Abstract
Aims
This work investigates the role of myoglobin in mediating the vascular relaxation induced by nitrite. Nitrite, previously considered an inert by-product of nitric oxide metabolism, is now believed to play an important role in several areas of pharmacology and physiology. Myoglobin can act as a nitrite reductase in the heart, where it is plentiful, but it is present at a far lower level in vascular smooth muscle—indeed, its existence in the vessel wall is controversial. Haem proteins have been postulated to be important in nitrite-induced vasodilation, but the specific role of myoglobin is unknown. The current study was designed to confirm the presence of myoglobin in murine aortic tissue and to test the hypothesis that vascular wall myoglobin is important for nitrite-induced vasodilation.
Methods and results
Aortic rings from wild-type and myoglobin knockout mice were challenged with nitrite, before and after exposure to the haem-protein inhibitor carbon monoxide (CO). CO inhibited vasodilation in wild-type rings but not in myoglobin-deficient rings. Restitution of myoglobin using a genetically modified adenovirus both increased vasodilation to nitrite and reinstated the wild-type pattern of response to CO.
Conclusion
Myoglobin is present in the murine vasculature and contributes significantly to nitrite-induced vasodilation.
Keywords: Vasodilation, Nitrite, Nitric oxide, Myoglobin
1. Introduction
Nitric oxide, derived from endothelial nitric oxide synthase (eNOS), acts as a paracrine regulator of vascular smooth muscle tone. Lumenal diffusion of nitric oxide results in the production of a number of metabolites including nitrite (NO2−), which was previously considered to have little vasoactive effect. However, it has recently become clear that under certain circumstances, nitrite may be reduced back to nitric oxide to elicit vasorelaxation1,2 and confer protection against ischaemia-reperfusion injury.3–6 While there is an increasing appreciation of the significance of these pathways, controversy exists as to whether they represent pharmacological or genuinely physiological phenomena.
Under conditions of profound hypoxia and/or acidosis, the conversion of nitrite to nitric oxide may occur spontaneously (via acid disproportionation).7 Under less extreme conditions, various factors have been shown to be capable of facilitating nitrite reduction. These include microsomal cytochrome P-450;8,9 red cell and vascular xanthine oxidase;10 red cell and vascular eNOS, mitochondrial aldehyde oxidase (ALDH2);10 neuroglobin;11 haemoglobin;12–18 and myoglobin.19 While all of these agents, alone and in combination, are capable of facilitating nitrite reduction, their physiological role remains unclear. In the heart, myoglobin is an important nitrite reductase, and the potent protective effect of nitrite against ischaemia-reperfusion injury seen in wild-type mice is absent in myoglobin knockout mice.20 Conversely, the role of myoglobin as a nitrite reductase in the vasculature is unknown. Myoglobin is plentiful in cardiac muscle but is present in very low concentrations in vascular smooth muscle—indeed some studies have failed to demonstrate its presence at all21—so a priori myoglobin might be considered an unlikely contributor to nitrite reductase activity in the vasculature. The present study was therefore designed to assess the role of vascular smooth muscle myoglobin as a nitrite reductase during conditions of minor (physiological) hypoxia.
2. Methods
2.1. Animals
Male NMRI and myo−/− mice were used at 6–7 weeks of age; Myo−/− mice were obtained from a breeding colony at the Heinrich-Heine-Universität, Düsseldorf. Animals were housed under standard lighting conditions (normal 12 h/12 h cycle) and provided normal rodent chow and water ad libitum. Tissue was harvested after euthanasia, using methods approved under Schedule 1 of the Animals (Scientific Procedures) Act 1986. The study was approved by the local University Review Committee for ethical and safety issues (The investigation also conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, NIH Publication No. 85-23, revised 1996).
2.2. Measurement of vasodilatation using murine aortic myography
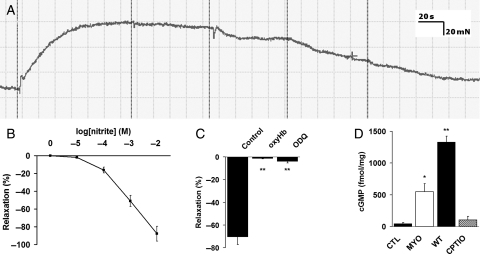
Six to 7-week-old male mice were sacrificed by cervical dislocation. The thoracic aorta from the arch to the diaphragm was removed en bloc and immediately immersed in ice-cold buffer. Krebs-Henseleit buffer was used throughout, with the following composition: NaCl 120.0 mM, KCl 4.7 mM, MgSO4 1.2 mM, K2PO4 1.2 mM, Glucose 11.0 mM, CaCl2 2.5 mM, NaHCO3 25 mM. All chemicals were obtained from Sigma-Aldrich Co (Gillingham, Dorset, UK), apart from sodium nitrite (Martindale Pharmaceuticals, Romford, UK) and carboxy-PTIO (Tocris Bioscience, Bristol, UK). All adherent fat and adventitia was stripped and the endothelium removed by bubbling with air. The aorta was cut into four 2 mm rings and hung in a four-well wire myograph (Danish Myo Technology model 610M, A/S, Denmark) in Krebs-Henseleit buffer. Myography wells were open to the air and bubbled with either carbogen (95% O2/5% CO2) or nitrogen (N2), with and without carbon monoxide, CO (95% N2/5% CO2 or 75% N2/5% CO2/20% CO, respectively). Buffer was allowed to reach steady state. Using this equipment, the nitrogen-containing gas mixtures produced a buffer oxygen tension of 83 ± 2.5 mmHg (11 kPa, i.e. between average arterial and venous conditions), which was stable over time. The rings were attached to an isometric force transducer and the resting tension was increased stepwise to a maximum of 20 mN, which was determined as optimal in preliminary experiments. A typical trace is shown in Figure 1A, and a concentration response curve in Figure 1B (carbogen gassing, wild-type aorta, 1 µM phenylephrine precontraction). Preliminary experiments revealed that the level of constriction achieved using phenylephrine was variable over different oxygen concentrations, and was inadequate under reduced oxygen tension (the precontractile tone achieved using 95% N2/5% CO2 reached ∼40% of that in the presence of 95% O2/5% CO2). Therefore, 50 mM KCl was substituted for phenylephrine for experiments performed under mildly hypoxic conditions. Each measure was taken as the average of at least two segments and is expressed as a percentage reversal of preconstriction. For inhibitor experiments, 50 µM oxypurinol and 50 nM raloxifene were incubated with the rings for 5 min before the experimental runs, which were otherwise performed in an identical fashion. All data are shown as the mean ± SEM, and P < 0.05 was taken to indicate statistical significance. Bonferroni post hoc correction was used for data with multiple comparisons.
Figure 1.
Nitrite-dependent vasorelaxation proceeds through liberation of NO. (A) A typical myography trace. (B) A concentration response curve under typical pharmacological conditions (1 µM phenylephrine constriction, 95% O2)/5% CO2. (C) Addition of 20 µM oxygenated haemoglobin (oxyHb) or 10 µM 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ) abolished nitrite-induced vasorelaxation (n = 4 for each group); **P < 0.001 compared with control by ANOVA. (D) Cyclic GMP (cGMP) accumulation was lower in myo−/− tissue than wild type (n = 6 for each), and was prevented by co-administration of carboxy-PTIO (n = 5); *P < 0.05 compared with control, **P < 0.001 compared with control and myo−/− by ANOVA.
2.3. Viral transduction of myoglobin into myo−/− aortic rings
Thoracic aortas from myo−/− mice were obtained as before. These rings were then exposed to either adenovirus expressing myoglobin (AdMYO) or control virus (AdCtl) (at a concentration of 10−8 PFU/mL) diluted in Opti-MEM (Sigma), supplemented with 11 mM glucose, for 120 min under continuous bubbling with carbogen gas. Rings were washed with Opti-MEM to remove adherent virus particles, and then incubated at 37°C in Krebs-Henseleit buffer for 270 min. This incubation time was determined in preliminary experiments to be sufficient for recapitulation of the low levels of myoglobin seen in wild-type aorta. Response to nitrite both in the presence and absence of CO was assessed as before. Nitrite was used at a final concentration of 9 mM (∼EC90) in order to focus solely on the effect of myoglobin (i.e. after saturating other pathways).
2.4. Measurement of cGMP accumulation in aortic tissue
Fresh aortic tissue was exposed to 10 mM nitrite in Krebs-Henseleit buffer at 37°C for 15 min, with and without the NO scavenger carboxy-PTIO. A phosphodiesterase inhibitor cocktail was used, as described previously.22 cyclic GMP (cGMP) was measured in aortic tissue after homogenization using a commercial ELISA kit (Amersham Biotech, UK).
2.5. Generation of replication-defective adenoviruses
Both AdMyo and AdCtl (control virus) were generated using the AdEasy XL Adenoviral vector system (Stratagene). Human wild-type myoglobin cDNA was cloned into the pShuttle-IRES-hrGFP-1 vector (Stratagene), allowing co-expression of green fluorescent protein (GFP) with myoglobin (a stop codon was introduced to prevent fusion of the FLAG tag). The vector was linearized by PmeI digestion and recombined with the pAdEasy-1 (viral backbone vector) in BJ5183 Escherichia coli. The recombined adenoviral constructs were transfected into DH10β E. coli to allow higher plasmid yields. After confirmation of recombination by BstXI and Pac-1 restriction digestion and sequencing, Pac1 linearized recombinant Ad plasmids were then transfected into AD293 cells (Stratagene) using oligofectamine (Invitrogen). AD293 cells were scraped from cell culture vessels and lysed by four freeze-thaw vortex cycles. Lysates containing recombinant adenoviruses used to infect further AD293 cells for amplification. The viruses were purified by caesium chloride gradient ultracentrifugation. The viral titre was estimated by infecting HEK293 cells (not expressing capsid proteins) and counting GFP expressing cells.
2.6. Endpoint PCR and western blotting
Descending thoracic aortas were removed after euthanasia as above. They were stripped of all adherent fat and adventitia and snap frozen in liquid nitrogen. Samples were homogenized using a small blade homogenizer in TriReagent (Sigma) and RNA was extracted. cDNA was transcribed from purified RNA using a standard kit (Applied Biosystems) and 40 cycles of PCR were performed (primer sequences available on request). Tissue samples for western blotting were snap frozen and subsequently homogenized in lysis buffer (25 mM Tris/HCl, pH 7.4, 1 mM EDTA, 1% SDS, 1 mM dithiothreitol, and complete-mini protease inhibitor cocktail tablet (Roche)) and then boiled with Laemlli buffer. Homogenates were separated by 15% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE). Proteins were transferred to PVDF membranes (BioRad), saturated with 5% fat-free milk in 0.1% Tween-20 in phosphate buffered saline (PBS) (blocking solution), and hybridized with α-myoglobin rabbit polyclonal antibody (Santa Cruz, (FL-154) sc-25607) at 1:1000 dilution overnight at 4°C. The membranes were washed four times (10 min each at RT) with 0.1% Tween-20 in PBS and then incubated with goat anti-rabbit IgG (HRP) (Abcam ab2721-1) at 1:4000 dilution for 1 h at RT, washed four times, and finally visualized with the ECL advance immunoblotting detection system (GE Healthcare).
3. Results
3.1. Vasorelaxation with nitrite depends upon the release of nitric oxide
Nitrite caused concentration-dependent relaxation of endothelium-denuded aortic rings, which was completely abolished by preincubation with 20 µM oxygenated haemoglobin (oxyHb) (Figure 1C) implying that nitrite acts through the generation of NO. Moreover, addition of 10 µM 1H-(1,2,4)oxadiazolo(4,3-a)quinoxalin-1-one (ODQ) to the organ bath prevented nitrite-induced relaxation (Figure 1C), suggesting the effects were mediated by stimulation of soluble guanylyl cyclase. This was confirmed in a separate set of experiments performed in the presence of a phosphodiesterase inhibitor cocktail. Under these conditions, the exposure of vascular tissue to nitrite caused accumulation of cyclic GMP (cGMP), which was lower in myo−/− tissue than in wild type and abolished by preincubation with the NO scavenger, carboxy-PTIO (Figure 1D).
3.2. Vascular myoglobin can mediate nitrite-dependent vasorelaxation and is inhibited by carbon monoxide
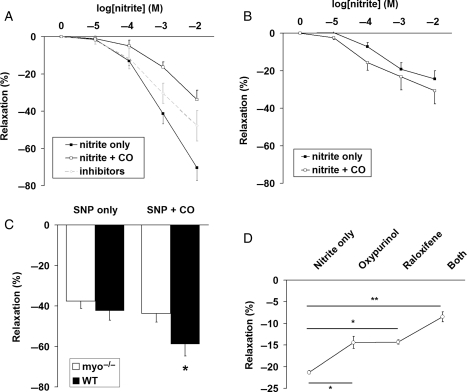
Acute vasorelaxation induced by increasing concentrations of sodium nitrite was assessed during exposure to 95% N2/5% CO2 and then to 20% CO/75% N2/5% CO2. The level of CO was chosen to inhibit only haem proteins, as previously described,23 and did not in itself cause vasorelaxation. CO inhibited nitrite-induced vasorelaxation in the murine wild-type aortas (P < 0.001 by two-way ANOVA) (Figure 2A) suggesting that an intrinsic vascular haem moiety significantly contributes to vascular nitrite bioconversion. The effect of 50 μM oxypurinol (an inhibitor of xanthine oxidase) and 50 nM raloxifene (an inhibitor of aldehyde dehydrogenase) is shown for comparison. To assess whether myoglobin was this haem moiety, myo−/− mice24 were compared with wild-type controls. Overall response to nitrite was markedly reduced in myo−/− rings (Figure 2B) (P < 0.001 by two-way ANOVA), implying that myoglobin significantly contributes to vascular nitrite bioconversion. In contrast to wild-type aortas, CO did not inhibit vasorelaxation in myo−/− rings (Figure 2B). To identify the sources of residual nitrite bioconversion, we assessed myo−/− aortic rings for their vasodilator response to an informative level of nitrite (9 mM; as derived from the concentration response curve) in our experimental system. Preincubation time of vascular rings with inhibitors was 5 min with each agent separately or together; optimal concentrations of inhibitors used were determined in preliminary experiments. Fifty micromolar oxypurinol caused a 32% inhibition of vasorelaxation (from 21.3 ± 0.4 to 14.4 ± 1.4%, P < 0.05 by ANOVA) whereas 50 nM raloxifene produced a 32% inhibition (from 21.3 ± 0.4 to 14.3 ± 0.5%, P < 0.05 by ANOVA). The two agents in combination inhibited nitrite-induced vasodilation by 60% (from 21.3 ± 0.4 to 8.5 ± 1.1%, P < 0.001 by ANOVA) (Figure 2C).
Figure 2.
Myoglobin is a major contributor to nitrite-dependent vasorelaxation. (A) Wild-type aorta shows an inhibition of nitrite-dependent vasorelaxation in response to CO, n = 5, P < 0.001 by two-way ANOVA. (B) This inhibition with CO is not seen in myo−/−, n = 5, P = 0.44 by two-way ANOVA. (C) Conversely, response to sodium nitroprusside is increased by CO in both wild-type and myo−/− rings, *P < 0.05 compared with the same tissue preparation without CO (repeated measures ANOVA). (D) The majority of the remainder of nitrite-dependent vasorelaxation in myo−/− aorta is due to xanthine oxidase and aldehyde dehydrogenase, n = 4, *P < 0.05, **P < 0.001 compared with nitrite alone (repeated measures ANOVA).
3.3. Relaxation to sodium nitroprusside is increased by carbon monoxide in both wild-type and myo−/− rings
Sodium nitroprusside (SNP) was used to assess the effect of CO on vasorelaxation by a nitrite-independent NO donor. Importantly, myo−/− aortic rings retained their response to NO (i.e. the extent of vasorelaxation to a fixed concentration of SNP (0.1 µM) was comparable in tissues from wt and myo−/− mice) and relaxation was increased from 37.7 ± 3.5 to 43.7 ± 4.2% by the addition of CO (P = 0.032 by ANOVA). In wild-type mice, relaxation was increased from 42.3 ± 4.7 to 58.7 ± 6.0% (P = 0.029 by ANOVA) (Figure 2D). The difference in magnitude of effect of CO is consistent with greater scavenging of NO by myoglobin in the wild-type mouse.
3.4. Viral transduction of myoglobin into myo−/− aortas increases nitrite response and reverses the response to CO
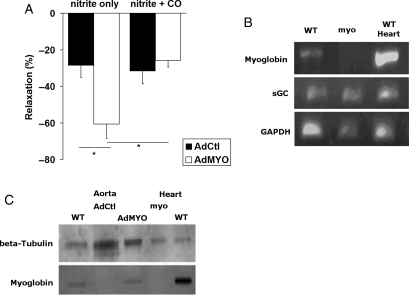
To confirm the role of myoglobin as a nitrite bioconvertor and to exclude the possibility that the changes noted in the myo−/− mice were artefactual adaptations to gene manipulation, we investigated the effect of restoring myoglobin in myo−/− mouse vessels. Transduction of myoglobin augmented the baseline response to nitrite from 28.6 ± 6.5 to 60.7 ± 7.6% (P = 0.024 by ANOVA) (Figure 3A), compared with control virus-treated rings. The pattern of response to CO was similar. Thus, treatment with control virus did not change the effect of CO (from 28.6 ± 6.5 to 31.6 ± 7.0%) (Figure 3A). Crucially, viral transduction of myoglobin reinstated the inhibition of nitrite relaxation by CO seen in wild-type mice (from 60.7 ± 7.6 to 25.8 ± 3.7%, P = 0.014 by ANOVA) (Figure 3A). The concentration of nitrite (∼EC90 at 9 mM) used in this study was chosen to facilitate accurate determination of the vasorelaxing effect of nitrite and the potentially subtle effect of interventions. As expected, endpoint RT–PCR confirmed that myoglobin is present at mRNA level in wild-type murine aortas and absent in myo−/− aortas (Figure 3B). Western blotting confirmed the presence of myoglobin in AdMYO-treated and wild-type rings, at a similarly low level, and its absence in AdCtl-treated rings (Figure 3C).
Figure 3.
Adenovirus-mediated restitution of myoglobin in myo−/− mouse aortic rings augments nitrite-dependent vasorelaxation and restores the inhibitory response to CO. (A) AdMYO-treated aortic rings show increased nitrite-dependent vasorelaxation compared with AdCtl-treated rings, n = 4 for both, P = 0.024 by ANOVA. Similar to untreated myo−/− aorta, AdCtl-treated rings show no decrease in nitrite-dependent vasorelaxation in response to CO, P = NS by ANOVA. Conversely, AdMYO-treated aortic rings show a diminution of nitrite response when exposed to CO, P = 0.014 by ANOVA. (B) Myoglobin was present at mRNA level in wild type but not in myo−/− aortas; myo−/− mice do not adapt to knockout by upregulation of soluble guanylate cyclase (sGC). Endpoint PCR of cDNA from wild-type control and myo−/− aorta, plus wild-type heart positive control; targets are myoglobin, sGC, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a control. (C) The presence of myoglobin (at a very low level) was confirmed by western blotting of wild-type and AdMYO-transduced myo−/− aortic rings. As expected, control virus-treated (AdCtl) myo−/− rings did not express myoglobin.
4. Discussion
In this study, we demonstrate for the first time that smooth muscle myoglobin is an important vascular nitrite reductase. This conclusion was based on the following observations: (a) vascular bioconversion of exogenous nitrite is sensitive to CO, and therefore is likely to depend upon a haem protein; (b) in contrast, in aortic rings from myoglobin-deleted mice vasorelaxation was unchanged or increased in the presence of CO, implying that the net contribution of haem proteins other than myoglobin is NO scavenging; (c) deletion of myoglobin substantially diminishes exogenous nitrite-mediated vasorelaxation, indicating that myoglobin is a major bioconvertor of exogenous nitrite in aortic rings; (d) restitution of myoglobin to myo−/− mouse aortas results in increased vasorelaxation; and restoration of the wild-type pattern of response to CO (i.e. inhibition of vasorelaxation). Further inhibitor studies imply that other contributors to nitrite-mediated blood vessel relaxation are xanthine oxidase and aldehyde dehydrogenase.
There is broad consensus that haem proteins may be involved in vasodilatation by nitrite,21,25 although the precise contribution of haemoglobin is controversial.13−15,17,26,27 Nitrite-induced vasorelaxation is inhibited by oxyhaemoglobin and, to a lesser extent, by deoxyhaemoglobin.17,21,28 There is also increasing evidence that the mechanism of nitrite-induced vasorelaxation is intrinsic to the vessel.21,28 From a chemical perspective, myoglobin is an excellent candidate for an intrinsic vascular nitrite reductase. First, the nitrite to haem ratio in vascular smooth muscle is ∼5000 times greater than in red blood cells.27,29,30 Secondly, myoglobin is a more potent reductase at low pH and at low oxygen tensions (deoxy state Km = 3.1 μM). Finally, the low haem redox potential of deoxymyoglobin enhances reductase activity such that it is probably equivalent in this regard to the R-state of haemoglobin.27 Nitrite reduction by myoglobin yields NO and metmyoglobin;27 metmyoglobin is a relatively inefficient NO scavenger, which allows NO to be liberated. By contrast, in red blood cells, methemoglobin is both more plentiful and a more efficient scavenger, which may hinder NO release. Myoglobin has been shown to be a very important nitrite reductase in the heart,20 but myoglobin concentrations are very high in cardiac muscle, whereas studies in vascular smooth muscle have reported very low or absent concentrations of myoglobin.21,31 While we confirm that the concentration of myoglobin in vascular rings is very low, we nevertheless demonstrated that it is sufficient to affect nitrite reductase activity in this model.
Nitrite is present in human plasma at a level of 0.3–1.0 µM,26,29,32,33 so the concentrations used in the present study are clearly supraphysiological. Thus, the results of our investigation may pertain more to nitrite pharmacology or pathophysiology than physiological homeostasis. Nonetheless, the potential role of myoglobin in the physiological reduction of nitrite is an exciting avenue for future research. A relative strength of the present study was that it was performed at physiological oxygen tension (normoxia to mild hypoxia). However, our aortic ring studies shed no light on the role of haemoglobin or the interactions between red blood cells and vessel walls,14,17 for example, the extent to which red blood cells scavenge or augment NO production by myoglobin, or the contribution of their S-nitrosothiols to nitrite-induced hypoxic vasodilation.21,25,28
It is tempting to speculate on a physiological role for this phenomenon, as smooth muscle myoglobin is perfectly positioned to sense and respond to oxygen deficiency. In hypoxia, nitrite is a more potent vasodilator,2 likely through increased liberation of NO. Through NO release, oxygen may be conserved by controlling oxidative phosphorylation34 and by modifying HIF-1α.4,19,20,35,36 The same mechanism could also restore tissue oxygenation through vasodilatation.2
Funding
This research was supported by the British Heart Foundation. Funding to pay the Open Access publication charge was provided by the British Heart Foundation.
Acknowledgements
The authors would like to thank Professor J. Schrader for the generous provision of myoglobin-deleted mice.
Conflict of interest: none declared.
References
- 1.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, et al. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 2.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, et al. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 3.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med. 2008;104:19144–19149. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci USA. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 8.Feelisch M, Fernandez BO, Bryan NS, Garcia-Saura MF, Bauer S, Whitlock DR, et al. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J Biol Chem. 2008;283:33927–33934. doi: 10.1074/jbc.M806654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozlov AV, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen MG, Dewilde S, Fago A. Reactions of ferrous neuroglobin and cytoglobin with nitrite under anaerobic conditions. J Inorg Biochem. 2008;102:1777–1782. doi: 10.1016/j.jinorgbio.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, et al. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 13.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 14.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grubina R, Huang Z, Shiva S, Joshi MS, Azarov I, Basu S, et al. Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy- and oxyhemoglobin. J Biol Chem. 2007;282:12916–12927. doi: 10.1074/jbc.M700546200. [DOI] [PubMed] [Google Scholar]
- 16.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, et al. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 17.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol. 2007;293:2565–2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 18.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 19.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 20.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alzawahra WF, Talukder MAH, Liu XP, Samouilov A, Zweier JL. Heme proteins mediate the conversion of nitrite to nitric oxide in the vascular wall. Am J Physiol Heart Circ Physiol. 2008;295:H499–H508. doi: 10.1152/ajpheart.00374.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, et al. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 23.Wunderlich C, Flogel U, Godecke A, Heger J, Schrader J. Acute inhibition of myoglobin impairs contractility and energy state of iNOS-overexpressing hearts. Circ Res. 2003;92:1352–1358. doi: 10.1161/01.RES.0000079026.70629.E5. [DOI] [PubMed] [Google Scholar]
- 24.Godecke A, Flogel U, Zanger K, Ding Z, Hirchenhain J, Decking UK, et al. Disruption of myoglobin in mice induces multiple compensatory mechanisms. Proc Natl Acad Sci USA. 1999;96:10495–10500. doi: 10.1073/pnas.96.18.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells. Evidence for an S-nitrosothiol-based signal. Circ Res. 2008;103:545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci USA. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol. 2007;292:H3072–H3078. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 29.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, et al. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez J, Maloney RE, Rassaf T, Bryan NS, Feelisch M. Chemical nature of nitric oxide storage forms in rat vascular tissue. Proc Natl Acad Sci USA. 2003;100:336–341. doi: 10.1073/pnas.0234600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunori M. Nitric oxide, cytochrome-c oxidase and myoglobin. Trends Biochem Sci. 2001;26:21–23. doi: 10.1016/s0968-0004(00)01698-4. [DOI] [PubMed] [Google Scholar]
- 32.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rassaf T, Bryan NS, Maloney RE, Specian V, Kelm M, Kalyanaraman B, et al. NO adducts in mammalian red blood cells: too much or too little? Nat Med. 2003;9:481–482. doi: 10.1038/nm0503-481. [DOI] [PubMed] [Google Scholar]
- 34.Benamar A, Rolletschek H, Borisjuk L, Velange-Macherel MH, Curien G, Mostefai HA, et al. Nitrite-nitric oxide control of mitochondrial respiration at the frontier of anoxia. Biochim Biophys Acta. 2008;1777:1268–1275. doi: 10.1016/j.bbabio.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, et al. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]