Abstract
Our previous studies indicated that recruitment and/or activation of dendritic cells (DCs) is important in enhancing the protective immune responses against rabies virus (RABV) (L. Zhao, H. Toriumi, H. Wang, Y. Kuang, X. Guo, K. Morimoto, and Z. F. Fu, J. Virol. 84:9642-9648). To address the importance of DC activation for RABV vaccine efficacy, the genes for several DC recruitment and/or activation molecules, e.g., granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage-derived chemokine (MDC), and macrophage inflammatory protein 1α (MIP-1α), were individually cloned into RABV. The ability of these recombinant viruses to activate DCs was determined in vitro and in vivo. Infection of mouse bone marrow-derived DCs with each of the recombinant viruses resulted in DC activation, as shown by increased surface expression of CD11c and CD86 as well as an increased level of alpha interferon (IFN-α) production compared to levels observed after infection with the parent virus. Intramuscular infection of mice with each of the viruses recruited and/or activated more DCs and B cells in the periphery than infection with the parent virus, leading to the production of higher levels of virus-neutralizing antibodies. Furthermore, a single immunization with recombinant RABV expressing GM-CSF or MDC protected significantly more mice against intracerebral challenge with virulent RABV than did immunization with the parental virus. Yet, these viruses did not show more virulence than the parent virus, since direct intracerebral inoculation with each virus at up to 1 × 107 fluorescent focus units each did not induce any overt clinic symptom, such as abnormal behavior, or any neurological signs. Together, these data indicate that recombinant RABVs expressing these molecules activate/recruit DCs and enhance protective immune responses.
Rabies virus (RABV) is a single-strand, negative-sense RNA virus in the family Rhabdoviridae and is the causative agent for rabies in many species of mammals (33). Its genome encodes five structural proteins in the following order: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and RNA-dependent RNA polymerase (L) (55). Despite the fact that rabies is one of the oldest human infections, it continues to present a public health threat worldwide. Each year, more than 55,000 humans die from rabies around the globe and millions more undergo postexposure prophylaxis (PEP) (38). Most of the human cases occur in the developing nations of Asia and Africa, where canine rabies remains the main source for human exposure (22). In developed countries, human rabies has dramatically declined during the past 50 years as a direct consequence of routine vaccination of pet animals. However, rabies in wildlife has emerged as a major threat (46). Therefore, controlling rabies and protecting humans from rabies requires multilayered control strategies, particularly vaccination of humans before or after exposure and routine vaccination of pet and wildlife animals.
Current human rabies vaccines are produced in cultured cells, and virions are then inactivated with β-propiolactone (21). Although these vaccines are safe and efficacious, multiple doses (at least four) must be administered over an extended period of time (14 days) to people who have been exposed to rabid animals or animals suspected of being rabid (45). In addition, the high cost (more than $600 for four doses) (40) associated with these inactivated RABV vaccines prevents their effective use in developing countries, where the vaccines are needed most (50). Routine vaccination of pet animals (dogs and cats) is carried out by using inactivated vaccines (12). Although these vaccines provide adequate protection, they induce local reactions, and multiple immunizations are required to maintain sufficient immunity throughout the life of the animal. Live attenuated RABV vaccines or recombinant live vaccines, particularly for wild animals, have been licensed. A recombinant vaccinia virus expressing the RABV G protein (VRG) has been used for large-scale elimination of fox rabies in Europe (6, 8) as well as coyote and raccoon rabies in North America (27). A live avirulent RABV, SAG-2, has also been used for immunization of wildlife against rabies in many parts of Europe (20). These vaccines are effective; however, they have problems. Human exposure to VRG has been associated with intensive skin inflammation and systemic vaccinia infection (9, 44). A low virus-neutralizing antibody (VNA) response has been reported after oral immunization with live attenuated SAG-2 (28). Therefore, more efficacious and affordable RABV vaccines are needed, particularly in developing nations.
Recently, attempts were made to develop avirulent live RABV vaccines by expressing multiple copies of the glycoprotein (G) (19) or other innate immune response-specific molecules (17, 58, 59). It has been found that recruitment/activation of dendritic cells (DCs) is important in inducing protective immunity (59). DCs are the most efficient antigen-presenting cells (APCs) and a key element of both innate and adaptive immune responses to viral infections (3). DCs are present in small quantities in tissues, and once activated, they migrate to the lymphoid organs, where they interact with T and B cells to initiate and shape the adaptive immune response (7). One of the cytokines, granulocyte-macrophage colony-stimulating factor (GM-CSF), plays an important role in the differentiation of monocytes into immature DCs as well as in the maturation and/or activation of DCs (14, 31). Activated DCs augment antigen-induced humoral and cellular immune responses (49). Thus, GM-CSF has been extensively used as an effective genetic and protein adjuvant to enhance the immunogenicity of tumor and pathogen antigens (15, 25, 42, 53).
In the present study, the genes for GM-CSF and other DC-stimulating molecules (macrophage-derived chemokine [MDC] or CCL22 and macrophage inflammatory protein 1α [MIP-1α] or CCL3) were individually cloned into the RABV SAD L16 strain. It was found that overexpression of DC-stimulating molecules further increases the immunogenicity of RABV.
MATERIALS AND METHODS
Cells, viruses, antibodies, and animals.
Mouse neuroblastoma (NA) cells were maintained in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY). BSR cells, a cloned cell line derived from BHK-21 cells, were maintained in Dulbecco's modified Eagle's medium (DMEM) (Mediatech) containing 10% FBS. Recombinant RABV (rRABV) strains were propagated in BSR cells. Challenge virus standard 11 (CVS-11) was propagated in NA cells. CVS-24 was propagated in suckling mouse brains. Fluorescein isothiocyanate (FITC)-conjugated antibody against the RABV N protein was purchased from Fujirebio Diagnostics, Inc. (Malvern, PA). Antibodies used for flow cytometric analysis, such as CD4 (GK1.5), CD8 (53-6.7), CD11b (M1/70), CD11c (HL3), CD19 (1D3), CD40 (3/23), CD45 (30-F11), CD80 (16-10A1), and CD86 (GL1), were purchased from BD Pharmingen (San Jose, CA). Female BALB/c and ICR mice were purchased from Harlan and housed in the animal facility of the College of Veterinary Medicine, University of Georgia. All animal experiments were carried out under Institutional Animal Care and Use Committee-approved protocols (animal welfare assurance no. A3085-01).
Construction of rRABV cDNA clones.
The rRABV vector pLBNSE, flanked by hammerhead ribozyme and hepatitis virus delta ribozyme sequences, was generated from an SAD L16 cDNA clone in pcDNA3.1(+) (Invitrogen, Carlsbad, CA) as described previously (48). A transcription unit with the BwsiI and NheI restriction sites was created between the G- and L-coding sequences by deleting the pseudogene. Site-directed mutagenesis was carried out to mutate the glycoprotein at amino acid positions 194 and 333 (18) by overlap PCR with the following primers: position 194 mutation primers 5′-TCTTGTGACATTTTTACCTCCAGTAGAGGGAAGAGAGCAT-3′ (forward) and 5′-ATGCTCTCTTCCCTCTACTGGAGGTAAAAATGTCACAAGA-3′ (reverse) and position 333 mutation primers 5′-TGCTCACTACAAGTCAGTCGAAACTTGGAATGAGATCCTC-3′ (forward) and 5′-GGAGGATCTCATTCCAAGTTTCGACTGACTTGTAGTGAGC-3′ (reverse) (boldface italics indicates positions 194 and 333, respectively). The RABV N, P, G, and L genes were individually cloned into pcDNA as helper plasmids. Primers used for the construction of these infectious clones and helper plasmids were designed by using Primer5.0, as listed in Tables 1 and 2, respectively. Murine GM-CSF (mGM-CSF) and murine MDC (mMDC) sequences were amplified from plasmids pORF9-mGMCSF and pORF5-mMDC, respectively (InvivoGen, San Diego, CA). The murine MIP-1α gene was amplified from mouse spleen by reverse transcription-PCR (RT-PCR) as described previously (58). Each of the genes was cloned into the transcript unit between the G- and L-coding sequences in the pLBNSE vector. All of the inserted genes were amplified using the following specific primers: (i) the GM-CSF upper primer (5′-GGTAGCGTACGAACATGTGGCTGCAGAATTTAC-3′) and lower primer (5′-TCGAGCTAGCTGGGCTTCCTCATTTTTGGC-3′), (ii) the MDC upper primer (5′-CAGAGCGTACGATGGCTACCCTGCGTGTCC-3′) and lower primer (5′-ATGTCGAGCTAGCATGGTCATCAGGTCCTC-3′), and (iii) the MIP-1α upper primer (5′-TGCTCGTACGCATGAAGGTCTCCACCACTGC-3′) and lower primer (5′-GCCTGCTAGCCTCTCAGGCATTCAGTTCCAG-3′) (a BsiWI site is underlined in the upper primers, and an NheI site is underlined in the lower primers) to introduce BsiWI and NheI recognition sites before and after the insert. The resulting plasmids were designated pLBNSE-GM-CSF, pLBNSE-MDC, and pLBNSE-MIP-1α, respectively (Fig. 1 A). Inserts were verified by restriction analysis and DNA sequencing.
TABLE 1.
Primers used for construction of recombinant RABV infectious clones
| Primer | Nucleotide sequence (5′-3′) | Sense | Enzyme |
|---|---|---|---|
| RP1 | TCCTCCGATCGTTGTCAGAAGTAAG | + | PvuI |
| RP2 | GATCTGGTTGTTAAGCGT | − | |
| RP3 | ACGCTTAACAACCAGATC | + | |
| RP4 | CTTTCCCTAGGGTTATACAGG | + | BlnI |
| RP5 | GTATAACCCTAGGAAAGGCTCCCGATTTAA | − | BlnI |
| RP6 | AACGTACGGGAGGGGTGTTAGTTTTTTTCATGGACTTG | + | BsiWI |
| GATCGTTGAAAGGACG | − | NheI | |
| RP7 | TTTTGCTAGCTTATAAAGTGCTGGGTCATCTAAGC | + | KpnI |
| RP8 | AGCCGGGTACCCGCCCTCCCTTAGCCATCCGAGT |
Bold letters (nucleotides) in sequences denote the restriction enzyme sites, and the underlined letters in the RP6 sequence indicate RABV transcription stop/start sites.
TABLE 2.
Primers used for construction of helper plasmids
| Primer | Nucleotide sequence (5′-3′) | Sense | Relative position in the genome |
|---|---|---|---|
| RPNf | GTAGCTAGCCTACAATGGATGCCGA | + | 62 |
| RPNr | TTAGGTACCTTCTTATGAGTCACTC | − | 1415 |
| RPPf | GAAGCTAGCCAAACATGAGCAAG | + | 1505 |
| RPPr | GAGAGGTACCGTTAGCAAGATG | + | 2401 |
| RPGf | GACGCTAGCAAAGATGGTTCCTCAG | − | 3309 |
| RPGr | AAAGGTACCCCAGTCCTTACAGTCT | + | 4887 |
| RPLf | GAGGCTAGCTTCAAGATGCTCGATC | − | 5404 |
| RPLr | CACAGGTACCTTAGCATGGGCAGGC | + | 11819 |
Upper primers have the BsiWI enzyme site, and lower primers have the NheI site (boldface).
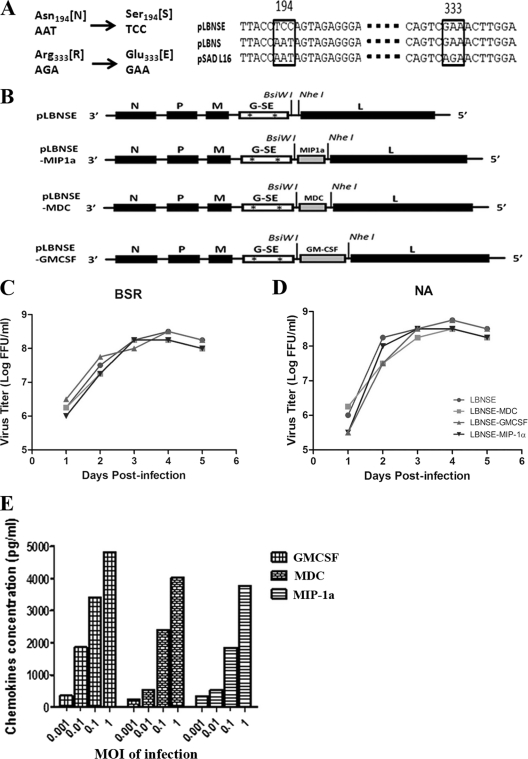
FIG. 1.
Construction and in vitro characterization of recombinant RABVs. (A) Construction of the RABV glycoprotein (G) with the Asn194→Ser194 and Arg333→Glu333 mutations (G-SE). (B) Schematic diagram for the construction of LBNSE-GM-CSF, LBNSE-MDC, and LBNSE-MIP-1α recombinant RABVs. The pLBNSE vector was derived from L16 by removing the pseudogene and introducing BsiWI and NheI sites between the G and L genes. The GM-CSF, MDC, or MIP-1α gene was individually cloned into the BsiWI and NheI sites. N, P, M, G-SE, and L indicate the nucleoprotein gene, phosphoprotein gene, matrix protein gene, G gene, and polymerase gene, respectively. Growth curves of recombinant RABV were assessed in BSR (C) or NA (D) cells. Cells were infected with LBNSE, LBNSE-MDC, LBNSE-GM-CSF, and LBNSE-MIP-1α at a multiplicity of infection (MOI) of 0.01 FFU per cell and incubated at 37°C. Viruses were harvested at 1, 2, 3, 4, and 5 dpi and viral titers determined as described in Materials and Methods. All titrations were carried out in quadruplicate, and titers are expressed as mean values ± standard errors of the means (SEM). (E) Production of chemokines or cytokines in NA cells by recombinant RABV. NA cells were infected with different viruses at MOIs of 0.001, 0.01, 0.1, and 1 FFU/cell. After incubation at 37°C for 24 h, the culture supernatants were collected and the concentrations of the indicated chemokines/cytokines were determined with a commercial ELISA kit.
Rescue of recombinant RABV.
Recombinant RABVs were rescued as described previously (32, 52). Briefly, BSR cells were transfected with 2.0 μg of the full-length infectious clone and 0.5 μg of N-, 0.25 μg of P-, 0.1 μg of L-, and 0.15 μg of G-expressing plasmids using the SuperFect transfection reagent (Qiagen, Valencia, CA) according to the manufacturer's protocol. After incubation for 4 days, the culture medium was removed and fresh medium added to the cells. After incubation for another 3 days, the culture medium was harvested and the cells were examined for the presence of rescued viruses by using FITC-conjugated antibody against the RABV N protein.
Virus titration.
Virus titration was carried out by using the direct fluorescent-antibody assay with NA cells. NA cells in a 96-well plate were inoculated with serial 10-fold dilutions of virus and incubated at 34°C for 2 days. The culture supernatant was removed, and the cells were fixed with ice-cold 80% acetone for 30 min. The cells were then stained with FITC-conjugated anti-RABV N antibodies. Antigen-positive foci were counted under a fluorescence microscope (Zeiss, Germany), and viral titers were calculated as numbers of fluorescent focus units (FFU) per milliliter. All titrations were carried out in quadruplicate.
RFFIT.
Blood was collected from each mouse for measurement of VNA using the rapid fluorescent focus inhibition test (RFFIT) as described previously (51). Briefly, 50 μl of serial 5-fold dilutions of serum were prepared in Lab-Tek chamber slides (Nalge Nunc International, Rochester, NY). Fifty 50% fluorescing-focus doses (FFD50) of CVS-11 was added to each chamber and incubated at 37°C for 90 min. NA cells (5 × 105 cells/ml) were added into each chamber, and the slides were incubated at 37°C for 20 h. Then the slides were fixed with ice-cold 80% acetone and stained with FITC-conjugated anti-RABV N antibodies. Twenty fields in each chamber were observed under a fluorescence microscope, and the 50% endpoint titers were calculated according to the Reed-Muench formula (43). The values were compared with those obtained with the reference serum (National Institute for Biological Standards and Control, Herts EN6 3QH, United Kingdom) and normalized to international units (IU)/ml.
ELISA.
Commercial mGM-CSF, mMDC, and mMIP-1α enzyme-linked immunosorbent assay (ELISA) kits (Quantikine M; R&D Systems, Minneapolis, MN) were used to quantify the amounts of GM-CSF, MDC, and MIP-1α in cell culture supernatants. A mouse alpha interferon (IFN-α) ELISA kit was purchased from Biomedical Laboratories (Piscataway, NJ). All assays were performed according to the manufacturer's instructions.
Real-time PCR.
A real-time (RT) SYBR green PCR assay was carried out in an Mx3000P apparatus (Stratagene, La Jolla, CA) to quantify the rate of viral replication and the expression of chemokines and cytokines. Muscle tissues at the site of immunization were removed from infected mice and flash frozen on dry ice before being stored at −80°C. RNA was extracted from the tissue with Trizol and used for quantitative RT PCR (qRT-PCR) as described previously (35). The reverse transcriptase and DNA polymerase were utilized from a one-step Brilliant II SYBR green qRT-PCR master mix kit (Stratagene). The primers of the inserted genes are listed in Table 3. Amplification was carried out at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles in two steps: 95°C for 15 s and 60°C for 1 min. For absolute quantification of viral genomic RNA, a standard curve was generated by using a serially diluted RNA in vitro transcribed from a plasmid expressing RABV N, and the copy numbers of viral genomic RNA were normalized to 1 μg of total RNA. For the expression of chemokines/cytokines and markers of immune cells, mRNA copy numbers of a particular gene were normalized to those of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Levels of gene expression in a test sample are presented as the fold increase over that detected in uninfected controls.
TABLE 3.
Primers used for amplifying chemokines, cytokines, and markers of immune cells
| Gene | Upper primer (5′-3′) | Lower primer (5′-3′) |
|---|---|---|
| GAPDH | GGAGAAGCTGCCAATGGATA | TTACGCTTGCACTTCTGGTG |
| GM-CSF | CAGTTGGAAGGCAGTATA | CAGTTGGAAGGCAGTATA |
| MDC | ATGGCTACCCTGCGTGTCC | ATGGTCATCAGGTCCTC |
| MIP-1α | CATGAAGGTCTCCACCACTGC | TCTCAGGCATTCAGTTCCAG |
| CD11b | ATTCTTCTGTTGAGGAGTA | ATTCTTCTGTTGAGGAGTA |
| CD11c | GGAAGTGAGAATAATGTA | GGAAGTGAGAATAATGTA |
| CD19 | TAGCCTGGACTTCGTTAG | TAGCCTGGACTTCGTTAG |
| IL-4 | TAGGTAGAGAACAAGATG | TAGGTAGAGAACAAGATG |
Cultivation of bone marrow-derived DCs.
Bone marrow-derived DCs were isolated as described previously (23, 37). Briefly, bone marrow was harvested and processed from euthanized BALB/c mice by cutting between the femur and hip joint. Bone marrow was transferred to a 6-well plate using a 10-ml syringe loaded with phosphate-buffered saline (PBS) and a needle and dissociated into single-cell suspensions. The DC precursors were counted on a hemocytometer and cultured at a density of 2 × 105 DC precursors per ml in DC medium (RPMI medium containing 0.1% 2-mercaptoethanol, 1× nonessential amino acids, and 1× sodium pyruvate) supplemented with 40 ng/ml recombinant mGM-CSF (Peprotech Inc., Princeton, NJ).
Flow cytometry.
Flow cytometry was carried out to quantify immune cells in the lymph nodes and in the peripheral blood as well as in in vitro-cultured DCs. Briefly, mouse lymph nodes were collected, pressed through a 40-μm nylon filter, and washed with 1× PBS. Red blood cells were lysed with ACK lysis buffer (BioSource International, Inc., Camarillo, CA) for 1 min at room temperature. Single-cell suspensions (at 106 cells) were prepared in Hanks balanced salt solution (HBSS) (Invitrogen) and stained for CD4, CD8, CD11b, CD11c, CD19, CD40, CD45, CD80, or CD86 with antibodies (BD PharMingen). After incubation on ice for 30 min, cells were washed twice in PBS containing 2% FBS and 0.02% NaN3. Then the cells were fixed with 1% paraformaldehyde. Data collection and analysis were performed with a BD LSR-II flow cytometer, BD FACSDiva software (BD Pharmingen), FlowJo software (TreeStar, San Carlos, CA), Prism software, and Microsoft Excel (Seattle, WA).
Statistical analyses.
The statistical significance of the differences between group values was determined using one-way analysis of variance (ANOVA) or Fisher's exact test (χ2; GraphPad).
RESULTS
Construction and selection of recombinant RABV (rRABV) expressing GM-CSF, MDC, or MIP-1α.
Our previous studies indicated that overexpression of the chemokine MIP-1α further attenuated RABV virulence yet increased its immunogenicity (58). One of the mechanisms for the increased immunogenicity is the recruitment of DC and B cells in the periphery, including the site of immunization (muscle tissue), draining lymph nodes, and blood (59). To further investigate the role of DCs in enhancing RABV immunogenicity, murine GM-CSF, MDC, and MIP-1α genes were individually cloned into the RABV SAD genome as described previously (11, 48) (Fig. 1B). The RABV L16 strain was selected over Flurry strain HEP (58) because L16 can grow to higher titers than HEP can in BSR cells. Insertion of the mouse GM-CSF, MDC, and MIP-1α genes was confirmed by sequencing these fragments within the infectious clones. The rRABVs were rescued using the procedures described by Inoue et al. (32) and designated LBNSE (the parent virus), LBNSE-GM-CSF, LBNSE-MDC, and LBNSE-MIP-1α, respectively. Since previous studies indicated that mutations at positions 194 and 333 in the glycoprotein attenuate and stabilize the recombinant RABV (18), overlap PCR was performed to introduce these mutations (Fig. 1A).
In vitro characterization of rRABVs.
To characterize the rRABVs in vitro, viral growth kinetics were examined in BSR and NA cells. As shown in Fig. 1C and D, no significant difference in values was observed between recombinant viruses and the parental virus, indicating that viral growth was not affected by the insertion of the GM-CSF, MDC, or MIP-1α gene. The ability of the rRABVs to produce GM-CSF, MDC, and MIP-1α was determined by measuring GM-CSF, MDC, and MIP-1α in virus-infected cells with ELISA kits. As shown in Fig. 1E, GM-CSF, MDC, and MIP-1α was produced by respective rRABVs in a dose-dependent manner. No GM-CSF, MDC, or MIP-1α product was detected in the supernatant of NA cells infected with the parent LBNSE virus.
Maturation and activation of bone marrow-derived DCs in vitro stimulated by rRABV.
To investigate whether expression of the DC stimulation molecules promotes maturation and/or activation of DCs in vitro, DCs were isolated from mouse bone marrow, cocultured with each of the rRABVs, and compared to those from a lipopolysaccharide (LPS)-positive control. As shown in Fig. 2 A and B, all of the rRABVs expressing DC-stimulating molecules promoted better maturation and/or activation of DCs than the parent virus when they were pretreated with GM-CSF (differentiation from monocytes to immature DCs), as shown by expression of CD11c and/or CD86, except that there was no significant difference in the numbers of CD11+/CD86+ doubly positive cells between the groups treated with recombinant virus expressing MIP-1α and the parent virus (Fig. 2B). Very few GM-CSF-treated bone marrow-derived DCs were activated by medium alone. On the other hand, only rRABV expressing GM-CSF stimulated DC maturation and/or activation without prior treatment with GM-CSF. To confirm that the DCs were activated, the expression of IFN-α was measured in the supernatant of DCs infected with each of the rRABVs with or without prior treatment with GM-CSF. All of the rRABVs encoding DC-stimulating molecules induced the production of significantly more IFN-α than the parent virus (Fig. 2C) when the DCs were pretreated in vitro with GM-CSF. Significantly more IFN-α was detected only in the cells infected with rRABV expressing GM-CSF without prior treatment with GM-CSF. These results indicate that all the rRABVs stimulated the maturation and/or activation of DCs. However, only the rRABVs expressing GM-CSF promoted differentiation from monocytes to DCs in vitro.
FIG. 2.
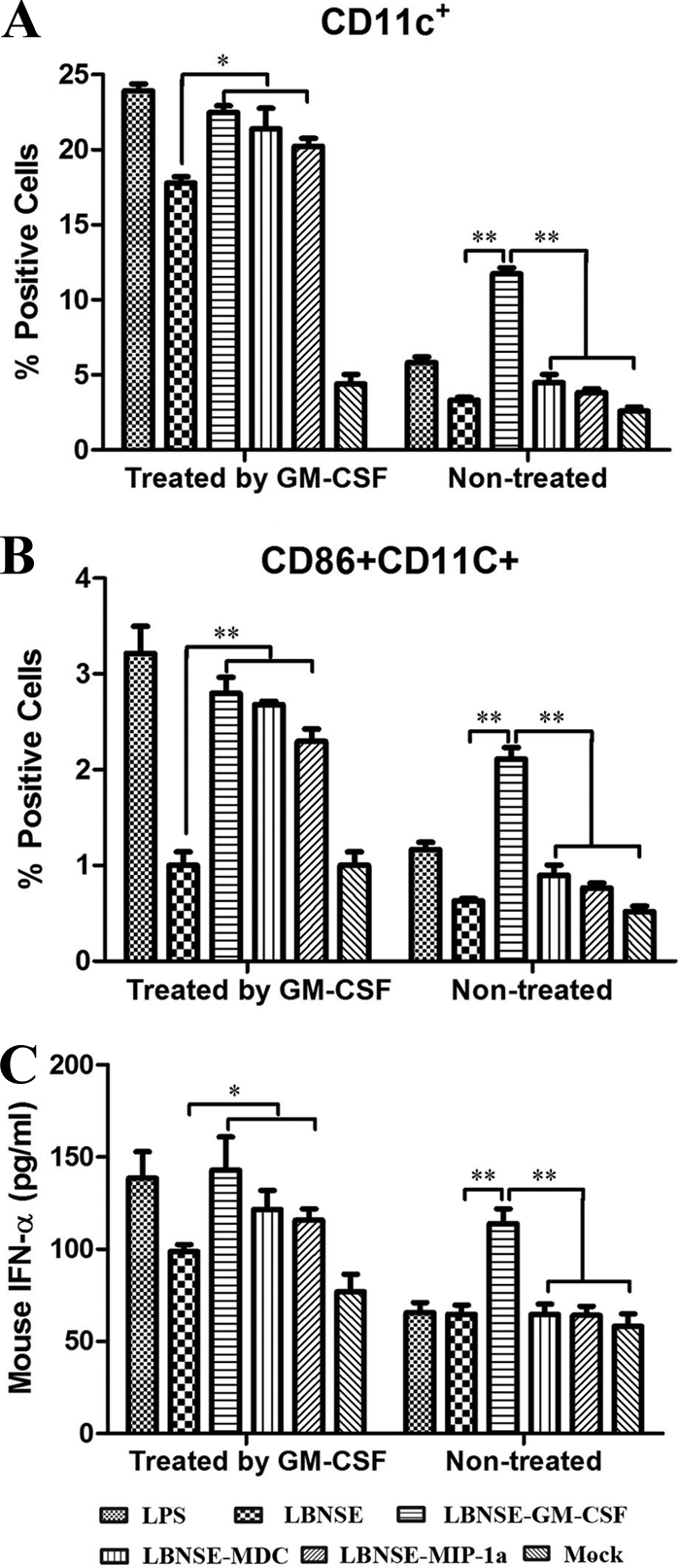
Maturation and/or activation of bone marrow-derived DCs by rRABV. Bone marrow was obtained from BALB/c mice, and DC precursors were cultured with or without GM-CSF. The cells were infected with each of the rRABVs. Expression of DC (CD11c) (A) or a DC activation marker (CD11c+ and CD86+) (B) as well as production of IFN-α (C) are shown. LPS was used as a positive control, and the medium from untreated cells (Mock) served as a negative control. Data are the means from four independent experiments with cells from different donors. The horizontal lines represent the geometric mean for each group, and statistical analysis was performed. *, P < 0.05; **, P < 0.01.
Recruitment and/or activation of DCs and other immune cells in vivo by rRABVs.
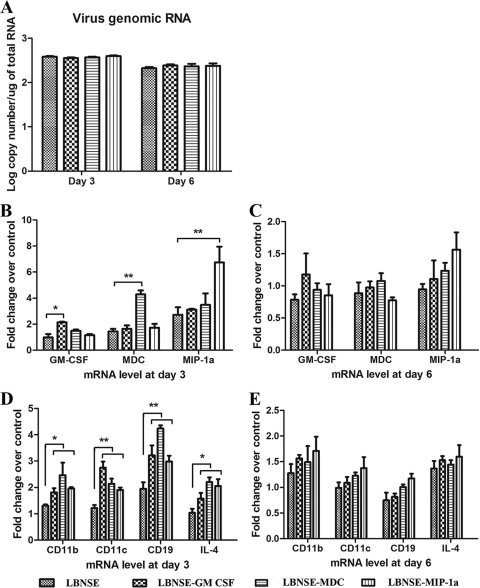
To investigate whether the rRABVs expressing DC-activating molecules recruit and/or activate DCs in vivo, mice were immunized by the intramuscular (i.m.) route with 1 × 105 FFU of each rRABV. Muscle tissues at the site of injection were harvested at 3 and 6 days postinfection (dpi) and used for total RNA extraction. qRT-PCR was performed to measure virus replication and expression of GM-CSF, MDC, MIP-1α, CD11c (markers of DCs), and interleukin 4 (IL-4; marker for Th cells), as well as CD19 (B cells). As shown in Fig. 3 A, quantification of viral genomic RNA by qRT-PCR showed no differences between mice infected with each of the rRABVs at 3 or 6 dpi. At 3 dpi, expression of the intended cytokine/chemokine was the highest in mice infected with the respective rRABVs (Fig. 3B). The differences were no longer significant among the mice infected with each of these viruses by 6 dpi, although the level of expression of these chemokines/cytokines were still high in all immunized mice (Fig. 3C). Significantly more CD11c, IL-4, and CD19 mRNA was detected at 3 dpi in mice infected with each of the rRABVs expressing DC-stimulating molecules than in mice infected with the parent virus (Fig. 3D). The differences were no longer significant by 6 dpi, although the levels of expression of these markers were still high (Fig. 3E). Our data suggest that rRABVs expressing DC-stimulating molecules induced more expression of chemokines/cytokines and recruited more DCs, B cells, and T cells to the site of immunization at an early stage (3 dpi) than the parent virus.
FIG. 3.
Quantification of viral genomic RNA as well as mRNA for chemokines/cytokines and markers of immune cells by qRT-PCR at the site of immunization. BALB/c mice were infected i.m. with rRABVs at 1 × 105 FFU per mouse, and muscle tissues were harvested from the site of immunization at 3 and 6 dpi. Total RNA was prepared and used in a qRT-PCR to determine levels of viral genomic RNA (A) or mRNA levels for chemokines/cytokines (B and C) as well as markers of immune cells (D and E). All data are from experiments with 4 mice per group. Asterisks indicate significant differences between the indicated experimental groups (*, P < 0.05; **, P < 0.01).
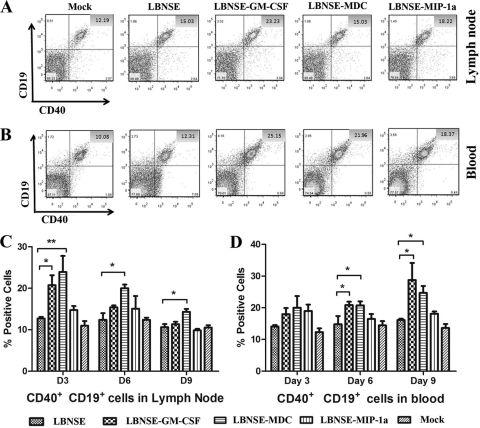
To investigate if local stimulation leads to systemic recruitment and activation of the innate and the adaptive immunity, flow cytometry was performed to quantify the immune cells (CD11c and CD86 for DCs, CD19 and CD40 for B cells, and CD4 and CD8 for T cells) in the draining lymph nodes and the blood at 3, 6, and 9 dpi. Figures 4 A and B and 5 A and B show representative flow cytometric plots of DCs (CD11c+ and/or CD86+) and B cells (CD19+ and/or CD40+) at 6 dpi after infection with each rRABV. Detailed data are presented in Fig. 4C and D and 5C and D. Overall, the recruitment and/or activation of immune cells was detected first in the lymph nodes and then in the peripheral blood. In addition, the duration of recruitment and activation of immune cells is shorter in the lymph nodes (3 and 6 dpi) than in the blood (3 to 9 dpi). Significantly more immune cells (DCs as detected by CD11c and CD86 and B cells as detected by CD19 and CD40) were detected in mice infected with rRABVs expressing GM-CSF or MDC than in mice infected with the parent virus. rRABV expressing MIP-1α also recruited and/or activated more immune cells in the lymph nodes and the blood than the parent virus did, but no statistical significance was detected except in the blood at 3 dpi, when a significant difference was detected between the group of mice immunized with rRABV expressing MIP-1α and the group immunized with the parent virus. A similar trend was also observed for T cells, as indicated by increased numbers of CD4+ and CD8+ cells in the lymph nodes and the peripheral blood (data not shown). Thus, our studies suggest that rRABV expressing DC-stimulating molecules recruited and activated more DCs as well as other immune cells in the lymph nodes and the peripheral blood than the parent virus.
FIG. 4.
Recruitment and/or activation of DCs in the lymph nodes and blood after infection with rRABVs. BALB/c mice were infected i.m. with 1 × 105 FFU of different rRABVs, and draining (inguinal) lymph nodes and blood were harvested after extensive perfusion at 3, 6, and 9 dpi. Single-cell suspensions were prepared, stained with antibodies against DCs and the DC activation markers CD11c and CD86, and analyzed by flow cytometry. Representative flow cytometric plots of DCs are shown from the lymph nodes (A) and the blood (B). The results of a detailed analysis for activated DCs (CD11c+ and CD86+) at 3, 6, and 9 dpi are presented for the lymph nodes (C) and blood (D). Asterisks indicate significant differences between the indicated experimental groups (*, P < 0.05; **, P < 0.01).
FIG. 5.
Recruitment and/or activation of B cells in the lymph nodes and blood after infection with rRABVs. BALB/c mice were infected i.m. with 1 × 105 FFU of different rRABVs, and draining (inguinal) lymph nodes and blood were harvested after extensive perfusion at 3, 6, and 9 dpi. Single-cell suspensions were prepared, stained with antibodies against B cells and the B cell activation markers CD19 and CD40, and analyzed by flow cytometry. Representative flow cytometric plots of B cells are shown from the lymph nodes (A) and the blood (B). The detailed analyses for activated B cells (CD19+ and CD40+) at 3, 6, and 9 dpi are presented for lymph nodes (C) and blood (D). Asterisks denote significant differences between the indicated experimental groups (*, P < 0.05; **, P < 0.01).
Immunogenicity of rRABV.
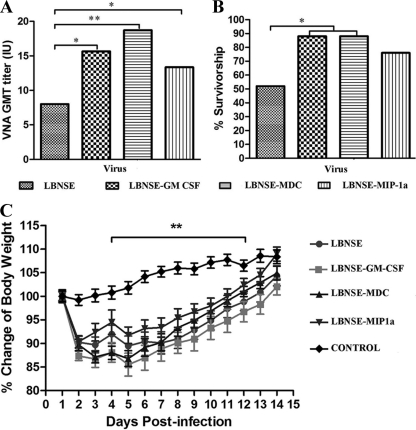
To determine if recruitment and/or activation of DCs and other immune cells in the periphery increases the immunogenicity of the rRABV, mice (10 in each group) were immunized once by the i.m. route with different doses of rRABV (1 × 103, 1 × 104, 1 × 105, or 1 × 106 FFU per mouse). Blood samples were collected 21 dpi, and sera were used for determination of VNA by the RFFIT (51). Overall, the level of VNA is dose dependent for all viruses (data not shown). Figure 6 A shows the level of VNA in mice immunized with 1 × 106 FFU of RABV. Significantly higher VNA titers were detected in mice immunized with the LBNSE-MDC (18.72 IU) (P < 0.01), LBNSE-GM-CSF (15.65 IU) (P < 0.05), and LBNSE-MIP-1α (13.36 IU) (P < 0.05) viruses than were induced by immunization with the parent virus, LBNSE (8.01 IU). To investigate if the higher VNA titers correlate with better protection, mice (25 in each group) immunized with 106 FFU of each virus were then challenged with 50 LD50 of virulent CVS-24 on day 21 after vaccination and observed for development of disease and death for 2 weeks. As depicted in Fig. 6B, significantly more survivors were observed among mice immunized with LBNSE-GM-CSF or LBNSE-MDC than among those immunized with the parent LBNSE virus. More survivors were also observed among mice immunized with LBNSE-MIP-1α than among mice immunized with the parent virus, but no significant difference was detected. Together, these data indicate that rRABV expressing GM-CSF or MDC provides better protective immunity than the parent virus.
FIG. 6.
Immunogenicity and pathogenicity of rRABVs in mice. (A) Groups of ICR mice (n = 10) were immunized with 1 × 106 FFU of rRABVs by the i.m. route. At 21 dpi, blood samples were obtained and VNA titers determined using the RFFIT. Titers were normalized to IU by using the WHO standard and are expressed as geometric mean titers. (B) Groups of ICR mice (n = 25) were immunized with 1 × 106 FFU of rRABVs by the i.m. route. Three weeks after immunization, mice were challenged i.c. with 50 LD50 of CVS-24 and observed for 2 weeks, and survivorship was recorded. (C) ICR mice (n = 10) were infected i.c. with 1 × 107 FFU of different rRABVs or DMEM (mock infection), and their body weights were monitored daily for 2 weeks. Data were obtained from all 10 mice in each group and are presented as mean values ± SEM. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) between the indicated experimental groups as analyzed by one-way ANOVA (A and C) or Fisher's exact test (χ2) (B).
Pathogenicity of rRABVs in mice.
To determine whether overexpression of GM-CSF, MDC, or MIP-1α has adverse effect in animals, three groups of 10 4- to 6-week-old ICR mice were infected with 1 × 105, 1 × 106, or 1 × 107 FFU of recombinant viruses by the intracerebral (i.c.) route. Infected mice were monitored daily for 2 weeks for weight loss as well as for development of disease and death. When infected at the low doses (1 × 105 and 1 × 106 FFU), infected mice lost 5 to 10% of their body weight (data not shown) but 10 to 15% of their body weight when they were infected with the high dose (1 × 107 FFU), which is significant compared to the value for sham-infected mice (Fig. 6C). Most of the mice regained their prechallenge body weight by 15 dpi. However, no overt clinic symptoms, such as abnormal behaviors, or any neurological signs were observed in these mice. These data indicate that expression of DC-stimulating molecules did not increase RABV virulence.
DISCUSSION
Previous studies with recombinant RABV expressing the chemokine MIP-1α revealed that it further attenuated RABV virulence by inducing a transient innate immune response in the central nervous system (CNS) (58). In addition, viral expression of MIP-1α enhanced RABV immunogenicity by inducing higher levels of VNA (59). This was achieved via recruitment and/or activation of DCs at the site of immunization, in the draining lymph nodes, and in the peripheral blood (59). To further confirm the role of DCs in enhancing RABV immunogenicity, more genes for DC recruitment/activation molecules (GM-CSF and MDC) in addition to MIP-1α were individually cloned into RABV and the immunogenicity and pathogenicity of these recombinant viruses were determined in a mouse model. Each of the viruses stimulated more maturation and activation of murine bone marrow-derived DCs in vitro and more recruitment and/or activation of DCs and mature B cells, as well as T cells, in the periphery than the parent virus, which led to higher levels of VNA and better protection. Most importantly, a single immunization with recombinant RABV expressing GM-CSF or MDC protected significantly more mice against intracerebral challenge with virulent RABV than did the parental virus. Yet, these viruses did not show more virulence than the parent virus, since direct intracerebral inoculation with each virus (up to 1 × 107 FFU) did not induce any overt clinic symptom, such as abnormal behavior, or any neurological signs. Thus, our data suggest that recruitment/activation of DCs is important in enhancing RABV immunogenicity and protection.
DCs probably arise from monocytes and white blood cells. These cells circulate in the body and, depending on the appropriate signal, can turn into either DCs or macrophages (16). Treatment of in vitro-derived monocytes with GM-CSF leads to differentiation of immature DCs in about a week (56). Prior studies showed that RABV can activate DCs in vitro (36). Our data indicate that rRABV expressing GM-CSF, MDC, or MIP-1α stimulated more maturation and/or activation of DCs in vitro than the parent virus. All RABVs stimulated maturation and/or activation of DCs when the cells were pretreated in vitro with GM-CSF. Activation of DCs is shown not only by expression of the DC markers CD86 and CD80 (data not shown) but also by production of IFN-α. However, only the rRABV expressing GM-CSF promoted differentiation from monocytes to DCs as well as the maturation and activation of DCs in vitro.
DCs are the most efficient APCs and thus play a key role in both innate and adaptive immune responses in vivo (3). Immature DCs constantly sample the surrounding environment for pathogens such as viruses and bacteria. Once they have come into contact with antigens, DCs become activated into mature DCs and begin to migrate to the lymph nodes, where they activate T and B cells via surface receptors such as CD80 (B7.1), CD86 (B7.2), and CD40 (39). In a recent report (36), it was found that RABV could induce the activation of DCs via the NF-κB signaling pathway. In this study, immunization with all rRABVs induced recruitment and/or activation of DCs at the site of immunization, as was shown by expression of the DC activation markers CD11c+ and CD86+, as well as in the draining lymph nodes and in the peripheral blood by infiltration of CD11c+ and CD86+ cells. Recruitment and/or activation of DCs resulted in recruitment and/or activation of T and B cells, thus stimulating the production of VNA and enhancing protection.
In our previous studies, it was found that MIP-1α enhanced RABV immunogenicity by recruiting and/or activating DCs (59). MIP-1α binds to CC chemokine receptor 5 on immature DCs and recruits DCs to the site of inoculation, resulting in enhanced cellular immune responses and increased antibody titers (59). To further enhance the immunogenicity of RABV, other DC recruitment and/or activation molecules were cloned into the RABV genome. GM-CSF is a cytokine responsible for the recruitment, activation, and maturation of APCs (26). GM-CSF regulates the production and functional activation of hematopoietic cells, such as monocyte/macrophages and all granulocytes (41). MDC is known to preferentially attract Th2 cells and regulatory T cells via CCR4 (29, 30, 57). It is also a potent chemoattractant for additional cell types, including DCs (10, 24). MDC produced by DCs attracts CCR4-bearing activated (or memory) T cells to enhance immune responses and increase effector functions (54), and it may allow for T cell-B cell interaction, with the subsequent formation of germinal centers (47). Immunization with these recombinant viruses resulted in the expression of the intended molecules and significantly more recruitment and/or activation of DCs, B cells, and T cells to the site of immunization than did immunization with the parent virus, particularly at 3 dpi, as shown by the expression of markers for each of the immune cell types. Once these cells are activated, they migrate to the lymph nodes and the peripheral blood. Indeed, significantly more recruited and/or activated DCs were detected in mice immunized with RABV expressing GM-CSF or MDC than in mice immunized with the parent virus. Mature, activated DCs are critical to the stimulation of T cells and the generation of the virus-specific adaptive immune responses (1, 2). The epitopes presented by APCs can be recognized by T cells, which can provide help for B cells to produce large quantities of antibodies (34). Our data demonstrate that recruitment and/or activation of DCs by recombinant RABVs resulted in recruitment and activation of B and T cells at the site of immunization and in the draining lymph nodes, as well as in the peripheral blood. Furthermore, we have shown that infection of DCs with these recombinant RABVs promoted the production of IFN-α, which can in turn promote the differentiation of B cells into plasma cells via increased expression of TLR7 in naïve B cells (4, 13). Taken together, these data indicate that recruitment and/or activation of more DCs leads to activation of T and B cells and results in the production of higher levels of VNA in mice immunized with rRABVs expressing DC-activating molecules than in mice immunized with the parent virus. Expression of DC-activating molecules, particularly GM-CSF, has been reported to enhance the immunogenicity of many other antigens, including viral and tumor antigens (15, 25, 42, 53). MDC has also been reported to enhance systemic and mucosal immune responses for HIV gp120 (5).
Our previous studies showed that recombinant RABV expressing MIP-1α activated significantly more DCs and stimulated more production of VNA than did the parent virus (59). In the previous study, RABV strain HEP was used, while in the present study, RABV strain L16 was used. The reason for the switch of vectors was that HEP can grow to titers up to 107 FFU (58), while L16 can grow to titers up to 108 FFU. High virus titers are needed for assessment of pathogenicity and immunogenicity. Despite the use of different vectors (HEP versus the L16 strain of RABV), similar findings were found for mice immunized with each of the viruses, as reported earlier (59) and in this study. Both viruses recruited/activated more immune cells in the periphery and induced higher VNA titers in mice than the parent virus. In fact, the VNA titers (about 10 IU) were similar in mice immunized with both viruses at high doses (5 × 105 for HEP-MIP-1α and 1 × 106 for LBNSE-MIP-1α). Mice immunized with the highest dose for each virus protected about 80% of the mice against challenge.
In summary, recombinant RABVs expressing DC activation molecules enhanced the immunogenicity of RABV via recruitment, maturation, and/or activation of DCs. Yet, the viruses did not increase RABV virulence, as direct infection by the i.c. route with up to 1 × 107 FFU did not induce any overt clinical symptoms in mice infected with the recombinant or the parental RABV. The weight loss in mice infected with recombinant RABVs by the intracerebral route could be related to RABV replication in the brain, expression of chemokines in the brain independent of viral replication, inflammatory responses induced by the increased expression of chemokines/cytokines, or a combination of all these factors. Indeed, previous experiments with recombinant RABV expressing RANTES or IP-10 induced extensive inflammation in the CNS (58). However, if these recombinant RABVs are to be used for vaccine development, further studies will be required to address the issue of the residual virulence of these viruses.
Acknowledgments
This work is supported partially by Public Health Service grant AI-051560 from the National Institute of Allergy and Infectious Diseases.
We thank Guoqing Zhang for technical help during the study.
Footnotes
Published ahead of print on 24 November 2010.
REFERENCES
- 1.Banchereau, J., et al. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 3.Becker, Y. 2003. Immunological and regulatory functions of uninfected and virus infected immature and mature subtypes of dendritic cells—a review. Virus Genes 26:119-130. [DOI] [PubMed] [Google Scholar]
- 4.Bekeredjian-Ding, I. B., et al. 2005. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J. Immunol. 174:4043-4050. [DOI] [PubMed] [Google Scholar]
- 5.Biragyn, A., et al. 2002. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood 100:1153-1159. [DOI] [PubMed] [Google Scholar]
- 6.Blancou, J., et al. 1989. Safety and efficacy of an antirabies vaccine consisting of recombinant vaccinia-rabies virus administered orally to the fox, dog and cat. Ann. Rech. Vet. 20:195-204. (In French.) [PubMed] [Google Scholar]
- 7.Brilot, F., T. Strowig, and C. Munz. 2008. NK cell interactions with dendritic cells shape innate and adaptive immunity. Front. Biosci. 13:6443-6454. [DOI] [PubMed] [Google Scholar]
- 8.Brochier, B., et al. 1991. Large-scale eradication of rabies using recombinant vaccinia-rabies vaccine. Nature 354:520-522. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2009. Human vaccinia infection after contact with a raccoon rabies vaccine bait—Pennsylvania. MMWR Morb. Mortal. Wkly. Rep. 58:3. [PubMed] [Google Scholar]
- 10.Chantry, D., et al. 1999. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3(+), CD4(+), CD8(low) thymocytes. Blood 94:1890-1898. [PubMed] [Google Scholar]
- 11.Conzelmann, K. K., J. H. Cox, L. G. Schneider, and H. J. Thiel. 1990. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175:485-499. [DOI] [PubMed] [Google Scholar]
- 12.Crick, J. 1973. The vaccination of man and other animals against rabies. Postgrad. Med. J. 49:551-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 14.Dieu, M. C., et al. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disis, M. L., et al. 1996. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood 88:202-210. [PubMed] [Google Scholar]
- 16.Dominguez, P. M., and C. Ardavin. 2010. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol. Rev. 234:90-104. [DOI] [PubMed] [Google Scholar]
- 17.Faber, M., et al. 2005. Overexpression of tumor necrosis factor alpha by a recombinant rabies virus attenuates replication in neurons and prevents lethal infection in mice. J. Virol. 79:15405-15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faber, M., et al. 2007. Dominance of a nonpathogenic glycoprotein gene over a pathogenic glycoprotein gene in rabies virus. J. Virol. 81:7041-7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faber, M., et al. 2009. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc. Natl. Acad. Sci. U. S. A. 106:11300-11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flamand, A., P. Coulon, F. Lafay, and C. Tuffereau. 1993. Avirulent mutants of rabies virus and their use as live vaccine. Trends Microbiol. 1:317-320. [DOI] [PubMed] [Google Scholar]
- 21.Frazatti-Gallina, N. M., et al. 2004. Vero-cell rabies vaccine produced using serum-free medium. Vaccine 23:511-517. [DOI] [PubMed] [Google Scholar]
- 22.Fu, Z. F. 1997. Rabies and rabies research: past, present and future. Vaccine 15(Suppl.):S20-S24. [DOI] [PubMed] [Google Scholar]
- 23.Gilboa, E. 2007. DC-based cancer vaccines. J. Clin. Invest. 117:1195-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godiska, R., et al. 1997. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J. Exp. Med. 185:1595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddad, D., et al. 2000. Plasmid vaccine expressing granulocyte-macrophage colony-stimulating factor attracts infiltrates including immature dendritic cells into injected muscles. J. Immunol. 165:3772-3781. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton, J. A., and G. P. Anderson. 2004. GM-CSF biology. Growth Factors 22:225-231. [DOI] [PubMed] [Google Scholar]
- 27.Hanlon, C. A., et al. 1998. First North American field release of a vaccinia-rabies glycoprotein recombinant virus. J. Wildl. Dis. 34:228-239. [DOI] [PubMed] [Google Scholar]
- 28.Hanlon, C. A., M. Niezgoda, P. Morrill, and C. E. Rupprecht. 2002. Oral efficacy of an attenuated rabies virus vaccine in skunks and raccoons. J. Wildl. Dis. 38:420-427. [DOI] [PubMed] [Google Scholar]
- 29.Iellem, A., et al. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 194:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai, T., et al. 1999. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int. Immunol. 11:81-88. [DOI] [PubMed] [Google Scholar]
- 31.Inaba, K., et al. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue, K., et al. 2003. An improved method for recovering rabies virus from cloned cDNA. J. Virol. Methods 107:229-236. [DOI] [PubMed] [Google Scholar]
- 33.Jackson, A. C. 2002. Rabies pathogenesis. J. Neurovirol. 8:267-269. [DOI] [PubMed] [Google Scholar]
- 34.Jelley-Gibbs, D. M., T. M. Strutt, K. K. McKinstry, and S. L. Swain. 2008. Influencing the fates of CD4 T cells on the path to memory: lessons from influenza. Immunol. Cell Biol. 86:343-352. [DOI] [PubMed] [Google Scholar]
- 35.Kuang, Y., S. N. Lackay, L. Zhao, and Z. F. Fu. 2009. Role of chemokines in the enhancement of BBB permeability and inflammatory infiltration after rabies virus infection. Virus Res. 144:18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, J., J. P. McGettigan, M. Faber, M. J. Schnell, and B. Dietzschold. 2008. Infection of monocytes or immature dendritic cells (DCs) with an attenuated rabies virus results in DC maturation and a strong activation of the NFkappaB signaling pathway. Vaccine 26:419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutz, M. B., et al. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 38.Martinez, L. 2000. Global infectious disease surveillance. Int. J. Infect. Dis. 4:222-228. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Fontecha, A., A. Lanzavecchia, and F. Sallusto. 2009. Dendritic cell migration to peripheral lymph nodes. Handb. Exp. Pharmacol. 2009:31-49. [DOI] [PubMed] [Google Scholar]
- 40.Meltzer, M. I. 1996. Assessing the costs and benefits of an oral vaccine for raccoon rabies: a possible model. Emerg. Infect. Dis. 2:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metcalf, D. 2008. Hematopoietic cytokines. Blood 111:485-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrissey, P. J., L. Bressler, L. S. Park, A. Alpert, and S. Gillis. 1987. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J. Immunol. 139:1113-1119. [PubMed] [Google Scholar]
- 43.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 27:493-497. [Google Scholar]
- 44.Rupprecht, C. E., et al. 2001. Human infection due to recombinant vaccinia-rabies glycoprotein virus. N. Engl. J. Med. 345:582-586. [DOI] [PubMed] [Google Scholar]
- 45.Rupprecht, C. E., et al. 2010. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm. Rep. 59:1-9. [PubMed] [Google Scholar]
- 46.Rupprecht, C. E., J. S. Smith, M. Fekadu, and J. E. Childs. 1995. The ascension of wildlife rabies: a cause for public health concern or intervention? Emerg. Infect. Dis. 1:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaniel, C., et al. 1998. Activated murine B lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J. Exp. Med. 188:451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnell, M. J., T. Mebatsion, and K. K. Conzelmann. 1994. Infectious rabies viruses from cloned cDNA. EMBO J. 13:4195-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi, Y., et al. 2006. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res. 16:126-133. [DOI] [PubMed] [Google Scholar]
- 50.Shim, E., K. Hampson, S. Cleaveland, and A. P. Galvani. 2009. Evaluating the cost-effectiveness of rabies post-exposure prophylaxis: a case study in Tanzania. Vaccine 27:7167-7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith, J. S., P. A. Yager, and G. M. Baer. 1996. A rapid fluorescence focus inhibition test (RFFIT) for determining rabies virus-neutralising antibody, p. 181-192. In F.-X. Meslin, M. M. Kaplan, and H. Koprowski (ed.), Laboratory techniques in rabies, 4th ed. World Health Organization, Geneva, Switzerland.
- 52.Takayama-Ito, M., et al. 2006. A highly attenuated rabies virus HEP-Flury strain reverts to virulent by single amino acid substitution to arginine at position 333 in glycoprotein. Virus Res. 119:208-215. [DOI] [PubMed] [Google Scholar]
- 53.Tarr, P. E. 1996. Granulocyte-macrophage colony-stimulating factor and the immune system. Med. Oncol. 13:133-140. [DOI] [PubMed] [Google Scholar]
- 54.Wu, M., H. Fang, and S. T. Hwang. 2001. Cutting edge: CCR4 mediates antigen-primed T cell binding to activated dendritic cells. J. Immunol. 167:4791-4795. [DOI] [PubMed] [Google Scholar]
- 55.Wunner, W. 1991. The chemical composition and molecular structure of rabies viruses, p. 31-67. In G. M. Baer (ed.), Natural history of rabies, 2nd ed. CRC Press, Inc., Boca Raton, FL.
- 56.Yi, H., L. Zhang, Y. Zhen, X. He, and Y. Zhao. 2007. Dendritic cells induced in the presence of GM-CSF and IL-5. Cytokine 37:35-43. [DOI] [PubMed] [Google Scholar]
- 57.Yoshie, O., T. Imai, and H. Nomiyama. 2001. Chemokines in immunity. Adv. Immunol. 78:57-110. [DOI] [PubMed] [Google Scholar]
- 58.Zhao, L., H. Toriumi, Y. Kuang, H. Chen, and Z. F. Fu. 2009. The roles of chemokines in rabies virus infection: overexpression may not always be beneficial. J. Virol. 83:11808-11818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao, L., et al. 2010. Expression of MIP-1α (CCL3) by a recombinant rabies virus enhances its immunogenicity by inducing innate immunity and recruiting dendritic cells and B cells. J. Virol. 84:9642-9648. [DOI] [PMC free article] [PubMed] [Google Scholar]