Abstract
West Nile virus (WNV) is transmitted to vertebrate hosts primarily by infected Culex mosquitoes. Transmission of arboviruses by the bite of infected mosquitoes can potentiate infection in hosts compared to viral infection by needle inoculation. Here we examined the effect of mosquito transmission on WNV infection and systematically investigated multiple factors that differ between mosquito infection and needle inoculation of WNV. We found that mice infected with WNV through the bite of a single infected Culex tarsalis mosquito exhibited 5- to 10-fold-higher viremia and tissue titers at 24 and 48 h postinoculation and faster neuroinvasion than mice given a median mosquito-inoculated dose of WNV (105 PFU) by needle. Mosquito-induced enhancement was not due to differences in inoculation location, because additional intravenous inoculation of WNV did not enhance viremia or tissue titers. Inoculation of WNV into a location where uninfected mosquitoes had fed resulted in enhanced viremia and tissue titers in mice similar to those in mice infected by a single infected mosquito bite, suggesting that differences in where virus is deposited in the skin and in the virus particle itself were not responsible for the enhanced early infection in mosquito-infected mice. In addition, inoculation of mice with WNV mixed with salivary gland extract (SGE) led to higher viremia, demonstrating that mosquito saliva is the major cause of mosquito-induced enhancement. Enhanced viremia was not observed when SGE was inoculated at a distal site, suggesting that SGE enhances WNV replication by exerting a local effect. Furthermore, enhancement of WNV infection still occurred in mice with antibodies against mosquito saliva. In conclusion, saliva from C. tarsalis is responsible for enhancement of early WNV infection in vertebrate hosts.
West Nile virus (WNV) is a mosquito-transmitted virus in the family Flaviviridae, genus Flavivirus. Since its introduction in New York in 1999, WNV has become the most prevalent arbovirus in the United States (5). WNV is transmitted in an enzootic cycle between mosquito vectors and avian hosts. In the United States, important enzootic vectors include Culex pipiens, C. quinquefasciatus, and C. tarsalis (12). Transmission of arboviruses to vertebrate hosts occurs through the bite of female mosquitoes that imbibe blood to acquire protein for egg development. After alighting on a potential host, a mosquito inserts its mouthparts into the skin, actively probes within the tissue for blood, and when blood is found, begins feeding either directly from the vessel or from the resulting hemorrhagic pool. Throughout the probing and blood feeding process, a mosquito ejects saliva into the host. Mosquito saliva not only contains pharmacologically active molecules that serve to counteract the host hemostatic response, reduce inflammation, and alter host immunity (reviewed in references 20 and 29) but may also contain pathogens, such as WNV.
Saliva from arthropods, including sand flies, ticks, and mosquitoes, can potentiate infection of arthropod-borne pathogens (reviewed in references 22 and 29). Several studies have shown that arboviruses transmitted by mosquito bite or associated with mosquito saliva produce enhanced infection in vertebrate hosts compared to infection with the same viruses by needle inoculation (9, 15, 17, 24, 27). In contrast, other studies have shown no effect on arbovirus infection due to mosquito transmission (13, 18, 21, 26).
The effect of mosquito transmission on WNV infection remains unclear. Two different studies used WNV-infected Culex mosquitoes and did not observe an effect of mosquito transmission compared to needle inoculation on WNV infection (13, 21), but different experimental parameters, such as viral dose, viral source, and route of inoculation, were not evaluated. On the other hand, two other studies did show an effect due to mosquitoes (24, 27). One of these studies was from our laboratory, and we demonstrated that young chickens infected with WNV by a single Culex mosquito have higher early viremia and greater viral shedding than chickens inoculated in the same location with 103 PFU of WNV (27). In addition, Schneider and coworkers (24) demonstrated that mice inoculated with WNV in an area where uninfected Aedes mosquitoes have fed (spot feeding) have lower survival rates, higher viremia, and faster neuroinvasion than mice infected with WNV without prior mosquito feeding. Although these two studies suggest that WNV infection is enhanced by mosquito transmission, the studies themselves have limitations. In our earlier study with chickens, enhancement by mosquito transmission occurs in comparison to needle inoculation at doses of ≤103 PFU, but not at higher doses. Later studies in our laboratory showed that the median dose inoculated by mosquitoes was actually 100-fold higher (105 PFU) (28). In addition, we did not address other potential differences between mosquito bite and needle inoculation (viral source and inoculation location) that may have caused the differences between mosquito and needle infections, and the use of 1- and 5-day-old chickens, which are not fully immunologically mature, may have affected our results. In the study performed by Schneider and coworkers (23), WNV was inoculated into a spot where approximately 11 Aedes aegypti mosquitoes had fed. Although WNV has been detected in field-caught A. aegypti, this species is not an important enzootic or bridge vector of WNV (30). Furthermore, salivary proteins and the biological effects of saliva differ between Aedes and Culex mosquitoes (4, 19, 32); therefore, the results of this study may not apply to the major enzootic vectors in the Culex genus. Additionally, the high dose of mosquito saliva (from approximately 11 mosquitoes) that was delivered into the WNV inoculation site in this study may not accurately reflect the amount of saliva that would be present during a natural WNV infection by mosquito bite.
The goals of our current study were (i) to determine if mosquito transmission enhances WNV infection by modeling natural transmission using an important enzootic vector (C. tarsalis) feeding on an immunocompetent host and (ii) to investigate the mechanism(s) responsible for enhancement. In contrast to previous studies with WNV and Culex species mosquitoes (13, 21), we demonstrated that a single WNV-infected C. tarsalis mosquito bite enhanced early viral infection. In addition, we systematically investigated the causes for mosquito-induced enhancement and demonstrated that enhancement was not due to differences in viral dose, viral source, location of the inoculation, or mechanical probing by the mosquito. We showed that mosquito saliva enhanced early infection in mice, characterized by higher viremia and higher tissue titers, using three methods—a single WNV-infected mosquito, inoculation of WNV into a spot where 2 or 3 uninfected mosquitoes had fed, and WNV mixed with salivary gland extract (SGE). Enhancement also occurred when virus and SGE were inoculated separately in the same location but did not occur when SGE was inoculated at a site distal from virus. We explored the robustness of mosquito saliva-induced enhancement in two additional studies. Since mosquitoes inoculate a wide range of viral doses while probing and feeding (28), we tested low viral doses and found that mosquito saliva did not affect the 50% infective dose (ID50) of WNV in mice. We also presensitized mice to mosquito saliva. Hosts in nature are bitten by uninfected mosquitoes and develop antibodies to mosquito salivary proteins. Previous studies have shown that presensitized hosts react differently than naïve hosts when infected with pathogens by mosquitoes (8, 11, 23); however, in our studies, presensitized hosts reacted to WNV infection by mosquito transmission in the same manner as naïve hosts. In summary, our studies demonstrate that mosquito saliva alters the response of WNV infection in vertebrate hosts.
MATERIALS AND METHODS
Cells.
Vero cells (African green monkey kidney; ATCC CCL-81) and BHK-21 cells (baby hamster kidney; ATCC CCL-10) were cultured in minimal essential medium (Gibco Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS), 2 mM l-glutamine, 1.5 g/liter sodium bicarbonate, 100 U/ml penicillin, and 100 μg/ml streptomycin. C6/36 cells (A. albopictus mosquito; ATCC CRL-1660) were cultured in minimal essential medium supplemented with 10% HI-FBS, 2 mM l-glutamine, 1.1 g/liter sodium bicarbonate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.1 mM nonessential amino acid. Mammalian cells were incubated at 37°C and 5% CO2, and C6/36 cells were incubated at 28°C and 5% CO2.
Virus.
Virus was produced from a full-length cDNA clone of a WNV isolate obtained from New York, NY, in 2000 (25). RNA was transcribed in vitro from the cDNA clone and electroporated into BHK-21 or C6/36 cells as previously described (14). The viral stocks were designated WNV-BHK for virus harvested from BHK-21 cells and WNV-C6/36 for virus harvested from C6/36 cells. Viral titers were determined by plaque assays on Vero cell monolayers.
Animals.
We used a C. tarsalis colony established in 2003 from mosquitoes collected in the Coachella Valley of California (originally graciously provided by W. K. Reisen). Five-week-old, female C57BL/6 (B6) and C3H/HeN (C3H) mice were purchased from Taconic (Germantown, NY), acclimatized for 1 week in a biosafety level 3 (BSL-3) animal facility, and given food and water ad libitum. All mice were 6 to 7 weeks old at the start of each study. All studies were approved by Institutional Animal Care and Use Committees and followed criteria established by the National Institutes of Health.
Blood and tissue processing.
Blood samples were centrifuged (5,000 × g, 5 min), and serum was removed and stored at −80°C for later titration. Tissues were weighed and processed as previously described (3). Virus was quantified in sera and clarified tissue homogenates by plaque assay on Vero cell monolayers.
Infection of mice with WNV by mosquito bite or needle inoculation.
Seven days prior to feeding on mice, mosquitoes were infected with WNV by intrathoracic inoculation of approximately 30 PFU of WNV-BHK. After inoculation, mosquitoes were held in 0.5-liter cardboard cartons with a mesh top at 27°C and provided with 10% sucrose via a soaked cotton pad. Forty to 48 h prior to feeding, infected mosquitoes were placed into clear plastic 18.5-ml vials with a mesh top (one mosquito per vial) and kept in a humid environment without access to sucrose or water. On the day of inoculation/mosquito feeding, mice were anesthetized with isoflurane (E-Z anesthesia; Euthanex Corp., Palmer, PA). Mice were either inoculated subcutaneously (s.c.) by needle with 105 PFU of WNV-BHK into the left rear footpad as previously described (3) or infected by the bite of a single infected mosquito. For mosquito infection, the mesh top of a vial containing a single infected mosquito was placed in contact with the left rear footpad. Probing time, feeding time, and blood engorgement status were recorded for each mosquito, and WNV infection was confirmed in all mosquitoes that fed or probed as previously described (28). Anesthesia times were matched for mosquito-bitten and needle-inoculated mice.
Two independent experiments were conducted to determine the viremia of the infected animals. In the first experiment, B6 mice (n = 16 per group) were inoculated or infected by the bite of a single infected mosquito with WNV as described above. Eight mice from each group were tail bled at 12 and 24 h postinoculation (hpi) and then euthanized at 96 hpi. The remaining mice from each group (n = 8) were tail bled at 24, 48, and 72 hpi and then euthanized at 120 hpi. In the second experiment, 13 mice were probed and/or fed upon by infected mosquitoes, and 22 mice were needle inoculated with WNV. All mice were tail bled at 24, 48, and 72 hpi and then euthanized at either 96 hpi or 120 hpi. Blood samples were harvested from all euthanized mice.
Three independent experiments were conducted to examine the viral load in the tissues. B6 mice (n = 6 to 18 per group) were inoculated with either WNV-BHK (replicates 1 and 2) or WNV-C6/36 (replicate 3) or infected by the bite of a single infected mosquito as described above. In the second replicate, an additional group of mice (n = 18) was inoculated with WNV-BHK by an s.c. inoculation of 105 PFU in the left rear footpad plus 200 PFU inoculated intravenously (i.v.) into the tail vein (see below). Six mice from each group were euthanized at 12, 24, and 48 hpi (replicate 1), at 24, 48, and 72 hpi (replicate 2), or at 12 hpi (replicate 3), and blood samples and the following tissues were harvested: ipsilateral (left) footpad (inoculation site), ipsilateral popliteal lymph node (draining lymph node), contralateral (right) footpad, contralateral popliteal lymph node, spleen, brain, and spinal cord. Prior to tissue harvest at >12 hpi, mice were perfused with phosphate-buffered saline (PBS) supplemented with 1% HI-FBS for 10 to 15 min at 6 ml/min.
Morbidity and mortality studies were performed by infecting B6 or C3H mice with WNV-BHK as described above (n = 12 in each group). In addition, mice (n = 4 per group) were either inoculated with diluent (low-endotoxin PBS [tissue culture grade; Gibco Invitrogen] with 1% HI-FBS) alone or fed upon by uninfected mosquitoes. All mice were observed for clinical signs, including ruffled fur, hunching, weakness, ataxia, and paralysis, at least once per day and weighed daily through 16 days postinoculation (dpi) and every 2 to 4 days until 29 dpi. A mouse was considered to be sick if at least one of the following criteria was met: (i) ≥10% weight loss or (ii) clinical signs for at least 2 days. Mice that exhibited severe disease were euthanized. All surviving mice were bled at >21 dpi, and viral infection was confirmed by seropositivity on an enzyme-linked immunosorbent assay (ELISA) for WNV-specific antibodies as described previously (3). No clinical signs, weight loss, or WNV-specific antibodies were observed in mock-inoculated mice or mice fed upon by uninfected mosquitoes.
Effect of i.v. inoculation on WNV infection in mice.
B6 mice were inoculated with 105 PFU of WNV-BHK by s.c. needle inoculation in the left rear footpad plus i.v. inoculation of either 200 PFU of WNV-BHK or diluent delivered into the lateral tail vein. Three studies were conducted to compare the routes of virus delivery. In the first study, mice were tail bled at 12, 24, and 72 hpi (n = 8 per group). At 120 hpi, mice were euthanized, and serum samples and the following tissues were collected: brain, spleen, ipsilateral footpad, and ipsilateral popliteal lymph node. In the second study, eight mice from each group were euthanized at 12 hpi, and serum samples and the following tissues were collected: ipsilateral footpad, ipsilateral popliteal lymph node, contralateral footpad, contralateral popliteal lymph node, and spleen. The remaining mice in each group were tail bled at 24, 48, and 72 hpi; at 96 hpi, these mice were euthanized, and blood samples were harvested. The third study had three treatment groups, s.c. inoculation, s.c. plus i.v. inoculation, and mosquito infection, and is described in the previous section.
Spot feeding studies.
B6 mice were anesthetized with isoflurane and then inoculated with WNV either by needle inoculation alone (control group) or by needle inoculation into a location or “spot” where uninfected mosquitoes had probed/fed and deposited saliva (spot-fed group). The mesh top of a clear plastic 18.5-ml vial containing five uninfected mosquitoes (starved for 44 h prior to feeding) was placed in contact with the left rear foot of the spot-fed mice. The feeding site was restricted to the footpad by placement of tape over the toes and heel. Mosquitoes were allowed to probe and feed on the footpad for 10 min. We recorded the number of mosquitoes that probed and the blood engorgement status of each mosquito. Immediately following mosquito feeding, the left rear footpad of each mouse was inoculated s.c. with 105 PFU of WNV-C6/36. Control mice were inoculated with WNV-C6/36 without prior mosquito feeding. Anesthesia times of control mice and spot-fed mice were matched.
Two independent experiments were performed to determine the viremia of the mice (n = 16 per group per experiment). For each experiment, half of the mice in each group (n = 8) were tail bled at 12 and 24 hpi and then euthanized at 48 hpi. The remaining mice in each group (n = 8) were tail bled at 24, 48, and 72 hpi and then euthanized at 96 hpi. Blood samples were harvested from all euthanized mice.
Mice (n = 18 per group) were infected with WNV as described above to determine the viral load in the tissues. Six mice from each group were euthanized at 12, 24, and 48 hpi, and serum samples and the following tissues were harvested: ipsilateral footpad, ipsilateral popliteal lymph node, contralateral footpad, contralateral popliteal lymph node, spleen, brain, and spinal cord. Prior to tissue harvest at 24 and 48 hpi, mice were perfused with buffer as described above.
A low-dose study was performed by inoculating mice with 1 or 10 PFU of WNV-C6/36 with or without prior mosquito feeding as described above. All mice (n = 12 per group) were tail bled at 24, 48, and 72 hpi. Mice were observed for clinical signs at least once per day for 4 weeks, and any mice that exhibited severe disease were euthanized. All surviving mice were bled at 28 dpi, and viral infection was confirmed by seropositivity on an ELISA for WNV-specific antibodies as described previously (3).
Preparation of SGE.
Uninfected C. tarsalis mosquitoes were immobilized with CO2, dipped into 70% ethanol, and placed onto a drop of PBS on a glass slide. Under a dissecting scope, the mosquito head was gently teased away from the body, causing the salivary glands to be exposed. Glands were dissected from the head or thorax tissues, placed into low-endotoxin PBS, and frozen at −20°C. The final concentration was 1.2 salivary glands/10 μl (replicate 1) or 2 salivary glands/10 μl (replicate 2). Salivary gland membranes for replicate 1 were disrupted by two −80°C freeze-thaw cycles. Salivary glands for replicate 2 were subjected to sonication in ice water at 100 mV for 3 bursts of 20 s with 1 min of cooling time between bursts and centrifugation at 4°C and 5,000 × g for 10 min. The SGE supernatant was collected and frozen at −80°C until use. Protein concentrations of the SGE were 37 μg/ml for replicate 1 and 90 μg/ml for replicate 2, as determined by a commercially available protein quantification kit (Micro BCA protein assay kit; Pierce Biotechnology, Rockford, IL).
Infection of mice with WNV and SGE.
SGE was prepared as described above. Two replicate experiments were performed by inoculating B6 mice (n = 6 to 10 per group) s.c. in the left rear footpad with 105 PFU of WNV-C6/36 in 10 μl of (i) SGE plus 1% HI-FBS or (ii) PBS plus 1% HI-FBS (diluent). In another experiment, a study was designed to determine if SGE exerted a local or systemic effect by injecting SGE into local or distal sites. For this study, all B6 mice were inoculated s.c. in the left rear footpad with 105 PFU of WNV-C6/36 in 5 μl of diluent. Immediately prior to the virus inoculation, the mice (n = 8 per group) were inoculated s.c. with 5 μl of (i) diluent in the left and right rear footpads, (ii) SGE in the left rear footpad (local site) and diluent in the right rear footpad, or (iii) diluent in the left rear footpad and SGE in the right rear footpad (distal site). For all experiments, ≤3 serial blood samples per mouse were collected via the tail or maxillary vein from 12 to 72 hpi. At various times (48 to 96 hpi), mice were euthanized, and terminal blood samples were collected.
WNV infection of mice that were presensitized to mosquito saliva.
B6 mice were presensitized to mosquito saliva by exposing them to mosquito bites every 2 weeks over a period of 6 weeks (i.e., three exposures) (presensitized mice). At each exposure, mice were anesthetized with isoflurane, and uninfected mosquitoes (n = 10 to 20) in a mesh-covered carton were allowed to feed on their shaved ventral abdomen for 20 min. Mice in the unexposed group (naïve control) were anesthetized for the same amount of time. The average number of mosquitoes that fed on each mouse was determined by counting the number of engorged mosquitoes at the end of the feeding period. Two weeks after the last feeding period, we performed spot feeding studies on the presensitized and naïve mice as described above. All mice were tail bled at 24, 48, and 72 hpi. At 96 hpi, mice were euthanized, and blood samples were harvested.
Determination of antibodies against SGE in presensitized and naïve mice.
Serum samples from presensitized and naïve mice at 96 hpi (described above) were heat inactivated for 1 h at 56°C, and an ELISA was performed to identify antibodies against mosquito salivary proteins. Plates (96-well Immulon 1B; Thermo Scientific, Milford, MA) were incubated with 10 μg/ml of SGE or bovine serum albumin (BSA) in 50 mM sodium carbonate at 4°C overnight. Wells were washed three times with PBS and blocked with PBS-3% BSA (wt/vol) for 1 h at room temperature (RT). Mouse serum (1:50 dilution in PBS-3% BSA) was added to each well and incubated at RT for 1 h. Wells were washed three times with PBS-3% BSA. Horseradish peroxidase-conjugated goat-anti mouse IgG (1:1,000 dilution in PBS-3% BSA, 0.1% [vol/vol] Tween 20; Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD) was added to each well, and plates were incubated at RT for 1 h. Wells were washed five times with PBS, 0.1% Tween 20. 3′,3′,5′,5′-tetramethylbenzidine substrate (Kirkegaard & Perry Laboratories, Inc.) was added to each well, and plates were incubated at RT for 8 min. HCl (1:20) was added to each well to stop the enzyme reaction, and the plates were read using a wavelength of 450 nm. Samples were run in triplicate. Positive/negative (P/N) values were calculated as the ratio of absorbance of wells coated with mosquito SGE to absorbance of wells coated with BSA. The P/N cutoff value for positive samples was determined by taking the average P/N value of control samples (naïve mice that were infected with WNV by needle inoculation) plus 3 standard deviations.
Statistical analysis.
Viral titers were log transformed and checked for normality. Ranks of nonnormal data were determined using PROC RANK (SAS, version 9.0). One-way or two-way analysis of variance (ANOVA) on ranked or nonranked data was used to test for differences between virus titers in serum or tissues between treatment groups and experimental replicates. Two-tailed Fisher's exact test was used to compare percentages of tissues positive for virus (GraphPad, San Diego, CA). A two-tailed Mann-Whitney U test was used to test for differences between viremic loads for studies with a single replicate and to determine if P/N values differed significantly between the presensitized and naïve groups (GraphPad). A log rank test was used to compare survival curves (GraphPad).
RESULTS
Establishment of mouse model for WNV infection by C. tarsalis.
Mosquitoes eject saliva during probing and blood feeding, and if infected, virus is also ejected. Furthermore, WNV enters the bloodstream during blood feeding (28). Thus, in order to study mosquito transmission of WNV, we characterized mosquito feeding and WNV infection of mice by infected C. tarsalis. The data for all studies were very similar. For the studies with results shown in Fig. 1 and 2, mosquitoes probed for an average of 5 min and fed on blood for an average of 4 min, and all mice that were exposed to probing and/or feeding of WNV-infected mosquitoes developed a productive infection as measured by virus in serum and/or tissues. Most mosquitoes (60/71, 85%) took a partial or full blood meal; the remaining 11 mosquitoes only probed. In the morbidity and mortality study (Table 1), again most mosquitoes (22/28, 79%) took a partial or full blood meal; the remaining 6 mosquitoes only probed. Average probing and feeding times were 6 min and 3 min, respectively. In this experiment, infection status of the mice was monitored by seroconversion to WNV at >21 dpi. All but two mice (one in each replicate) seroconverted following exposure to WNV-infected mosquitoes. The mosquitoes that fed on these two “uninfected” mice probed and fed for normal lengths of time and were confirmed to be infected with WNV.
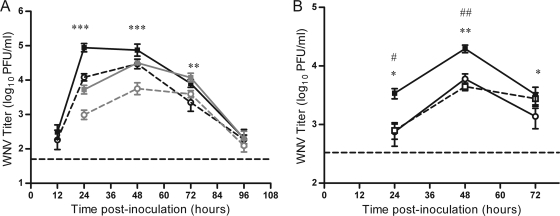
FIG. 1.
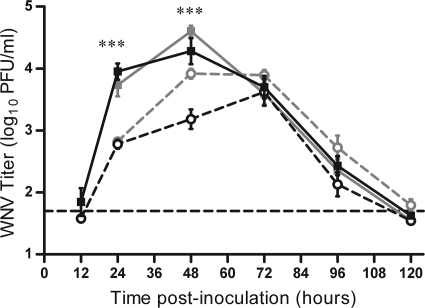
Mice infected with WNV by mosquito bite have higher early viremia than mice infected with WNV by needle. Mice were infected through the bite of a single WNV-infected C. tarsalis mosquito in the left rear footpad (solid lines, filled symbols) or were needle inoculated s.c. with 105 PFU of WNV-BHK in the same location (dashed lines, open symbols). Serial serum samples were harvested at various times postinoculation (n = 6 to 22 mice per group per time point), and plaque assays were performed to determine viremia. Two replicates of this experiment were performed; black lines indicate data from the first replicate, and gray lines indicate data from the second replicate. A two-way ANOVA was performed on ranked data to determine the effect of treatment (mosquito versus needle) and replicate. Significant treatment effect P values are indicated as follows: ***, P ≤ 0.001. A significant effect of replicate (P < 0.0001) was observed at 48 hpi. Error bars represent standard errors of the means. The horizontal dashed line indicates an LOD of 50 PFU/ml for the assay.
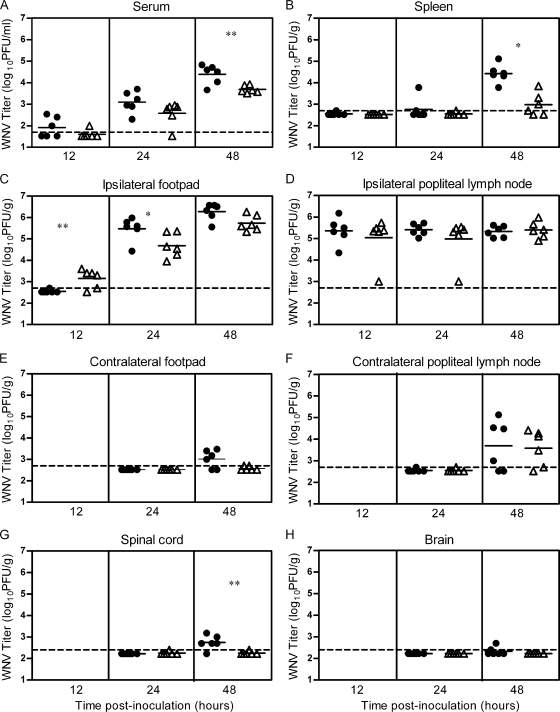
FIG. 2.
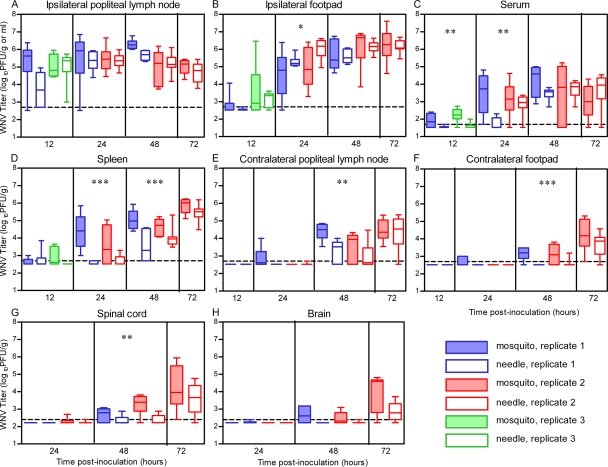
WNV spreads more quickly to peripheral tissues in mice when delivered by mosquito bite than by needle. Mice were infected through the bite of a single WNV-infected C. tarsalis mosquito in the left rear footpad (mosquito, shaded boxes) or were needle inoculated s.c. with 105 PFU of WNV-BHK (1st and 2nd replicates) or WNV-C6/36 (3rd replicate) in the same location (needle, open boxes) (n = 6 to 12 mice per group per time point). Serum samples and tissues were harvested at various times postinoculation, and plaque assays were performed to determine viral load. Data are shown as whisker-box plots, with the middle two quartiles (25th to 75th percentiles) represented by the box, the median represented as the horizontal line inside the box, and the highest and lowest values represented at the ends of the bars. Data from the 1st, 2nd, and 3rd replicates are indicated by blue, red, and green symbols, respectively. A two-way ANOVA was performed on ranked data to determine effect of treatment (mosquito versus needle) and replicate. Significant treatment effect P values are indicated as follows: *, P = 0.01 to 0.05; **, P = 0.001 to 0.01; ***, P ≤ 0.001. A significant replicate effect was observed at 48 hpi (A) and 24 hpi (B) (P = 0.01 to 0.05), and significant treatment by replicate effects were observed at 12 hpi (A), 24 hpi (C), and 24 hpi (E) (P = 0.01 to 0.05). The horizontal dashed line indicates an LOD of 500 PFU/g (A, B, D, E, and F), 50 PFU/ml (C), or 250 PFU/g (G and H).
TABLE 1.
Method of WNV infection does not affect morbidity or mortality in mice
| Mouse strain | Mode of infectiona | No. infectedb/no. exposed | No. sickc/no. infected | No. diedc/no. infected | Avg day of disease onset (SD)d | Avg survival time, days (SD)e |
|---|---|---|---|---|---|---|
| B6 | Mosquito | 11/12 | 2/11 | 1/11 | 7.0 (0.0) | 12.0 (NAf) |
| Needle | 12/12 | 5/12 | 3/12 | 6.8 (0.8) | 10.7 (2.3) | |
| C3H | Mosquito | 15/16 | 14/15 | 14/15 | 6.6 (0.8) | 7.5 (1.2) |
| Needle | 16/16 | 16/16 | 13/16 | 7.4 (1.5) | 8.2 (1.6) |
Mice were infected by the bite of a single WNV-infected C. tarsalis mosquito or by s.c. needle inoculation with 105 PFU of WNV-BHK in the left rear footpad. After feeding, all mosquitoes were confirmed to be infected by plaque assay.
Number of infected mice includes mice that died of WNV disease and mice that seroconverted to WNV at >21 dpi.
Mice were monitored for weight loss and clinical signs and were euthanized upon severe disease. No morbidity or mortality was observed in control mice inoculated with diluent or fed upon by uninfected mosquitoes.
Average day of disease onset in days, calculated for mice that were sick only.
Average survival time in days, calculated for mice that died only.
NA, not applicable.
Mice infected by mosquito bite exhibit higher viremia early in infection than mice inoculated by needle.
In order to study the effect of mosquito transmission on WNV infection, it was important to use an appropriate viral dose. We chose a dose of 105 PFU of WNV, which is equivalent to the median dose inoculated by a single C. tarsalis mosquito (28). Thus, on average, the mice fed upon by an infected mosquito received the same dose as mice inoculated by needle. We compared the levels of viremia in mice infected by the bite of a single WNV-infected mosquito in the left rear footpad and in mice infected by s.c. needle inoculation in the left rear footpad with 105 PFU of WNV. Early after infection (12 hpi), virus was detected in the serum of a few mice in both groups, and by 24 hpi, all mice developed viremia (Fig. 1). For two independent studies, mice infected by mosquito bite had serum titers that were 10-fold higher at 24 hpi and 5- to 10-fold higher at 48 hpi than those of needle-inoculated mice (two-way ANOVA on ranked data: 24 h, P < 0.0001; 48 h, P < 0.0001). Additionally, viremia of mosquito-infected mice peaked earlier and at a higher level than viremia of mice infected by needle inoculation (peak viremia: mosquito, 104.5 PFU/ml at 48 hpi; needle, 103.8 PFU/ml at 72 hpi). Viral titers were close to or below the limit of detection (LOD) by 120 hpi, and there was no difference in viral clearance between mice infected by the two methods.
Mice infected by mosquito bite had faster viral spread to peripheral and central nervous system tissues than mice inoculated by needle.
Viral kinetics and viral loads in tissues and serum following a single infected mosquito bite or s.c. needle inoculation of WNV (105 PFU) were compared (Fig. 2). Three independent studies were performed: the “1st replicate,” from 12 to 48 hpi; the “2nd replicate,” from 24 to 72 hpi; and the “3rd replicate,” at 12 hpi. We examined initial targets of WNV replication (skin at the inoculation site and draining lymph node) as well as other known targets of WNV, including distal skin sites, lymphoid tissues, and the central nervous system (CNS) (3).
In the initial targets of replication, WNV was detected in the ipsilateral popliteal lymph node (the draining lymph node for the inoculation site) and ipsilateral footpad (inoculation site) at 12 hpi (Fig. 2A and B). At this time point, the WNV titer in the draining lymph node of mosquito-infected mice was 30-fold higher than that in the draining lymph node of needle-inoculated mice in the first replicate (Fig. 2A); however, titers were similar in the third replicate (treatment by replicate, P = 0.03). There were significant differences in WNV titer in the ipsilateral footpad at 24 hpi (Fig. 2B); mosquito-infected mice had lower WNV titers than needle-inoculated mice (treatment, P = 0.04). No significant titer differences were observed between mosquito-infected and needle-inoculated mice in the draining lymph node or skin at the inoculation site after 24 hpi.
Viremia titers were significantly higher in the mosquito-infected mice than in the needle-inoculated mice at 12 hpi in the 1st and 3rd replicates and at 24 hpi in the 1st replicate (Fig. 2C). These results support the data shown in Fig. 1 demonstrating that viremia was enhanced in mosquito-infected mice; however, the enhancement was shifted to 12 to 24 hpi (Fig. 2C) instead of 24 to 48 hpi (Fig. 1). This shift may be due to differences in experimental design. The viremia titers shown in Fig. 1 were from four serial bleeds of the same mice over time, whereas the viremia titers shown in Fig. 2C were from different mice at each time point (i.e., at time of sacrifice).
Viral spread from the initial sites of replication to other tissues occurred earlier in mice infected by mosquito bite than in those infected by needle inoculation. Mosquito-infected mice had significantly higher titers than needle-inoculated mice in the spleen (>10-fold higher; P ≤ 0.0001) at 24 and 48 hpi (Fig. 2D). Viral titers were also significantly higher (P < 0.01) at 48 hpi in the contralateral popliteal lymph node, contralateral footpad, and spinal cord of mosquito-infected mice than in the same tissues in needle-inoculated mice (Fig. 2E to G). In addition to these differences in viral titers, the proportion of mice with detectable virus in the brain and contralateral footpad at 48 hpi was significantly higher in mosquito-infected mice than in mice infected by needle (brain: mosquito, 42%, versus needle, 6%; Fisher's exact test, P = 0.03; contralateral footpad: mosquito, 75%, versus needle, 11%; Fisher's exact test, P = 0.0012).
In summary, these results suggest that mosquito transmission of WNV results in higher viremia, earlier spread to peripheral tissues, and earlier neuroinvasion than needle inoculation.
Infection with WNV by mosquito bite does not affect morbidity and mortality.
We hypothesized that mice infected with WNV by mosquito bite would have higher morbidity and mortality than mice inoculated by needle, due to the higher viral load and earlier neuroinvasion observed in the former group. In fact, we saw the opposite effect; B6 mice infected with WNV by mosquito bite exhibited lower morbidity and mortality than needle-inoculated mice (Table 1). However, these differences were not statistically significant (survival log rank test, P = 0.31). We explored further the possibility that there was a protective effect due to mosquito bite infection by using C3H mice, which are more susceptible to WNV disease (3). Thus, any protective effect due to mosquito infection would be detected more readily. There were no differences in morbidity, mortality, disease onset, or survival time for C3H mice infected with WNV by needle inoculation or by mosquito bite (Table 1) (survival log rank test, P = 0.13), suggesting that the method of infection does not impact WNV disease in mice.
Location of virus inoculation is not responsible for enhanced early infection in mosquito-infected mice.
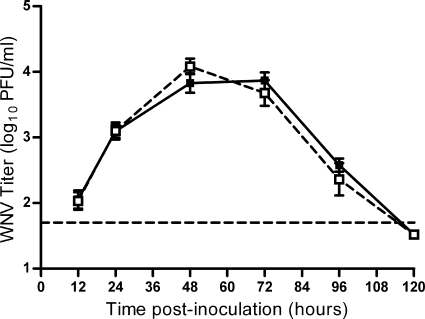
We hypothesized that the enhanced early infection in mosquito-infected mice was due to differences in location of virus inoculation into the host. In a previous study, we found that although mosquitoes inoculate almost all WNV extravascularly, approximately half of the mosquitoes also inoculate a small amount of virus (median of 200 PFU) directly into the blood, which is correlated with successful blood feeding (28). Direct inoculation of virus into the blood could result in higher early viremia and faster spread to peripheral tissues. We tested this possibility by needle inoculating mice s.c. with 105 PFU of WNV with or without an additional i.v. inoculation of 200 PFU of WNV, the median intravascular dose inoculated by a single C. tarsalis mosquito (28). There were no differences in viremia (Fig. 3) or tissue titer at 12 or 120 hpi (data not shown), suggesting that the small amount of virus inoculated intravenously by mosquitoes during blood feeding does not account for enhanced infection in mosquito-infected mice.
FIG. 3.
Inoculation location does not affect WNV viremia in mice. Mice were needle inoculated in two locations: s.c. with 105 PFU of WNV-BHK in the left rear footpad and i.v. with diluent alone (solid line, filled symbols) or 200 PFU of WNV (dashed line, open symbols) into the tail vein (n = 15 or 16 mice per group per time point). Serial serum samples were harvested at various times postinoculation, and plaque assays were performed. Error bars represent standard errors of the means. The horizontal dashed line indicates an LOD of 50 PFU/ml.
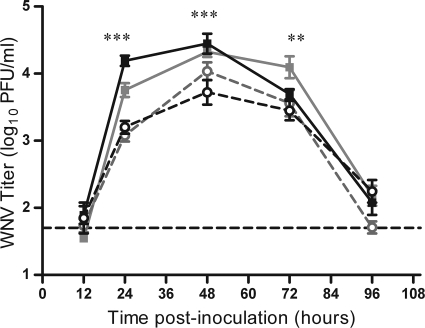
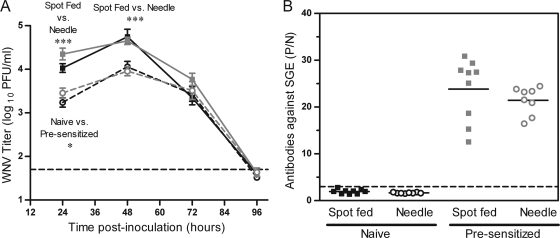
In addition, we tested the hypothesis that differences in where the virus was deposited in the skin were responsible for enhanced infection in mosquito-infected mice. Mosquitoes deposit saliva and virus in the dermis and subdermis, whereas we inoculated virus by needle s.c. (subdermis). Thus, we separated virus deposition from mosquito feeding by using a spot feeding technique. In these studies, five uninfected mosquitoes were allowed to feed in a spot on the left rear footpad of mice for 10 min, and WNV was inoculated into the feeding site by needle inoculation (spot-fed group). Control mice were inoculated with the same dose of WNV without prior mosquito feeding. Viremia of the spot-fed group was 4- to 10-fold higher at 24 hpi (Fig. 4) (ANOVA: treatment, P < 0.001; replicate, P < 0.01), 2- to 5-fold higher at 48 hpi (ANOVA: treatment, P < 0.001; treatment by replicate, P < 0.05), and 2- to 4-fold higher at 72 hpi (ANOVA: treatment, P < 0.01) than that of the control group. An average of 3 (range of 1 to 5) mosquitoes probed and/or fed on each footpad prior to virus inoculation. Viremia of spot-fed mice at 24 and 48 hpi did not vary based on the number of mosquitoes that probed or fed on the footpad (linear regression, P > 0.05). In an independent viral kinetics and viral load study, spot-fed mice exhibited higher serum (Fig. 5 A) (P < 0.005) and spleen (Fig. 5B) (P = 0.013) titers at 48 hpi, higher titers in the ipsilateral footpad (inoculation site) at 24 hpi (Fig. 5C) (P < 0.05), and earlier detection of the virus in the spinal cord (Fig. 5G) (P < 0.01). There were no significant differences in viral load in the contralateral footpad, lymph nodes, or brain between the two groups.
FIG. 4.
Spot feeding causes higher early viremia in mice. Mice were needle inoculated s.c. with 105 PFU of WNV-C6/36 in the left rear footpad without mosquito exposure (dashed lines, open symbols) or in the left rear footpad at a spot where up to 5 uninfected C. tarsalis mosquitoes had probed and/or fed (spot feeding) (solid lines, filled symbols). Serial serum samples were harvested at various times postinoculation, and plaque assays were performed. Two replicates of this experiment were performed (n = 8 to 16 mice per group per time point). Black lines indicate data from the 1st replicate, and gray lines indicate data from the 2nd replicate. A two-way ANOVA was performed on ranked data to determine effect of treatment and replicate. Significant treatment effect P values are indicated as follows: **, P = 0.001 to 0.01; ***, P ≤ 0.001. A significant replicate effect was observed at 24 hpi (P = 0.001 to 0.01), and a significant treatment by replicate effect was observed at 48 hpi (P = 0.01 to 0.05). Error bars represent standard errors of the means. The horizontal dashed line indicates an LOD of 50 PFU/ml.
FIG. 5.
Spot feeding causes faster spread of WNV to peripheral tissues in mice. Mice were needle inoculated s.c. with 105 PFU of WNV-C6/36 in the left rear footpad without mosquito exposure (open triangles) or in the left rear footpad at a spot where up to 5 uninfected C. tarsalis mosquitoes had probed and/or fed (filled circles). Serum samples and tissues were harvested at various times postinoculation, and plaque assays were performed to determine viral loads. An ANOVA on ranked data was performed to determine the effect of infection method at each time point and tissue type. Significant P values are indicated as follows: *, P = 0.01 to 0.05; **, P = 0.001 to 0.01. Horizontal solid lines indicate the geometric means of each group. The horizontal dashed line indicates an LOD of 50 PFU/ml (A), 500 PFU/g (B, C, D, E, and F), or 250 PFU/g (G and H).
Overall, these data indicate that the location where WNV is deposited by the mosquito (either intravenously or within the skin) is not responsible for the enhancement of WNV infection observed in mice infected by mosquito bite. Furthermore, these spot feeding studies controlled for dose and any difference in the source of virus (e.g., virus produced in cell culture versus salivary gland of a mosquito). Thus, these data suggest that either mosquito saliva or the mechanical act of mosquito probing at the inoculation site results in enhanced infection.
Mosquito saliva causes enhanced infection in mosquito-infected mice due to a local effect.
We tested the hypothesis that mosquito saliva deposited by feeding mosquitoes was responsible for enhanced infection in mosquito-infected mice by mixing virus directly with SGE or diluent and inoculating the mixture into mice. For two independent studies, mice inoculated with WNV and SGE exhibited viremia levels that were approximately 10-fold higher at 24 hpi (P < 0.001), 7-fold higher at 48 hpi (P < 0.001), and 3-fold higher at 72 hpi (P < 0.01) than those in mice inoculated with WNV in diluent (Fig. 6 A). We further examined whether SGE exerts a local or systemic effect on enhanced viremia by inoculating SGE and WNV in the same or opposite foot. The viremia was approximately 4-fold higher in mice inoculated with WNV and SGE at the same site (P = 0.01 at 24 hpi and P = 0.001 to 0.002 at 48 hpi) than in mice inoculated with SGE at a distal site or mice inoculated with diluent alone (Fig. 6B). There were no significant differences in viremia for mice inoculated with SGE at a distal site compared to mice inoculated with diluent alone (P > 0.05). These results suggest that mosquito saliva, not the mechanical act of probing, causes enhanced infection in mosquito-infected mice and that the effect is local, not systemic. Furthermore, enhancement occurred even when WNV was not mixed directly with mosquito saliva (Fig. 4 and 6B), which strongly suggests that the effect of saliva is not on the virus particle itself.
FIG. 6.
Salivary gland extract causes higher viremia in mice during early infection, due to a local effect. (A) Mice were needle inoculated s.c. in the left rear footpad with 105 PFU of WNV-C6/36 mixed with diluent alone (dashed lines, open symbols) or with SGE from C. tarsalis mosquitoes (solid lines, filled symbols). Serial serum samples (n = 6 to 12 mice per group per time point) were harvested. Black lines indicate data from the first replicate, and gray lines indicate data from the second replicate. A two-way ANOVA was performed on ranked data to determine the effect of treatment and replicate. Significant treatment effect P values are indicated as follows: **, P = 0.001 to 0.01; ***, P ≤ 0.001. Significant replicate effects were observed at 24 hpi (P ≤ 0.001) and at 48 hpi (P = 0.001 to 0.01). (B) Mice were needle inoculated s.c. in the left rear footpad with 105 PFU of WNV-C6/36 mixed with diluent alone. Additional separate s.c. inoculations of diluent in left and right rear footpads (open circle, solid line), SGE in the right rear footpad (open square, dashed line), or SGE in the left rear footpad (filled square, solid line) were given. Serial serum samples (n = 8 mice per group per time point) were harvested. Mann-Whitney tests were performed to compare treatment groups. Significant differences between diluent inoculation and SGE inoculation in the left rear footpad are indicated by an asterisk (*), and significant differences between SGE inoculation in the left rear footpad and SGE inoculation in the right rear footpad are indicated by a number symbol (#). P values are indicated as follows: * or #, P = 0.01 to 0.05; ** or ##, P = 0.001 to 0.01. Plaque assays were performed to determine viremia. Error bars represent standard errors of the means. The horizontal dashed line indicates an LOD of 50 PFU/ml.
Robustness of enhanced WNV infection by mosquito saliva.
We conducted two additional studies to explore the robustness of WNV enhancement due to mosquito saliva. First, previous studies in our laboratory showed that C. tarsalis females inoculate a median dose of 105 PFU (28); however, these same studies also showed that a wide range of doses (5 to 500,000 PFU) is inoculated by mosquitoes as they probe and feed on vertebrate hosts. We therefore determined whether spot feeding lowers the infectious dose of WNV by enhancing early infection. The ID50 of needle-inoculated WNV in our mouse model is approximately 1 PFU (14). Thus, we inoculated 1 or 10 PFU of WNV with and without spot feeding. The ID50 (1 PFU) was the same in both groups, suggesting that mosquito saliva does not alter virus infectivity in mice (Table 2). In addition, we found that spot feeding had no effect on viremia at 48 or 72 hpi when either 1 or 10 PFU was inoculated into the feeding site (Table 2). At these low doses, virus was not detected in the serum at 24 hpi. Overall, these results demonstrate that saliva does not enhance infection when low doses of WNV are inoculated into mice.
TABLE 2.
WNV infection rate and geometric mean viremia do not differ in mice inoculated with low doses of WNV with or without prior feeding by uninfected mosquitoes
| Groupa | Viral dose (PFU) | No. infected/no. exposed | WNV titer in serum, log PFU/ml (SD)b |
||
|---|---|---|---|---|---|
| 24 hpi | 48 hpi | 72 hpi | |||
| Spot fed + WNV | 1 | 6/12 | <LODc | 3.7 (0.6) | 4.0 (0.8) |
| WNV | 1 | 6/12 | <LOD | 3.2 (0.8) | 3.7 (1.01) |
| Spot fed + WNV | 10 | 12/12 | <LOD | 3.7 (0.6) | 4.4 (0.2) |
| WNV | 10 | 12/12 | <LOD | 3.7 (0.7) | 4.0 (0.7) |
Mice were needle inoculated s.c. with WNV-C6/36 in the left rear footpad at a spot where up to 5 uninfected C. tarsalis females had probed and/or fed (spot fed + WNV) or without prior mosquito exposure (WNV).
Only mice that became infected with WNV (as determined by positive IgG ELISA on 28 dpi serum) were used in calculation of geometric mean viremia.
LOD, 50 PFU/ml.
In nature, most vertebrate hosts have previously been exposed to mosquito bites, thus presensitizing their immune systems. Since presensitization results in antibodies against mosquito salivary proteins (reviewed in reference 2), we hypothesized that presensitization to mosquitoes would inhibit the enhancing effect of mosquito saliva on WNV infection. We presensitized mice by exposing them to up to 20 uninfected mosquitoes three times, 2 weeks apart, and an average of 7 to 9 uninfected mosquitoes fed per mouse during each of the 3 presensitization feedings. Two weeks after the last exposure, the mice were inoculated with WNV by needle with or without spot feeding (average of 2 mosquitoes fed per mouse). Spot-fed mice in both presensitized and naïve control groups had significantly higher viremia at 24 and 48 hpi (P < 0.0001) than mice inoculated with WNV without spot feeding (Fig. 7 A). Thus, presensitization did not inhibit the enhancing effect of mosquito saliva. In fact, there was a small (approximately 2-fold) but significant increase in viremia at 24 hpi for presensitized mice in both the spot-fed and needle-only-inoculated groups (Fig. 7A) (P = 0.02). We confirmed by ELISA that all presensitized mice had antibodies to mosquito salivary proteins. The P/N values for presensitized mice were approximately 10-fold higher than they were for control mice (Fig. 7B) (P < 0.0001).
FIG. 7.
Presensitization to mosquito saliva does not alter the enhancing effect of spot feeding, despite the development of antibodies. Mice were exposed to up to 20 uninfected C. tarsalis mosquito bites three times, 2 weeks apart (presensitized; gray lines), or were not exposed to mosquitoes (naïve; black lines). Two weeks after the last exposure, mice were needle inoculated s.c. with 105 PFU of WNV-C6/36 in the left rear footpad without mosquito exposure (needle, dashed lines) or in the left rear footpad at a spot where up to 5 uninfected C. tarsalis mosquitoes had probed and/or fed (spot, solid lines) (n = 8 mice per group per time point). Serial serum samples were harvested at various times postinoculation, and plaque assays were performed to determine viremia (A). Sera from 96 hpi were also tested for antibodies to C. tarsalis mosquito SGE by use of an ELISA (B). For the viremia data, a two-way ANOVA on ranked data was performed to determine the effect of presensitization (naïve versus presensitized) and infection method (needle versus spot feeding) on viremia at each time point. Significant P values are indicated as follows: *, P = 0.01 to 0.05; ***, P ≤ 0.001. Error bars represent standard errors of the means. In panel A, the horizontal dashed line indicates an LOD of 50 PFU/ml. In panel B, the horizontal solid line represents the mean P/N value for each group, and the horizontal dashed line indicates the cutoff value for positivity. A Mann-Whitney t test was used to compare P/N values between presensitized and naïve mice (P < 0.0001).
DISCUSSION
Mosquito transmission can affect arbovirus infection; however, the outcome varies depending on the mosquito-virus-host system. To our knowledge, we are the first to demonstrate that the bite of a single WNV-infected mosquito enhances viral infection in an immunologically mature animal compared to infection from needle inoculation of WNV. Enhanced infection in mice infected by mosquito was characterized by higher early viremia, faster spread of the virus to peripheral tissues, and earlier neuroinvasion. Furthermore, this study is the first to explore multiple possible mechanisms of enhancement by mosquito-transmitted arboviruses in the same experimental system. Using this systematic approach, we demonstrated that enhancement by mosquito transmission was due to a local effect of mosquito saliva on the virus-host interaction.
Mosquitoes inoculate saliva into the host while probing for and feeding on blood, and saliva contains a variety of bioactive molecules that maintains blood flow by inhibiting the hemostatic response (reviewed in reference 20) and alters the local immune response (6, 7). Secreted salivary proteins differ in various mosquito species (4) and exert different biological effects on vertebrate hosts and cells (19, 32). Thus, it is important to examine biologically relevant mosquito species in the study of the mosquito-virus-host interaction. We observed enhancement of WNV using C. tarsalis mosquitoes, an important enzootic vector of WNV in the United States (10, 12, 30, 31), which also transmits the virus to humans and other mammals (1, 16). Enhanced infection due to mosquito saliva has been observed in previous studies with Aedes mosquitoes (9, 24), but we are the first to demonstrate that saliva from Culex mosquitoes enhances arboviral infections.
Our results differ from other studies that examined mosquito enhancement of WNV infection. Viremia in hamsters that are infected with WNV by the bite of a single C. quinquefasciatus mosquito does not differ from that in hamsters inoculated intraperitoneally with 104 PFU of WNV (21). Similarly, viremia in adult chickens that are infected with WNV by the bites of 3 to 5 infected C. tritaeniorhynchus mosquitoes does not differ from that in chickens inoculated s.c. with 104 PFU of WNV (13). These researchers did not conduct other studies with mosquito saliva or spot feeding; therefore, it is difficult to determine if the lack of enhancement by mosquitoes was due to inherent differences in mosquito-virus-host interaction or in experimental methodology. Another study with WNV used A. aegypti mosquitoes (24), which are not important vectors of WNV (30). These researchers did not examine the effect of infected mosquitoes but rather used spot feeding of uninfected mosquitoes (average of 11.5) and a mixture of WNV and SGE. Similarly to results from our studies with C. tarsalis, these researchers (24) observed higher viremia and earlier neuroinvasion in mice that were inoculated with WNV after spot feeding with A. aegypti than in mice given needle inoculation alone. In contrast to our studies, they observed greater mortality in mice inoculated with WNV mixed with SGE or after spot feeding with A. aegypti. We found no significant differences in morbidity, mortality, disease onset, or survival time in two different strains of mice infected with a single WNV-infected C. tarsalis mosquito and in mice inoculated with WNV by needle. Similarly to results from our studies, morbidity and mortality do not differ between needle inoculation and mosquito infection of WNV in hamsters (21) or chickens (13). The differences in survival between our studies and those of Schneider et al. (24) are most likely due to differences in the mosquito species used, but the method of infection (single infected mosquito versus spot feeding with an average of 11.5 mosquitoes or mixture with SGE) may have also had an effect on survival. Further studies that directly compare the effects of saliva from A. aegypti and C. tarsalis on WNV infection in mice are needed to resolve these contradictory results.
We conducted additional studies to investigate the robustness of saliva-induced enhancement. First, we determined if this enhancement occurred when low viral doses were inoculated into the host. Although most mosquitoes inoculate high doses of virus (105 PFU) while feeding on a host, a few inoculate low doses (28). We found that when low doses of virus (1 and 10 PFU) were inoculated into a host, spot feeding had no effect on the infection rate or WNV viremia, i.e., saliva-induced enhancement was not detected. However, due to the low inoculation dose, viremia was not detected in these mice until 48 hpi. Thus, it is possible that enhancement did occur but that our detection method was not sensitive enough to detect the low levels of virus. Alternatively, there may be a threshold level of virus that is needed for saliva-induced enhancement to occur. Perhaps when virus titers are below this threshold, the immune system is able to effectively control viral spread, whereas above this threshold, virus can escape immune control and, due to the effects of saliva, spreads more quickly to the draining lymph nodes and peripheral tissues. Further studies are needed to investigate the dose dependence of saliva-induced enhancement.
We also investigated if saliva-induced enhancement occurred in mice with preexisting antibodies to mosquito salivary proteins. Most vertebrate hosts in nature are previously exposed to bites from uninfected mosquitoes and have antibodies to mosquito salivary proteins (reviewed in reference 2) prior to becoming infected with WNV by mosquito bite. These antibodies could potentially alter the enhancing effect of mosquito saliva. However, we found that the preexposure of mice to uninfected mosquito bites and the development of antibodies to mosquito salivary proteins had no effect on saliva-induced enhancement. Both naïve and presensitized mice exhibited enhanced early WNV infection following spot feeding. A study by Schneider et al. (23) reported that WNV-infected mice that are preexposed to uninfected A. aegypti mosquito bites exhibit greater mortality than naïve mice. We did not measure mortality in our studies with preexposed mice, and we used a different mosquito species. Therefore, it is unclear whether we would have seen a similar phenomenon of greater mortality in mice preexposed to C. tarsalis. Similarly to results from our study, Schneider et al. (23) observed no significant differences in viremia between presensitized and naïve mice at 3 dpi; viremia earlier than 3 dpi was not measured in their study. More research is needed to determine the importance of preexposure to uninfected mosquito bites in the ultimate response of vertebrate hosts to infection with mosquito-borne viruses.
Several possible mechanisms could explain the enhancing effect of mosquito saliva on WNV infection in mice. Whatever the mechanism, it must occur early in infection, as significant differences were observed in mosquito-exposed mice at 12 to 24 hpi. In addition, our results suggest that saliva exerts a local effect on the host, which is not due to a direct interaction with the virus particle. Mosquito saliva is known to alter cytokine levels and other components of innate immunity, which can lead to immunosuppression or immune dysregulation (reviewed in reference 22). Therefore, one possible mechanism is that mosquito saliva modulates the immune response at the bite site, which could allow virus to replicate to higher levels at that site. Alternatively, leukocytes have been shown to infiltrate the skin at sites bitten by mosquitoes (6), suggesting that mosquito saliva can affect the composition of host cells at the bite site. This could alter the cell tropism of WNV, increase cell susceptibility to WNV, or increase the number of WNV-susceptible cells at the bite site, leading to higher levels of virus production. Future studies will be conducted to further understand how mosquito saliva induces enhancement of WNV infection in mice.
In conclusion, we demonstrated that saliva from C. tarsalis, a major enzootic and epizootic vector of WNV, is the primary factor that contributes to mosquito-enhanced WNV infection in mice. To our knowledge, this is the first paper that systematically examines the differences between transmission of WNV via a single infected mosquito and transmission via needle inoculation. Although we found that mosquito saliva affects only the early phase of infection in our mouse model, it is possible that mosquito saliva may have a more dramatic impact on pathogenesis in immunocompromised individuals. In addition, the enhanced viremia may impact the transmission cycle in nature by increasing the rate of transmission to naïve mosquitoes. Therefore, comprehension of the mechanism(s) by which mosquito saliva potentiates WNV infection and identification of the salivary components important for this effect are crucial. Knowledge obtained from these studies will aid in possible control strategies for WNV and other mosquito-borne pathogens.
Acknowledgments
We acknowledge the excellent technical assistance provided by Kim Appler, Chrystal Chadwick, Corey Bennett, and Pamela Chin. We thank the Wadsworth Center's Tissue Culture Core for providing cell culture support.
R. G. Albright was supported in part by an appointment to the Wadsworth Center Emerging Infectious Diseases (EID) Fellowship Program, administered and funded by the New York State Department of Health's Wadsworth Center. This project was funded in part by the Centers for Disease Control and Prevention, grant R01-CI000232-01, and the National Institutes of Health, National Institute of Allergy and Infectious Diseases, contract no. N01-AI25490. The BSL-3 vivarium at the Wadsworth Center was used and is funded in part as a core facility by National Institutes of Health, National Institute of Allergy and Infectious Diseases, U54-AI057158 (Northeast Biodefense Center).
Footnotes
Published ahead of print on 8 December 2010.
REFERENCES
- 1.Bell, J. A., N. J. Mickelson, and J. A. Vaughan. 2005. West Nile virus in host-seeking mosquitoes within a residential neighborhood in Grand Forks, North Dakota. Vector Borne Zoonotic Dis. 5:373-382. [DOI] [PubMed] [Google Scholar]
- 2.Billingsley, P. F., J. Baird, J. A. Mitchell, and C. Drakeley. 2006. Immune interactions between mosquitoes and their hosts. Parasite Immunol. 28:143-153. [DOI] [PubMed] [Google Scholar]
- 3.Brown, A. N., K. A. Kent, C. J. Bennett, and K. A. Bernard. 2007. Tissue tropism and neuroinvasion of West Nile virus do not differ for two mouse strains with different survival rates. Virology 368:422-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvo, E., et al. 2010. An insight into the sialotranscriptome of the West Nile mosquito vector, Culex tarsalis. BMC Genomics 11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2010. Surveillance for human West Nile virus disease—United States, 1999-2008. Surveillance summaries. MMWR Morb. Mortal. Wkly. Rep. 59:1-17. [PubMed] [Google Scholar]
- 6.Demeure, C. E., et al. 2005. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J. Immunol. 174:3932-3940. [DOI] [PubMed] [Google Scholar]
- 7.Depinay, N., F. Hacini, W. Beghdadi, R. Peronet, and S. Mecheri. 2006. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J. Immunol. 176:4141-4146. [DOI] [PubMed] [Google Scholar]
- 8.Donovan, M. J., et al. 2007. Uninfected mosquito bites confer protection against infection with malaria parasites. Infect. Immun. 75:2523-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards, J. F., S. Higgs, and B. J. Beaty. 1998. Mosquito feeding-induced enhancement of Cache Valley virus (Bunyaviridae) infection in mice. J. Med. Entomol. 35:261-265. [DOI] [PubMed] [Google Scholar]
- 10.Goddard, L. B., A. E. Roth, W. K. Reisen, and T. W. Scott. 2002. Vector competence of California mosquitoes for West Nile virus. Emerg. Infect. Dis. 8:1385-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes, R., et al. 2008. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc. Natl. Acad. Sci. U. S. A. 105:7845-7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes, E. B., et al. 2005. Epidemiology and transmission dynamics of West Nile virus disease. Emerg. Infect. Dis. 11:1167-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langevin, S. A., M. Bunning, B. Davis, and N. Komar. 2001. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerg. Infect. Dis. 7:726-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim, P. Y., K. L. Louie, L. M. Styer, P. Y. Shi, and K. A. Bernard. 2010. Viral pathogenesis in mice is similar for West Nile virus derived from mosquito and mammalian cells. Virology 400:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limesand, K. H., S. Higgs, L. D. Pearson, and B. J. Beaty. 2000. Potentiation of vesicular stomatitis New Jersey virus infection in mice by mosquito saliva. Parasite Immunol. 22:461-467. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen, C. F., W. K. Reisen, M. V. Armijos, N. J. Maclachlan, and T. W. Scott. 2008. High subclinical West Nile virus incidence among nonvaccinated horses in northern California associated with low vector abundance and infection. Am. J. Trop. Med. Hyg. 78:45-52. [PubMed] [Google Scholar]
- 17.Osorio, J. E., M. S. Godsey, G. R. DeFoliart, and T. M. Yuill. 1996. La Crosse viremias in white-tailed deer and chipmunks exposed by injection or mosquito bite. Am. J. Trop. Med. Hyg. 54:338-342. [DOI] [PubMed] [Google Scholar]
- 18.Reisen, W. K., R. E. Chiles, L. D. Kramer, V. M. Martinez, and B. F. Eldridge. 2000. Method of infection does not alter response of chicks and house finches to western equine encephalomyelitis and St. Louis encephalitis viruses. J. Med. Entomol. 37:250-258. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro, J. M. 2000. Blood-feeding in mosquitoes: probing time and salivary gland anti-haemostatic activities in representatives of three genera (Aedes, Anopheles, Culex). Med. Vet. Entomol. 14:142-148. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro, J. M. C., and I. M. B. Francischetti. 2003. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 48:73-88. [DOI] [PubMed] [Google Scholar]
- 21.Sbrana, E., et al. 2005. Oral transmission of West Nile virus in a hamster model. Am. J. Trop. Med. Hyg. 72:325-329. [PubMed] [Google Scholar]
- 22.Schneider, B. S., and S. Higgs. 2008. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans. R. Soc. Trop. Med. Hyg. 102:400-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider, B. S., et al. 2007. Prior exposure to uninfected mosquitoes enhances mortality in naturally-transmitted West Nile virus infection. PLoS One 2:e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider, B. S., et al. 2006. Potentiation of West Nile encephalitis by mosquito feeding. Viral Immunol. 19:74-82. [DOI] [PubMed] [Google Scholar]
- 25.Shi, P. Y., M. Tilgner, M. K. Lo, K. A. Kent, and K. A. Bernard. 2002. Infectious cDNA clone of the epidemic West Nile virus from New York City. J. Virol. 76:5847-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, D. R., et al. 2006. Venezuelan equine encephalitis virus transmission and effect on pathogenesis. Emerg. Infect. Dis. 12:1190-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Styer, L. M., K. A. Bernard, and L. D. Kramer. 2006. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: effect of viral dose. Am. J. Trop. Med. Hyg. 75:337-345. [PubMed] [Google Scholar]
- 28.Styer, L. M., et al. 2007. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 3:e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Titus, R. G., J. V. Bishop, and J. S. Mejia. 2006. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 28:131-141. [DOI] [PubMed] [Google Scholar]
- 30.Turell, M. J., et al. 2005. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 42:57-62. [DOI] [PubMed] [Google Scholar]
- 31.Turell, M. J., M. L. O'Guinn, D. J. Dohm, J. P. Webb, Jr., and M. R. Sardelis. 2002. Vector competence of Culex tarsalis from Orange County, California, for West Nile virus. Vector Borne Zoonotic Dis. 2:193-196. [DOI] [PubMed] [Google Scholar]
- 32.Wanasen, N., R. H. Nussenzveig, D. E. Champagne, L. Soong, and S. Higgs. 2004. Differential modulation of murine host immune response by salivary gland extracts from the mosquitoes Aedes aegypti and Culex quinquefasciatus Med. Vet. Entomol. 18:191-199. [DOI] [PubMed] [Google Scholar]