Abstract
In cancer screening, it is essential to undertake effective screening with appropriate methodology, which should be supported by evidence of a reduced mortality rate. At present, mammography is the only method for breast cancer screening with such evidence. However, mammography does not achieve sufficient accuracy in breasts with high density at ages below 50. Although ultrasonography achieves better accuracy in Breast Cancer detection even in dense breasts, the effectiveness has not been verified. We have planned a randomized controlled trial to assess the effectiveness of ultrasonography in women aged 40–49, with a design to study 50 000 women with mammography and ultrasonography (intervention group), and 50 000 controls with mammography only (control group). The participants are scheduled to take second round screening with the same modality 2 years on. The primary endpoints are sensitivity and specificity, and the secondary endpoint is the rate of advanced breast cancers.
Keywords: breast cancer screening, mammography, ultrasonography, randomized controlled trial
INTRODUCTION
Breast cancer is one of the most common cancers worldwide (1). The age-standardized incidence rate is the first among all female cancers, and it is continuously increasing in Japan (2,3), although Japan has a lower risk of breast cancer in comparison with Western countries. The incidence peaks at ages 45–49, and the mortality peaks at ages 55–59 in Japan (2). In breast cancer screening, it is essential to undertake effective screening with appropriate methodology. Effective screening should be supported by evidence of a reduced mortality rate. At present, mammography (MG) is the only method for breast cancer screening that has such evidence. However, MG does not achieve sufficient screening accuracy in breasts with high mammary gland density. Dense breasts are common at ages below 50 and are more common in Japanese populations than in Western populations (4). As the US Preventive Services Task Force (USPSTF) recommends against routine screen MG in women aged 40–49 years, the issue of breast imaging to screen women aged 40–49 still remains unclear (5).
Since ultrasonography (US) achieves better accuracy in breast cancer detection even in dense breasts (6) and supplemental screening US has the potential to depict early breast cancers not seen on MG (6–8), several single-institution observational studies in screening setting began. As mentioned in the WHO guidelines, ‘population-based cancer screening’ conducted as a public health program should be undertaken only when there is evidence of a reduced mortality rate (9). Before introducing any new technology in population-based breast cancer screening, it is essential to evaluate the effectiveness. However, randomized controlled trials (RCTs), cohort studies or case–control studies have not been completed to assess the efficacy of screening US to reduce breast cancer mortality, and the effectiveness has not been verified.
Therefore, we have planned an RCT to assess effectiveness of screening US for breast cancer, the Japan Strategic Anti-cancer Randomized Trial (J-START) in 2006. The defined study population is women aged 40–49 years, because this is the age range at which breast cancer peaks in Japan (2) and because a high percentage of Japanese women aged 40s have dense breast. This is a large-scale controlled trial, designed to study 50 000 women with MG and US (intervention group) and 50 000 controls with MG only (control group).
The primary endpoints of this trial are the inter-group comparisons of the sensitivity and specificity, and the secondary endpoint is the inter-group comparison of the accumulated incidence rate of advanced breast cancer during the follow-up period. The most important index in the evaluation of the effectiveness of cancer screening is the mortality rate from the cancer in question in the target population. However, in view of the natural history of breast cancer, the 4-year period scheduled in the strategic study grant is too short to observe a significant inter-group difference. Although the rate of advanced breast cancer could be a surrogate for mortality reduction, it is necessary to have a system that has the long-term follow-up of the survival status of individuals even after the completion of the strategic study, J-START.
This study may have several limitations. First, the screening interval is 2 years, despite evidence that screening MG at age 40–49 years is more effective with annual screening. The recent USPSTF, however, recommends biannual MG screening in view of reducing ‘harm’, i.e. higher recall rate at age 40–49 years (5). Secondly, the study population, which is so different from that in Western countries, may limit the generalization of study outcomes. Most countries in Asia, however, demonstrate the similar trend of breast cancer incidence as observed in Japan; therefore, this trial may influence their health strategy against breast cancer. Nevertheless, for women aged 40–49 years even in Western countries, there is a limitation of MG screening as the USPSTF recommends against the routine use of screening MG for this age group. Thirdly, the study may be underpowered to provide follow-up data on breast cancer deaths because of the low breast cancer risk of native Japanese women. In this context, as much as 100 000 women are targeted in this trial to ensure the statistical power be sufficient enough in comparison between the two groups.
PROTOCOL DIGEST OF THE STUDY
Purpose
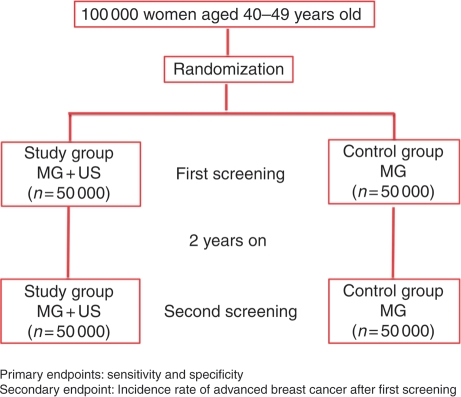
The aim of this study is to assess the effectiveness of screening US for breast cancer in women aged 40–49 (Fig. 1).
Figure 1.
J-START study design. MG, mammography; US, ultrasonography.
Study Setting
This study is a multi-institutional prospective RCT, with 42 participating centers in 23 prefectures in Japan as of 31 March 2011.
Endpoints
The primary endpoints of this trial are sensitivity and specificity, based on the data of each incremental cancer detection rate, false-positives and false-negatives should be forthcoming in 2 years. The secondary endpoint is the rate of advanced breast cancers, as this has been demonstrated in the screening MG RCTs to be a surrogate for mortality reduction (10).
Eligibility Criteria
Inclusion criteria are as follows:
women aged 40–49 years when registered;
women signed the informed consent to participate in the study.
Exclusion criteria are as follows:
women with a history of breast cancer;
women with a history of malignant disease other than breast cancer within 5 years;
women in severe condition, who are not expected to live for 5 years.
Treatment Methods
Patient Assignments
Each participating center confirms the participants’ eligibility and screening methods are assigned according to the random number provided by the Japan Clinical Research Supporting Unit (J-CRSU) Data Center. Cluster randomization is also used in some institutions.
Screening Method and Assessment
For the intervention arm, US and MG are performed at the same time. For the control arm, MG is performed. The technologists and the physicians involved in this trial are asked to finish 2-day, 16-h education program for the standardization of US screening for breast cancer. Regarding the procedure in screening with US, the handheld US is performed by a technologist or by a physician, and later, the US image is interpreted by a physician. An interpretation of MG is performed by a physician who is not regulated to be the same doctor interpreting US image or not, although the categorization of the two modalities are defined separately in the protocol. The findings of MG and/or US are subsequently evaluated by authorized screeners and are classified into five categories as follows: Category 1, negative; Category 2, benign finding(s); Category 3, probably benign finding(s); Category 4, suspicious abnormality; and Category 5, malignancy. The women who are rated in Category 3 or higher by the MG and/or US are referred for further diagnostic examinations.
Statistical Analysis
The sample size was calculated on the hypothesis that adjunct US is expected to improve sensitivity of the intervention group compared with the control group. Our previous data demonstrated the lower sensitivity of MG screening, 71% in women aged 40–49, when compared with those in women aged 50–59 and 60–69, 85 and 86%, respectively (11). Assuming that the sensitivity increases from 71 to 86% by adding US to MG, 42 500 subjects for each arm is needed to make it 5% statistical significance (two-sided) with 80% power. Thus, the number of 100 000 subjects (two arms combined) is set to be a targeted sample size to verify the primary endpoint, a sensitivity improvement in the intervention group when compared with the control group.
Follow-up Period
The participants are invited to be screened 2 years after the first recruitment or asked to answer questionnaires of health status, history of receiving other screening program, incidence of breast cancer, and history of hospital consultation with any breast symptoms within 2 years. For evaluating the actual evidence of a reduced mortality rate of the intervention group compared with the control group, there must be needed to establish follow-up strategies for a long time period and systematic, nationwide population-based cancer registries.
Registration of the Protocol
The J-START was registered on the University Hospital Medical Information Network Clinical Trial Registration (UMIN-CTR), Japan (registration number: UMIN000000757), on 2007. Details are available at the following address: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000000910&language=E.
Funding
This work was supported by the 3rd term comprehensive control research for cancer (grant number: H-18-Senryaku-001), the Ministry of Health, Labour and Welfare of Japan.
Conflict of interest statement
None declared.
References
- 1.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer Incidence in Five Continents, Vol. IX. Lyon: IARC; 2007. IARC Scientific Publications No. 160. [Google Scholar]
- 2.Matsuda T, Marugame T, Kamo KI, Katanoda K, Ajiki W, Sobue T. Cancer incidence and incidence rates in Japan in 2005: based on data from 12 population-based cancer registries in the Monitoring of Cancer Incidence in Japan (MCIJ) Project. Jpn J Clin Oncol. 2010 doi: 10.1093/jjco/hyq169. doi:10.1093/jjco/hyq169 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 3.Minami Y, Tsubono Y, Nishino Y, Ohuchi N, Shibuya D, Hisamichi S. The increase of female breast cancer incidence in Japan: emergence of birth cohort effect. Int J Cancer. 2004;108:901–6. doi: 10.1002/ijc.11661. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa S, Ohnuki K, Nagakubo J, Mizukubo J, Kitami J, Oyama K, et al. Evaluation of breast cancer visualization ability of mammography by age group and by breast constitution (mammary gland/fat ratio) J Jpn Assoc Breast Cancer Screen. 2003;12:101–7. (in Japanese) [Google Scholar]
- 5.Screening for Breast Cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–26. doi: 10.7326/0003-4819-151-10-200911170-00008. W-236. [DOI] [PubMed] [Google Scholar]
- 6.Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. Am J Roentgenol. 2003;181:177–82. doi: 10.2214/ajr.181.1.1810177. [DOI] [PubMed] [Google Scholar]
- 7.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–75. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 8.Leconte I, Feger C, Galant C, Berliere M, Berg BV, D'Hoore W, et al. Mammography and subsequent whole-breast sonography of nonpalpable breast cancers: the importance of radiologic breast density. Am J Roentgenol. 2003;180:1675–9. doi: 10.2214/ajr.180.6.1801675. [DOI] [PubMed] [Google Scholar]
- 9.WHO [Internet] National Cancer Control Programmes: Policies and Managerial Guidelines. 2nd edn. 2002. [cited 21 October 2010]. http://www.who.int/cancer/media/en/408.pdf .
- 10.Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42:793–806. doi: 10.1016/j.rcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki A, Kuriyama S, Kawai M, Amari M, Takeda M, Ishida T, et al. Age-specific interval breast cancers in Japan: estimation of the proper sensitivity of screening using a population-based cancer registry. Cancer Sci. 2008;99:2264–7. doi: 10.1111/j.1349-7006.2008.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]