Abstract
Aims
Glycosylation with β-N-acetylglucosamine (O-GlcNAcylation) is one of the most complex post-translational modifications. The cycling of O-GlcNAc is controlled by two enzymes: UDP-NAc transferase (OGT) and O-GlcNAcase (OGA). We recently reported that endothelin-1 (ET-1) augments vascular levels of O-GlcNAcylated proteins. Here we tested the hypothesis that O-GlcNAcylation contributes to the vascular effects of ET-1 via activation of the RhoA/Rho-kinase pathway.
Methods and results
Incubation of vascular smooth muscle cells (VSMCs) with ET-1 (0.1 μM) produces a time-dependent increase in O-GlcNAc levels. ET-1-induced O-GlcNAcylation is not observed when VSMCs are previously transfected with OGT siRNA, treated with ST045849 (OGT inhibitor) or atrasentan (ETA antagonist). ET-1 as well as PugNAc (OGA inhibitor) augmented contractions to phenylephrine in endothelium-denuded rat aortas, an effect that was abolished by the Rho kinase inhibitor Y-27632. Incubation of VSMCs with ET-1 increased expression of the phosphorylated forms of myosin phosphatase target subunit 1 (MYPT-1), protein kinase C-potentiated protein phosphatase 1 inhibitor protein (protein kinase C-potentiated phosphatase inhibitor-17), and myosin light chain (MLC) and RhoA expression and activity, and this effect was abolished by both OGT siRNA transfection or OGT inhibition and atrasentan. ET-1 also augmented expression of PDZ-Rho GEF (guanine nucleotide exchange factor) and p115-Rho GEF in VSMCs and this was prevented by OGT siRNA, ST045849, and atrasentan.
Conclusion
We suggest that ET-1 augments O-GlcNAcylation and this modification contributes to increased vascular contractile responses via activation of the RhoA/Rho-kinase pathway.
Keywords: β-N-acetylglucosamine, Endothelin-1, Vascular reactivity, RhoA/Rho-kinase pathway
1. Introduction
The attachment of β-O-linked 2-acetamido-2-deoxy-d-glycopyranose (O-GlcNAc) in nuclear and cytoplasmic proteins, known as O-GlcNAcylation, is one of the most common post-translational modifications.1–6 The cycling of O-GlcNAc is controlled by two highly conserved enzymes, O-GlcNAc transferase (OGT or uridine diphospho-N-acetyl glucosamine: polypeptide β-N-acetylglucosaminyl transferase; UDP-NAc transferase) and β-N-acetylglucosaminidase [O-GlcNAcase (OGA)]. Whereas OGT catalyses the addition of O-GlcNAc to the hydroxyl group of serine or threonine residues of a target protein, OGA catalyses the hydrolytic cleavage of O-GlcNAc from post-translationally modified proteins.3,6,7
Over the past decade, a surge of discoveries in O-GlcNAcylation has revealed new roles for this modification in the cardiovascular system. Many proteins important in vascular function are targets for O-GlcNAcylation,3,4,8–10 and recent studies have shown that this post-translational modification interferes with vascular processes.10–13 However, one of the central challenges, specific to the vasculature, is to understand the unique molecular and cellular O-GlcNAcylation signalling as it relates to function.
Endothelin-1 (ET-1), a peptide that plays a crucial role in the vascular function of most organ systems, activates several signalling pathways that include protein kinase C (PKC), mitogen-activated protein kinases (MAPKs), and RhoA/Rho kinase, which is important in Ca+2 sensitization of smooth muscle cells.14–16 ET-1 also activates transcriptional factors responsible for the coordinated increase in many cytokines and enzymes, thus enhancing inflammation, oxidative stress, fibrosis, and tissue damage,17–20 all important in the regulation of vascular tone, vascular injury, and remodelling.
Recent evidence from our laboratory suggests that ET-1 augments O-GlcNAcylation of vascular proteins and that this post-translational modification contributes to ET-1-induced vascular effects. Accordingly, incubation of arterial segments with ET-1 produced a time-dependent increase in O-GlcNAcylation and augmented reactivity to constrictor stimuli. ET-1 effects on O-GlcNAcylated-protein levels and vascular reactivity were prevented by neutralizing antibodies against OGT, the enzyme that catalyses the addition of O-GlcNAc to a target protein, as well as by the OGT inhibitor ST045849 [3-(2-adamantanylethyl)-2-[(4-chlorophenyl) azamethylene]-4-oxo-1,3-thiazaperhyd roine-6-carboxylic acid; 100 µM].11,12 The effects of ET-1 on O-GlcNAcylation levels and vascular reactivity were mediated by activation of ETA receptors,11 the predominant subtype in vascular smooth muscle cells (VSMCs).18,21
However, our current understanding of the unique molecular O-GlcNAcylation signalling in VSMCs and its relation to vascular function is still incomplete. One of the many central questions to be answered is how changes in O-GlcNAcylation modify vascular reactivity to contractile agents. A recent study has shown that increased flux through the hexosamine biosynthesis pathway, which induces O-GlcNAcylation, increases Rho activity in VSMCs, although a specific role for O-GlcNAcylation was not assessed in that paper.22 In addition, some proteins from the RhoA/Rho-kinase pathway, such as myosin light chains (MLC-1 and MLC-2), Rho GDP-dissociation inhibitor, and PDZ-GEF (guanine nucleotide exchange factor), are targets of O-GlcNAcylation.23–26
In the present study, we hypothesized that ET-1 increases O-GlcNAcylation levels in VSMCs, which leads to increased RhoA/Rho kinase activation, increasing vascular reactivity to constrictor stimuli. To test our hypothesis, we determined whether proteins of the RhoA/Rho-kinase pathway in VSMCs are regulated by O-GlcNAcylation and whether these changes contribute to increased vascular responses to constrictor stimuli.
2. Methods
2.1. Animals
Male Wistar rats (8–10 weeks old, 230–250 g; Harlan Laboratories, Indianapolis, IN, USA) were used in this study. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Medical College of Georgia Committee on the Use of Animals in Research and Education and in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The animals were housed on a 12 h light/dark cycle and fed a standard chow diet with water ad libitum.
2.2. ET-1 incubation procedures
After euthanasia, thoracic aortas were removed and cleaned from fat tissue in an ice-cold physiological salt solution. Endothelium was removed and arterial segments were incubated in Eagle's Minimum Essential Medium containing l-glutamine (1%), fetal bovine serum (FBS, 10%), penicillin (0.5%), and streptomycin (0.5%). Rings were incubated for 24 h with either vehicle [H2O or dimethyl sulfoxide (DMSO)]; PugNAc [O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate; 100 µM, from Toronto Research Chemicals, Inc, North York, Canada], which blocks OGA activity by mimicking the enzyme-stabilized transition state; or ET-1 (0.1 µM, from Tocris Bioscience, Ellisville, MO, USA). After incubation, aortas were used to evaluate reactivity to phenylephrine (PE, from Sigma-Aldrich; concentration–response curves, 1 nM to 100 µM from Sigma-Aldrich), Vascular reactivity to PE was determined in the presence and absence of 1 µM Y-27632 (from Tocris Bioscience, Ellisville, MO, USA), a specific Rho kinase inhibitor.27
2.3. VSMCs isolation and culture
VSMCs were isolated from rat thoracic aortas by explant, as previously described.28 Cultures were maintained in Dulbecco Modified Eagle's Medium (GIBCO-BRL, Gaithersburg, MD, USA) supplemented with 10% FBS (Invitrogen, Grand Island, NY, USA). Only fourth-passage cells were used. Immunoreactivity assays were used to characterize the VSMCs and to confirm the absence of other cell types in the cultures. The cells expressed α-smooth muscle actin and calponin, contractile proteins that are indicative of VSMCs. No positive immunoreactivity, to von Willebrand factor VIII or CD31 (platelet endothelial cell adhesion molecule-1), markers of endothelial cells, was detected (data not shown).
After maximum confluence and 24 h removal of serum, cells were incubated with vehicle (H2O or DMSO), or ET-1 (0.1 nM to 1 μM) for 5 min, 1, 3, 6, 12 or 24 h, to verify the optimal time-point and concentration for ET-1-induced O-GlcNAcylation of VSMCs. In further experiments, incubations were performed for 24 h with: vehicle (H2O), or ET-1 (0.1 µM). In some experiments, VSMCs were previously incubated with the OGT inhibitor ST045849 [3-(2-adamantanylethyl)-2-[(4-chlorophenyl) azamethylene]-4-oxo-1,3-thiazaperhyd roine-6-carboxylic acid; 100 µM, for 3 h from TimTec, Newark, DE, USA) or the ETA antagonist atrasentan (1 μM, for 3 h; kindly provided by Dr Adviye Ergul) or transfected with OGT siRNA (100 nM, as described below).
2.4. Transfection of siRNA
The siRNA for OGT and the siRNA negative control were purchased from Dharmacon, Lafayette, CO, USA. The siRNA (On-Target plus Smart pool; 100 nM) was incubated with Lipofectamine 2000 (Invitrogen) in 1.0 mL of serum-free medium for 30 min. The siRNA–Lipofectamine 2000 complex was then added to the cells in 4.0 mL of serum-free medium and maintained for 2 days. In the third day cells were incubated with ET-1 (0.1 µM) for 24 h.
2.5. Western blot analysis
Proteins (30 µg) extracted from VSMCs were separated by electrophoresis, and western blots performed as previously described.10 Antibodies used were: anti-O-GlcNAc antibody, CTD 110.6 (1:2000; Pierce Biotechnology, Rockford, IL, USA), OGT [1:400, Santa Cruz antibodies], total myosin phosphatase target subunit 1 (MYPT-1), rho-kinase (ROCK)-α, ROCK-β, protein kinase C-potentiated phosphatase inhibitor (CPI)-17, MLC, RhoA [(1:1000) BD Biosciences], PDZ-Rho GEF, p115-Rho GEF [(1:500) Abcam, Inc.,] and Rho-GDI (guanine dissociation inhibitor) [(1:1000) Cell Signaling]. Immunoblots for non-phosphoproteins were carried out in the same membranes used to evaluate the phosphorylated (-P) forms: P-MYPT-1 (Thr853), P-CPI-17 (Thr38), and P-MLC (Thr18/Ser19) [(1:500); BD Biosciences]. After incubation with secondary antibodies, signals were developed for chemiluminescence, visualized by autoradiography, and quantified densitometrically. Results were normalized to β-actin [(1:10000), Sigma-Aldrich, Inc.,] protein and expressed as arbitrary units.
2.6. Assessment of RhoA activity
RhoA activity was also determined by using the absorbance-based G-LISA RhoA activation assay kit (Cytoskeleton; Cat. # BK124). The method detects RhoA-GTP bound in cell lysates.
2.7. Data analysis
The results are shown as mean ± SEM and n represents the number of animals used in the experiments. Contractions were recorded as changes in the displacement (mN) from baseline. Concentration–response curves were fitted using a non-linear interactive fitting program (Graph Pad Prism 4.0; GraphPad Software, Inc., San Diego, CA, USA) and two pharmacological parameters were obtained: the maximal effect generated by the agonist (or Emax) and the negative logarithm of the concentration of agonist that produces 50% of the maximum response [−log EC50 (or pD2)]. Statistical analyses of Emax and pD2 values were performed using one-way ANOVA or Student's t-test. Post hoc comparisons were performed using Newman–Keuls's test. Western blot data were analysed by one-sample t-test and the P-value was computed from the t ratio and the numbers of degrees of freedom. Values of P < 0.05 were considered statistically significant.
3. Results
Different time points and concentrations of ET-1 were used in order to determine whether ET-1 elicits changes in O-GlcNAcylation levels in rat aortic VSMCs. ET-1 produced a time-dependent increase in O-GlcNAcylation in VSMCs (see Supplementary material online, Figure S1A). The greatest time-effect of ET-1 incubation on the O-GlcNAcylation levels was observed at 24 h. Incubation of VSMCs with ET-1, from 0.1 nM to 1 μM (for 24 h) augmented O-GlcNAcylation levels in comparison with cells that were incubated with vehicle (for 24 h). No significant differences in O-GlcNAcylation levels were observed with the different concentrations of ET-1 (see Supplementary material online, Figure S1B). Therefore, the functional and molecular experiments were performed in VSMCs incubated with 0.1 µM of ET-1, for 24 h. This concentration of ET-1 increases vascular O-GlcNAcylation levels in rat aortas11 and is largely used in the literature for stimulation of cultured cells, allowing comparisons between these two experimental conditions.29,30
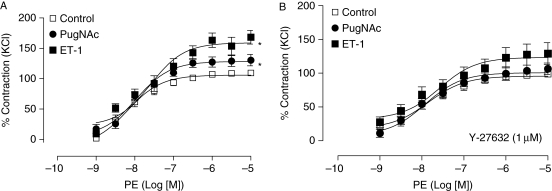
Endothelium-denuded aortas incubated with ET-1 displayed increased vascular reactivity to PE, an α1-adrenergic agonist, and developed similar contractions to those exhibited by aortas incubated with PugNAc, an inhibitor of OGA, the enzyme that catalyses the hydrolytic cleavage of O-GlcNAc from post-translationally-modified proteins (Figure 1A). Augmented aortic contractions to PE after treatment with PugNAc or ET-1 were abolished by the Rho kinase inhibitor Y-27632 (1 μM, 40 min incubation, Figure 1B).
Figure 1.
Augmented vascular contraction to PE, after incubation with ET-1 or PugNAc, is abolished by the Rho kinase inhibitor Y-27632. (A) Incubation with ET-1 (0.1 µM) or PugNAc (100 µM) for 24 h increases contractions to PE in endothelium-denuded rat aortas vs. control (n = 8 for each group). (B) ET-1 effects on PE responses were abolished by the Rho kinase inhibitor Y-27632 (1 μM, 40 min). Experimental values of contraction were calculated relative to the contractile response produced by KCl 120 mM, which was taken as 100% (n = 8). Results are presented as mean ± SEM in each experimental group. *P < 0.05 vs. control.
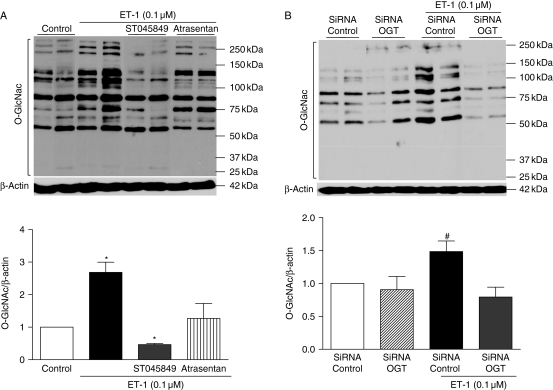
ET-1 increased O-GlcNAcylation levels in proteins from VSMCs. This effect was not observed when cells were incubated with ST045849, a selective OGT inhibitor, or with atrasentan, an ETA receptor antagonist (Figure 2A). A similar effect was observed when OGT expression was knocked down by transfection with the OGT siRNA. At this condition, ET-1 effects on O-GlcNAcylated-protein levels were completely abolished (Figure 2B). The siRNA targeting O-GlcNAc transferase (siOGT) decreased levels of OGT mRNA (data not shown) and protein (see Supplementary material online, Figure 2) by ∼60% when compared with the negative siRNA control.
Figure 2.
ET-1 effects on O-GlcNAcylated-proteins are not observed when cells are previously transfected with OGT siRNA, incubated with an OGT inhibitor or with an ETA antagonist. Treatment with (A) the OGT inhibitor (ST045849) or the ETA antagonist (atrasentan) as well as (B) transfection with the OGT siRNA decrease vascular O-GlcNAcylation, upon ET-1 incubation for 24 h in comparison with VSMCs that were incubated with vehicle (DMSO) or control siRNA for 24 h. On the top, western blot image of O-GlcNAcylated-proteins; on the bottom, corresponding bar graphs showing the relative O-GlcNAcylated-proteins after normalization to β-actin expression (n = 6). Results are presented as mean ± SEM in each experimental group (n = 6). *P < 0.05 vs. vehicle (DMSO); #P < 0.05 vs. control siRNA.
Considering that O-GlcNAcylation is a highly dynamic post-translational modification that plays a key role in signal transduction pathways and that ET-1 stimulates the RhoA/Rho-kinase pathway, which is important in Ca+2 sensitization of smooth muscle cells,11,15,31 we investigated whether ET-1-induced augmented vascular O-GlcNAc-modified proteins modulate RhoA/Rho kinase activation.
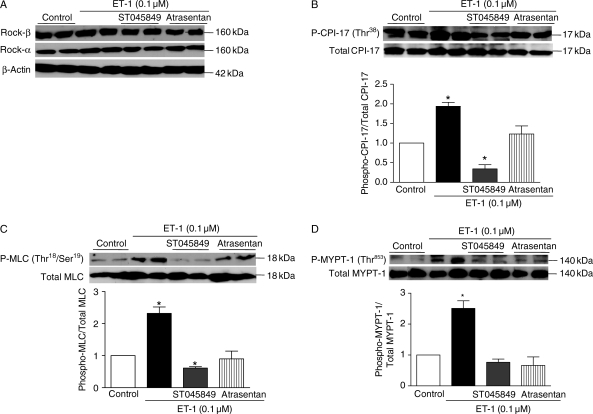
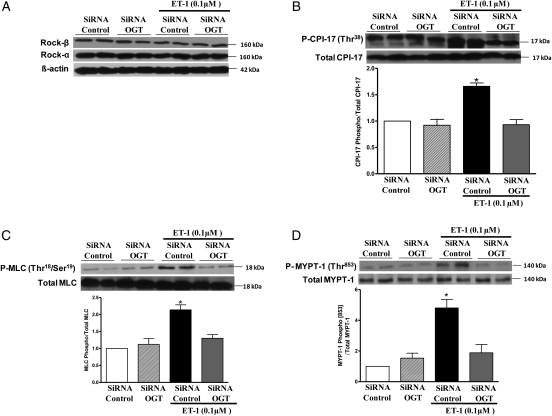
Our data show that incubation of VSMCs with ET-1 (0.1 μM) did not change expression of ROCK-α, ROCK-β, CPI-17, MYPT-1, or MLC, but increased expression of the phosphorylated forms of MYPT-1 (Thr853) and CPI-17 (Thr38), which are involved in RhoA/ROCK-mediated inhibition of myosin phosphatase. ET-1 incubation also augmented levels of P-MLC (Thr18/Ser19), which is correlated with smooth muscle contraction. The effects of ET-1 on CPI-17 (Figure 3B), MLC (Figure 3C), and MYPT-1 (Figure 3D) expression and activation were not observed when VSMCs were treated with the OGT inhibitor (ST045849) or previously transfected with the OGT siRNA (Figure 4). In addition, treatment with the ETA antagonist (atrasentan) prevented ET-1-induced increased expression of the phosphorylated forms of CPI-17 (Figure 3B), MLC (Figure 3C), and MYPT-1 (Figure 3D).
Figure 3.
ET-1-induced phosphorylation of MYPT-1 (Thr853), CPI-17 (Thr38), and MLC (Thr18/Ser19) is not observed when VSMCs are treated with the OGT inhibitor ST045849 or the ETA antagonist atrasentan. (A) Incubation of VSMCs with ET-1 (0.1 μM), ST045849 or atrasentan did not change expression of ROCK-α, ROCK-β, MYPT-1, MLC, or CPI-17. ET-1 increased expression of the phosphorylated forms of (B) CPI-17 (Thr38), (C) MLC (Thr18/Ser19), and (D) MYPT-1 (Thr853), an effect that was not observed when VSMCs were treated with the OGT inhibitor [ST045849]. In addition, treatment with the ETA antagonist (atrasentan) prevented ET-1-induced increased expression of the phosphorylated forms of (A) CPI-17, (B) MLC and (C) MYPT-1. Bar graphs show the relative expression of P-MYPT-1 (Thr853), P-CPI-17 (Thr38), or P-MLC (Thr18/Ser19) after normalization to corresponding total protein expression (n = 6). Results are presented as mean ± SEM in each experimental group. *P < 0.05 vs. control.
Figure 4.
Transfection with OGT siRNA prevents ET-1-induced increased expression of P-MYPT-1 (Thr853), P-CPI-17 (Thr38), and P-MLC (Thr18/Ser19). The transfection with OGT siRNA, for 2 days, did not change expression of (A) ROCK-α, ROCK-β, total MYPT-1, MLC, or CPI-17. The effects of ET-1 on (B) P-CPI-17, (C) P-MLC, and (D) P-MYPT-1 expression were not observed when VSMCs were previously transfected with OGT siRNA. Bar graphs show the relative expression of phosphorylated forms of P-MYPT-1 (Thr853), P-CPI-17 (Thr38), and P-MLC (Thr18/Ser19) after normalization to corresponding total protein expression (n = 5). Results are presented as mean ± SEM in each experimental group. *P < 0.05 vs. siRNA control.
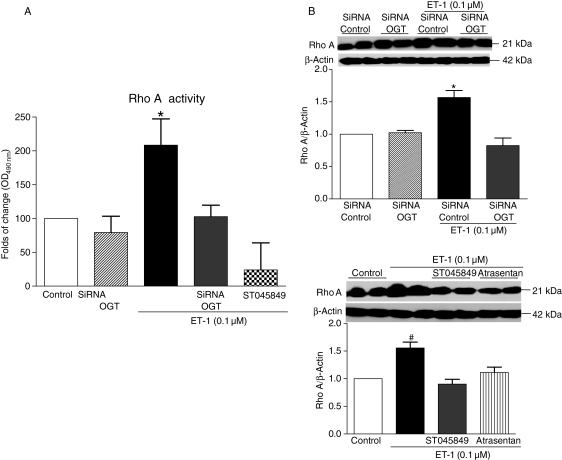
All together, these results suggest that ET-1-induced O-GlcNAcylation of VSMCs proteins leads to activation of the RhoA/Rho-kinase pathway. This post-translational modification and the associated activation of the RhoA/Rho-kinase pathway may be functionally translated into augmented vascular reactivity to constrictor stimuli. This hypothesis is further supported by additional experiments showing that ET-1 increases RhoA activation (Figure 5A) and expression (Figure 5B) in VSMCs, and these effects were abolished in VSMCs previously transfected with OGT siRNA or treated with the OGT inhibitor.
Figure 5.
ET-1 increases RhoA activity and expression in VSMCs, and this effect was abolished by previous transfection with OGT siRNA or treatment with the OGT inhibitor and atrasentan. (A) Bar graphs show fold of change in RhoA activity with absorbance at 490 nm. (B) Bar graphs show the relative expression of RhoA after normalization to β-actin expression (n = 6). Results are presented as mean ± SEM in each experimental group. *P < 0.05 vs. siRNA control; #P < 0.05 vs. control.
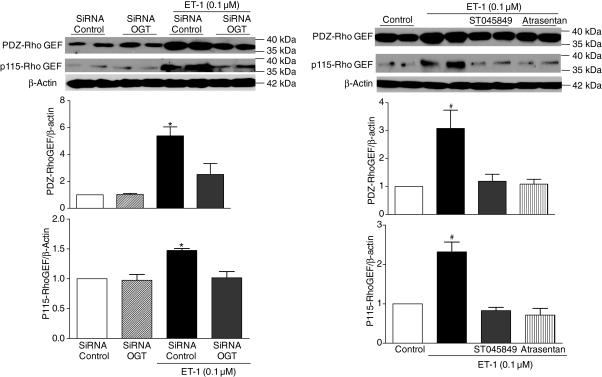
In order to clarify how augmented ET-1-induced O-GlcNAcylation contributes to augmented activation of the RhoA/Rho-kinase pathway, we determined the expression of PDZ-Rho GEF and p115-Rho GEF, which leads to RhoA activation in VSMCs. We also determined Rho-GDI protein expression. ET-1 treatment did not change GDI expression (data not shown), but increased PDZ-Rho GEF and p115-Rho GEF protein expression. This effect was prevented when VSMCs were previously transfected with OGT siRNA (Figure 6A), or incubated with the OGT inhibitor or ETA antagonist (Figure 6B).
Figure 6.
ET-1-induced augmented expression of PDZ-Rho GEF and p115-Rho GEF is not observed when VSMCs are previously transfected with OGT siRNA, or incubated with OGT inhibitor and ETA antagonist. On the top, representative western blot images of PDZ-Rho GEF, p115-Rho GEF, and β-actin; on the bottom, bar graphs show the relative PDZ-Rho GEF and p115-Rho GEF proteins after normalization to β-actin expression. Results are presented as mean ± SEM in each experimental group (n = 6). *P < 0.05 vs. siRNA control; #P < 0.05 vs. control.
4. Discussion
Important insights into the pathological processes leading to vascular dysfunction can be learned from elucidating the mechanisms by which O-GlcNAcylation modifies vascular function. Recent results from our laboratory indicate that elevated vascular levels of O-GlcNAcylated-proteins represent a common mechanism underlying the adverse effects of ET-1 on VSMCs.11
Several studies have reported that increased O-GlcNAcylation decreases production of NO as a consequence of decreased activity of endothelial NO synthase, reinforcing the concept that O-GlcNAcylation modulates vascular reactivity partially via impairment of endothelial cell function.12,13,32 However, our data provide evidence, for the first time, that ET-1 produces a time-dependent increase in O-GlcNAcylated-modified proteins in VSMCs, leading to the activation of the RhoA/Rho-kinase pathway, which is involved in the maintenance of the vascular contractile status. Additionally, we showed that ET-1 as well as PugNAc incubation increases vascular reactivity to PE in the absence of endothelium, suggesting that this post-translational modification increases vascular reactivity by directly altering VSMCs function.
We performed experiments with VSMCs transfected with an OGT siRNA to knock down OGT expression. The effects of ET-1 on O-GlcNAcylation levels were not observed when VSMCs were previously transfected with OGT siRNA. This result is further supported by experiments where ET-1 effects were prevented in cells incubated with a selective OGT inhibitor (ST045849). Together, these results strongly suggest that ET-1 effects on O-GlcNAcylation levels are mediated by OGT activation.
ETA receptors are highly expressed in VSMCs and exhibit high affinity for ET-1.21,33 Accordingly, the ETA antagonist atrasentan abrogated ET-1-induced O-GlcNAcylation in VSMCs, indicating that ETA receptor activation is required for ET-1-induced vascular O-GlcNAcylation.
Several hundred proteins from diverse functional groups can be directly modified by O-GlcNAcylation.3,4 Proteins with an important role in the vascular function, such as endothelial nitric oxide synthase, sarcoplasmic reticulum Ca2+-ATPase, phospholipase C, PKC, are targets for this post-translational modification. Considering that augmented aortic contractions to PE, after treatment with PugNAc or ET-1, were abolished by the Rho kinase inhibitor Y-27632 and that ET-1 activates the RhoA/Rho kinase signalling,34 we decided to investigate whether increased levels of O-GlcNAcylation modify the RhoA/Rho-kinase pathway.
The process of smooth muscle contraction is principally regulated by the phosphorylation of MLC, activated by Ca2+ calmodulin, enabling the molecular interaction of myosin with actin.27,35 The state of MLC phosphorylation is further regulated by myosin phosphatase, which removes the phosphate from MLC to promote smooth muscle relaxation. Myosin phosphatase isolated from smooth muscle is a holoenzyme consisting of three subunits: a small subunit, a catalytic subunit, and a MYPT-1. Most recently, some studies have shown that MLC is also a target for O-GlcNAcylation.26,36
Our data show that ET-1 increases expression of the phosphorylated forms of MYPT-1 (Thr853), CPI-17 (Thr38), and MLC (Thr18/Ser19). The effects of ET-1 on the activation of those proteins are not observed when cells are previously transfected with OGT siRNA, treated with an ETA antagonist (atrasentan) or an OGT inhibitor (ST045849). It is known that stimulation of ETA receptor by ET-1 induces RhoA/Rho kinase activation.15,37 Here we show for the first time, that this activation can be mediated by increased O-GlcNAcylated-proteins. It has already been proposed that O-GlcNAcylation may counteract the effect of phosphorylation leading to increased Ca2+ sensitivity of muscle fibres and myofilament assembly. Thus, an imbalance between phosphorylation/O-GlcNAcylation may alter actin–myosin interactions and myosin assembly, and therefore, favour/promote contraction.26,38
Inhibition of MYPT-1, by phosphorylation at the Thr853 site, decreases the enzymatic activity of myosin phosphatase, allowing MLC to remain phosphorylated, and therefore, promoting contraction.35 In addition, the small protein CPI-17, once phosphorylated, is able to bind to the catalytic subunit of myosin phosphatase inhibiting the enzyme's activity, and prolonging smooth muscle contraction.
Cheung et al. have shown that changes in protein activity, such as MYPT-1, as well as changes in cell physiology are associated with transient and dramatic alterations in O-GlcNAcylation levels.9,39 Thus, one may speculate that increased vascular O-GlcNAcylation by ET-1 may result in increased RhoA/Rho kinase activation, increasing vascular reactivity to constrictor stimuli. In support to this hypothesis, our data show that RhoA expression and activation is increased by ET-1 in VSMCs, and this effect is abolished by previous transfection with OGT siRNA or treatment with the OGT inhibitor.
There are three Rho-guanine nucleotide exchange factors (Rho-GEFs) identified in smooth muscle cells: PDZ-Rho GEF, leukaemia-associated RhoGEF, and p115-RhoGEF. Rho GEFs promote RhoA activation by enabling the exchange of nucleotides between RhoA-GDP and RhoA-GTP. Increased expression or activation of Rho GEF proteins augments contractile activation of smooth muscle, via RhoA-dependent mechanisms. We observed that ET-1 treatment increases PDZ-Rho GEF and p115-Rho GEF protein expression and this effect was prevented when VSMCs were previously transfected with OGT siRNA, incubated with the OGT inhibitor or ETA antagonist. Therefore, it seems that OGT inhibition prevents activation of RhoA by ET-1, in accordance with our hypothesis.
The mechanisms by which O-GlcNAcylation lead to activation of components of the RhoA/Rho-kinase pathway are not known, but one possibility includes changes in phosphorylation levels of these components. Accordingly, both modifications occur on serine and threonine residues, are dynamically added and removed from proteins in response to cellular signals, and alter the function and association of the modified proteins.32,40 It has been shown that increased O-GlcNacylation results in lower phosphorylation at 280 sites and causes increased phosphorylation at 148 sites, which implies that the interaction between these two translational modifications is large.41 We may speculate that O-GlcNAc modification on sites close or adjacent to where phosphorylation occurs may increase activation of contractile proteins and components of the ET-1/ETA receptor pathway, or decrease the activity of proteins involved in the relaxation process or in the desensitization of ET-1 receptors.
Hence, we cannot exclude the possibility that increased activation of ET-1 receptors modulates the transcriptional machinery to induce or to suppress expression of proteins that regulates O-GlcNAc levels. We have recently shown that ET-1 infusion for 14 days reduces OGA expression and that incubation of arterial segments with ET-1 for 24 h modifies OGT and OGA expression at different time points.11
In summary, our data strongly suggest that ET-1 augments vascular O-GlcNAcylation and this modification increases vascular reactivity via activation of the RhoA/Rho-kinase pathway. We speculate that the modulatory effect of ET-1 on vascular O-GlcNAcylation may represent a novel mechanism underlying the effects of the peptide on vascular function. Our results add to the understanding of the mechanisms by which O-GlcNAcylation alters vascular function and open the possibility that O-GlcNAcylation may represent a novel therapeutic target for the treatment of pathological conditions associated with augmented vascular ET-1 expression, such as salt-sensitive forms of hypertension.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This study was supported by grants from the National Institutes of Health (HL-74167) and Fundação de Amparo à Pesquisa do Estado de Sao Paulo (FAPESP), Brazil.
References
- 1.Hart GW, Haltiwanger RS, Holt GD, Kelly WG. Glycosylation in the nucleus and cytoplasm. Annu Rev Biochem. 1989;58:841–874. doi: 10.1146/annurev.bi.58.070189.004205. [DOI] [PubMed] [Google Scholar]
- 2.Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986;261:8049–8057. [PubMed] [Google Scholar]
- 3.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 4.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 6.Lima VVRC, Hardy DM, Webb RC, Tostes RC. O-GlcNAcylation: a novel post-translational mechanism to alter vascular cellular signaling in health and disease: focus on hypertension. J Am Soc Hypertens. 2009;3:374–387. doi: 10.1016/j.jash.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells L, Gao Y, Mahoney JA, Vosseller K, Chen C, Rosen A, et al. Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem. 2002;277:1755–1761. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- 8.Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE. 2005;312 doi: 10.1126/stke.3122005re13. re13. [DOI] [PubMed] [Google Scholar]
- 9.Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MHC, et al. Increased vascular O-GlcNAcylation augments reactivity to constrictor stimuli. J Am Soc Hypertens. 2008;2:410–417. doi: 10.1016/j.jash.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Saleh MA, Pollock DM, et al. O-GlcNAcylation contributes to augmented vascular reactivity induced by endothelin 1. Hypertension. 2010;55:180–188. doi: 10.1161/HYPERTENSIONAHA.109.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, et al. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension. 2009;53:166–174. doi: 10.1161/HYPERTENSIONAHA.108.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 14.Allahdadi KJ, Walker BR, Kanagy NL. ROK contribution to endothelin-mediated contraction in aorta and mesenteric arteries following intermittent hypoxia/hypercapnia in rats. Am J Physiol Heart Circ Physiol. 2007;293:H2911–2918. doi: 10.1152/ajpheart.00217.2007. [DOI] [PubMed] [Google Scholar]
- 15.Homma N, Nagaoka T, Morio Y, Ota H, Gebb SA, Karoor V, et al. Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol. 2007;50:697–702. doi: 10.1097/FJC.0b013e3181593774. [DOI] [PubMed] [Google Scholar]
- 16.Scherer EQ, Herzog M, Wangemann P. Endothelin-1-induced vasospasms of spiral modiolar artery are mediated by rho-kinase-induced Ca(2+) sensitization of contractile apparatus and reversed by calcitonin gene-related Peptide. Stroke. 2002;33:2965–2971. doi: 10.1161/01.str.0000043673.22993.fd. [DOI] [PubMed] [Google Scholar]
- 17.Hynynen MM, Khalil RA. The vascular endothelin system in hypertension–recent patents and discoveries. Recent Pat Cardiovasc Drug Discov. 2006;1:95–108. doi: 10.2174/157489006775244263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Schiffrin EL, Touyz RM. Vascular biology of endothelin. J Cardiovasc Pharmacol. 1998;32:S2–13. [PubMed] [Google Scholar]
- 20.Touyz RM, Schiffrin EL. Role of endothelin in human hypertension. Can J Physiol Pharmacol. 2003;81:533–541. doi: 10.1139/y03-009. [DOI] [PubMed] [Google Scholar]
- 21.Yazawa H, Iida-Kubota E, Honma Y, Honda K. Characterization of the endothelin receptor in primary cultures of human aortic smooth muscle cells. Jpn J Pharmacol. 1993;63:313–318. doi: 10.1254/jjp.63.313. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura H, Yokote K, Asaumi S, Kobayashi K, Fujimoto M, Maezawa Y, et al. High glucose-induced upregulation of osteopontin is mediated via Rho/Rho kinase pathway in cultured rat aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:276–281. doi: 10.1161/01.ATV.0000112012.33770.2a. [DOI] [PubMed] [Google Scholar]
- 23.Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Exploring the O-GlcNAc proteome: direct identification of O-GlcNAc-modified proteins from the brain. Proc Natl Acad Sci USA. 2004;101:13132–13137. doi: 10.1073/pnas.0403471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark PM, Dweck JF, Mason DE, Hart CR, Buck SB, Peters EC, et al. Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. J Am Chem Soc. 2008;130:11576–11577. doi: 10.1021/ja8030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart GW, Akimoto Y. The O-GlcNAc modification. In: Varki ACR, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor NY: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 26.Hedou J, Cieniewski-Bernard C, Leroy Y, Michalski JC, Mounier Y, Bastide B. O-linked N-acetylglucosaminylation is involved in the Ca2+ activation properties of rat skeletal muscle. J Biol Chem. 2007;282:10360–10369. doi: 10.1074/jbc.M606787200. [DOI] [PubMed] [Google Scholar]
- 27.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 28.Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971;50:172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yogi A, Callera GE, Montezano AC, Aranha AB, Tostes RC, Schiffrin EL, et al. Endothelin-1, but not Ang II, activates MAP kinases through c-Src independent Ras-Raf dependent pathways in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1960–1967. doi: 10.1161/ATVBAHA.107.146746. [DOI] [PubMed] [Google Scholar]
- 30.Sakai H, Chiba Y, Misawa M. Role of Rho kinase in endothelin-1-induced phosphorylation of CPI-17 in rat bronchial smooth muscle. Pulm Pharmacol Ther. 2007;20:734–739. doi: 10.1016/j.pupt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Cain AE, Tanner DM, Khalil RA. Endothelin-1-induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent Ca(2+)(i) sensitization pathways. Hypertension. 2002;39:543–549. doi: 10.1161/hy0202.103129. [DOI] [PubMed] [Google Scholar]
- 32.Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA. 2005;102:11870–11875. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouallegue A, Daou GB, Srivastava AK. Endothelin-1-induced signaling pathways in vascular smooth muscle cells. Curr Vasc Pharmacol. 2007;5:45–52. doi: 10.2174/157016107779317161. [DOI] [PubMed] [Google Scholar]
- 34.Ivey ME, Osman N, Little PJ. Endothelin-1 signalling in vascular smooth muscle: pathways controlling cellular functions associated with atherosclerosis. Atherosclerosis. 2008;199:237–247. doi: 10.1016/j.atherosclerosis.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Hilgers RH, Webb RC. Molecular aspects of arterial smooth muscle contraction: focus on Rho. Exp Biol Med (Maywood) 2005;230:829–835. doi: 10.1177/153537020523001107. [DOI] [PubMed] [Google Scholar]
- 36.Cieniewski-Bernard C, Bastide B, Lefebvre T, Lemoine J, Mounier Y, Michalski JC. Identification of O-linked N-acetylglucosamine proteins in rat skeletal muscle using two-dimensional gel electrophoresis and mass spectrometry. Mol Cell Proteomics. 2004;3:577–585. doi: 10.1074/mcp.M400024-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Chiba Y, Sakai H, Misawa M. Endothelin-1-induced translocation of RhoA is mediated by endothelin ET(A) receptors in rat bronchial smooth muscle. Eur J Pharmacol. 2005;517:182–185. doi: 10.1016/j.ejphar.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 38.Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, et al. Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system. Am J Physiol Heart Circ Physiol. 2009;296:H13–H28. doi: 10.1152/ajpheart.01056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung WD, Sakabe K, Housley MP, Dias WB, Hart GW. O-linked beta-N-acetylglucosaminyltransferase substrate specificity is regulated by myosin phosphatase targeting and other interacting proteins. J Biol Chem. 2008;283:33935–33941. doi: 10.1074/jbc.M806199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Comer FI, Hart GW. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry. 2001;40:7845–7852. doi: 10.1021/bi0027480. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Gucek M, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc Natl Acad Sci USA. 2008;105:13793–13798. doi: 10.1073/pnas.0806216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.