Abstract
The 2009 H1N1 pandemic influenza virus represents the greatest incidence of human infection with an influenza virus of swine origin to date. Moreover, triple-reassortant swine (TRS) H1N1 viruses, which share similar host and lineage origins with 2009 H1N1 viruses, have been responsible for sporadic human cases since 2005. Similar to 2009 H1N1 viruses, TRS viruses are capable of causing severe disease in previously healthy individuals and frequently manifest with gastrointestinal symptoms; however, their ability to cause severe disease has not been extensively studied. Here, we evaluated the pathogenicity and transmissibility of two TRS viruses associated with disease in humans in the ferret model. TRS and 2009 H1N1 viruses exhibited comparable viral titers and histopathologies following virus infection and were similarly unable to transmit efficiently via respiratory droplets in the ferret model. Utilizing TRS and 2009 H1N1 viruses, we conducted extensive hematologic and blood serum analyses on infected ferrets to identify lymphohematopoietic parameters associated with mild to severe influenza virus infection. Following H1N1 or H5N1 influenza virus infection, ferrets were found to recapitulate several laboratory abnormalities previously documented with human disease, furthering the utility of the ferret model for the assessment of influenza virus pathogenicity.
In March 2009, a novel swine origin H1N1 influenza virus (2009 H1N1) suddenly emerged and caused a worldwide pandemic. By the time the World Health Organization (WHO) declared the pandemic over in August 2010, the virus had spread to over 215 countries, with over 18,000 deaths reported worldwide (3). While the majority of infected individuals have experienced uncomplicated upper respiratory tract illness, 2009 H1N1 viruses caused a greater rate of severe or complicated illness in healthy young adults and children than seasonal H1N1 influenza (5, 8, 14). In particular, a wide spectrum of influenza-related complications characterized by signs of lower respiratory tract disease and gastrointestinal symptoms have been observed in some children and pregnant women (5, 26). Fatal infections have been distinguished histopathologically with diffuse alveolar damage in lung tissue and bacterial coinfection in >25% of cases (27). Although individuals with underlying medical conditions account for many of the severe cases reported (23), it is not entirely clear why some 2009 H1N1-infected patients developed severe disease whereas others did not. Data from mammalian animal models will increase our understanding of virus-host interactions that determine the outcome of H1N1 virus infection.
Use of the ferret model allowed for rapid initial characterization of 2009 H1N1 viruses (10, 17, 20). These studies revealed that, compared to infection with seasonal H1N1 influenza viruses, infection of ferrets with 2009 H1N1 viruses causes enhanced morbidity, increased lung pathology, and higher viral titers in the upper and lower respiratory tract. Seasonal and 2009 H1N1 viruses transmit to naïve animals with equal efficiency in a direct contact (DC) model and are capable of transmitting by respiratory droplets (RD); however, the efficiency of respiratory droplet transmission of 2009 H1N1 viruses varies among laboratories and likely depends on experimental conditions (10, 17, 20). Overall, these findings indicate that disease caused by 2009 H1N1 viruses, while more severe than disease caused by seasonal viruses, is predominantly less severe than that caused by highly pathogenic avian influenza (HPAI) viruses (18). While severe disease has been noted among select ferrets infected with 2009 H1N1 viruses, a detailed examination of fatal disease caused by 2009 H1N1 as it compares with H5N1 viruses in this model has not been conducted to date (17).
Triple-reassortant swine (TRS) H1N1 viruses have caused sporadic human infections in North America, most often following human exposure to swine (28). Similar to 2009 H1N1 viruses, TRS H1N1 viruses are capable of causing severe disease in previously healthy individuals, with occasional gastrointestinal symptoms. The hemagglutinin (HA) gene segment of TRS H1N1 viruses is derived from the classic North American swine lineage, which is comprised of three distinct phylogenetic groups currently circulating in swine: swH1α, swH1β, and swH1λ (32). Viruses from two of these phylogenetic groups, swH1β and swH1λ, have been isolated from human cases of TRS H1N1 (28). Genetically, TRS H1N1 and 2009 H1N1 viruses share similar host and generally similar lineage origins, with the exception of Eurasian and not classical swine lineage neuraminidase and matrix genes present in 2009 isolates (7, 28). Unlike 2009 H1N1 viruses, TRS H1N1 viruses have not been associated with human-to-human spread.
While 2009 H1N1 and TRS H1N1 viruses share common host and partial genetic origins, it was unknown if they also shared a similar capacity to cause disease in mammalian species. A swH1λ TRS H1N1 virus was recently shown to possess enhanced pathogenicity compared with that of 2009 H1N1 and seasonal influenza viruses in the mouse model (2). However, the potential influence of phylogenetic differences between TRS H1N1 viruses on pathogenicity has not been studied, and the evaluation of TRS H1N1 viruses in the ferret model has not been performed. Furthermore, the transmissibility of triple-reassortant H1N1 viruses associated with human disease has not been examined in experimental laboratory models. Laboratory abnormalities in hematologic and serum chemistry parameters are frequently observed in hospitalized and severe human cases of 2009 H1N1, TRS H1N1, and H5N1 virus infection (1, 5, 11, 28, 33) but have not been experimentally studied in vivo, limiting our ability to delineate lymphohematopoietic parameters affected by infection with these virus subtypes.
In the present study, we used the ferret model to evaluate the pathogenicity and transmissibility of two phylogenetically distinct TRS H1N1 viruses isolated from humans in 2007 and 2008. Histopathology, hematologic, and blood serum analyses were employed to more closely understand the scope of disease caused by TRS H1N1 or 2009 H1N1 viruses in this model as they compared to seasonal H1N1 or HPAI H5N1 viruses. We found that TRS H1N1 and 2009 H1N1 viruses were similarly pathogenic in the ferret, but TRS H1N1 viruses failed to transmit efficiently by respiratory droplets. We further identified several serologic and hematologic parameters which were associated with mild to severe disease following influenza A virus infection in this model.
MATERIALS AND METHODS
Viruses.
H1N1 viruses used in this study were grown in Madin-Darby canine kidney (MDCK) cells, and the H5N1 virus A/Vietnam/1203/04 was grown in the allantoic cavity of 10-day-old embryonated hens' eggs at 37°C for 26 h as previously described (18, 31). Pooled cell supernatant or allantoic fluid was clarified by centrifugation and frozen in aliquots at −70°C. Cell-grown stocks were titrated by standard plaque assay in MDCK cells as previously described for determination of PFU titer (34). The 50% egg infectious dose (EID50) titer for egg-grown virus was calculated by the method of Reed and Muench, following serial titration in eggs (24). All animal experiments with H5N1 virus were conducted under biosafety level 3 containment, including enhancements required by the U.S. Department of Agriculture and the Select Agent Program, and in accordance with guidelines of the WHO (https://www.who.int/csr/resources/publications/swineflu/Laboratorybioriskmanagement.pdf) (25). Experiments using 2009 H1N1 or TRS H1N1 viruses were conducted under biosafety conditions similar to those indicated above.
Ferret pathogenesis and transmission experiments.
Male Fitch ferrets (Triple F Farms, Sayre, PA), 6 to 12 months of age and serologically negative by hemagglutination inhibition (HI) assay for currently circulating influenza viruses, were used in this study. Ferrets were housed for the duration of each experiment in a Duo-Flo BioClean environmental enclosure (Lab Products, Seaford, DE) which provides HEPA-filtered air (directional top to bottom) with uniform airflow circulated at 150 to 180 air changes per hour. Temperature and relative humidity were maintained at ambient conditions (23°C ± 3°C and 35 to 50% relative humidity) throughout the duration of each experiment. The pathogenesis of each virus following intranasal (i.n.) inoculation of 106 PFU or EID50 was determined as previously described (18). Respiratory droplet and contact transmission experiments were conducted as previously described (16). Postexposure serum was collected from H1N1 virus-inoculated (day 14 postinoculation [p.i.]) and contact (day 21 postcontact) ferrets and tested for H1-specific antibodies against homologous virus by HI assay using 0.5% turkey erythrocytes (30). Statistical significance of viral titers was determined using Student's t test.
Histological analysis.
Necropsies were performed on infected ferrets euthanatized on day 3 p.i. Tissues were fixed in formalin, representative samples from the trachea (one cassette), hilar region (one cassette), and each lung lobe (one cassette per side) and additional representative samples from the small intestine (one cassette), spleen, kidney, liver, heart (one cassette), and a coronal section, including the parietal and temporal brain cortex (in one cassette), were paraffin embedded, and sections were stained with hematoxylin and eosin (H&E) or by immunohistochemistry (IHC). IHC assays were performed on 3-μm sections of tissue using a monoclonal antibody against the nucleoprotein (NP) of influenza A virus (9). Detection of the attached primary antibody was carried out with the UltraVision LP system with naphthol phosphate substrate (Thermo Scientific/Lab Vision). Only cells with red-staining nuclei were considered positive with the IHC assay. Negative controls consisted of sequential tissue sections incubated with an isotype control monoclonal antibody. Histopathology was assessed on H&E evaluation using the following scoring: 0, no lesions; 1, rare (focal) mild lesions; 2, widespread mild lesions; 3, moderate lesions; and 4, extensive or severe lesions. The IHC results were scored as follows: 0, no positive cells; 1, rare positive cells; 2, infrequent positive cells; 3, common positive cells; and 4, extensive positive cells.
Ferret hematologic and blood chemistry analyses.
On days 0, 3, 7, and 14 to 16 p.i., blood was collected from three inoculated ferrets from each virus group. Complete blood counts were quantified from blood collected in EDTA Vacutainer tubes (BD, Franklin Lakes, NJ) using a Hemavet HV950FS instrument per the manufacturer's instructions (Drew Scientific, Inc., Oxford, CT). Ferret blood chemistry analysis was performed using a VetScan classic analyzer and comprehensive diagnostic profile rotors (Abaxis, Union City, CA), with serum collected at each indicated time point after infection per the manufacturer's instructions. All blood and serum samples were processed and analyzed the same day as sample collection. Statistical significance of differences observed was determined using Student's t test (total complete blood counts) or the Mann-Whitney test (blood chemistry).
RESULTS
Pathogenesis of triple-reassortant swine influenza viruses in ferrets.
TRS H1N1 influenza viruses have caused sporadic mild to severe human disease in recent years, but their pathogenicity in the ferret model has not been closely examined (28). We studied the virulence of two TRS H1N1 viruses from separate phylogenic lineages currently circulating in North American swine. A/Ohio/2/07 (OH/2) virus was isolated from a 10-year-old female and belongs to the swH1λ lineage; A/Texas/14/08 (Tx/14) virus was isolated from a 14-year-old male with asthma and belongs to the swH1β lineage. Both patients exhibited uncomplicated, upper respiratory illness following direct contact with swine (28). Nine ferrets were inoculated with 106 PFU of each virus, a dose which resulted in consistent infection of ferrets with seasonal and 2009 H1N1 viruses (17). Six ferrets were observed for 14 days p.i. for clinical signs of illness, and three ferrets were euthanatized on day 3 p.i. for assessment of virologic and histopathologic parameters. Infection with either OH/2 or Tx/14 virus caused moderate weight loss in all animals and mild lethargy in 2/6 ferrets (Table 1); sneezing and increased nasal secretions were rarely observed. Due to severe weight loss (>25%), one ferret inoculated with each virus was euthanatized during the observation period (day 12 p.i., OH/2 virus; day 9 p.i., Tx/14 virus).
TABLE 1.
Infection of ferrets with triple-reassortant swine or 2009 H1N1 influenza virus
| Virus | No. of ferrets with clinical signs through day 14 p.i./total no. of ferrets |
Virus titer on day 3 p.i.a |
||||||
|---|---|---|---|---|---|---|---|---|
| Weight loss (% mean maximum weight loss)b | Lethargy (relative inactivity index) | Lethalityc | Virus in rectal swabd | Lung | Trachea | Nasal turbinates | Intestinal tracte | |
| OH/2 | 6/6 (9.4) | 2/6 (1.0) | 2/6 | 0/6 | 4.8 ± 1.0 | 4.3 ± 0.4 | 5.0 ± 0.7 | 1.4 |
| Tx/14 | 6/6 (11.7) | 2/6 (1.0) | 2/6 | 1/6 | 6.0 ± 0.1 | 4.1 ± 1.9 | 4.7 ± 1.0 | 1.9 ± 0.7 |
| 2009 H1N1f | 18/18 (12.3) | 9/18 (1.2) | 3/18 | 5/18 | 5.6 ± 0.7 | 5.2 ± 0.8 | 5.4 ± 0.7 | 2.5 ± 0.6 |
Virus titers are expressed as the mean number of log10 PFU/g or the number of PFU/ml of tissue ± standard deviations.
Numbers in parentheses represent the percentages of mean maximum weight loss observed during the first 10 days p.i.
Number of animals euthanatized before the end of the experimental period due to excessive weight loss.
Number of ferrets with detectable virus in rectal swabs collected on days 3 to 5 p.i.
Mean intestinal tract tissue virus titers ± standard deviations from positive samples only are shown. OH/2, 1/6 ferrets positive; Tx/14, 3/6 ferrets positive; 2009 H1N1, 8/18 ferrets positive.
A summary of three 2009 H1N1 strains (CA/4, Tx/15, Mex/4482) is shown for comparison purposes (17).
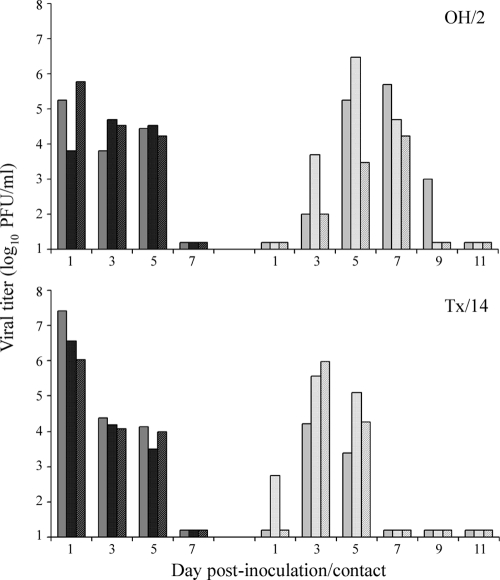
Kinetics and titers of replicating virus in ferrets following TRS virus infection were generally similar to those of 2009 H1N1 pandemic virus-infected ferrets. OH/2 and Tx/14 viruses replicated efficiently in the upper respiratory tract of inoculated ferrets through day 5 p.i., with peak mean nasal wash (NW) viral titers of 4.9 ± 1.0 and 6.7 ± 0.7 log10 PFU/ml on day 1 p.i., respectively (Fig. 1). Also similar to pandemic H1N1, both OH/2 and Tx/14 viruses replicated to high titers in the ferret lung and nasal turbinates and were intermittently detected in multiple regions of the intestinal tract (Table 1). Infectious virus was not detected in the blood, spleen, kidney, liver, or brain of any animals at this time p.i. In summary, compared to 2009 H1N1 viruses, TRS H1N1 viruses generally caused a similar disease in ferrets; both viruses were moderately more pathogenic than seasonal influenza viruses in the ferret model (17).
FIG. 1.
Direct contact transmissibility of triple-reassortant swine influenza viruses. Three ferrets were inoculated i.n. with 106 PFU of OH/2 (A) or Tx/14 (B) virus, and a naïve ferret was placed in the same cage at 24 h p.i. Nasal washes were collected from inoculated (dark bars) and contact (light bars) ferrets on alternate days p.i. or p.c., respectively, and titrated for the presence of infectious virus. Results from individual ferrets are presented. The limit of virus detection was 10 PFU.
Histological pathology observed in H1N1-infected ferrets.
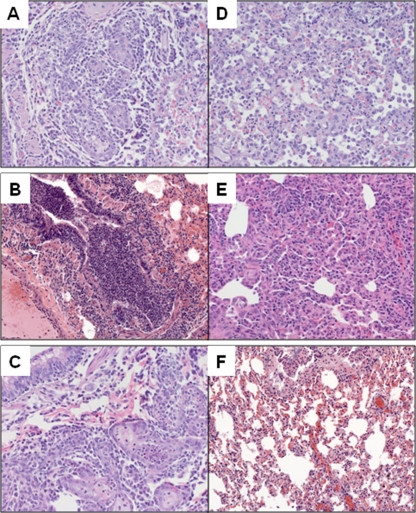
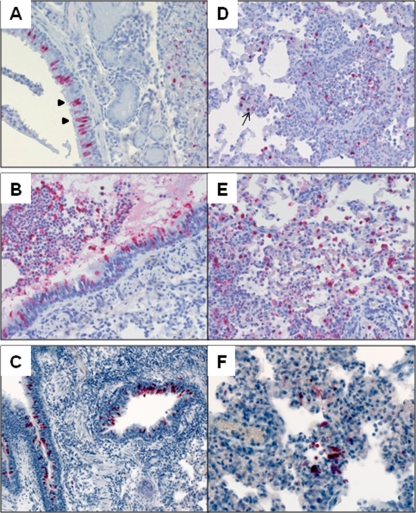
Previous studies have found that 2009 H1N1 viruses cause more extensive lung pathology in ferrets, including a greater distribution of nucleoprotein-positive cells in the lower respiratory tract, compared with that caused by seasonal H1N1 virus (10, 20). However, histopathology following TRS virus infection has not been described. At day 3 p.i., all ferrets inoculated with TRS and 2009 H1N1 viruses had lesions consistent with changes observed with influenza A infection (Fig. 2). There was focal necrosis of the bronchial epithelium and infiltration of inflammatory cells peribronchiolar and within the lumen. Additionally, fibrin, edema, and detached epithelial cells were evident within a few bronchi (Fig. 2B). The most frequent histopathologic changes in the lung parenchyma consisted of edema and interstitial and intra-alveolar inflammatory cell infiltrates. These histopathologic changes corresponded to the detection of viral antigen by immunohistochemistry staining. In large airways, virus antigen was seen in submucosal glands and bronchial epithelial cells, intact and detached in lumen (Fig. 3A to C). Pneumocytes were positive for viral antigen in the lung parenchyma following infection with all swine lineage H1N1 viruses examined (Fig. 3D to F).
FIG. 2.
Histopathology in large airway and parenchyma. Ferrets were inoculated i.n. with 106 PFU of each virus, and tissues were collected on day 3 p.i. for analysis. Inflammation and epithelial necrosis is seen in the large airways (A to C) and interstitial and intraalveolar inflammation in the parenchyma (D to F) of ferrets inoculated with Mx/4482 virus (representative of 2009 H1N1 viruses) (A, D), OH/2 virus (B, E), or TX/14 virus (C, F).
FIG. 3.
Immunohistochemistry in the lungs. Ferrets were inoculated i.n. with 106 PFU of each virus, and tissues were collected on day 3 p.i. for analysis. Viral antigen was present in the nuclei of bronchial epithelial cells (arrowheads in panel A) present in large airways (A to C) and in bronchioles and pneumocytes (arrows in panel D) in lung parenchyma (D to F) of ferrets inoculated with Mx/4482 virus (representative of 2009 H1N1 viruses) (A, D), OH/2 virus (B, E), or TX/14 virus (C, F).
Histopathologic lesions and viral antigen seen in ferrets infected with TRS and 2009 H1N1 viruses (17) were more extensive in the large airway than in lung parenchyma (Table 2). Compared with 2009 H1N1 viruses, ferrets with TRS viruses of either phylogenetic group displayed slightly less pathology in lung parenchyma than that seen in ferrets inoculated with 2009 H1N1. However, all swine lineage H1N1 viruses exhibited greater histopathology in these tissues than seasonal H1N1 virus-infected ferrets (Table 2). Despite detection of virus in the intestinal tract of TRS- and 2009 H1N1-infected ferrets, significant histopathological changes were not observed in intestinal tissue (data not shown). In summary, we found that TRS viruses generally caused lung pathology in ferrets similar to that caused by 2009 H1N1 viruses.
TABLE 2.
Severity of histopathology following infection of ferrets with H1N1 viruses
| Virus | Abbreviation in study | H1N1 subtype | Histopathology score at day 3 p.i.a |
|||
|---|---|---|---|---|---|---|
| Bronchial histopathology | Bronchial IHC | Parenchyma histopathology | Parenchyma IHC | |||
| A/Ohio/2/07 | OH/2 | swH1γ triple reassortant | 3.0 | 2.7 | 2.0 | 1.7 |
| A/Texas/14/08 | Tx/14 | swH1β triple reassortant | 1.7 | 2.0 | 1.7 | 1.7 |
| A/California/4/09 | CA/4 | 2009 pandemic | 2.7 | 2.7 | 2.7 | 0.6 |
| A/Texas/15/09 | Tx/15 | 2009 pandemic | 2.7 | 3.0 | 2.7 | 0.6 |
| A/Mexico/4482/09 | Mx/4482 | 2009 pandemic | 2.7 | 2.7 | 2.7 | 1.7 |
| A/Brisbane/59/07 | Brisbane | Contemporary | 1.6 | 0 | 1.0 | 0 |
Values represent the average scores for three ferrets/virus. All scores were within one step of each other on the scoring scale.
Transmissibility of triple-reassortant swine influenza viruses in ferrets.
To evaluate the transmissibility of TRS influenza viruses, respiratory droplet (RD) and direct contact (DC) transmission experiments with both OH/2 and Tx/14 strains were performed. In this ferret transmission model, six ferrets were inoculated with 106 PFU of virus. Twenty-four hours p.i., three ferrets were placed in separate modified cages with a perforated side wall, allowing air exchange with a naïve ferret in an adjacent cage but preventing direct or indirect contact between animals to assess RD transmission. The remaining three inoculated ferrets were each pair housed with a naïve ferret to facilitate transmission in the presence of DC. NWs were collected from both inoculated and contact ferrets (either RD or DC) on alternate days p.i. or postcontact (p.c.), respectively, for virus titration. Detection of virus in NWs and seroconversion of RD or DC ferrets were considered evidence of virus transmission.
Both TRS influenza viruses tested transmitted efficiently to three of three ferrets in the direct contact model by day 3 p.c. (Fig. 1). Peak mean NW viral titers in DC animals reached >5 log10 PFU/ml (Fig. 1), and seroconversion of all animals to homologous virus occurred by day 21 p.c. (data not shown). Weight loss was observed in one of three OH/2 DC ferrets (6.9% change from baseline) and three of three Tx/14 DC ferrets (mean, 7.3%), with maximum weight loss observed 4 days after virus detection in NWs in all instances (data not shown). Efficient transmission of OH/2 and Tx/14 viruses by respiratory droplets did not occur, as virus was not detected in NW samples from RD contact animals. However, HI antibody titers against homologous virus were detected in serum from one of three OH/2 RD contact ferrets and two of three Tx/14 RD contact ferrets by day 21 p.c., indicating that these viruses were capable of limited transmission by respiratory droplets in the absence of detectable virus in nasal secretions (data not shown). For comparison, 2009 H1N1 influenza viruses transmitted efficiently to nine of nine ferrets in a direct contact model but only to six of nine ferrets by respiratory droplets (17). Overall, these results demonstrate that TRS influenza viruses can transmit readily by direct contact but lack the capacity for efficient transmission by respiratory droplets observed with contemporary seasonal influenza viruses (16).
Hematologic and serum chemistry analyses of H1N1-infected ferrets.
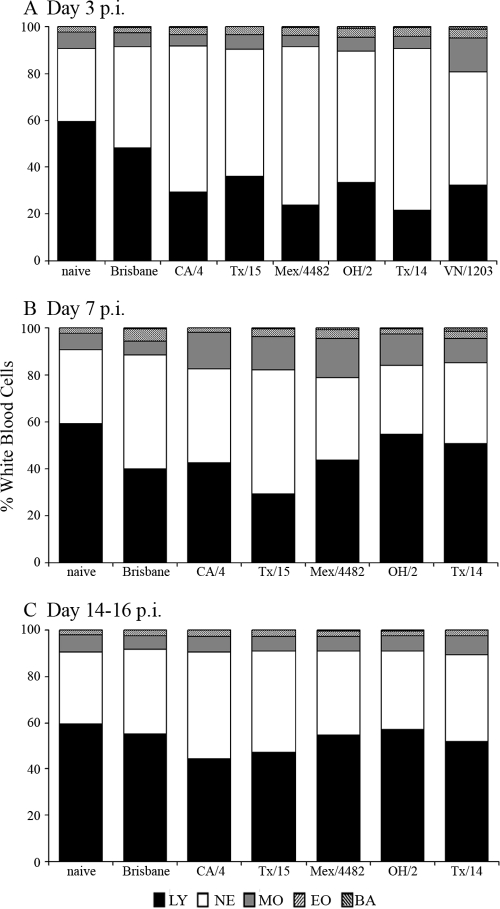
Uncomplicated human infection with seasonal, TRS, or 2009 H1N1 influenza viruses can result in transient and mild lymphopenia, whereas severe effects to the lymphohemopoietic system can follow infection with highly pathogenic avian H5N1 viruses and in some cases with swine origin H1N1 viruses (1, 5, 28, 33). While ferrets inoculated with highly pathogenic H5N1 viruses will exhibit lymphopenia during acute infection (35), the ability of the ferret model to recapitulate these and other hematologic parameters of H1N1 viral infection has not been previously studied. To better understand the effect of H1N1 virus infection on the lymphohemopoietic system, complete blood counts were performed on peripheral blood samples collected from ferrets inoculated with either seasonal, TRS, or 2009 H1N1 viruses (listed in Table 2) and compared with those from ferrets inoculated with a highly pathogenic H5N1 virus (A/Vietnam/1203/04 [VN/1203]) known to elicit lymphopenia following virus infection in the ferret model (Table 3; Fig. 4) (18).
TABLE 3.
Complete blood counts of influenza virus-infected ferrets
| Cell typea | Day p.i. | Blood count ± SD (K/μl)b |
||||||
|---|---|---|---|---|---|---|---|---|
| Brisbane | CA/4 | Tx/15 | Mx/4482 | OH/2 | Tx/14 | VN/1203 | ||
| WBC | 0 | 6.15 ± 2.5 | 7.60 ± 1.70 | 4.70 ± 2.39 | 7.54 ± 1.21 | 7.83 ± 3.06 | 7.16 ± 0.63 | 7.06 ± 2.12 |
| 3 | 6.12 ± 1.12 | 4.95± 1.14 | 7.21 ± 0.39 | 7.5 ± 1.66 | 7.50 ± 0.50 | 7.54 ± 0.85 | 4.01 ± 2.38 | |
| 7 | 12.12± 2.69 | 8.31 ± 3.16 | 9.3± 0.54 | 11.69± 2.82 | 7.65 ± 2.10 | 11.86± 3.76 | 4.3 ± 0.23c | |
| 14-16 | 7.27 ± 1.4 | 8.83 ± 1.66 | 7.95 ± 2.17 | 7.81 ± 1.88 | 7.21 ± 1.57 | 6.50 ± 1.43 | ||
| LY | 0 | 3.59 ± 1.46 | 4.46 ± 1.31 | 2.73 ± 1.07 | 4.23 ± 0.74 | 5.15 ± 2.40 | 4.18 ± 0.81 | 4.01 ± 2.03 |
| 3 | 3.05 ± 0.89 | 1.44± 0.26 | 2.60 ± 0.78 | 1.77± 0.44 | 2.48 ± 0.31 | 1.61± 0.57 | 1.30 ± 0.78 | |
| 7 | 4.97 ± 1.84 | 3.45 ± 1.02 | 2.67 ± 0.79 | 4.82 ± 2.36 | 4.08 ± 1.20 | 6.04 ± 1.96 | 0.73 ± 0.38c | |
| 14-16 | 4.00 ± 0.81 | 3.88 ± 0.54 | 3.18 ± 1.38 | 4.15 ± 0.43 | 4.13 ± 1.11 | 3.41 ± 0.95 | ||
| NE | 0 | 2.02 ± 1.14 | 2.57 ± 0.55 | 1.47 ± 1.34 | 2.64 ± 0.60 | 1.97 ± 0.41 | 2.20 ± 0.76 | 2.37 ± 0.35 |
| 3 | 2.6 ± 0.06 | 3.12 ± 0.90 | 3.92± 0.76 | 5.08± 1.27 | 4.25± 0.95 | 5.24± 0.76 | 1.94 ± 1.20 | |
| 7 | 5.78± 0.80 | 3.58 ± 2.14 | 4.98± 1.34 | 4.69± 0.20 | 3.14± 0.57 | 4.03± 1.21 | 3.04 ± 0.57c | |
| 14-16 | 2.67 ± 0.62 | 3.88± 1.11 | 4.07± 1.22 | 2.95 ± 1.34 | 2.34 ± 0.51 | 2.41 ± 0.52 | ||
| MO | 0 | 0.38 ± 0.11 | 0.37 ± 0.11 | 0.38 ± 0.11 | 0.47 ± 0.09 | 0.56 ± 0.26 | 0.60 ± 0.11 | 0.52 ± 0.24 |
| 3 | 0.39 ± 0.28 | 0.22± 0.02 | 0.43 ± 0.13 | 0.38 ± 0.07 | 0.41 ± 0.42 | 0.40± 0.16 | 0.53 ± 0.20 | |
| 7 | 0.72± 0.14 | 1.11± 0.17 | 1.31± 0.23 | 1.61± 0.43 | 0.95± 0.19 | 1.19± 0.35 | 0.24 ± 0.57c | |
| 14-16 | 0.42 ± 0.08 | 0.62± 0.21 | 0.50 ± 0.16 | 0.49 ± 0.24 | 0.51 ± 0.23 | 0.41 ± 0.20 | ||
| EO | 0 | 0.14 ± 0.10 | 0.20 ± 0.08 | 0.08 ± 0.09 | 0.20 ± 0.05 | 0.13 ± 0.08 | 0.14 ± 0.07 | 0.13 ± 0.04 |
| 3 | 0.14 ± 0.03 | 0.16 ± 0.05 | 0.24± 0.12 | 0.25 ± 0.04 | 0.29± 0.09 | 0.28± 0.05 | 0.18 ± 0.17 | |
| 7 | 0.62± 0.15 | 0.16 ± 0.16 | 0.3± 0.07 | 0.36± 0.08 | 0.19 ± 0.11 | 0.38± 0.16 | 0.28 ± 0.08c | |
| 14-16 | 0.17 ± 0.06 | 0.21 ± 0.05 | 0.19± 0.59 | 0.21 ± 0.09 | 0.15 ± 0.04 | 0.14 ± 0.04 | ||
| BA | 0 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.00 ± 0.00 | 0.01 ± 0.05 | 0.03 ± 0.07 | 0.04 ± 0.06 | 0.02 ± 0.04 |
| 3 | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.00 ± 0.01 | 0.25 ± 0.04 | 0.05 ± 0.03 | 0.01 ± 0.01 | 0.05 ± 0.06 | |
| 7 | 0.03± 0.01 | 0.01 ± 0.02 | 0.03± 0.02 | 0.36± 0.08 | 0.02 ± 0.01 | 0.18 ± 0.16 | 0.02 ± 0.01c | |
| 14-16 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.21 ± 0.09 | 0.03 ± 0.05 | 0.14 ± 0.04 | ||
WBC, total white blood cells; LY, total lymphocytes; NE, total neutrophils; MO, total monocytes; EO, total eosinophils; BA, total basophils.
Boldface denotes values which are significantly different from baseline counts on day 0 (P < 0.05).
Values collected on day 5 p.i. prior to euthanasia; VN/1203-infected ferrets did not survive to day 7 or 14 p.i.
FIG. 4.
Kinetic analysis of circulating lymphocytes following influenza virus infection. Three ferrets each were inoculated i.n. with 106 PFU of virus. Blood was collected on days 3 (A), 7 (B), and 14 to 16 (C) p.i. in EDTA Vacutainer tubes and analyzed with a hematology scanner. Blood collected immediately prior to inoculation was included as a baseline control (naïve). The average percentages of lymphocytes (LY), neutrophils (NE), monocytes (MO), eosinophils (EO), and basophils (BA) in whole blood are shown. Ferrets inoculated with VN/1203 virus did not survive to day 7 or 14 p.i.
Ferrets infected with Brisbane, a representative seasonal H1N1 virus, exhibited a mild, transient lymphopenia (11% and 19% decrease in circulating lymphocytes on days 3 and 7, respectively) compared with that of uninfected ferrets (Fig. 4A and B). A more pronounced lymphopenia was observed on day 3 p.i. with three 2009 pandemic H1N1 viruses, CA/4, Tx/15, and Mx/4482, previously shown to exhibit enhanced morbidity and replicate to higher titers compared to those of Brisbane virus in the ferret model (17). On average, infection with these 2009 H1N1 viruses resulted in a 30% decrease in lymphocytes at day 3 p.i., comparable to the lymphopenia observed with the TRS viruses OH/2 (26%) and Tx/14 (38%) and also to the level (27% decrease versus controls) observed with the highly virulent H5N1 VN/1203 virus (Fig. 4; Table 3). Transient lymphopenia following infection with any of the H1N1 viruses was detected on day 3 p.i. compared to that of naïve ferrets and persisted through day 7 p.i. but returned to preinfection levels by day 14 p.i. Significant differences in total erythrocyte counts, including hemoglobin and hematocrit levels, were not observed in ferrets following infection with any virus tested (data not shown). However, a significant reduction in the number of total platelets in peripheral blood was observed 3 days following VN/1203 virus inoculation (from 592 to 191 K/μl, P < 0.001) but not following infection with any H1N1 virus tested.
To further evaluate ferret health following H1N1 virus infection, sera were isolated from peripheral blood samples from the ferrets described above to identify any changes in serum chemistry parameters (Table 4). Influenza virus infection was found to impact several serum analytes associated with liver, kidney, and heart disease in addition to other metabolic and nutritional disorders. Blood urea nitrogen levels were unchanged following seasonal Brisbane virus infection but were significantly reduced on days 3 and 7 following infection with any of the 2009 H1N1 and TRS H1N1 viruses tested (Table 4) (P < 0.05). Significantly decreased serum albumin, calcium, and sodium levels were also frequently observed following H1N1 virus infection through day 14 p.i. (P < 0.05). Significantly altered levels of total protein and globulin were sporadically observed following H1N1 virus infection (P < 0.05). All serum analytes which differed significantly from baseline levels in H1N1-infected ferrets also were altered significantly following VN/1203 virus infection, with the addition of elevated levels of amylase, which were unaffected by H1N1 virus infection (P < 0.05). Concentrations of alkaline phosphatase, alanine aminotransferase, total bilirubin, phosphorus, creatinine, glucose, and potassium in ferrets infected with all viruses fell within baseline levels (data not shown). Taken together, we found that 2009 H1N1 and TRS influenza viruses resulted in greater levels of transient lymphopenia than a seasonal H1N1 virus early after infection in ferrets. We further observed significant alterations in ferret serum chemistry following infection with H1N1 seasonal and swine lineage viruses, although not to the extent observed following HPAI H5N1 virus infection.
TABLE 4.
Serum chemistry analysis of influenza virus-infected ferrets
| Analyte | Baseline range for naive ferrets | Day p.i. | Range for virus-infected ferretsc |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Brisbane | CA/4 | Tx/15 | Mx/4482 | OH/2 | Tx/14 | VN/1203 | |||
| Albumin (ALB) (g/dl) | 3.4-4.1a | 3 | 2.7-3.3 | 3.1-3.9 | 2.9-3.2 | 3.4-4 | 3.3-3.8 | 3.2-3.4 | 1.8-3 |
| 3.4-4.2b | 7 | 3-3.4 | 3.2-3.7 | 3.4-3.8 | 3-3.9 | 1.6-3.2 | 2.7-3 | ||
| 14 | 3.4-3.9 | 3.2-4.3 | 3.2-3.5 | 3.6-4 | 3.3-3.8 | 3.1-3.7 | |||
| Amylase (AMY) (U/liter) | <5-23 | 3 | 19-23 | 17-24 | 15-27 | 18-24 | 20-24 | 13-25 | 30-89 |
| 7 | 13-20 | 14-20 | 19-28 | 11-23 | 10-15 | 18-26 | |||
| 14 | 16-20 | 16-23 | 18-22 | 18-23 | 13-20 | 13-18 | |||
| Blood urea nitrogen (BUN) (mg/dl) | 27-38 | 3 | 25-33 | 18-23 | 22-23 | 27-30 | 16-24 | 24-30 | 19-27 |
| 11-42 | 7 | 23-38 | 19-28 | 15-23 | 23-30 | 16-17 | 25-30 | ||
| 14 | 28-38 | 30-43 | 26-36 | 32-47 | 26-42 | 28-39 | |||
| Calcium (CA2+) (mg/dl) | 9-10.6 | 3 | 8.4-9.1 | 8.3-9 | 8.4-8.9 | 8.4-9.2 | 8.6-8.8 | 9.2-9.5 | 7.8-9.6 |
| 8.6-10.6 | 7 | 8.8-9.3 | 8.6-9.5 | 9-9.7 | 9.3-9.5 | 5.5-8.7 | 8.6-9.3 | ||
| 14 | 9-9.1 | 9-10 | 8.6-9.2 | 9.2-9.5 | 8.9-9.3 | 8.5-9.8 | |||
| Sodium (NA+) (nmol/liter) | 150-161 | 3 | 149-153 | 145-148 | 143-152 | 152-157 | 144-154 | 148-151 | 136-146 |
| 146-151 | 7 | 146-154 | 146-151 | 151-154 | 153-160 | 110-152 | 145-149 | ||
| 14 | 152-154 | 149-161 | 149-152 | 153-156 | 148-154 | 148-150 | |||
| Total protein (TP) (g/dl) | 5.7-6.6 | 3 | 5.2-5.6 | 5.5-6.3 | 5.5-6.6 | 6.4-7.4 | 6.1-6.3 | 6.1-6.9 | 5-6.2 |
| 5.4-7.1 | 7 | 5.5-5.9 | 5.7-6.1 | 6-6.2 | 5.9-7 | 3.9-6.2 | 5.7-6.3 | ||
| 14 | 6-6.1 | 5.4-6.7 | 4.6-6 | 6-6.4 | 5.5-6.1 | 5.7-6.8 | |||
| Globulin (GLOB) (g/dl) | 1.9-3 | 3 | 2.3-2.5 | 2.3-2.5 | 2.5-3.4 | 3-3.3 | 2.3-3 | 2.8-3.5 | 2.7-3.9 |
| 1.9-3 | 7 | 2.3-2.5 | 2.4-2.6 | 2.4-3 | 2.6-3.2 | 2.3-3 | 3-3.5 | ||
| 14 | 2.1-2.7 | 1.5-2.7 | 1.4-2.8 | 2-2.8 | 1.7-2.4 | 2.6-3 | |||
Baseline range for naïve ferrets tested prior to infection.
Bold denotes ranges which extend outside the baseline range for naïve ferrets tested here and are significant by the Mann-Whitney test (P < 0.05). Ferrets inoculated with VN/1203 virus did not survive to day 7 or 14.
Hematologic and serum chemistry analysis of severe H1N1 virus infection in ferrets.
While the majority of ferrets infected with 2009 H1N1 and TRS H1N1 viruses exhibited mild to moderate disease and recovered from illness, some ferrets were euthanatized midexperiment due to excessive weight loss and lethargy, indicating that these animals presented with a more severe illness than other infected animals (17). To more closely examine systemic parameters associated with severe disease following H1N1 virus infection, blood and serum samples were collected from Tx/15-, Mx/4482-, OH/2-, or Tx/14-infected ferrets immediately prior to euthanasia and evaluated as described above (17). Samples collected from ferrets which succumbed to H5N1 virus infection were also evaluated as a comparison of known severe disease. Serum chemistry analysis detected sporadic abnormal peak readings among Tx/15-, Mx/4482-, OH/2-, and Tx/14-infected ferrets, including levels of alanine aminotransferase, phosphorus, and glucose which were not significantly altered following mild infection with these viruses (Table 5). Notably, serum analyzed from one ferret euthanized day 5 p.i. following Mx/4482 virus infection contained abnormal levels of several analytes. Postmortem necropsy revealed that this ferret possessed a single kidney, which may have contributed to the altered phosphorus and albumin levels detected. In contrast, multiple abnormal values were detected among both VN/1203 virus-infected ferrets day 5 p.i. at the time of necropsy, including albumin, amylase, alanine aminotransferase, and globulin levels. These findings identify several blood chemistry parameters which are associated with both mild and severe infection in this model. Furthermore, ferrets with severe H5N1 disease or underlying conditions (e.g., renal agenesis) presented with the greatest number of abnormal serum readings, a feature shared with severe human cases of H5N1 virus infection (1, 12, 21).
TABLE 5.
Blood and serum chemistry analysis of ferrets euthanized prior to day 14 p.i.
| Analysisc | Baseline rangea | Readings from virus-infected ferretsb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Tx/15 (10) | Mx/4482 (5) | Mx/4482 (10) | Mx/4482 (10) | OH/2 (12) | Tx/14 (9) | VN/1203 (5) | VN/1203 (5) | ||
| Serum | |||||||||
| ALB (g/dl) | 3.4-4.2 | 3.8 | 2.9c | 3.9 | 4.1 | 3.8 | 3.6 | 2.8 | 2.4 |
| AMY (U/liter) | <5-23 | 14.0 | 17.0 | 13.0 | 19.0 | 12.0 | 21.0 | 43.0 | 27.0 |
| ALT (U/liter) | 72-287 | 86.0 | 110.0 | 230.0 | 209.0 | 454.0 | 160.0 | 306.0 | 346.0 |
| PHOS (mg/dl) | 5.2-9.5 | 7.8 | 15.7 | 8.4 | 11.1 | 6.9 | 8.3 | 6.4 | 7.8 |
| GLU (mg/dl) | 63-237 | 181.0 | 263.0 | 208.0 | 292.0 | 186.0 | 180.0 | 173.0 | 302.0 |
| NA+ (nmol/liter) | 146-161 | 166.0 | 151.0 | 156.0 | 159.0 | 148.0 | 156.0 | 144.0 | 148.0 |
| TP (g/dl) | 5.4-7.1 | 6.5 | 7.9 | 6.5 | 6.3 | 5.2 | 6.3 | 5.9 | 5.7 |
| GLOB (g/dl) | 1.9-3 | 2.7 | 5.0 | 2.6 | 2.2 | 1.4 | 2.7 | 3.1 | 3.3 |
| Blood | |||||||||
| WBC (K/μl) | 6.2 | 6.1 | 24.7 | 9.68 | 5.54 | 6.6 | 3.86 | 4.14 | 4.46 |
| LY (%) | 59.3 | 30.4 | 11.5 | 51.2 | 36.3 | 51.8 | 41.7 | 24.2 | 10.2 |
| NE (%) | 31.4 | 50.8 | 73.3 | 33.1 | 52.6 | 33.4 | 38.8 | 63.7 | 77.2 |
| MO (%) | 7.2 | 16.1 | 7.9 | 12.4 | 8.9 | 10.5 | 17.5 | 6.6 | 4.4 |
| PLT (K/μl) | 426 | 200.0 | 480.0 | 328.0 | 448.0 | 346.0 | 272.0 | 348.0 | 280.0 |
Established baseline range of naïve ferrets tested here and from published reports (6, 13) (serum analysis) or mean values from naïve ferrets prior to infection (blood analysis).
Values in parentheses after the strain names indicate the day the ferret was euthanized. Bold denotes values which fall outside the baseline range for naïve ferrets tested here and in published reports (serum analysis) or values which lie outside the means ± 1 SD from baseline measurements collected from each group of ferrets preinfection (blood analysis).
ALB, albumin; AMY, amylase; ALT, alanine aminotransferase; PHOS, phosphorus; GLU, glucose; NA+, sodium; TP, total protein; GLOB, globulin; WBC, white blood cells; LY, total lymphocytes; NE, total neutrophils; MO, total monocytes; PLT, platelets.
As expected, lymphopenia was frequently observed among ferrets euthanatized due to severe disease with either H1N1 or H5N1 viruses. Both leukopenia and leukocytosis were detected following severe H1N1 virus infection in ferrets. Thrombocytopenia, previously reported among TRS H1N1-, 2009 H1N1-, and H5N1-infected patients, was also present at the time of necropsy among some ferrets (1, 11, 28). Similar to ferrets exhibiting mild disease, anemia (as measured by hemoglobin or hematocrit levels) was not observed among any ferrets euthanatized midexperiment (data not shown). In conclusion, we consistently observed abnormalities in the lymphohemopoietic system among H5N1- and H1N1-infected ferrets presenting with severe disease. Additionally, infected ferrets with severe disease possessed levels of several serum analytes which fell outside healthy baseline ranges prior to euthanasia.
DISCUSSION
TRS H1N1 influenza viruses circulating in North America have caused only sporadic and isolated human infection since 2005, in contrast to the 2009 H1N1 virus, which was responsible for the first pandemic of the 21st century. In this study, we utilized the ferret model to assess the relative virulence and transmissibility of two TRS H1N1 influenza viruses associated with human illness compared with those of both seasonal and 2009 H1N1 virus infection. Overall, TRS H1N1 viruses isolated from humans in 2007 and 2008 exhibited pathogenicities in ferrets similar to that of the 2009 H1N1 viruses but displayed a limited capacity for transmission by respiratory droplets compared with that of either seasonal or 2009 H1N1 viruses. To further expand the utility of the ferret model in the study of influenza virus pathogenesis, we analyzed complete blood counts and serum chemistries to identify detrimental lymphohematopoietic effects of H1N1 and H5N1 virus infection in this mammalian model. By providing comprehensive analyses of both baseline and virus-infected ferrets, we describe for the first time the potentiation of the ferret model to recapitulate many of these parameters not previously studied in an outbred laboratory mammalian model following influenza virus infection.
Virus was detected throughout respiratory and intestinal tissues of ferrets infected with TRS or 2009 H1N1 viruses, unlike seasonal influenza viruses, which are typically not detected in the lungs or intestinal tract of infected ferrets (17, 35). Both swH1β and swH1λ lineage TRS viruses replicated to generally similar titers in respiratory tract tissues; however, Tx/14 virus was detected in the intestinal tissue of 50% of infected ferrets (3/6) compared to only 17% (1/6) following OH/2 virus infection. Both TRS viruses elicited lung pathology that was more pronounced than that elicited by seasonal H1N1 infection and generally similar to that observed following 2009 H1N1 infection in ferrets (10, 20). Histological features characteristic of pandemic 2009 H1N1 infection, including necrosis of the bronchial epithelium and alveolar edema with inflammatory cell infiltrate, were also observed in TRS H1N1-infected lungs. A recent study of fatal 2009 H1N1 human cases identified a range of histopathological changes in autopsy tissues indicative of diffuse alveolar damage following infection of the lower respiratory tract (27). The detection of high titers of virus on day 3 p.i. in ferret lung tissue following 2009 H1N1 and TRS virus infection further establishes the ability of these swine origin H1N1 viruses to cause extensive damage to the mammalian lung (17, 27).
Unlike seasonal influenza virus infection in ferrets, clinical signs of respiratory symptoms, including sneezing and nasal secretions, were rarely observed among TRS H1N1-infected ferrets. Moreover, TRS H1N1 viruses did not possess the capacity for efficient aerosolized respiratory droplets, which is consistent with the limited, nonsustained transmission of TRS H1N1 viruses observed in humans (17, 20). It is important to note that, like 2009 H1N1 viruses which possess the capacity to spread efficiently between cohoused ferrets (17, 35), TRS H1N1 viruses were readily transmitted by direct contact in this animal model. It is reasonable to speculate that such a transmission phenotype could possess an advantage over viruses that transmit poorly or not at all, even in the presence of direct contact (e.g., H5N1 virus [17, 35]). Thus, virus passage from animal to animal by direct contact may ultimately result in the generation of mutant viruses conferring enhanced transmissibility. Experimentally, such natural virus variants have been isolated from contact ferrets following a rare transmission event in which an H2N2 mutant influenza strain demonstrated improved respiratory droplet transmission to naïve ferrets (22). Moreover, Sorrell et al. demonstrated that after 10 passages of an H9N2 virus in ferrets, a mutant influenza virus emerged that conferred efficient transmission via respiratory droplets (29), further demonstrating that minimal molecular changes, often those affecting receptor binding, will result in enhanced transmissibility of some influenza strains. An avian/swine reassortant H2N3 virus isolated from pigs exhibiting respiratory disease was found to possess molecular changes associated with increased affinity for α2-6-linked sialic acid receptors and was capable of direct contact transmission in the ferret model (15). As such, the capacity for direct contact transmission of TRS H1N1 viruses underscores the public health threat posed by this subtype despite the absence of documented human-to-human transmission of these viruses to date (28). Identification of the genetic determinants that govern airborne transmission and the receptor binding properties of influenza viruses isolated from swine is needed to understand the factors that may lead to the emergence of future swine origin pandemic influenza viruses.
There is a need to establish additional markers of virulence associated with virus-host interactions that may help identify viruses which possess the capacity to cause severe disease. The use of blood chemistry analysis has been employed to assess ferret health following severe acute respiratory syndrome coronavirus (SARS-CoV) infection; however, investigation of serum chemistries following influenza virus infection in the ferret model has not been previously reported (4). Several parameters associated with influenza virus infection in humans were also present in ferrets, notably decreased levels of serum albumin following infection with any H1N1 virus tested here. Serum albumin levels were even further reduced from baseline levels following H5N1 infection in ferrets, at a magnitude in accordance with reports of hypoalbuminemia among H5N1 virus-infected patients (12). Atypical levels of alanine aminotransferase and amylase, detected in some ferrets exhibiting severe H1N1 and H5N1 disease, have also been reported following severe human cases of H5N1, 2009 H1N1, and TRS H1N1 influenza virus infection in humans (1, 11, 21). The abnormal levels of these and other analytes observed in our hands are indicative of numerous systemic disruptions in severely infected ferrets, including liver and kidney disease and metabolic and nutritional disorders, indicating that severe H5N1 or H1N1 virus infection in this model can result in dysfunction of multiple organ systems in the absence of detectable infectious virus in these tissues. Elevated levels of creatinine and total bilirubin, reported among H5N1 and hospitalized H1N1 virus-infected patients, respectively, were not observed here (11, 21). Examination of serum chemistries from ferrets infected with an expanded range of influenza viruses, such as additional highly pathogenic avian influenza viruses, will provide a greater understanding of the biological relevance of these findings as they pertain to ferret health following severe infection. Furthermore, increased use of these analyses will allow for a more thorough assessment of ferret health prior to experimental use, potentially identifying ferrets with congenital abnormalities which otherwise would not be detected until postmortem necropsy.
With the exception of transient lymphopenia, there were no significant changes in white blood cell or erythrocyte populations detected among ferrets surviving infection with any H1N1 viruses tested here, similar to what was observed in BALB/c mice following 2009 H1N1 and TRS virus infection (2). However, complete blood counts performed on ferrets with severe disease requiring euthanasia revealed several abnormalities, including leukopenia, leukocytosis, and lymphopenia, all of which have been observed following severe human infection with H5N1, H1N1, and TRS viruses (1, 11, 21, 28). While there is currently no established absolute lymphocyte cutoff value associated with lymphopenia in the ferret model, these lymphocytic abnormalities are associated with a poor prognosis in both human infection and mammalian models (19). Anemia has been reported among H1N1 and H5N1 virus-infected patients, but consistent levels of hemoglobin and hematocrit levels observed in ferrets exhibiting mild to severe disease suggest that the ferret model may not be an ideal model to study erythrocyte parameters following influenza virus infection (11, 21). Despite the altered lymphohematopoietic parameters discussed here, systemic tissues collected from ferrets euthanatized during the course of the observational period due to severe disease did not possess more extensive histopathological changes than infected ferrets exhibiting milder disease (data not shown).
While the 2009 H1N1 pandemic virus has caused severe and fatal disease in previously healthy individuals, the majority of cases worldwide have resolved without the need for hospitalization or medical treatment. Likewise, 2009 H1N1 and TRS virus infection in ferrets generally resulted in moderate illness, with only few ferrets exhibiting severe disease. By differentiating between mild to moderately ill ferrets and those animals exhibiting severe disease, a more comprehensive evaluation of ferret health following infection with these H1N1 viruses was possible and provides a greater understanding of lymphohematopoietic involvement during influenza virus infection in this model. Determination of TRS H1N1 virus pathogenicity and transmissibility further places in context those features shared by swine lineage influenza viruses associated with disease in humans. Given the ability of swine lineage influenza viruses to jump the species barrier to infect humans, continuous surveillance and monitoring of influenza viruses that circulate among swine remain of critical importance to public health.
Acknowledgments
We thank P. Blair (Naval Health Research Center, San Diego, CA), G. J. Demmler (Texas Children's Hospital, Houston, TX), and C. Alpuche-Aranda (Instituto de Diagnóstico y Referencia Epidemiológicos, Mexico) for facilitating access to viruses.
The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency.
Footnotes
Published ahead of print on 1 December 2010.
REFERENCES
- 1.Abdel-Ghafar, A. N., et al. 2008. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358:261-273. [DOI] [PubMed] [Google Scholar]
- 2.Belser, J. A., et al. 2010. Pathogenesis of pandemic influenza A (H1N1) and triple-reassortant swine influenza A (H1) viruses in mice. J. Virol. 84:4194-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. 2010. 2009 H1N1 flu: international situation update. Centers for Disease Control and Prevention, Atlanta, GA.
- 4.Darnell, M. E., et al. 2007. Severe acute respiratory syndrome coronavirus infection in vaccinated ferrets. J. Infect. Dis. 196:1329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawood, F. S., et al. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605-2615. [DOI] [PubMed] [Google Scholar]
- 6.Fox, J. G. 1988. Normal clinical and biologic parameters, p. 183-210. In J. G. Fox (ed.), Biology and diseases of the ferret, 2nd ed. Williams & Wilkins, Baltimore, MD.
- 7.Garten, R. J., et al. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Gomez, A., et al. 2010. Severe pneumonia associated with pandemic (H1N1) 2009 outbreak, San Luis Potosi, Mexico. Emerg. Infect. Dis. 16:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarner, J., et al. 2000. Immunohistochemical and in situ hybridization studies of influenza A virus infection in human lungs. Am. J. Clin. Pathol. 114:227-233. [DOI] [PubMed] [Google Scholar]
- 10.Itoh, Y., et al. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain, S., et al. 2009. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N. Engl. J. Med. 361:1935-1944. [DOI] [PubMed] [Google Scholar]
- 12.Kandun, I. N., et al. 2006. Three Indonesian clusters of H5N1 virus infection in 2005. N. Engl. J. Med. 355:2186-2194. [DOI] [PubMed] [Google Scholar]
- 13.Lee, E. J., W. E. Moore, H. C. Fryer, and H. C. Minocha. 1982. Haematological and serum chemistry profiles of ferrets (Mustela putorius furo). Lab. Anim. 16:133-137. [DOI] [PubMed] [Google Scholar]
- 14.Louie, J. K., et al. 2009. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 302:1896-1902. [DOI] [PubMed] [Google Scholar]
- 15.Ma, W., et al. 2007. Identification of H2N3 influenza A viruses from swine in the United States. Proc. Natl. Acad. Sci. U. S. A. 104:20949-20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maines, T. R., et al. 2006. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc. Natl. Acad. Sci. U. S. A. 103:12121-12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maines, T. R., et al. 2009. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325:484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maines, T. R., et al. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 79:11788-11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maines, T. R., et al. 2008. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol. Rev. 225:68-84. [DOI] [PubMed] [Google Scholar]
- 20.Munster, V. J., et al. 2009. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 325:481-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oner, A. F., et al. 2006. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N. Engl. J. Med. 355:2179-2185. [DOI] [PubMed] [Google Scholar]
- 22.Pappas, C., et al. 2010. Receptor specificity and transmission of H2N2 subtype viruses isolated from the pandemic of 1957. PLoS One 5:e11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pebody, R. G., et al. 2010. Pandemic influenza A (H1N1) 2009 and mortality in the United Kingdom: risk factors for death, April 2009 to March 2010. Euro Surveill. 15:19571. [PubMed] [Google Scholar]
- 24.Reed, L. J., and H. A. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. (Lond.) 27:493-497. [Google Scholar]
- 25.Richmond, J. Y., and R. W. M. McKinney. 2007. Laboratory biosafety level criteria. In J. Y. Richmond and R. W. McKinney (ed.), Biosafety in microbiological and biomedical laboratories, 5th ed. Centers for Disease Control and Prevention, Atlanta, GA.
- 26.Riquelme, A., et al. 2009. Gastrointestinal manifestations among Chilean patients infected with novel influenza A (H1N1) 2009 virus. Gut 58:1567-1568. [DOI] [PubMed] [Google Scholar]
- 27.Shieh, W. J., et al. 2010. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am. J. Pathol. 177:166-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinde, V., et al. 2009. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005-2009. N. Engl. J. Med. 360:2616-2625. [DOI] [PubMed] [Google Scholar]
- 29.Sorrell, E. M., H. Wan, Y. Araya, H. Song, and D. R. Perez. 2009. Minimal molecular constraints for respiratory droplet transmission of an avian-human H9N2 influenza A virus. Proc. Natl. Acad. Sci. U. S. A. 106:7565-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephenson, I., J. M. Wood, K. G. Nicholson, A. Charlett, and M. C. Zambon. 2004. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 103:91-95. [DOI] [PubMed] [Google Scholar]
- 31.Tumpey, T. M., et al. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310:77-80. [DOI] [PubMed] [Google Scholar]
- 32.Vincent, A. L., et al. 2009. Characterization of an influenza A virus isolated from pigs during an outbreak of respiratory disease in swine and people during a county fair in the United States. Vet. Microbiol. 137:51-59. [DOI] [PubMed] [Google Scholar]
- 33.Yuen, K. Y., et al. 1998. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351:467-471. [DOI] [PubMed] [Google Scholar]
- 34.Zeng, H., et al. 2007. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type I interferon response in polarized human bronchial epithelial cells. J. Virol. 81:12439-12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zitzow, L. A., et al. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76:4420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]