Abstract
To investigate the efficacy of endocrine parathyroid hormone treatment on tissue-engineered bone regeneration, massive femoral defects in C57Bl/6 mice were reconstructed with either 100:0 or 85:15 poly-lactic acid (PLA)/beta-tricalcium phosphate (β-TCP) scaffolds (hereafter PLA or PLA/βTCP, respectively), which were fabricated with low porosity (<30%) to improve their structural rigidity. Experimental mice were treated starting at 1 week postop with daily subcutaneous injections of 40 μg/kg teriparatide until sacrifice at 9 weeks, whereas control mice underwent the same procedure but were injected with sterile saline. Bone regeneration was assessed longitudinally using planar X-ray and quantitative microcomputed tomography, and the reconstructed femurs were evaluated at 9 weeks either histologically or biomechanically to determine their torsional strength and rigidity. Teriparatide treatment increased bone volume and bone mineral content significantly at 6 weeks and led to enhanced trabeculated bone callus formation that appeared to surround and integrate with the scaffold, thereby establishing union by bridging bone regeneration across the segmental defect in 30% of the reconstructed femurs, regardless of the scaffold type. However, the bone volume and mineral content in the PLA reconstructed femurs treated with teriparatide was reduced at 9 weeks to control levels, but remained significantly increased in the PLA/βTCP scaffolds. Further, bridged teriparatide-treated femurs demonstrated a prototypical brittle bone torsion behavior, and were significantly stronger and stiffer than control specimens or treated specimens that failed to form bridging bone union. Taken together, these observations suggest that intermittent, systemic parathyroid hormone treatment can enhance bone regeneration in scaffold-reconstructed femoral defects, which can be further enhanced by mineralized (βTCP) particles within the scaffold.
Introduction
Segmental defects in long bones, secondary to tumor resection, trauma, and osteomyelitis, are a challenge for the reconstructive orthopedic surgeon. Traditionally used cadaveric bone allografts are marred by poor incorporation (with delayed union or nonunion), microdamage accumulation, graft failure, and the risk of disease transmission.1 Therefore, engineered biomaterial scaffolds have emerged as an alternative to biological grafts for reconstructive bone surgery and limb salvage. An ideally engineered bone scaffold should be nonimmunogenic, osteoconductive, osteoinductive and have porosity properties permissive of tissue ingrowth and host incorporation, while providing structural rigidity and stability.2 The engineered scaffold could also be used as a delivery vehicle for bone anabolic factors such as proteins or gene delivery vectors (plasmids, viral vectors, etc.).3 Alternatively, endocrine hormones that enhance bone formation, such as parathyroid hormone (PTH), which have been reported to augment bone repair and regeneration,4 could be used as a systemic adjuvant therapy.
Intermittent, systemic PTH treatment is an effective therapy to increase bone mass density and reduce fracture risk in osteoporosis patients.5–7 A growing body of preclinical data has supported the conclusion that PTH can also be effective in accelerating bone repair after fracture, especially in cases of delayed healing due to persistent fibrosis and nonunion.8,9 Among the most potent forms of PTH is teriparatide (Forteo™; Eli-Lilly Co.), which is approved by FDA for the treatment of osteoporosis. Teriparatide is a recombinant fragment of human PTH (rhPTH) that has an identical sequence to the biologically active region (the 34 N-terminal amino acids) of the full 84-amino acid hPTH. The simplicity of the daily systemic injection of teriparatide overcomes many of the challenges and limitations of localized cellular-, cytokine-, or gene delivery-based and controlled-release therapies. Therefore, off-label clinical use of teriparatide is becoming an increasingly used option in challenging fractures and delayed nonunions with early reports of remarkable success.10
We have recently demonstrated that dense poly-lactic acid (PLA) scaffolds, with or without embedded mineral (beta-tricalcium phosphate [β-TCP]) particles, could be designed to withstand in vivo stresses in an established murine femoral defect model,11 and could be processed for delivery of growth factors and gene transfer particles, in vitro and in vivo, to enhance their osteogenic potential.12 In this article, we investigate the effects of intermittent daily dosing of teriparatide on the reconstruction of murine femoral segmental defects with PLA and PLA/β-TCP scaffolds. We hypothesized that teriparatide would induce a robust healing response around the scaffold–femur interface, and that this response will be further potentiated in scaffolds containing the osteoinductive β-TCP particles.
Materials and Methods
Mouse femoral defect reconstruction surgery
PLA scaffolds or PLA impregnated with a nominal 15% of medical-grade β-TCP particle (PLA/β-TCP) scaffolds (generously donated for this study by Kensey Nash Corporation, Exton, PA) were custom fabricated using a rapid volume expansion phase separation technique that suspends uncoated β-TCP particles in the porous polymer,12 and were made to fit the dimensions of the mouse femur and the segmental defect (2 mm diameter × 4 mm length), with a predrilled hole longitudinally to allow intramedullary stabilization (Fig. 1). Animal surgery and care protocols were performed in accordance with the regulations of the University of Rochester's Committee on Animal Resources as previously described.11 Briefly, the left femurs of 8-week-old female C57BL/6 mice were exposed with a direct lateral approach. The musculature was elevated at the femoral mid-shaft, then retracted with curved forceps. The surgical wound and area for osteotomy was irrigated with sterile normal saline, whereas a 4 mm segmental defect was created using a diamond-tipped rotary blade. The scaffold was inserted in the defect and fixed with an intramedullary titanium pin. The muscle and skin layers were repaired with single layer of interrupted 4-0 silk sutures and then reinforced with surgical staples as previously described.11
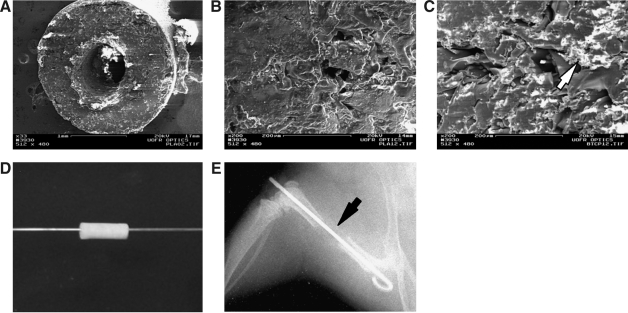
FIG. 1.
Tissue-engineered scaffolds for long bone reconstruction. (A) Low-power scanning electron microscopy of the cross section of a 100:0 PLA/β-TCP (PLA) scaffold (scale bar represents 1 mm). High-power scanning electron microscopy images of the PLA (B) and 85:15 PLA/β-TCP (C) scaffolds with the arrow head pointing to β-TCP particles (scale bar represents 200 μm). Titanium pins were passed through the lumen of the scaffolds (D) to be used for fixation of the scaffolds when implanted as stand-alone femoral graft substitutes in massive 4 mm femoral defects in our previously established mouse model. The grafted animals were either treated by daily (5 days/week) injections of PTH or left untreated (controls). (E) Radiographic image of a PLA scaffold-grafted femur on day 0. Arrowhead points to the radio-translucent scaffold filling the femoral defect. β-TCP, beta-tricalcium phosphate; PLA, poly-lactic acid; PTH, parathyroid hormone.
Experimental mice (n = 13 PLA scaffolds and n = 13 PLA/β-TCP scaffolds) were treated starting 1 week postop with daily subcutaneous injections of rhPTH (40 μg/kg teriparatide, Forteo; Eli-Lilly Co.) for 9 weeks until sacrifice.8 Control mice (n = 13 PLA scaffolds and n = 13 PLA/β-TCP scaffolds) underwent the same injection schedule, but were injected with sterile saline.
Histology
Specimens processed for histology (n = 3 per group) were first dehydrated through steps of graded alcohols. Technovit 7100 (2-hydroxyethylmethacrylate [GMA]-embedding polymer; Heraeus Kulzer GmbH) was then used to infiltrate and embed the samples. The GMA was mixed 1:1 with ethanol as a preinfiltration step. Infiltration was performed in open glass vials inside of a vacuum chamber for a total of 48 h and three changes of GMA. The samples were then embedded in high density polyethylene (HDPE) molds with aluminum block holders. Once the GMA fully polymerized, the blocks were cut on a heavy-duty rotary microtome with D-profile tungsten-carbide knife. Sections were cut at 4 μm thickness and mounted on glass slides, which were then stained in a 1:100 alcian blue/hematoxylin solution and counterstained with an eosin/pholoxine B/orange G solution.
Microcomputed tomography imaging
X-rays of the mice were taken weekly beginning the day of surgery until sacrificed at 9 weeks. X-rays were evaluated qualitatively for callus formation and scaffold stability. Immediately after surgery and at 6 weeks postop, in vivo microcomputed tomography (micro-CT) scans were performed using the VivaCT 40 imager (Scanco Medical). The mice (n = 20 mice; 5 mice randomly selected from each group) were anesthetized using 1.5% isoflurane and an oxygen flow rate of 0.6 L/min. High-resolution (17.5 μm) scans were performed on each mouse using an integration time of 300 ms, 145 microA current, and 55 kV energy setting. A region of interest spanning 11 mm, or 628 slices, was selected to include the scaffold within the reconstructed femur as well as the nonoperated femur as a control. Analysis was performed to determine the bone (i.e., mineralized callus) volume, bone mineral density (BMD), and bone mineral content (BMC) of the repair tissue.
After 9 weeks, the animals were sacrificed and the reconstructed femurs were harvested and stored at −20°C. All samples were micro-CT imaged before mechanical testing was performed. Briefly, a region of interest spanning 14.8 mm, or 846 slices, was scanned using the same in vivo scan settings to assess the scaffold and callus of each sample. Analysis was then performed to determine repair bone volume, BMD, and BMC.
Biomechanical (torsional) testing
Isolated femurs were thawed at room temperature while wrapped in saline-soaked gauze. The femurs were then dissected of soft and muscle tissues and the intramedullar pins carefully removed. The ends of the femurs were cemented into 6.35 mm2 square aluminum tube holders using polymethylmethacrylate bone cement in a custom jig to ensure axial alignment and to maintain a gage length of 7.1 ± 0.9 mm, allowing at least 3 mm to be potted at each end. After potting, the samples were re-hydrated in PBS for 2 h while the polymethylmethacrylate hardened. Testing was performed on the EnduraTec TestBench™ system (200 N.mm torque cell; Bose Corporation). A rotation rate of 1°/s was used to twist the samples to failure or up to 80°. Torque versus rotation data were collected and analyzed in MATLAB to determine maximum torque, maximum rotation, and torsional rigidity, as previously described.13
Statistical data analysis
Two-way analysis of variance with Bonferonni post hoc multiple comparisons were performed to compare the effects scaffold type and teriparatide treatments (alpha = 0.05).
Results
Teriparatide and β-TCP enhance bone regeneration in scaffold reconstructed femurs
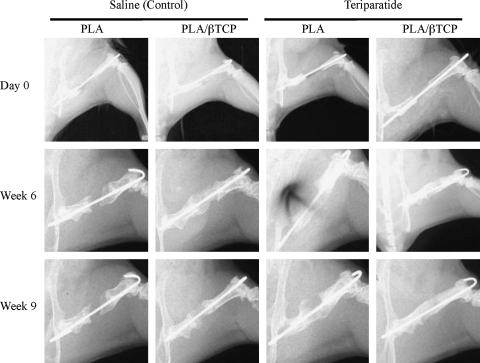
Radiographic evaluation of the transplanted load-bearing scaffolds showed no incidence of collapse throughout the study duration (up to 9 weeks), and suggested that the rate and extent of bone regeneration was enhanced in the teriparatide-treated animals compared to controls (Fig. 2). Two-way analysis of variance and post hoc multiple comparisons of repair bone volume (mineralized callus) determined from micro-CT imaging, confirmed the significant increase in bone volume due to teriparatide treatment regardless of scaffold type at both 6 and 9 weeks (Fig. 3). At 6 weeks the new bone volume in the teriparatide-treated group was 3-fold and 2.1-fold greater than untreated controls (p < 0.05) in both PLA and PLA/β-TCP groups, respectively (Fig. 3A, B). After 9 weeks of healing, the new bone volume in the teriparatide-treated PLA/β-TCP group remained significantly greater (1.8-fold) than untreated controls (p < 0.05), but there was no difference between the teriparatide and control groups reconstructed with the PLA scaffolds (Fig. 3A, B). Interestingly, the new bone volume decreased significantly between 6 and 9 weeks for the teriparatide-treated femurs reconstructed with the PLA scaffolds (Fig. 3A), whereas the callus volume for the treated PLA/β-TCP scaffolds remained unchanged between 6 and 9 weeks (Fig. 3B).
FIG. 2.
Representative planar X-ray images depicting the effects of scaffold type and teriparatide (PTH) treatment on bone regeneration over time.
FIG. 3.
Teriparatide (PTH) treatment increases early callus formation regardless of the scaffold type, which is only sustained in mature callus of PLA/β-TCP scaffolds. Quantitative micro-CT segmentation of the mineralized callus volume and BMC at 6 weeks and at 9 weeks for the PLA scaffolds (A, D) and the PLA/β-TCP scaffolds (B, E). These data were reanalyzed for callus volume (C) and BMC (F) after separating the PTH-treated samples based on their bridging outcome. Data are presented as mean ± standard error of the mean. Asterisk indicates significant differences from control (p < 0.05); **indicates p < 0.01. BMC, bone mineral content; micro-CT, microcomputed tomography.
There were no treatment or scaffold effects on the BMD of the callus. However, when integrated over the new bone volume, the BMC of the teriparatide-treated group at 6 weeks was 2.9-fold and 1.8-fold greater than the saline controls (p < 0.05) in both PLA and PLA/β-TCP groups, respectively (Fig. 3D, E). At 9 weeks, the BMC in the teriparatide-treated PLA/β-TCP group remained significantly greater (1.6-fold) than untreated controls (p < 0.05), but no differences could be detected due to the teriparatide treatment in the PLA scaffolds (Fig. 3D, E).
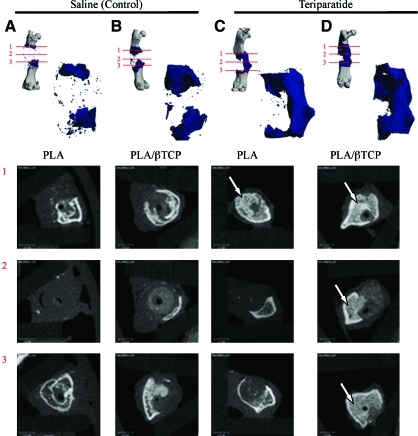
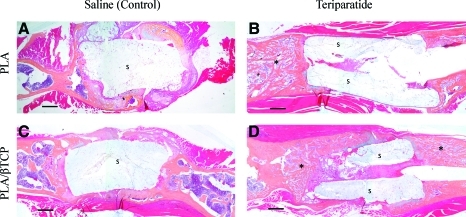
By 6 weeks, 3 out of 10 specimens or 30% of the teriparatide-treated scaffolds, in each of the PLA and PLA/β-TCP groups, had already established union by bridging bone regeneration across the segmental defect, which was not observed in the untreated controls of either scaffold groups. The incidence of union did not increase at 9 weeks. Examination of the femur–scaffold interface in the untreated control specimens showed mineralized cortical shell emanating from the femur periosteal surface, similar to the healing response seen in the femur–allograft interface in mouse segmental defects.13 In contrast, the femur–scaffold interface in the teriparatide-treated specimens was characterized by dense and trabecular bone-like mineralized tissue that was distinctly different from the cortical shell seen in the saline controls (Fig. 4). Histology confirmed these observations and demonstrated that there was very limited and insignificant cellular infiltration into the pore spaces of the scaffold, but on average, the PLA/β-TCP scaffolds produced larger bone collars around the scaffold, compared to PLA scaffolds (Fig. 5). Further, remarkable trabeculated bone formation was observed in the teriparatide-treated specimens and appeared to emanate from the host bone ends to surround and integrate with the either scaffold (Fig. 5B, D), which was not observed in saline-treated controls (Fig. 5A, C).
FIG. 4.
Teriparatide treatment enhances osteointegration of PLA and PLA/β-TCP scaffolds by increasing callus volume, trabecular bone formation, and bridging. Representative micro-CT segmentation of the mineralized callus in femurs reconstructed with PLA scaffolds (A, C) or (B, D) at 9 weeks postsurgery. Control animals were untreated (A, B), whereas treated animals received daily teriparatide injections (C, D). Note that 30% of the teriparatide-treated animals developed a mineralized callus that bridged the defect for both scaffolds resulting in union. In contrast, none of the scaffolds in the nontreated controls developed a bridging union. Lower panels show the corresponding micro-CT slices of each specimen at the proximal end (1), midline (2), and distal end (3) of the callus. Arrows indicate the dense and trabecular nature of the mineralized callus in the teriparatide-treated groups (C, D) compared to the mostly cortical shell in the controls (A, B). Color images available online at www.liebertonline.com/ten.
FIG. 5.
Teriparatide treatment increases trabeculated bone callus emanating from the host bone to provide better integration with the scaffold. Representative sagittal sections of scaffold-reconstructed femurs treated with saline (A, C) and 40 μg/kg teriparatide (B, D) at 9 weeks. (A, B) The PLA scaffold-reconstructed femur and (C, D) the PLA/β-TCP scaffold-reconstructed femur. 2-Hydroxyethylmethacrylate-embedded sections were stained with orange G and alcian blue. Scale bars represent 0.5 mm. Asterisks label the dense trabeculated bone regeneration in the teriparatide-treated specimens. S indicates the scaffold. Color images available online at www.liebertonline.com/ten.
When the repair in teriparatide-treated PLA specimens that developed bridging union across the segmental defect was compared to controls and nonunion-treated specimens reconstructed with PLA scaffolds, there was no difference in bone volume or BMC (Fig. 3C). However, in the PLA/β-TCP reconstructed group, the repair bone volume in the teriparatide-treated specimens establishing union was 2-fold and 1.3-fold greater than the bone volume of the controls and nonunion teriparatide-treated specimens, respectively (p < 0.05, Fig. 3C). Similarly, the BMC in the teriparatide-treated specimens establishing union was 1.8-fold greater than the controls (p < 0.05), but was not significantly different from the nonunion teriparatide-treated specimens (Fig. 3F).
Teriparatide improves torsional strength and rigidity independent of the scaffold type
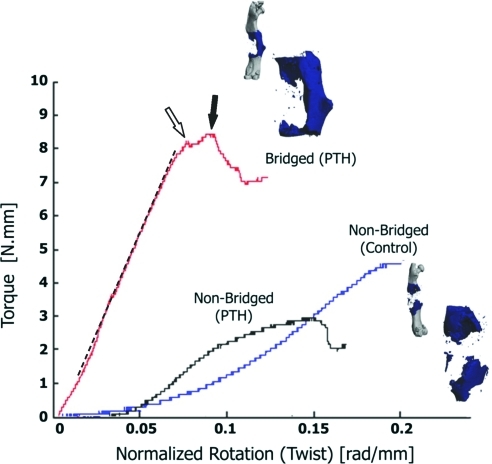
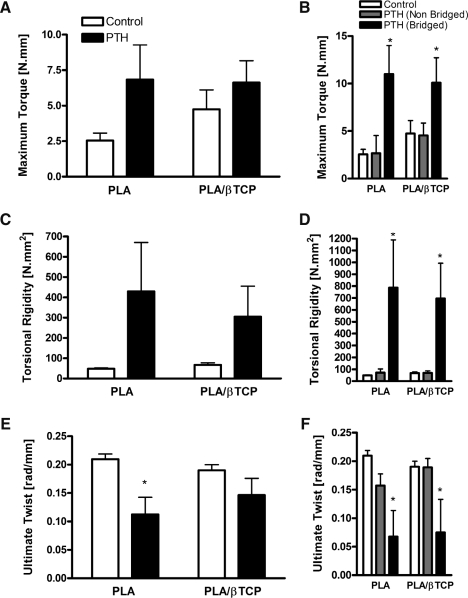
Biomechanical testing of specimens in torsion was performed to determine the torsional strength and rigidity of the reconstructed femurs. Figure 6 depicts representative torque-twist data for PLA-reconstructed femurs at 9 weeks postsurgery, highlighting the fact that specimens with bridging bone union had torsion characteristics similar to bone, with a clearly defined elastic (linear) region, yield, and ultimate failure points, and brittle fracture failure. There were no scaffold-related effects on the torsional strength, rigidity, or ultimate twist, regardless of the teriparatide treatment. On average, there was a trend toward increased maximum torque and torsional rigidity and a decrease in ultimate twist in the teriparatide-treated groups compared to controls, but in general the differences were not statistically significant (Fig. 7A, C, E).
FIG. 6.
Bridged teriparatide (PTH)-treated specimens demonstrate a prototypical brittle bone torsion behavior. Scaffold-grafted femurs were harvested at 9 weeks, imaged via micro-CT, and then tested in torsion at a rate of 1°/s. The representative torque-normalized rotation curve shown demonstrates that teriparatide-treated samples that developed a bridging union exhibit a torsion behavior characteristic of bone with clearly defined linear region (dashed line), yield (open arrow), and ultimate failure torque (closed arrow), and a relatively brittle fracture. In contrast, nonunion specimens for the most part did not have defined failure points and were quite ductile. Color images available online at www.liebertonline.com/ten.
FIG. 7.
Teriparatide-induced bone bridging is required for improved biomechanical properties of the scaffold-grafted femurs. The scaffold-grafted femurs were tested in torsion to determine their biomechanical properties, including maximum torque (A, B), torsional rigidity (C, D), and ultimate normalized rotation or twist (E, F). (B, D, F) The maximum torque, torsional rigidity, and ultimate twist, respectively, of specimens that developed a bridging union compared to nonunion control and teriparatide-treated specimens. Data are presented as mean ± standard error of the mean. Asterisk indicates significant differences from control (p < 0.05).
When compared to controls and nonunion teriparatide-treated specimens, the maximum torque of the teriparatide-treated femurs that developed bridging union across the segmental defect were >4-fold or >2-fold greater for the PLA and the PLA/β-TCP scaffolds, respectively (p < 0.05, Fig. 7B). Similarly, the torsional rigidity of the teriparatide-treated femurs that developed bridging union across the segmental defect were >11-fold and >9.9-fold greater than nonunion PLA and the PLA/β-TCP scaffolds, respectively (p < 0.05, Fig. 7D). Bridging union also significantly reduced the ultimate twist of teriparatide-treated specimens to <40% of the nonunion specimens (Fig. 7F).
Discussion
Reconstruction of long-bone segmental defects remains a difficult problem for orthopedic surgeons. While metallic implants and allografts are two clinically available options for reconstruction, they are fraught with complications such as implant loosening, nonunion, and microfracture. Tissue engineering may soon introduce alternative treatment options by offering osteoinductive biomaterial scaffolds in lieu of the metallic implants or biologic grafts. There are at least two strategies by which osteoinductive properties can be conferred onto biomaterial scaffolds. The first strategy involves engraftment of mesenchymal stem cells on the scaffolds to provide a viable bone substitute. While the efficacy of this approach has been demonstrated,14 many concerns regarding the cell sourcing, effective and reproducible engraftment protocols, and cost have hindered its clinical applicability. The second strategy is to introduce osteogenic factors (recombinant or animal-derived)15–18 or genes (plasmids or vectors) onto the scaffold for localized delivery.3,19 The localized delivery approach has shown promising results in preclinical studies and has come to fruition in surgical applications such as spinal fusion scaffolds (cages) augmented by bone morphogenetic proteins.20 However, the complications associated with the need for high local doses of bone morphogenetic proteins21–25 and the safety concerns surrounding viral-based gene delivery26–28 have thus far limited wide clinical applicability of this strategy. In this study, we tested the alternative approach to enhance bone regeneration around a structural biomaterial scaffold implanted in a massive segmental femoral defect via systemic injections of teriparatide, and demonstrated that this treatment, independent of scaffold's mineral content, led to significant enhancements of mineralized tissue volume, mineral content, union incidence, and biomechanical (torsional) strength and rigidity.
Although teriparatide is primarily approved in the United States for the treatment of osteoporosis in postmenopausal women and in men at high risk for fracture,29 there are ample preclinical and clinical evidence to suggest that different forms of PTH can accelerate fracture healing, and could be beneficial in treating nonunions and delayed unions,10 and in enhancing bony ingrowth in orthopedic implants.30 The anabolic effects of intermittent PTH treatment on the repair of long bone fractures in an animal model were first reported in a rat tibial fracture model in 1999.31 Similar results have since been published confirming that intermittent PTH treatment increases fracture callus volume and improves the biomechanical properties of the fractured bone,9,32–38 even after withdrawal of treatment.9,33
The effects of hPTH(1,34) on bone regeneration in critical-sized osteotomy models were first reported by Bonadio et al.,3 wherein DNA plasmids encoding the active fragment of hPTH(1,34) were loaded onto lyophilized collagen sponges, and then implanted in critical-size (up to 2.0 cm long) defects in the tibiae of beagle dogs. This study reported that high doses (100 mg) of hPTH(1,34) encoding plasmids were necessary for progressive bone filling in the defect, although no incidence of complete gap filling were reported, whereas lower doses (1.0–20 mg) showed no substantial bone regeneration.3 In a follow-up study, Chen et al. investigated the effects of daily injections (40 μg/kg body weight) of rhPTH(1,34) on bone regeneration in a rat model of critical size (4 mm) femoral defects, with or without localized, collagen matrix-mediated gene delivery of hPTH(1,34).4 At 6 weeks postsurgery, neither treatment on its own resulted in increased BMD and BMC at the osteotomy site. However, compared to local gene delivery of hPTH(1,34), the systemic injections of rhPTH(1,34) resulted in increased defect filling based on planar X-ray quantification. Interestingly, the combined treatment of systemic and localized PTH resulted in significant increases in BMD, BMC, and percent bone regeneration in the defect, compared to either treatment alone, or untreated controls.4 However, as in the previous work, no complete defect filling or union was observed. While these studies bear similarities to our study, there are major differences in the femoral defect model, the scaffold, the timing of treatment, and the functional assessment of the treatment outcome. In our study, we utilized a mouse model of a 4 mm femoral defect, which measures close to 20%–25% of the entire length of the femur, and thus models a massive segmental defect as opposed to critical nonunion defects in larger animals. In addition, our PLA and PLA/β-TCP scaffolds are structurally rigid and load bearing as they were stabilized using intramedullar pins, compared to the nonstructural collagen matrices stabilized with external fixators.3,4 This is a significant advantage of our model since the combination of PTH and in vivo mechanical stimulation has been shown to synergistically increase bone formation rates.39 Further, the systemic injections of teriparatide in our study were initiated at 1 week postreconstruction, and continued for 8 weeks until sacrifice, to simulate delayed onset of treatment that would commence when nonunion is clinically evident. Finally, and to the best of our knowledge, our study is the first to report functional outcomes that demonstrated increased bone regeneration and incidence of union based on 3D micro-CT data, and enhanced biomechanical properties (torsional strength and rigidity) in massive femoral defects following tissue-engineered limb reconstruction and daily systemic injections of teriparatide.
Regardless of the scaffold used in the femoral reconstruction, only a subset of specimens (30%) treated with teriparatide formed a bridging bone union across the segmental defect, with significant torsional rigidity and strength. None of the saline-injected controls, on the other hand, displayed union or comparable torsional stability. The reasons for this variability in the response to teriparatide treatment are unclear at this time, but might be related to slight variability in animal weights, which may have led to undocumented dosing variability, surgical factors related to the rigidity of the scaffold stabilization using an intramedullary pin or inadvertent injury to the periosteum of the femur during surgical osteotomy and reconstruction, and/or the inherent variability in experimental animals. It is possible that optimizing the teriparatide dose and window of treatment (i.e., timing of onset and withdrawal of treatment) might lead to more consistent and reproducible bridging bone repair in the treated animals, which warrants formal investigation in future experiments.
Interestingly, the observed teriparatide-induced increases in the callus volume of the PLA scaffolds were lost between 6 and 9 weeks, but remained persistent in the PLA/β-TCP scaffolds. This might suggest a synergistic effect of the embedded β-TCP particles with teriparatide. The mechanism of this phenomenon is not clear but may be related to enhanced osteoblast adhesion on the β-TCP particles,40 which might increase their mechanosensitivity to the in vivo loading environment. This possibility is supported by reports that suggest that the activity of transcriptional attenuators of the anabolic response of osteoblasts to PTH such as the nuclear matrix protein 4/cas interacting zinc finger or Nmp4/CIZ is sensitive to cell adhesion and can be suppressed in response to mechanical perturbations.41
One of the limitations in our study is that we did not probe the mechanism of action of PTH and the reason of the observed synergy with scaffold mineral content using molecular and cellular assays over time. This should be the subject of future studies, especially since the anabolic pathways in bone regeneration at sites of fracture or osteotomy in response to PTH have yet to be fully identified. Endogenous PTH acts as a regulatory hormone for serum calcium homeostasis. The N-terminus of endogenous PTH activates the PTH-1 receptor (a G protein-coupled receptor), and activates the cyclic AMP-dependent protein kinase A and calcium-dependent protein kinase C pathways to regulate osteoblast function.29 Interestingly, continuous treatment with PTH is reportedly associated with a decrease in BMD and hypercalcemia (which mimics chronic elevation of endogenous PTH levels due to hyperparathyroidism). Sclerostin (a negative regulator of osteogenesis transcribed by the Sost gene) is a Wnt/β-catenin antagonist produced by osteocytes.42,43 Recent findings have shown that continuous elevation of PTH downregulates Sost, leading to increased osteoblast apoptosis. On the other hand, the anabolic effects of intermittent PTH dosing are associated with attenuation of osteoblast apoptosis independent of Sost.42 Whether Sost regulation plays a role in PTH-stimulated bone repair has yet to be investigated. However, the likely targets of the early effects of PTH during repair might include mesenchymal stem cells, periosteal cells, and osteoblast progenitors. Chen et al. reported positive mRNA expression of PTH-1 receptor at the site of femoral osteotomy, regardless of local or systemic PTH treatment. However, systemic PTH treatment led to increased osteocalcin (Ocn) mRNA expression in sera and callus tissue at the site of femoral osteotomy.4 Kaback et al. reported that systemic teriparatide upregulates the transcription factor Osterix (Osx) in femoral fracture callus.8 Others have shown that PTH effects on fracture repair are associated with enhanced chondrogenesis (chondrocyte recruitment and maturation) in the early fracture callus, mediated in part by canonical Wnt signaling.44
Interestingly, while intermittent PTH treatment stimulates bone growth in vivo, possibly by increasing local bone factors, its effects on angiogenesis are much less studied despite the critical role of angiogenesis in the process of bone repair. It has been shown that continuous treatment with PTHrP(1–34) increases vascular endothelial growth factor mRNA in human osteoblastic cells from trabecular bone,45 whereas other reports have shown that vascular endothelial growth factor-A production during osteoblast differentiation is inhibited by PTH-related peptide that also inhibited preosteoblast differentiation.46 Others have reported anti-angiogenic effects of PTH in the skin.47 These reports suggest an intriguing mechanism of action for systemic PTH in bone regeneration that might involve negative regulation of angiogenesis, which merits further investigation in future studies.
In conclusion, the current findings suggest that the effects of intermittent, systemic PTH in scaffold mediated bone repair and regeneration could be enhanced by mineralized scaffold components such as β-TCP. Further studies delineating the mechanism of the observed synergy between PTH and β-TCP are warranted using in vitro and in vivo model systems that can potentially lead to tissue-engineered alternatives to bone graft reconstruction.
Acknowledgments
The authors thank Mr. Patrick Hearn (Kensey Nash) for scaffold fabrication, and Mr. Ryan Tierney and the Histology Core at the Center for Musculoskeletal Research for their excellent help with the histology. This work was funded in part by grants from the Wallace H. Coulter Foundation, the Aircast Foundation, and the National Institutes of Health (1R01AR056696-01A1, 5P50AR054041-04, and 1R01DE019902-01).
Disclosure Statement
No competing financial interests exist.
References
- 1.Akkus O. Rimnac C.M. Fracture resistance of gamma radiation sterilized cortical bone allografts. J Orthop Res. 2001;19:927. doi: 10.1016/S0736-0266(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 2.Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 3.Bonadio J. Smiley E. Patil P. Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 4.Chen H. Frankenburg E.P. Goldstein S.A. McCauley L.K. Combination of local and systemic parathyroid hormone enhances bone regeneration. Clin Orthop Relat Res. 2003;416:291. doi: 10.1097/01.blo.0000079443.64912.18. [DOI] [PubMed] [Google Scholar]
- 5.Holick M.F. PTH (1–34): a novel anabolic drug for the treatment of osteoporosis. South Med J. 2005;98:1114. doi: 10.1097/01.smj.0000140864.78760.8d. [DOI] [PubMed] [Google Scholar]
- 6.Deal C. Gideon J. Recombinant human PTH 1–34 (Forteo): an anabolic drug for osteoporosis. Cleve Clin J Med. 2003;70:585–589. doi: 10.3949/ccjm.70.7.585. 592 passim. [DOI] [PubMed] [Google Scholar]
- 7.Reeve J. PTH: a future role in the management of osteoporosis? J Bone Miner Res. 1996;11:440. doi: 10.1002/jbmr.5650110404. [DOI] [PubMed] [Google Scholar]
- 8.Kaback L.A. Soung D.Y. Naik A. Geneau G. Schwarz E.M. Rosier R.N., et al. Teriparatide (1–34 human PTH) regulation of osterix during fracture repair. J Cell Biochem. 2008;105:219. doi: 10.1002/jcb.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkhiary Y.M. Gerstenfeld L.C. Krall E. Westmore M. Sato M. Mitlak B.H., et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34) J Bone Joint Surg Am. 2005;87:731. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- 10.Puzas J.E. Houck J. Bukata S.V. Accelerated fracture healing. J Am Acad Orthop Surg. 2006;14:S145. doi: 10.5435/00124635-200600001-00033. [DOI] [PubMed] [Google Scholar]
- 11.Tiyapatanaputi P. Rubery P.T. Carmouche J. Schwarz E.M. O'Keefe R.J. Zhang X. A novel murine segmental femoral graft model. J Orthop Res. 2004;22:1254. doi: 10.1016/j.orthres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Yanoso-Scholl L. Jacobson J.A. Bradica G. Lerner A.L. Zuscik M.J. Schwarz E.M. O'Keefe R.J. Awad H.A. Evaluation of polylactic acid/beta-tricalcium phosphate scaffolds for bone tissue engineering. J Biomed Mat Res A. 2010 Aug 19; doi: 10.1002/jbm.a.32868. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds D.G. Hock C. Shaikh S. Jacobson J. Zhang X. Rubery P.T., et al. Micro-computed tomography prediction of biomechanical strength in murine structural bone grafts. J Biomech. 2007;40:3178. doi: 10.1016/j.jbiomech.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Bruder S.P. Fink D.J. Caplan A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niu X. Feng Q. Wang M. Guo X. Zheng Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J Control Release. 2009;134:111. doi: 10.1016/j.jconrel.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Nie H. Soh B.W. Fu Y.C. Wang C.H. Three-dimensional fibrous PLGA/HAp composite scaffold for BMP-2 delivery. Biotechnol Bioeng. 2008;99:223. doi: 10.1002/bit.21517. [DOI] [PubMed] [Google Scholar]
- 17.Saito A. Suzuki Y. Kitamura M. Ogata S. Yoshihara Y. Masuda S., et al. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an alpha-tricalcium phosphate scaffold. J Biomed Mater Res A. 2006;77:700. doi: 10.1002/jbm.a.30662. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y. Zhang C. Zhang S. Xiong Z. Xu J. Development of a porous poly(L-lactic acid)/hydroxyapatite/collagen scaffold as a BMP delivery system and its use in healing canine segmental bone defect. J Biomed Mater Res A. 2003;67:591. doi: 10.1002/jbm.a.10070. [DOI] [PubMed] [Google Scholar]
- 19.Kofron M.D. Laurencin C.T. Bone tissue engineering by gene delivery. Adv Drug Deliv Rev. 2006;58:555. doi: 10.1016/j.addr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 20.White A.P. Vaccaro A.R. Hall J.A. Whang P.G. Friel B.C. McKee M.D. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31:735. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leach J. Bittar R.G. BMP-7 (OP-1) safety in anterior cervical fusion surgery. J Clin Neurosci. 2009;16:1417. doi: 10.1016/j.jocn.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Brower R.S. Vickroy N.M. A case of psoas ossification from the use of BMP-2 for posterolateral fusion at L4-L5. Spine (Phila Pa 1976) 2008;33:E653. doi: 10.1097/BRS.0b013e31817c4f1c. [DOI] [PubMed] [Google Scholar]
- 23.Allen R.T. Lee Y.P. Stimson E. Garfin S.R. Bone morphogenetic protein-2 (BMP-2) in the treatment of pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976) 2007;32:2996. doi: 10.1097/BRS.0b013e31815cde3e. [DOI] [PubMed] [Google Scholar]
- 24.Wong D.A. Kumar A. Jatana S. Ghiselli G. Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) Spine J. 2008;8:1011. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Perri B. Cooper M. Lauryssen C. Anand N. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J. 2007;7:235. doi: 10.1016/j.spinee.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Levicoff E.A. Kim J.S. Sobajima S. Wallach C.J. Larson J.W., 3rd Robbins P.D., et al. Safety assessment of intradiscal gene therapy II: effect of dosing and vector choice. Spine (Phila Pa 1976) 2008;33:1509. doi: 10.1097/BRS.0b013e318178866c. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haviernik P. Bunting K.D. Safety concerns related to hematopoietic stem cell gene transfer using retroviral vectors. Curr Gene Ther. 2004;4:263. doi: 10.2174/1566523043346174. [DOI] [PubMed] [Google Scholar]
- 28.Kho S.T. Pettis R.M. Mhatre A.N. Lalwani A.K. Safety of adeno-associated virus as cochlear gene transfer vector: analysis of distant spread beyond injected cochleae. Mol Ther. 2000;2:368. doi: 10.1006/mthe.2000.0129. [DOI] [PubMed] [Google Scholar]
- 29.Canalis E. Giustina A. Bilezikian J.P. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 30.Skripitz R. Aspenberg P. Implant fixation enhanced by intermittent treatment with parathyroid hormone. J Bone Joint Surg Br. 2001;83:437. doi: 10.1302/0301-620x.83b3.10256. [DOI] [PubMed] [Google Scholar]
- 31.Andreassen T.T. Ejersted C. Oxlund H. Intermittent parathyroid hormone (1–34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999;14:960. doi: 10.1359/jbmr.1999.14.6.960. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima A. Shimoji N. Shiomi K. Shimizu S. Moriya H. Einhorn T.A., et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34) J Bone Miner Res. 2002;17:2038. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- 33.Andreassen T.T. Willick G.E. Morley P. Whitfield J.F. Treatment with parathyroid hormone hPTH(1–34), hPTH(1–31), and monocyclic hPTH(1–31) enhances fracture strength and callus amount after withdrawal fracture strength and callus mechanical quality continue to increase. Calcif Tissue Int. 2004;74:351. doi: 10.1007/s00223-003-0093-6. [DOI] [PubMed] [Google Scholar]
- 34.Komatsubara S. Mori S. Mashiba T. Nonaka K. Seki A. Akiyama T., et al. Human parathyroid hormone (1–34) accelerates the fracture healing process of woven to lamellar bone replacement and new cortical shell formation in rat femora. Bone. 2005;36:678. doi: 10.1016/j.bone.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Manabe T. Mori S. Mashiba T. Kaji Y. Iwata K. Komatsubara S., et al. Human parathyroid hormone (1–34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone. 2007;40:1475. doi: 10.1016/j.bone.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Nakazawa T. Nakajima A. Shiomi K. Moriya H. Einhorn T.A. Yamazaki M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1–34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37:711. doi: 10.1016/j.bone.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Pettway G.J. Meganck J.A. Koh A.J. Keller E.T. Goldstein S.A. McCauley L.K. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42:806. doi: 10.1016/j.bone.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettway G.J. Schneider A. Koh A.J. Widjaja E. Morris M.D. Meganck J.A., et al. Anabolic actions of PTH (1–34): use of a novel tissue engineering model to investigate temporal effects on bone. Bone. 2005;36:959. doi: 10.1016/j.bone.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Kim C.H. Takai E. Zhou H. von Stechow D. Muller R. Dempster D.W., et al. Trabecular bone response to mechanical and parathyroid hormone stimulation: the role of mechanical microenvironment. J Bone Miner Res. 2003;18:2116. doi: 10.1359/jbmr.2003.18.12.2116. [DOI] [PubMed] [Google Scholar]
- 40.Lu Z. Zreiqat H. Beta-tricalcium phosphate exerts osteoconductivity through alpha2beta1 integrin and down-stream MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 2010;394:323. doi: 10.1016/j.bbrc.2010.02.178. [DOI] [PubMed] [Google Scholar]
- 41.Childress P. Robling A.G. Bidwell J.P. Nmp4/CIZ: road block at the intersection of PTH and load. Bone. 2010;46:259. doi: 10.1016/j.bone.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellido T. Ali A.A. Gubrij I. Plotkin L.I. Fu Q. O'Brien C.A., et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 43.van Bezooijen R.L. Roelen B.A. Visser A. van der Wee-Pals L. de Wilt E. Karperien M., et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kakar S. Einhorn T.A. Vora S. Miara L.J. Hon G. Wigner N.A., et al. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res. 2007;22:1903. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- 45.Esbrit P. Alvarez-Arroyo M.V. De Miguel F. Martin O. Martinez M.E. Caramelo C. C-terminal parathyroid hormone-related protein increases vascular endothelial growth factor in human osteoblastic cells. J Am Soc Nephrol. 2000;11:1085. doi: 10.1681/ASN.V1161085. [DOI] [PubMed] [Google Scholar]
- 46.Deckers M.M. Karperien M. van der Bent C. Yamashita T. Papapoulos S.E. Lowik C.W. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- 47.Diamond A.G. Gonterman R.M. Anderson A.L. Menon K. Offutt C.D. Weaver C.H., et al. Parathyroid hormone hormone-related protein and the PTH receptor regulate angiogenesis of the skin. J Invest Dermatol. 2006;126:2127. doi: 10.1038/sj.jid.5700338. [DOI] [PubMed] [Google Scholar]