. 147:1431-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimball, S. R., R. L. Horetsky, D. Ron, L. S. Jefferson, and H. P. Harding. 2003. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am. J. Physiol. Cell Physiol. 284:C273-C284. [DOI] [PubMed] [Google Scholar]
- 35.Kozak, S. L., M. Marin, K. M. Rose, C. Bystrom, and D. Kabat. 2006. The anti-HIV-1 editing enzyme APOBEC3G binds HIV-1 RNA and messenger RNAs that shuttle between polysomes and stress granules. J. Biol. Chem. 281:29105-29119. [DOI] [PubMed] [Google Scholar]
- 36.Kwon, S., Y. Zhang, and P. Matthias. 2007. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 21:3381-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung, A. K., J. M. Calabrese, and P. A. Sharp. 2006. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc. Natl. Acad. Sci. U. S. A. 103:18125-18130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, C. H., T. Ohn, P. Ivanov, S. Tisdale, and P. Anderson. 2010. eIF5A promotes translation elongation, polysome disassembly and stress granule assembly. PLoS One 5:e9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, W., et al. 2002. Cell proteins TIA-1 and TIAR interact with the 3′ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J. Virol. 76:11989-12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, J., F. V. Rivas, J. Wohlschlegel, J. R. Yates III, R. Parker, and G. J. Hannon. 2005. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 7:1261-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loschi, M., C. C. Leishman, N. Berardone, and G. L. Boccaccio. 2009. Dynein and kinesin regulate stress-granule and P-body dynamics. J. Cell Sci. 122:3973-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazroui, R., et al. 2006. Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2α phosphorylation. Mol. Biol. Cell 17:4212-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McInerney, G. M., N. L. Kedersha, R. J. Kaufman, P. Anderson, and P. Liljestrom. 2005. Importance of eIF2α phosphorylation and stress granule assembly in alphavirus translation regulation. Mol. Biol. Cell 16:3753-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mokas, S., et al. 2009. Uncoupling stress granule assembly and translation initiation inhibition. Mol. Biol. Cell 20:2673-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mollet, S., et al. 2008. Translationally repressed mRNA transiently cycles through stress granules during stress. Mol. Biol. Cell 19:4469-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montero, H., M. Rojas, C. F. Arias, and S. Lopez. 2008. Rotavirus infection induces the phosphorylation of eIF2α but prevents the formation of stress granules. J. Virol. 82:1496-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathans, R., et al. 2009. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Mol. Cell 34:696-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nayak, A., et al. 2010. Cricket paralysis virus antagonizes Argonaute 2 to modulate antiviral defense in Drosophila. Nat. Struct. Mol. Biol. 17:547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohn, T., N. Kedersha, T. Hickman, S. Tisdale, and P. Anderson. 2008. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat. Cell Biol. 10:1224-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker, R., and U. Sheth. 2007. P bodies and the control of mRNA translation and degradation. Mol. Cell 25:635-646. [DOI] [PubMed] [Google Scholar]
- 51.Piotrowska, J., et al. 2010. Stable formation of compositionally unique stress granules in virus-infected cells. J. Virol. 84:3654-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin, Q., C. Hastings, and C. L. Miller. 2009. Mammalian orthoreovirus particles induce and are recruited into stress granules at early times postinfection. J. Virol. 83:11090-11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raaben, M., M. J. Groot Koerkamp, P. J. Rottier, and C. A. de Haan. 2007. Mouse hepatitis coronavirus replication induces host translational shutoff and mRNA decay, with concomitant formation of stress granules and processing bodies. Cell Microbiol. 9:2218-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rehwinkel, J., I. Behm-Ansmant, D. Gatfield, and E. Izaurralde. 2005. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA 11:1640-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider, M. D., et al. 2006. Gawky is a component of cytoplasmic mRNA processing bodies required for early Drosophila development. J. Cell Biol. 174:349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoecklin, G., et al. 2004. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 23:1313-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teixeira, D., U. Sheth, M. A. Valencia-Sanchez, M. Brengues, and R. Parker. 2005. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 11:371-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas, M. G., L. J. Martinez Tosar, M. A. Desbats, C. C. Leishman, and G. L. Boccaccio. 2009. Mammalian Staufen 1 is recruited to stress granules and impairs their assembly. J. Cell Sci. 122:563-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian, Q., M. Streuli, H. Saito, S. F. Schlossman, and P. Anderson. 1991. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell 67:629-639. [DOI] [PubMed] [Google Scholar]
- 60.Tourriere, H., et al. 2003. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160:823-831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.van Rij, R. P., et al. 2006. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 20:2985-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80:5059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White, J. P., A. M. Cardenas, W. E. Marissen, and R. E. Lloyd. 2007. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe 2:295-305. [DOI] [PubMed] [Google Scholar]
- 64.Wilson, J. E., M. J. Powell, S. E. Hoover, and P. Sarnow. 2000. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Mol. Cell. Biol. 20:4990-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]









