Abstract
Exercise training has been shown to reduce many risk factors related to cardiovascular disease, including high blood pressure, high cholesterol, obesity, and insulin resistance. More importantly, exercise training has been consistently shown to confer sustainable protection against myocardial infarction in animal models and has been associated with improved survival following a heart attack in humans. It is still unclear how exercise training is able to protect the heart, but some studies have suggested that it increases a number of classical signalling molecules. For instance, exercise can increase components of the endogenous antioxidant defences (i.e. superoxide dismutase and catalase), increase the expression of heat shock proteins, activate ATP-sensitive potassium (KATP) channels, and increase the expression and activity of endothelial nitric oxide (NO) synthase resulting in an increase in NO levels. This review article will provide a brief summary of the role that these signalling molecules play in mediating the cardioprotective effects of exercise. In particular, it will highlight the role that NO plays and introduce the idea that the stable NO metabolite, nitrite, may play a major role in mediating these cardioprotective effects.
Keywords: Exercise, Nitric oxide, Nitrite, Cardioprotection
1. Introduction
Despite numerous advances in health care practices, cardiovascular disease remains the number one killer in the USA with an estimated 81.1 million American adults having one or more types of cardiovascular disease resulting in about $503 billion being spent a year to cover the associated health care-related costs. Acute myocardial infarction (AMI), which is a direct result of cardiovascular disease, is responsible for approximately 141 462 or 17% of the nearly 831 272 deaths related to cardiovascular disease in the USA. It is also estimated that nearly 1 million Americans will have a new or recurrent myocardial infarction this year.1 Among patients who survive an AMI, the major determinant of the long-term prognosis is the amount of myocardium that is destroyed as a result of ischaemic injury (i.e. the size of infarction). Thus, it is believed that a significant reduction in myocardial infarct size will decrease subsequent morbidity and mortality. Therefore, it is critically important to develop and implement therapeutic strategies that will attenuate myocardial infarct size.
One such infarct-lowering strategy that has been intensely studied since its discovery is the preconditioning (PC) phenomenon. PC refers to the observation that one or several short intermittent periods of ischaemia protects tissue against the injury caused by a subsequent, prolonged period of ischaemia, usually lasting at least 30 min.2 Ischaemic PC was first described in the heart in 1986 when Murry et al.3 demonstrated that short, intermittent periods of ischaemia paradoxically limited infarct sizes when the hearts of dogs were subjected to subsequent prolonged ischaemic insults. Since this first groundbreaking report, ischaemic PC has been observed in all species from mice to man4 and has been observed in other organ systems besides the heart.5 Importantly, exposing tissue to various drugs can mimic the protective effects of brief ischaemic insults; a phenomenon termed pharmacological PC.6 Some of the drugs that have been reported to have PC effects include K+ channel openers, volatile anaesthetics, opiods, bradykinin, and nitric oxide (NO) donors.2 The exact mechanism(s) by which PC exerts its protective effects is incomplete, although several molecules have been implicated7,8: protein kinase C, heat shock proteins (HSPs), tyrosine kinases, mitogen activated protein kinases, protein kinase A, nuclear factor kB, adenosine, and NO.
Despite the clear cytoprotective effects of PC reported in experimental studies, there are two drawbacks to PC strategies that lessen their ability to be used in clinical practice. First, it is unknown when patients will experience a myocardial infarction. Therefore, individuals would have to be on medication chronically to protect them from the myocardial infarction that may or may not be imminent. Second, while many factors appear to be potent in reducing myocardial infarct size when administered acutely, repetitive administration of the PC stimuli results in a loss of efficacy. For these two reasons, the clinical applicability of PC as infarct-sparing strategies continues to be in question.9 However, it appears that there is always an exception to the rule. Once such strategy that falls under the umbrella of PC and that has been advocated to reduce the risk of cardiovascular disease is the concept of a healthy lifestyle consisting of a healthy diet and regular exercise.
Numerous studies have reported that exercise training confers sustainable protection against myocardial infarction in animal models9–13 and has been associated with improved survival following an ischaemic event in humans.14,15 The mechanisms that underlie these protective effects are not fully understood, but it is clear that exercise is associated with reducing many risk factors related to cardiovascular disease, including high blood pressure, high cholesterol, obesity, and insulin resistance.12 However, the beneficial effects of exercise are not solely related to the reduction of these risk factors, as the association of reduced mortality is independent of other coronary risk factors.16 Importantly, the infarct sparing effects of exercise have been reported following both long-term (>10 weeks) and short-term (1–5 days) training regimens. Therefore, exercise training seems to be unique, in that unlike other PC modalities there does not appear to be a de-sensitization to its cytoprotective effects. Additionally, exercise is unlike other treatment strategies (i.e. pharmacological interventions) in that it is safe, inexpensive, and widely accessible to all patient populations.
This article will provide a brief summary of the signalling mechanisms that are thought to mediate exercise-induced cardioprotection. In particular, it will highlight the role that NO plays and introduce the idea that the stable NO metabolite, nitrite, may play a major role in mediating these cardioprotective effects.
2. Cardioprotective signalling molecules activated by exercise
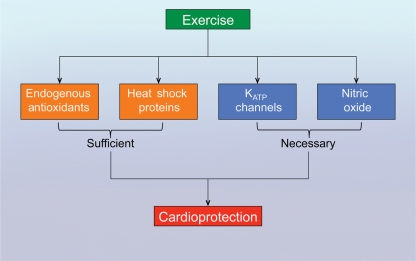
Although the physiological and cardioprotective effects of exercise training have previously been documented, the signalling mechanisms that mediate these effects have not been fully elucidated. However, it is known that exercise training increases a number of classical signalling molecules. For instance, exercise can increase (Figure 1) components of the endogenous antioxidant defences [i.e. superoxide dismutase (SOD) and catalase], increase the expression of HSPs, activate ATP-sensitive potassium (KATP) channels, and increase the expression and activity of endothelial nitric oxide synthase (eNOS) resulting in an increase in NO levels.9 Evidence for the involvement of each of these mechanisms is discussed below.
Figure 1.
Cardioprotective signalling molecules activated by exercise. Short-term and long-term exercise training has been shown to increase the expression and activity of components of the endogenous antioxidant defense system (catalase, SODs), increase the expression of heat shock proteins (HSPs), activate ATP-sensitive potassium (KATP) channels, and increase the expression and activity of endothelial nitric oxide synthase (eNOS) resulting in an increase in nitric oxide (NO) levels. Although all of these molecules have been associated with the cardioprotection afforded by exercise training, studies have shown that endogenous antioxidants and HSPs are not necessary for the observed cardioprotection, whereas KATP channels and eNOS are necessary.
3. Exercise and endogenous antioxidant defences
Under physiological conditions, small amounts of reactive oxygen species (ROS) produced as a consequence of electron transfer reactions in mitochondria, peroxisomes, and cytosol are quenched by cellular antioxidant defence systems. Antioxidants act by scavenging oxidative species and their precursors, inhibiting their formation and enhancing endogenous antioxidant defences.17 The main endogenous antioxidant is SOD, which catalyses the dismutation of two superoxide radicals to form hydrogen peroxide and molecular oxygen.18 Three distinct types of SODs have been found in mammalian tissues. The two most studied SODs are the mitochondrial, tetrameric manganese-containing enzyme (MnSOD) and the cytosolic, dimeric copper/zinc-containing enzyme (CuZnSOD).19 The third SOD, extracellular SOD, is a tetrameric glycoprotein containing Cu and Zn atoms at the active site20 and is found predominantly in intravascular and extra-cellular fluids. The hydrogen peroxide generated by the dismutation reaction is further scavenged by catalase to water and oxygen.21 During myocardial I/R, the activity of these systems becomes reduced or even abolished,22 suggesting that increasing the activity of the cellular antioxidant enzymes should protect tissues from reperfusion damage.23 Indeed, the administration of exogenous SOD,24 the overexpression of CuZnSOD,25 the overexpression of MnSOD,26 and the overexpression of catalase27 have all been shown to be cardioprotective.
There are some conflicting reports regarding the activation of endogenous antioxidant defences by exercise training. Long durations of exercise training (∼20 weeks) have been reported to increase the expression12 and the activity of both MnSOD and CuZnSOD.28,29 However, there are several studies, which have not observed an increase in activity or expression of either SOD and yet reported cardioprotection.30,31 For instance, Lennon et al.32 reported that 8 days of exercise training provided cardioprotection against myocardial I/R injury, but failed to increase the expression of either SOD, suggesting that short durations of exercise training are not sufficient to increase SOD expression. Lennon et al.32 did, however, report that catalase activity was increased in response to 8 days of exercise training. This is in agreement with other studies, which have noted an increase in catalase activity in response to both short and long durations of exercise training.33,34 However, the study by Lennon et al.32 also found that an increase in catalase activity might not be essential for exercise-induced cardioprotection. In this study, rats were allowed to rest for 1, 3, or 9 days after the 8-day training period. As noted, catalase activity was increased after 1 day of rest and also after 3 days of rest. By 9 days of rest, catalase activity had returned to baseline levels. Interestingly, the rats were still protected against myocardial infarction at this time point. Together, these results suggest two things. First, the duration of the training factors into determining which endogenous antioxidants are increase by exercise. Second, an increase in endogenous antioxidants may be sufficient to provide cardioprotection in response to exercise, but is not necessary.
4. Exercise and heat shock proteins
HSPs form the most ancient defence system in all living organisms on earth.35 Heat or other stressors induce HSPs in a variety of cell types, thus protecting cells from insults such as ischaemia, oxidative stress, and noxious chemicals.36 Both HSP70 and HSP27 have been demonstrated to provide cardioprotection in the setting of I/R.37,38 The mechanism of cytoprotection afforded by HSP70 and HSP27 has been attributed to their ability to function as anti-apoptotic agents in both caspase-dependent and -independent pathways.39 Several different isoforms of HSPs are increased in response to exercise training. For instance, Powers et al.40 reported that the expression of HSP72 (aka HSP70) was increased in the hearts of female rats following 10 weeks of endurance training. Demirel et al.41 also demonstrated that 5 days of treadmill exercise increased the expression of myocardial HSP72. Hamilton et al.42 found that other isoforms such as HSP90 and HSP40, but not HSP10, HSP60, and HSP73 were also increased following exercise training. In all of these studies, protection against myocardial injury was reported, suggesting that HSPs play a role in mediating exercise-induced cardioprotection. However, as noted for the endogenous antioxidants above, there are several reports, which provide evidence suggesting that HSPs are not the main contributors to exercise-mediated cardioprotection. For example, two different studies have demonstrated that exercise in a cold (4–8°C) environment provides the same cardioprotection as exercise in a warm environment (25°C), but does not increase the expression of HSP72 or any other isoform investigated.42,43 Hence, it appears that HSPs are also sufficient to induce exercise-induced cardioprotection, but not necessary.
5. Exercise and KATP channels
KATP channels are found on the surface membranes and mitochondria of many different cell types, including pancreatic β-cells, neurons, cardiac myocytes, liver, skeletal, and smooth muscle cells.44 These channels are weakly inwardly rectifying K+ (Kir) channels that stabilize the membrane potential close to the equilibrium potential for K+. At the molecular level, functional KATP channels are understood to be multi-subunit protein complexes. Transmembrane Kir6 pore forming subunits allow K+ ions to permeate the channel complex, whereas sulphonylurea (SUR) accessory subunits serve to act as receptors for a variety of pharmacological compounds that either activate or inhibit KATP channel opening. The Kir6.x subunit family consists of two members, Kir6.1 and Kir6.2, both of which are expressed in the heart.45 KATP channels are known to play an important role in the cardioprotective signalling of ischaemic PC.46 In addition, pharmacological agents that selectively open the KATP channel have also been shown to have infarct limiting effects.47
There is now considerable evidence that KATP channels are centrally involved in the cardioprotection afforded by exercise.48 Brown et al.49 first reported that 5 days of treadmill running significantly reduced myocardial infarction in both male and female rats. The observed cardioprotection was associated with an increase in the expression of both SUR2A and Kir6.2 in the male hearts and an increase in the expression of SUR2A in the female hearts. This indicates that although there is a sex-dependent difference in the regulation of KATP channel components by exercise, there is an increase in KATP expression in response to short-term exercise training. The role of KATP channels in exercise-mediated cardioprotection was further supported by the findings of Brown et al.9 In this study, the authors found that 12 weeks of exercise training increased the expression of SUR and Kir6.2 in the hearts of female rats. Additionally, the authors found that inhibition of sarcolemmal KATP channels abolished the protective effects of exercise, whereas the inhibition of mitochondrial KATP channels did not, suggesting that sarcolemmal KATP channels play a central role in mediating the cardioprotective effects of exercise. However, a recent study by Quindry et al.50 found that mitochondrial KATP channels provide anti-arrhythmic protection as part of exercise-mediated cardioprotection against ischaemia–reperfusion injury. Therefore, it appears that both sarcolemmal and mitochondrial KATP channels have a role to play in the cardioprotection afforded by exercise training. Moreover, unlike HSPs and antioxidants, it appears that KATP channels play a direct role in exercise-mediated cardioprotection. As noted above, blockade of KATP channels has been shown to abolish the cardioprotective effects of both short-term10 and long-term9 exercise training.
6. Exercise and nitric oxide
Recent studies have indicated that the endothelium plays a critical role in mediating the cardioprotective effects associated with exercise. Specifically, these studies have demonstrated that during exercise the expression and activity of eNOS is increased in response to shear stress.51 Sessa et al.52 were the first to report that 10 days of treadmill running increased the gene expression of eNOS and increased NO production in coronary arterioles from dogs. Chronic exercise for as long as 16 weeks has also been reported to alter eNOS gene expression in a porcine model.53 Furthermore, exercise has also been reported to alter eNOS expression and phosphorylation status in humans.54 Hambrecht et al.51 investigated the effects of exercise training on the endothelial function in relation to the expression of eNOS and Akt-dependent eNOS phosphorylation in the left internal mammary artery of patients with coronary artery disease. They found that exercise training resulted in a two-fold increase in the expression of eNOS and a four-fold increase in the expression of phosphorylated eNOS at serine residue 1117 (eNOS-PSer1177).
Matching tissue oxygen and substrate supply to demand during exercise is controlled by both blood delivery and the capacity of cells to extract these substances.55 It appears that NO plays a role in both of these processes. First, the release of NO from endothelial cells in response to shear stress induces vasodilatation of arteries in both the skeletal muscle and the heart to increase blood flow.55 Second, NO has been reported to alter carbohydrate metabolism in skeletal muscle through an enhancement of glucose uptake and inhibition of glyceraldehyde-3-phosphate dehydrogenase.55 In addition to its effects on matching blood supply to metabolic demands during exercise, NO is also responsible for some of the atheroprotective effects of exercise through its ability to inhibit inflammatory cells and platelets from adhering to the vascular surface.56 Furthermore, NO possesses a number of physiological properties not associated with reducing risk factors associated with cardiovascular disease that make it a potent cardioprotective-signalling molecule in the setting of myocardial ischaemia–reperfusion injury.57 For instance, NO reversibly inhibits mitochondrial respiration.58 The inhibition of mitochondrial respiration during early reperfusion counterintuitively leads to a decrease in mitochondrial-driven myocardial injury by extending the zone of adequate tissue cellular oxygenation away from vessels.59 NO also inhibits apoptosis60 either directly or indirectly by inhibiting caspase-3-like activation via a cGMP-dependent mechanism and by direct inhibition of caspase-3-like activity through protein S-nitrosylation.61 Given this diverse physiological profile, it is likely that NO contributes to the cardioprotective effects of exercise by first reducing cardiovascular risk and second by reducing injury in the event of myocardial ischaemia.
A central role for eNOS in the physiology of exercise was established in studies employing the use of eNOS deficient mice (eNOS−/−). Momken et al.62 found that over a training period of 8 weeks, eNOS−/− mice averaged a running distance almost two times lower than wild-type controls, as well as a mean running distance that was half that of the controls. This was further confirmed by Ojaimi et al.,63 who reported that eNOS−/− mice of different ages ran an average of up to 60% less than age-matched wild-type mice. A role for eNOS in mediating the protective effects of exercise against a component of cardiovascular disease was first reported in the brain, where it was found that exercise-induced neuroprotection against a stroke was lost in eNOS−/− mice.64 Additionally, a recent study also found that eNOS is required for the cardioprotective effects of exercise. de Waard et al.65 found that the beneficial effects of exercise on post-myocardial infarction remodelling, hypertrophy, fibrosis, and apoptosis were lost in both heterozygous and homozygous eNOS-deficient mice.
7. Exercise and nitrite
The anion nitrite is an oxidative breakdown product of NO that has traditionally served as a diagnostic marker of NO formation in biological systems.66 As such, nitrite has long been considered an inert oxidation product of NO metabolism. Recently, there has been a paradigm shift in nitrite biology with the discovery that nitrite is a physiologically relevant storage reservoir of NO67 in the blood and tissues that can readily be reduced to NO under pathological conditions, such as ischaemia or hypoxia.68 Nitrite reductase activity in mammalian tissues has been linked66 to the mitochondrial electron transport system, non-enzymatic acidic disproportionation, deoxyhaemoglobin, xanthine oxidase, and more recently myoglobin.
Nitrite therapy has been shown to provide cardioprotection in animal models of myocardial I/R injury.69,70 These experimental studies have provided important insights into the cardioprotective effects of nitrite therapy and have demonstrated nitrite therapy to be equally effective when it is administered before, during, or after ischaemia through either systemic or oral administration.71 In terms of mechanisms of action, the cytoprotective effects of nitrite therapy have not been fully elucidated. However, it has been shown that nitrite-mediated protection is independent of eNOS and dependent on NO generation.70 Nitrite can also transiently form nitrosothiols in a first-order reaction requiring haem and thiols under both normoxic and hypoxic conditions.72 Since both NO and nitrosothiols have been shown to be protective in the setting of I/R,70,73 nitrite is a critical signalling molecule in that it can form both NO and nitrosothiols. This suggests that nitrite can serve two functions in the setting of I/R. It first serves as a NOS-independent source of NO by which nitrite is reduced to NO during ischaemia when NOS is inactive due to low oxygen tensions. Secondly, nitrite reacts with critical thiols to form nitrosothiols. This nitroso modification acts as a reversible protective shield, which prevents irreversible oxidation of proteins and lipids during the early oxidative burst of reperfusion. Aside from ‘capping’ critical thiols from oxidation, the nitroso products can then release NO during the reperfusion phase and act on a redox sensitive NO donor.74
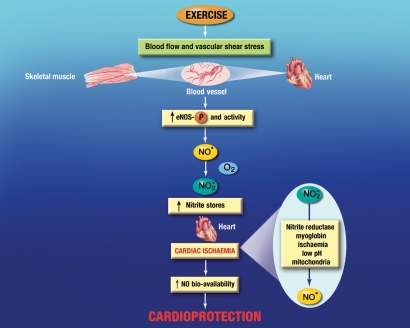
Exercise has been associated with increasing plasma nitrite levels in both rodents and humans.9,75,76 However, the increased circulating levels of nitrite have traditionally been considered only as an acute marker of NO production and a surrogate for endothelial function.75 As such, the role of nitrite in mediating the cardioprotective effects of exercise has not been suggested or investigated. Given the recent paradigm shift in NO biology, the role of nitrite in exercise should be reconsidered. Previous studies suggest that the circulating levels of nitrite directly regulate its tissue storage. The two main sources of circulating nitrite are: (i) the oxidative breakdown of NO generated by the NOS isoforms (mainly eNOS) and (ii) the dietary consumption of foods containing nitrite and nitrate. Both sources have a profound influence on nitrite tissue storage and also influence the severity of ischaemia–reperfusion injury. For example, mice supplemented with nitrite (50 mg/L) in their drinking water for 7 days exhibited higher plasma and heart levels of nitrite and displayed a reduction in infarct size following myocardial I/R injury.77 Similarly, the overexpression of eNOS, either systemically or specifically in the heart, results in higher plasma and heart nitrite levels and less injury following myocardial I/R.78 More importantly, mice that overexpress eNOS only in the heart have higher levels of nitrite in the liver and reduced injury following hepatic ischaemia,78 suggesting that nitrite can be transported in the blood and stored in remote organs. Based on this evidence and other literature, it can be hypothesized that nitrite generated from NO during exercise training can play a role in mediating the cardioprotective effects of exercise in the event myocardial ischaemia (Figure 2). In this scenario, the NO generated from the endothelium during exercise has two fates. First, it is used to induce vasodilatation to match blood flow to metabolic demands as noted above. Second, some of the NO is oxidized to nitrite. The nitrite can then be transported in the blood from its site of origin and stored in the heart. This can continue with each passing exercise period until the steady-state levels of nitrite in the heart are elevated above normal baseline levels. This would be analogous to the effects of oral nitrite supplementation on the heart that was observed in a previous study.77 Increasing nitrite stores in the heart prior to myocardial ischaemia is important because the bioavailability of NO is decreased during ischaemia. The cause of this decrease is still not completely understood, but it has been suggest that NO levels are reduced during myocardial ischaemia due to a decrease in production from eNOS because of low oxygen and diminished substrate delivery69,79 and/or an increase in ROS production.80 In any event, the stored nitrite could be reduced to NO during myocardial ischaemia by any of the identified nitrite reductases found in the heart, thereby providing an increase in the bioavailability of NO. The increase in NO could then serve as a signalling molecule and protect the heart by any of the mechanisms discussed above.
Figure 2.
Hypothesized fate of stored cardiac nitrite during myocardial ischaemia. Nitrite (NO2−) represents a physiologically relevant storage reservoir of NO in blood and tissues that can readily be reduced to NO under pathological conditions such as ischaemic or hypoxic events. Previous studies have indicated that nitrite levels are increased in the plasma following exercise in both rodents and humans. Given that nitrite can be stored in the heart and provide cardioprotection in the setting of myocardial ischaemia by being reduced to NO, it can be hypothesized that the NO generated during exercise from the endothelium can be oxidized to nitrite, transported in the plasma, and stored in the heart or vasculature. In the event of myocardial ischaemia, the nitrite can be reduced back to NO by any of the known reductases found in the heart, thereby increasing the bioavailability of NO and providing cardioprotection in an NO-dependent manner.
Currently, there is only one published abstract from a recent meeting, which reports that 4 weeks of voluntary exercise training increases cardiac nitrite and nitrosothiols levels in mice.81 Given the paucity of evidence regarding the role of nitrite in exercise-mediated cardioprotection, studies aimed at testing the proposed hypothesis are definitely warranted. Specifically, studies should address some of the following questions: (i) what is the minimum duration of exercise training needed to achieve an increase in the levels of nitrite in the heart and provide cardioprotection? (ii) How high can exercise training increase tissue levels of nitrite and how long does it take to reach a new steady-state level? (iii) Will the new steady-state levels of nitrite be maintained over time? Additionally, since there are different types of exercise training, it is also important to determine the type(s) of exercise that can increase nitrite levels in the heart.
8. Exercise and nitrate
As stated above, exercise training can increase the plasma levels of nitrite in both humans and rodents. In most cases, these studies also reported an increase in plasma nitrate levels. Much like nitrite, nitrate was once considered to be an inert oxidative by-product of NO that only served as a measure of endothelial function. Now there is evidence to suggest that nitrate is a physiologically relevant storage reservoir of NO just like nitrite.82 In terms of cardioprotection, nitrate has been reported to be equally effective as nitrite, as it has been reported that mice supplemented with nitrate (1 g/L) in their drinking water for 7 days exhibited higher plasma and heart levels of NO metabolites and displayed a reduction in infarct size following myocardial I/R injury.77 Therefore, it can be hypothesized that nitrate generated from NO during exercise training can also play a role in mediating the cardioprotective effects of exercise in the event of myocardial ischaemia.
9. Interaction of the proposed signalling mechanisms
Although the protective effects of exercise have been studied for quite some time now, the exact mechanisms responsible have not been fully elucidated. In the current article, a role for endogenous antioxidant defences, HSPs, KATP channels, and NO was discussed as individual components of a cardioprotective-signalling cascade. Interestingly, although, there are a number of studies, which suggest that all of these components are associated with exercise and cardioprotection, KATP channels and NO are the only two that have been shown to be necessary for cardioprotection. It is also interesting to speculate that NO may be the most critical factor due to the role it plays in mediating many of the physiological responses to exercise. First, NO is responsible for the vasodilatation to match blood flow to metabolic demands of the tissue. NO is also inhibits inflammatory cells and platelets from adhering to the vascular surface, which may be responsible for the atheroprotective effects of exercise. Additionally, NO can increase the expression of HSP7083 and activate KATP channels84 through cGMP-PKG signalling,85 suggesting that NO could be responsible for activating these factors during exercise.
With that being said, it does not mean that HSPs, KATP channels, and antioxidant defences do not play an important role in mediating the cardioprotective effects of exercise, since all of these components can provide cardioprotection in their own right. Additionally, when discussing the cardioprotective mechanisms of exercise, it is important to separate the events of exercise training into two phases: the exercise period prior to myocardial ischaemia and the period during myocardial ischaemia and reperfusion. During the period prior to myocardial ischaemia, it may seem like NO predominates due to its role in mediating the physiological response to exercise. However, the role that HSPs, KATP channels, and antioxidant defences play cannot be underestimated. It is during the period following the onset of myocardial ischaemia and reperfusion where the importance of these components and the convergence of these signalling mechanisms, especially in regards to NO bioavailability, become readily apparent. As mentioned above, the continuous generation of NO is essential for the integrity of the cardiovascular system86 and a decreased production and/or bioavailability of NO is central to the development of cardiovascular disorders. Without an adequate delivery of oxygen, substrate, and co-factors (conditions that exist during ischaemia), the production of NO from NOS can be diminished due to uncoupling and replaced by the production of ROS.87,88 Moreover, ROS can scavenge NO resulting in a decrease in the bioavailability of NO. The opening of KATP channels, especially mitochondrial KATP channels, during exercise can influence the bioavailability of NO by inducing low-level ROS production during the period prior to myocardial ischaemia that triggers protection by limiting the production of higher levels of ROS following ischaemia and reperfusion.89 Similarly, increasing antioxidant defences during exercise can increase the bioavailability of NO by reducing ROS levels and preventing the scavenging of NO. Additionally, the association of eNOS with HSP90 is an important step in controlling eNOS activity and eNOS coupling,90 which can result in NO rather than ROS being produced from eNOS. Additionally, reducing ROS levels through the opening of mitochondrial KATP channels and through an increase in endogenous antioxidants can lead to cardioprotection in a manner independent of NO.
10. Conclusion
The high incidence of cardiovascular disease in western societies is attributable to the contemporary lifestyle, which is often sedentary in nature and includes a diet high in saturated fats and sugar and devoid of fruits, vegetables, and fibre.91 As such, the recommended standard of care aimed at reducing the risk factors associated with cardiovascular disease is a combination of pharmacological interventions and a change in diet and physical activity. Given the growing costs associated with pharmacological agents, changes in lifestyle may be a more economical way for some individuals to reduce risk factors associated with cardiovascular disease. As such, exercise remains an intriguing strategy to combat the development of cardiovascular disease in that it is unlike other treatment strategies (i.e. pharmacological interventions) given that it is safe, inexpensive, and widely accessible to patients. Therefore, a better understanding of the mechanisms responsible for the cytoprotective effects of exercise will allow scientists and physicians the ability to design safe and efficient treatment modalities to effectively treat patients who are at risk for cardiovascular diseases or who have already suffered the effects of cardiovascular disease, such as a heart attack, stroke, or heart failure.
Conflict of interest: none declared.
Funding
Supported by grants from the American Diabetes Association (7-09-BS-26) and the National Institutes of Health (NIH) National Heart Lung and Blood Institute (1R01HL098481-01). This work was also supported by funding from the Carlyle Fraser Heart Center (CFHC) of Emory University Hospital Midtown.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart Disease and Stroke Statistics—2010 Update. A Report from the American Heart Association. Circulation. 2009;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Hanley PJ, Daut J. K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J Mol Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. doi:10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 4.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. doi:10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 5.Peralta C, Serafin A, Fernandez-Zabalegui L, Wu ZY, Rosello-Catafau J. Liver ischemic preconditioning: a new strategy for the prevention of ischemia-reperfusion injury. Transplant Proc. 2003;35:1800–1802. doi: 10.1016/s0041-1345(03)00571-2. doi:10.1016/S0041-1345(03)00571-2. [DOI] [PubMed] [Google Scholar]
- 6.Kukreja RC, Salloum F, Das A, Ockaili R, Yin C, Bremer YA, et al. Pharmacological preconditioning with sildenafil: basic mechanisms and clinical implications. Vascul Pharmacol. 2005;42:219–232. doi: 10.1016/j.vph.2005.02.010. doi:10.1016/j.vph.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Carini R, Grazia De Cesaris M, Splendore R, Domenicotti C, Nitti MP, Pronzato MA, et al. Signal pathway responsible for hepatocyte preconditioning by nitric oxide. Free Radic Biol Med. 2003;34:1047–1055. doi: 10.1016/s0891-5849(03)00039-x. doi:10.1016/S0891-5849(03)00039-X. [DOI] [PubMed] [Google Scholar]
- 8.Doi Y, Hamazaki K, Yabuki M, Tanaka N, Utsumi K. Effect of HSP70 induced by warm ischemia to the liver on liver function after partial hepatectomy. Hepatogastroenterology. 2001;48:533–540. [PubMed] [Google Scholar]
- 9.Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, et al. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol. 2005;569:913–924. doi: 10.1113/jphysiol.2005.095729. doi:10.1113/jphysiol.2005.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chicco AJ, Johnson MS, Armstrong CJ, Lynch JM, Gardner RT, Fasen GS, et al. Sex-specific and exercise-acquired cardioprotection is abolished by sarcolemmal KATP channel blockade in the rat heart. Am J Physiol Heart Circ Physiol. 2007;292:H2432–H2437. doi: 10.1152/ajpheart.01301.2006. doi:10.1152/ajpheart.01301.2006. [DOI] [PubMed] [Google Scholar]
- 11.Akita Y, Otani H, Matsuhisa S, Kyoi S, Enoki C, Hattori R, et al. Exercise-induced activation of cardiac sympathetic nerve triggers cardioprotection via redox-sensitive activation of eNOS and upregulation of iNOS. Am J Physiol Heart Circ Physiol. 2007;292:H2051–H2059. doi: 10.1152/ajpheart.01102.2006. doi:10.1152/ajpheart.01102.2006. [DOI] [PubMed] [Google Scholar]
- 12.Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J Appl Physiol. 2003;95:2510–2518. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- 13.Freimann S, Scheinowitz M, Yekutieli D, Feinberg MS, Eldar M, Kessler-Icekson G. Prior exercise training improves the outcome of acute myocardial infarction in the rat. Heart structure, function, and gene expression. J Am Coll Cardiol. 2005;45:931–938. doi: 10.1016/j.jacc.2004.11.052. doi:10.1016/j.jacc.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 14.Hull SS, Jr, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation. 1994;89:548–552. doi: 10.1161/01.cir.89.2.548. [DOI] [PubMed] [Google Scholar]
- 15.Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet. 1980;2:1207–1210. doi: 10.1016/s0140-6736(80)92476-9. doi:10.1016/S0140-6736(80)92476-9. [DOI] [PubMed] [Google Scholar]
- 16.Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation. 1999;99:963–972. doi: 10.1161/01.cir.99.7.963. [DOI] [PubMed] [Google Scholar]
- 17.Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation—the two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86:41–59. doi: 10.1016/s0167-5273(02)00189-4. doi:10.1016/S0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 18.Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. doi:10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- 19.Weisiger RA, Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973;248:3582–3592. [PubMed] [Google Scholar]
- 20.Marklund SL, Holme E, Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982;126:41–51. doi: 10.1016/0009-8981(82)90360-6. doi:10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- 21.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- 22.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. doi:10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, et al. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. doi:10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 24.Kilgore KS, Friedrichs GS, Johnson CR, Schasteen CS, Riley DP, Weiss RH, et al. Protective effects of the SOD-mimetic SC-52608 against ischemia/reperfusion damage in the rabbit isolated heart. J Mol Cell Cardiol. 1994;26:995–1006. doi: 10.1006/jmcc.1994.1120. doi:10.1006/jmcc.1994.1120. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Oberley TD, Ho Y, Chua CC, Siu B, Hamdy RC, et al. Overexpression of CuZnSOD in coronary vascular cells attenuates myocardial ischemia/reperfusion injury. Free Radic Biol Med. 2000;29:589–596. doi: 10.1016/s0891-5849(00)00363-4. doi:10.1016/S0891-5849(00)00363-4. [DOI] [PubMed] [Google Scholar]
- 26.Jones SP, Hoffmeyer MR, Sharp BR, Ho YS, Lefer DJ. Role of intracellular antioxidant enzymes after in vivo myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H277–H282. doi: 10.1152/ajpheart.00236.2002. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Chen Y, Saari JT, Kang YJ. Catalase-overexpressing transgenic mouse heart is resistant to ischemia-reperfusion injury. Am J Physiol. 1997;273:H1090–H1095. doi: 10.1152/ajpheart.1997.273.3.H1090. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton KL, Staib JL, Phillips T, Hess A, Lennon SL, Powers SK. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med. 2003;34:800–809. doi: 10.1016/s0891-5849(02)01431-4. doi:10.1016/S0891-5849(02)01431-4. [DOI] [PubMed] [Google Scholar]
- 29.Husain K, Hazelrigg SR. Oxidative injury due to chronic nitric oxide synthase inhibition in rat: effect of regular exercise on the heart. Biochim Biophys Acta. 2002;1587:75–82. doi: 10.1016/s0925-4439(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 30.Ji LL, Stratman FW, Lardy HA. Antioxidant enzyme response to selenium deficiency in rat myocardium. J Am Coll Nutr. 1992;11:79–86. doi: 10.1080/07315724.1992.10718200. [DOI] [PubMed] [Google Scholar]
- 31.Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol. 1997;272:R363–R369. doi: 10.1152/ajpregu.1997.272.1.R363. [DOI] [PubMed] [Google Scholar]
- 32.Lennon SL, Quindry J, Hamilton KL, French J, Staib J, Mehta JL, et al. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol. 2004;96:1299–1305. doi: 10.1152/japplphysiol.00920.2003. doi:10.1152/japplphysiol.00920.2003. [DOI] [PubMed] [Google Scholar]
- 33.Harris MB, Starnes JW. Effects of body temperature during exercise training on myocardial adaptations. Am J Physiol Heart Circ Physiol. 2001;280:H2271–H2280. doi: 10.1152/ajpheart.2001.280.5.H2271. [DOI] [PubMed] [Google Scholar]
- 34.Somani SM, Frank S, Rybak LP. Responses of antioxidant system to acute and trained exercise in rat heart subcellular fractions. Pharmacol Biochem Behav. 1995;51:627–634. doi: 10.1016/0091-3057(94)00427-k. doi:10.1016/0091-3057(94)00427-K. [DOI] [PubMed] [Google Scholar]
- 35.Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther. 2004;101:227–257. doi: 10.1016/j.pharmthera.2003.11.004. doi:10.1016/j.pharmthera.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Kogure K, Kato H. Altered gene expression in cerebral ischemia. Stroke. 1993;24:2121–2127. doi: 10.1161/01.str.24.12.2121. [DOI] [PubMed] [Google Scholar]
- 37.Jayakumar J, Suzuki K, Sammut IA, Smolenski RT, Khan M, Latif N, et al. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation. 2001;104:I303–I307. doi: 10.1161/hc37t1.094932. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Wu XJ, Lu XY, Zhu L, Wang LP, Yang HT, et al. Phosphorylated heat shock protein 27 is involved in enhanced heart tolerance to ischemia in short-term type 1 diabetic rats. Acta Pharmacol Sin. 2005;26:806–812. doi: 10.1111/j.1745-7254.2005.00113.x. doi:10.1111/j.1745-7254.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 39.Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, et al. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:899–910. doi: 10.1038/sj.jcbfm.9600080. doi:10.1038/sj.jcbfm.9600080. [DOI] [PubMed] [Google Scholar]
- 40.Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, et al. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol. 1998;275:R1468–R1477. doi: 10.1152/ajpregu.1998.275.5.R1468. [DOI] [PubMed] [Google Scholar]
- 41.Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, et al. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol. 2001;91:2205–2212. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton KL, Powers SK, Sugiura T, Kim S, Lennon S, Tumer N, et al. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol. 2001;281:H1346–H1352. doi: 10.1152/ajpheart.2001.281.3.H1346. [DOI] [PubMed] [Google Scholar]
- 43.Taylor RP, Harris MB, Starnes JW. Acute exercise can improve cardioprotection without increasing heat shock protein content. Am J Physiol. 1999;276:H1098–H1102. doi: 10.1152/ajpheart.1999.276.3.H1098. [DOI] [PubMed] [Google Scholar]
- 44.Baukrowitz T, Fakler B. KATP channels gated by intracellular nucleotides and phospholipids. Eur J Biochem. 2000;267:5842–5848. doi: 10.1046/j.1432-1327.2000.01672.x. doi:10.1046/j.1432-1327.2000.01672.x. [DOI] [PubMed] [Google Scholar]
- 45.Tong X, Porter LM, Liu G, Dhar-Chowdhury P, Srivastava S, Pountney DJ, et al. Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am J Physiol Heart Circ Physiol. 2006;291:H543–H551. doi: 10.1152/ajpheart.00051.2006. doi:10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernardo NL, D'Angelo M, Okubo S, Joy A, Kukreja RC. Delayed ischemic preconditioning is mediated by opening of ATP-sensitive potassium channels in the rabbit heart. Am J Physiol. 1999;276:H1323–H1330. doi: 10.1152/ajpheart.1999.276.4.H1323. [DOI] [PubMed] [Google Scholar]
- 47.Gross ER, Hsu AK, Gross GJ. Delayed cardioprotection afforded by the glycogen synthase kinase 3 inhibitor SB-216763 occurs via a KATP- and MPTP-dependent mechanism at reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H1497–H1500. doi: 10.1152/ajpheart.01381.2007. doi:10.1152/ajpheart.01381.2007. [DOI] [PubMed] [Google Scholar]
- 48.Brown DA, Moore RL. Perspectives in innate and acquired cardioprotection: cardioprotection acquired through exercise. J Appl Physiol. 2007;103:1894–1899. doi: 10.1152/japplphysiol.00464.2007. doi:10.1152/japplphysiol.00464.2007. [DOI] [PubMed] [Google Scholar]
- 49.Brown DA, Lynch JM, Armstrong CJ, Caruso NM, Ehlers LB, Johnson MS, et al. Susceptibility of the heart to ischaemia-reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol. 2005;564:619–630. doi: 10.1113/jphysiol.2004.081323. doi:10.1113/jphysiol.2004.081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quindry JC, Schreiber L, Hosick P, Wrieden J, Irwin JM, Hoyt E. Mitochondrial KATP channel inhibition blunts arrhythmia protection in ischemic exercised hearts. Am J Physiol Heart Circ Physiol. 2010;299:H175–H183. doi: 10.1152/ajpheart.01211.2009. doi:10.1152/ajpheart.01211.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. doi:10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 52.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 53.Woodman CR, Muller JM, Laughlin MH, Price EM. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am J Physiol. 1997;273:H2575–H2579. doi: 10.1152/ajpheart.1997.273.6.H2575. [DOI] [PubMed] [Google Scholar]
- 54.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. doi:10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kingwell BA. Nitric oxide as a metabolic regulator during exercise: effects of training in health and disease. Clin Exp Pharmacol Physiol. 2000;27:239–250. doi: 10.1046/j.1440-1681.2000.03232.x. doi:10.1046/j.1440-1681.2000.03232.x. [DOI] [PubMed] [Google Scholar]
- 56.Ma XL, Weyrich AS, Lefer DJ, Lefer AM. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res. 1993;72:403–412. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- 57.Lefer AM. Attenuation of myocardial ischemia-reperfusion injury with nitric oxide replacement therapy. Ann Thorac Surg. 1995;60:847–851. doi: 10.1016/0003-4975(95)00423-I. doi:10.1016/0003-4975(95)00423-I. [DOI] [PubMed] [Google Scholar]
- 58.Loke KE, Laycock SK, Mital S, Wolin MS, Bernstein R, Oz M, et al. Nitric oxide modulates mitochondrial respiration in failing human heart. Circulation. 1999;100:1291–1297. doi: 10.1161/01.cir.100.12.1291. [DOI] [PubMed] [Google Scholar]
- 59.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci USA. 2001;98:355–360. doi: 10.1073/pnas.011379598. doi:10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J, Bombeck CA, Yang S, Kim YM, Billiar TR. Nitric oxide suppresses apoptosis via interrupting caspase activation and mitochondrial dysfunction in cultured hepatocytes. J Biol Chem. 1999;274:17325–17333. doi: 10.1074/jbc.274.24.17325. doi:10.1074/jbc.274.24.17325. [DOI] [PubMed] [Google Scholar]
- 61.Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. doi:10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 62.Momken I, Lechene P, Ventura-Clapier R, Veksler V. Voluntary physical activity alterations in endothelial nitric oxide synthase knockout mice. Am J Physiol Heart Circ Physiol. 2004;287:H914–H920. doi: 10.1152/ajpheart.00651.2003. doi:10.1152/ajpheart.00651.2003. [DOI] [PubMed] [Google Scholar]
- 63.Ojaimi C, Li W, Kinugawa S, Post H, Csiszar A, Pacher P, et al. Transcriptional basis for exercise limitation in male eNOS-knockout mice with age: heart failure and the fetal phenotype. Am J Physiol Heart Circ Physiol. 2005;289:H1399–H1407. doi: 10.1152/ajpheart.00170.2005. doi:10.1152/ajpheart.00170.2005. [DOI] [PubMed] [Google Scholar]
- 64.Endres M, Gertz K, Lindauer U, Katchanov J, Schultze J, Schrock H, et al. Mechanisms of stroke protection by physical activity. Ann Neurol. 2003;54:582–590. doi: 10.1002/ana.10722. doi:10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 65.de Waard MC, van Haperen R, Soullie T, Tempel D, de Crom R, Duncker DJ. Beneficial effects of exercise training after myocardial infarction require full eNOS expression. J Mol Cell Cardiol. 2010;48:1041–1049. doi: 10.1016/j.yjmcc.2010.02.005. doi:10.1016/j.yjmcc.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, et al. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. doi:10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 67.Lefer DJ. Nitrite therapy for protection against ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2006;290:F777–F778. doi: 10.1152/ajprenal.00470.2005. doi:10.1152/ajprenal.00470.2005. [DOI] [PubMed] [Google Scholar]
- 68.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. doi:10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 69.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci USA. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. doi:10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. doi:10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, et al. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–297. doi: 10.1038/nchembio734. doi:10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 73.Hogg N, Broniowska KA, Novalija J, Kettenhofen NJ, Novalija E. Role of S-Nitrosothiol transport in the cardioprotective effects of S-Nitrosocysteine in rat hearts. Free Radic Biol Med. 2007;43:1086–1094. doi: 10.1016/j.freeradbiomed.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 74.Hogg N. Biological chemistry and clinical potential of S-nitrosothiols. Free Radic Biol Med. 2000;28:1478–1486. doi: 10.1016/s0891-5849(00)00248-3. doi:10.1016/S0891-5849(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, et al. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. doi:10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 76.Napoli C, Williams-Ignarro S, de Nigris F, Lerman LO, D'Armiento FP, Crimi E, et al. Physical training and metabolic supplementation reduce spontaneous atherosclerotic plaque rupture and prolong survival in hypercholesterolemic mice. Proc Natl Acad Sci USA. 2006;103:10479–10484. doi: 10.1073/pnas.0602774103. doi:10.1073/pnas.0602774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. doi:10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc Natl Acad Sci USA. 2008;105:11430–11435. doi: 10.1073/pnas.0800700105. doi:10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giraldez RR, Panda A, Xia Y, Sanders SP, Zweier JL. Decreased nitric-oxide synthase activity causes impaired endothelium-dependent relaxation in the postischemic heart. J Biol Chem. 1997;272:21420–21426. doi: 10.1074/jbc.272.34.21420. doi:10.1074/jbc.272.34.21420. [DOI] [PubMed] [Google Scholar]
- 80.Khan M, Mohan IK, Kutala VK, Kumbala D, Kuppusamy P. Cardioprotection by sulfaphenazole, a cytochrome p450 inhibitor: mitigation of ischemia-reperfusion injury by scavenging of reactive oxygen species. J Pharmacol Exp Ther. 2007;323:813–821. doi: 10.1124/jpet.107.129486. doi:10.1124/jpet.107.129486. [DOI] [PubMed] [Google Scholar]
- 81.Calvert JW, Elston M, Sindler A, Gundewar S, Grinsfelder DB, Aragon JP, et al. The generation and storage of nitric oxide metabolites during exercise training contributes to exercise-mediated cardioprotection. J Am Coll Cardiol. 2010;55 A116.E1081. [Google Scholar]
- 82.Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–417. doi: 10.1038/nchembio.92. doi:10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 83.Xu Q, Hu Y, Kleindienst R, Wick G. Nitric oxide induces heat-shock protein 70 expression in vascular smooth muscle cells via activation of heat shock factor 1. J Clin Invest. 1997;100:1089–1097. doi: 10.1172/JCI119619. doi:10.1172/JCI119619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baker JE, Contney SJ, Singh R, Kalyanaraman B, Gross GJ, Bosnjak ZJ. Nitric oxide activates the sarcolemmal K(ATP) channel in normoxic and chronically hypoxic hearts by a cyclic GMP-dependent mechanism. J Mol Cell Cardiol. 2001;33:331–341. doi: 10.1006/jmcc.2000.1305. doi:10.1006/jmcc.2000.1305. [DOI] [PubMed] [Google Scholar]
- 85.Cuong DV, Kim N, Youm JB, Joo H, Warda M, Lee JW, et al. Nitric oxide-cGMP-protein kinase G signaling pathway induces anoxic preconditioning through activation of ATP-sensitive K+ channels in rat hearts. Am J Physiol Heart Circ Physiol. 2006;290:H1808–H1817. doi: 10.1152/ajpheart.00772.2005. doi:10.1152/ajpheart.00772.2005. [DOI] [PubMed] [Google Scholar]
- 86.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. doi:10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 87.Bauersachs J, Schafer A. Tetrahydrobiopterin and eNOS dimer/monomer ratio—a clue to eNOS uncoupling in diabetes? Cardiovasc Res. 2005;65:768–769. doi: 10.1016/j.cardiores.2004.12.011. doi:10.1016/j.cardiores.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 88.Fukai T. Endothelial GTPCH in eNOS uncoupling and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1493–1495. doi: 10.1161/ATVBAHA.107.148239. doi:10.1161/ATVBAHA.107.148239. [DOI] [PubMed] [Google Scholar]
- 89.Garlid KD. Opening mitochondrial K(ATP) in the heart—what happens, and what does not happen. Basic Res Cardiol. 2000;95:275–279. doi: 10.1007/s003950070046. doi:10.1007/s003950070046. [DOI] [PubMed] [Google Scholar]
- 90.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. doi:10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 91.Ignarro LJ, Balestrieri ML, Napoli C. Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc Res. 2007;73:326–340. doi: 10.1016/j.cardiores.2006.06.030. doi:10.1016/j.cardiores.2006.06.030. [DOI] [PubMed] [Google Scholar]