Abstract
Tissue engineering utilizes scaffolds containing chondrogenic cells to promote cartilage development at a clinically relevant scale, yet there remains a limited understanding of the optimal conditions for inducing differentiation and matrix production. We investigated how cell density and temporal exposure to chondrogenic factors impacted chondrogenesis of human mesenchymal stem cells (hMSCs) encapsulated in poly(ethylene glycol) diacrylate hydrogels. We found maximal proteoglycan and collagen production in constructs seeded between 10 and 25 × 106 cells/mL. Matrix deposition was significantly less per cell in constructs seeded at either higher or lower densities, indicating that paracrine communications may remain important despite loss of direct cell–cell contact. In vitro chondrogenesis of hMSCs was first accomplished using pellet cultures and a defined medium containing transforming growth factor (TGF)-β1 and dexamethasone. The differentiation of hMSCs in hydrogels also required initial exposure to TGF-β1, with no chondrogenic matrix produced in its absence. If TGF-β1 was initially included for at least 7 days, its removal impacted collagen production per cell but also lead to an increase in cell number, such that total collagen deposition was equivalent to controls when TGF-β1 was included for at least 3 weeks. Further, proteoglycan content per construct was higher at 6 weeks after removal of TGF-β1 at any time. In contrast to TGF-β1, dexamethasone was not required for chondrogenesis of hMSCs in hydrogels: there was no difference in matrix deposition between hydrogels cultured with or without dexamethasone. Further, without dexamethasone, SOX9 gene expression was higher during early chondrogenesis and there was a significant reduction in collagen I deposition, suggesting that a more hyaline cartilage phenotype is achieved without dexamethasone. Collagen content at 6 weeks was lower if dexamethasone was excluded after the first 7 days, but was equivalent to control if dexamethasone was included for 2 weeks or longer. Proteoglycan deposition was unaffected by dexamethasone exclusion. These results indicate that modulating exposure to TGF-β1 is beneficial for cell survival/proliferation and matrix production from hMSCs in hydrogels, and that not only is dexamethasone dispensable but also its exclusion may be advantageous for forming hyaline cartilage.
Introduction
Mesenchymal stem cells (MSCs) are chondroprogenitors that offer a possible alternative to chondrocytes in cartilage engineering.1–3 They are more accessible than native chondrocytes, expand readily in vitro, and are capable of differentiating along multiple lineages, including toward chondrocytes.4 In vitro chondrogenesis of bone marrow-derived MSCs was first accomplished by our group in 1998 utilizing scaffold-free pellet culture to approximate the mesenchymal condensation observed during limb development.5,6 The defined medium that facilitated differentiation included transforming growth factor-β1 (TGF-β1) and dexamethasone. Although TGF-β1 or dexamethasone alone was sufficient to promote chondrogenesis in some preparations, the combination of these chondrogenic factors provided consistent differentiation and matrix production.2,6 Since these initial findings, other groups have validated this relationship7 and/or studied the effect of these chondrogenic factors on MSCs derived from other sources such as adipose tissue and periosteum.8–12
Pellet culture, in its original form, had a size limitation that precluded this technology from translating directly to use in human articular cartilage defects. In 2003, Williams et al. showed that goat MSCs could be photoencapsulated into hydrogel scaffolds to facilitate chondrogenesis on a much larger scale13 despite the loss of direct cell–cell communication that was postulated to be very important for differentiation in the pellet culture system.5,6 Since this work, scaffold design has become more sophisticated by tuning the physical (pore size and stiffness) and chemical (bioactive, biomimetic, and biodegradable) properties of the scaffold to enhance matrix production during chondrogenesis (recently reviewed in refs.14–16). We previously described the use of a poly(ethylene glycol) diacrylate (PEGDA)-based semiinterpenetrating network (sIPN) for improved elaboration and distribution of extracellular matrix from differentiating human MSCs (hMSCs).17 The purpose of the present study was to examine the effect of hMSC cell density and the timing of exposure to chondrogenic factors on subsequent matrix development in this PEGDA-based sIPN.
Materials and Methods
MSC isolation and culture
MSCs were isolated and expanded from human iliac crest bone marrow aspirates as previously described.5,6,17 Briefly, bone marrow was obtained from the iliac crests of consenting male and female donors with an age range of 21–79. Marrow aspirates were fractionated on a Percoll density gradient and plated in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum. Adherent cells were cultured at 37°C, 5% CO2 with medium changes every 4 days. Once primary cells were confluent, serum-containing DMEM was supplemented with basic fibroblast growth factor (10 ng/mL) to facilitate postprimary passage expansion with retention of chondrogenic potential. Expanded hMSCs were used after 3–8 population doublings (passage 1 or 2).
Preparation of PEGDA
PEGDA (6 kDa) was kindly provided by Jennifer West, Rice University, TX. It was synthesized as previously described.18 Briefly, PEGDA was prepared by combining 0.1 mM dry PEG with 0.4 mM acryloyl chloride and 0.2 mM triethylamine in anhydrous dichloromethane and stirred under argon overnight. The mixture was then lyophilized and frozen.
Cellular encapsulation and culture
Expanded hMSCs were photoencapsulated in a PEGDA-based sIPN with final concentration of 16% (w/v) PEGDA and 32% (w/v) PEG-n-dimethyl ether (PEG, n = 2000, MW 88 kDa) as previously described.17 Briefly, 32% (w/v) PEGDA and 64% (w/v) PEG was swollen in phosphate-buffered saline and filter sterilized using a 0.2 μm syringe filters. The monomer solution was then combined in equal volume with expanded cells for final cell densities ranging from 1.25 to 50 × 106 cells/mL. The cell–monomer mixture was photopolymerized into disk shaped hydrogels (7 mm diameter by 2 mm thick) after 6 min exposure to UV light (365 nm, 6mW) with 0.06% (w/v) Irgacure™ D2959 (Ciba) initiator. Hydrogels were cultured at 37°C, 5% CO2 for 6 weeks in a defined chondrogenic medium.5,6 Complete defined medium consisted of high-glucose DMEM with ITS+ Premix (Collaborative Biomedical Products), sodium pyruvate (1 mM), ascorbate-2-phosphate (37.5 μg/mL), dexamethasone (10−7 M), TGF-β1 (10 ng/mL, recombinant human; Peprotech), and l-glutamine (4 mM). For some experiments, dexamethasone or TGF-β1 was excluded from the culture medium, or withdrawn after 7, 14, 21, or 28 days of culture. For comparison, control hydrogels were fabricated from hMSCs from the same human preparation and continuously exposed to the complete chondrogenic medium containing both TGF-β1 and dexamethasone.
Histology and immunohistochemistry
Hydrogels were fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned onto slides at 7 μm. Representative slides were deparaffinized and stained with toluidine blue to observe sulfated proteoglycans. Immunohistochemistry was used to detect collagens I (kind gift from Anthony Hollander, University of Bristol, United Kingdom), II (II-II6B3, NIH Hybridoma Bank, University of Iowa), and X (kind gift from Gary Gibson, Henry Ford Hospital, Detroit, MI). For collagen II and X staining, sections were deparaffinized, blocked in 5% bovine serum albumin for 1 h at room temperature, and exposed to a 20 min pronase (1 mg/mL in phosphate-buffered saline) digestion at room temperature before reaction with monoclonal antibodies. Slides were exposed to the primary antibody at a 1:200 dilution in 1% bovine serum albumin and incubated overnight at 4°C. Collagen I staining required 1 h of antigen retrieval (Dako Target Retrieval Solution, 75°C water bath) before the blocking step, followed by sequential hyaluronidase (1%, 15 min at 37°C) and pronase (1 mg/mL, 15 min at 37°C) digestions. The collagen I antibody was diluted 1:400. AlexaFluor™ 594-linked secondary antibodies were used for detection of all collagen antibodies. Images were converted to grayscale using Photoshop.
Biochemical assays
After 6 weeks of in vitro culture, hydrogels were removed from the culture media and digested with 0.1 N NaOH overnight at 60°C. Samples were neutralized with 0.1 N HCl and then digested for an additional 18 h at 60°C with 125 μg/mL papain in 10 mM ethylenediaminetetraacetic acid and 2 mM cysteine, pH 6.0 (Sigma). Sulfated proteoglycans in the digested cell–polymer construct were quantified spectrophotometrically using the 1,9-dimethylmethylene blue (DMMB) dye assay (pH 3.0; Polysciences).19 Sample proteoglycan content was compared to shark cartilage chondroitin sulfate standards (Sigma-Aldrich). DNA content was determined spectrofluorimetrically using the PicoGreen fluorescent DNA binding dye assay according to manufacturer's instructions (P-7581; Molecular Probes). Sample fluorescence was compared to calf thymus DNA standards (Sigma). Total collagen content was determined by oxidation of hydroxyproline residues in collagen with chloramine T trihydrate (ICN Biomedicals), developed with p-dimethylaminobenzaldehyde (Ehrlich's reagent; ICN Biomedicals).20 Hydroxyproline content represents total collagen synthesis.20 Sample concentrations were compared to hydroxyproline standard solutions made from trans-4-hydroxy-L-proline (Fluka). Each series of experiments was run using a minimum of three different marrow cell isolates and at least three replicate hydrogels for each condition. Data represent a mean ± standard deviation. Statistical significance was set at a value of p < 0.05 and data were analyzed with ANOVA and Dunnett's statistical tests.
mRNA isolation and quantitative reverse transcriptase-polymerase chain reaction
Hydrogels were harvested into 1 mL TRIzol reagent (Invitrogen) and homogenized with the Ultramax IKA-T10 homogenizer. Homogenates were left at room temperature for 5 min to facilitate mRNA extraction and then centrifuged at 12,000 g for 15 min. The supernatant was removed and stored at −80°C until all samples had been harvested; mRNA was extracted per manufacturer's instructions. cDNA was reverse transcribed using a cDNA synthesis kit (Quanta qScript™ cDNA Synthesis) with 1 μg mRNA/20 μL reaction. Quantitative real-time reverse transcriptase (RT)-polymerase chain reaction (PCR) analysis utilized the TaqMan Assay primer/probes and the PCR master mix (Quanta PerfeCta® qPCR FastMix®). Results were analyzed using gene expression relative to endogenous control, 18S, and MSC gene expression before photoencapsulation (ΔΔCT). RT-PCR data were expressed as mean fold change (2ΔΔCT) ± 95% confidence. Significance was set as p-values <0.05 and tested using an unpaired two-tailed t-test.
Results
hMSC concentration
To explore the relationship between cell concentration and matrix production in hydrogel scaffolds, hMSCs were photoencapsulated into PEGDA-based sIPNs at concentrations between 1.25 and 50 × 106 cells/mL (Figs. 1 and 2). DNA content (Fig. 1A) and extracellular matrix production (Fig. 1B, C) was quantified after 6 weeks of culture in defined chondrogenic medium containing both TGF-β1 and dexamethasone. Proteoglycan and collagen matrix deposition per cell was highest at concentrations between 10 and 25 × 106 cells/mL, with the highest total matrix content at 25 × 106 cells/mL. Encapsulating cells at a higher density of 50 × 106 cells/mL did not result in greater total matrix elaboration, but rather inhibited matrix production on a per cell basis. Similarly, at cell concentrations below 10 × 106 cells/mL, both proteoglycan and collagen production per cell were significantly decreased. Toluidine blue-stained sections confirmed differences in proteoglycan content and also indicated differences in distribution (Fig. 2). At lower concentrations matrix deposition was predominantly pericellular, but more interterritorial matrix staining was observed as hMSC concentration increased. Further, pericellular staining at the lowest concentrations—5 × 106 cells/mL and lower—appeared less intense. In all cases, proteoglycan deposition was detectable around cells throughout the constructs after 6 weeks of in vitro culture.
FIG. 1.
Comparison of extracellular matrix content at 6 weeks in semiinterpenetrating network scaffolds with human mesenchymal stem cells photoencapsulated at initial concentrations ranging from 1.25 to 50 × 106 cells/mL. (A) DNA, (B) proteoglycan, and (C) hydroxyproline content. Data represent mean ± standard deviation. *p < 0.001 compared with per construct results for 50 × 106 cells/mL, and +p < 0.001 compared with 50 × 106 cells/mL measurements normalized for DNA content.
FIG. 2.
Toluidine blue staining of constructs seeded at (A, E) 25 × 106 cell/mL, (B, F) 10 × 106 cell/mL, (C, G) 5 × 106 cell/mL, (D, H) and 2.5 × 106 cells/mL. Scale bars: A–D = 500 μm, E–H = 200 μm.
TGF-β1 withdrawal during hydrogel culture
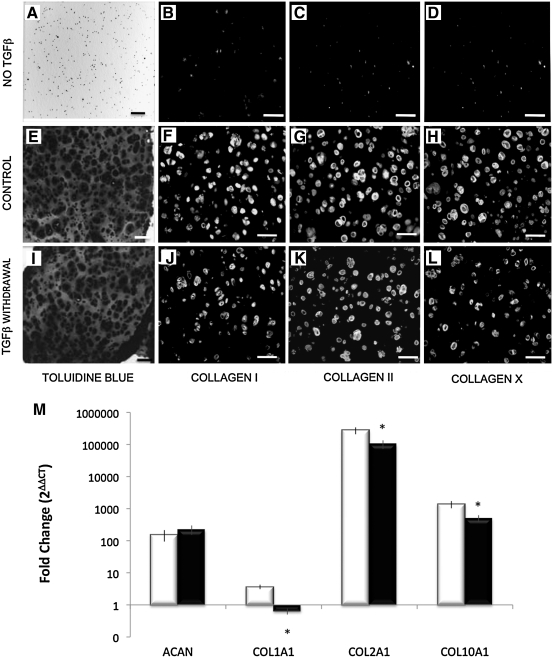
On the basis of the optimized cell concentration of 25 × 106 cells/mL, a series of experiments were run to determine the relationship between matrix deposition and temporal exposure to chondrogenic factors TGF-β1 and dexamethasone. To study the continued requirement for TGF-β1, the growth factor was withdrawn from chondrogenic medium at day 7, 14, 21, or 28 (Figs. 3 and 4). We found constructs cultured without TGF-β1 underwent minimal chondrogenesis, indicating that this growth factor is essential for initiation of hMSC differentiation (Fig. 4A–D). Constructs had higher total DNA and proteoglycan content at 6 weeks when TGF-β1 was withdrawn at any time point when compared with controls that received continuous exposure to TGF-β1 (Fig. 3A, B). Proteoglycan content normalized to DNA was unchanged by TGF-β1 withdrawal (Fig. 3B). In contrast, hydroxyproline content, a quantification of total collagen, was significantly decreased after TGF-β1 withdrawal at any time point when normalized to DNA content. Given the relative increase in cell number, collagen content per construct was comparable to control provided hydrogels received TGF-β1 for at least 21 days (Fig. 3C). Immunohistochemistry and quantitative real-time PCR of hydrogel constructs cultured for 6 weeks were used to analyze the individual contribution of collagens I, II, and X in the neocartilage construct after TGF-β1 withdrawal at 21 days (Fig. 4I–L). A clear reduction in both collagen I (Fig. 4F, J) and X (Fig. 4H, L) deposition is evident from immunohistochemistry, but RT-PCR showed a significant decrease in gene expression of all three collagens (Fig. 4M).
FIG. 3.
Hydrogels were grown in vitro for 42 days in chondrogenic medium including TGF-β1 (control) or TGF-β1 was omitted after 7, 14, 21, or 28 days. Hydrogels were quantified for (A) DNA, (B) sulfated proteoglycans, and (C) hydroxyproline; (gray) per construct or (black) normalized for DNA content. *p < 0.05 compared with per construct control, and +p < 0.05 compared with control per DNA. TGF, transforming growth factor.
FIG. 4.
Toluidine blue and collagens I, II, and X immunohistochemistry of constructs cultured for 42 days (A–D) without TGF-β1, (E–H) with continuous TGF-β1 and dexamethasone supplementation, control, or (I–L) TGF-β1 withdrawal at 21 days; scale bars = 100 μm. (M) Quantitative reverse transcriptase (RT)-PCR for fold change in gene expression of ACAN, COL1A1, COL2A1, and COL10A1 in control versus TGF-β1 withdrawal at 21 days; *p < 0.05 compared with control per DNA. PCR, polymerase chain reaction.
Hydrogel culture without dexamethasone
In contrast to hMSCs cultured in hydrogels without TGF-β1 supplementation, those cultured without dexamethasone consistently underwent chondrogenesis (Fig. 5). Deposition of proteoglycans and hydroxyproline was not significantly different between hydrogels cultured without dexamethasone and control constructs receiving both dexamethasone and TGF-β1 (Fig. 5A). However, differences were observed from a more detailed study of the chondrogenic phenotype in the resultant neocartilage using immunohistochemistry (Fig. 5B–I) and gene expression analysis (Fig. 5J, K). Cartilage markers, aggrecan and collagen II, were similar at both a protein (Fig. 5B, F, D, H) and gene level (Fig. 5J, K) in constructs cultured with or without dexamethasone. In contrast, immunohistochemistry revealed a decrease in deposition of both collagens I (Fig. 5C, G) and X (Fig. 5E, I) in constructs cultured without dexamethasone (Fig. 5G, I). COL1A expression was also significantly decreased at both 1 and 6 weeks (Fig. 5J, K) in the absence of dexamethasone, but the decrease in COL10A1 was not statistically significant (p = 0.06, Fig. 5J, K). To investigate the differentiation potential of hMSCs cultured with and without dexamethasone, we also looked at gene expression of SOX9, an early marker of chondrogenic potential. SOX9 was significantly increased in hMSCs cultured without dexamethasone at 1 week (Fig. 5J), but returned to control levels late in culture (Fig. 5K).
FIG. 5.
(A) Proteoglycan and hydroxyproline content per construct for hydrogels cultured with or without dexamethasone. (B, F) Toluidine blue, (C, G) collagen I, (D, H) collagen II, and (E, I) collagen X staining of hydrogels cultured (B–E) with or (F–I) without dexamethasone for 6 weeks; scale bars = 100 μm. Quantitative RT-PCR for ACAN, COL1A1, and COL2A1 relative gene expression after (J) 1 or (K) 6 weeks of culture either (white) with or (black) without dexamethasone. *p < 0.05 compared with dexamethasone treated samples.
Dexamethasone withdrawal during hydrogel culture
The withdrawal of dexamethasone (Figs. 6 and 7) was tested in a similar fashion to the TGF-β1 withdrawal experiments described above (Figs. 3 and 4). Total DNA and proteoglycan content were unaltered by dexamethasone withdrawal at days 7, 14, 21, or 28 (Fig. 6A, B). However, collagen content was decreased in constructs that received a 7 day exposure to dexamethasone (Fig. 6C). Collagen content was equivalent to control levels, provided that dexamethasone was included in the medium for 14 days or more. Histological images show similar proteoglycan staining and collagen immunohistochemistry for hydrogels that received continuous exposure to dexamethasone (Fig. 7A–D) and those from which dexamethasone was withdrawn after 21 days of culture (Fig. 7E–H). However, RT-PCR indicated a significant decrease in COL2A1 gene expression (Fig. 7I).
FIG. 6.
Hydrogels were grown in vitro for 42 days in chondrogenic medium including dexamethasone (control), or dexamethasone was omitted after 7, 14, 21, and 28 days. Hydrogels were quantified for (A) DNA, (B) sulfated proteoglycans, and (C) hydroxyproline, per construct or normalized to DNA. *p < 0.05 compared with control per construct, +p < 0.05 compared with control per DNA.
FIG. 7.
Toluidine blue and collagens I, II, and X immunohistochemistry of constructs at cultured for 42 days either with (A–D) continuous TGF-β1 and dexamethasone supplementation, control, or (E–H) with withdrawal of dexamethasone at 21 days; scale bars = 100 μm. (I) Quantitative RT-PCR for fold change in gene expression of ACAN, COL1A1, COL2A1, and COL10A1 in control versus dexamethasone withdrawal at 21 days. *p < 0.05 compared with control.
Discussion
Engineering a clinically useful cartilage regenerate offers a potential therapeutic treatment for joint degeneration, which currently accounts for a high percentage of the disability in the U.S. population.21,22 Presently, there is no clear understanding of the characteristics required of a neocartilaginous implant to induce repair of joint cartilage. Neocartilage that has production and assembly of an extracellular matrix with ultrastructural similarity to native articular cartilage would seem to be advantageous. An important step in this process is maximizing production of extracellular matrix proteins and facilitating interterritorial assembly within implants. We previously published data demonstrating increased extracellular matrix production and improved interterritorial distribution of proteoglycans by manipulation of the physical parameters of a PEGDA-based sIPN scaffold.17 In the present study we investigate how the cell density and temporal exposure to chondrogenic factors affect differentiation and in vitro neocartilage production in this sIPN scaffold. These data can be applied to engineer more effective implants for cartilage repair.
The first series of experiments were designed to investigate the relationship between hMSC concentration and matrix production. By seeding scaffolds with initial cell concentrations ranging from 1.25 to 50 × 106 cells/mL and analyzing matrix production after 6 weeks of in vitro culture, we found 25 × 106 cells/mL provided the optimal balance between matrix production per cell and per construct. Matrix production on a per cell basis was similar at seeding densities between 10 and 25 × 106 cells/mL; however, the lower cell density resulted in reduced total matrix content in the construct. This concentration range corresponds to the cell density of human femoral condyle cartilage, which was measured at 14.1 ± 3.2 × 106 cells/mL.23 At cell concentrations below 10 × 106 cells/mL in the hydrogel, there was a significant decrease in matrix production on a per cell basis, indicating that paracrine communication may still be important for optimal chondrogenesis within scaffolds. Interestingly, cellular density above 25 × 106 cells/mL did not improve total proteoglycan or collagen content. Rather, at 50 × 106 cells/mL we observed reduced production of matrix elements on a per cell basis. This decreased cellular productivity may be the result of nutrient limitations due to restricted transport to the cells through the elaborated extracellular matrix, and/or because of rapid depletion of medium nutrients in higher density cultures. For example, chondrocytes are highly glycolytic cells and glucose metabolism is important for maintaining cell homeostasis and stimulating anabolic production of glycosaminoglycan for the extracellular matrix.24–26 Alternatively, the increased cellular density and local accumulation of extracellular matrix may result in paracrine or autocrine feedback signals that cause cells to attenuate protein production. Kavalkovich et al. has also investigated the relationship between hMSC density and matrix production in hydrogels using an alginate layer system. They reported a similar relationship between differentiation and initial seeding density, finding that 25 × 106 cells/mL resulted in optimal proteoglycan synthesis per cell.27 The importance of cell density to matrix production in both of these systems indicates that despite loss of direct cell–cell contact in scaffolds, paracrine communication likely influences differentiation and matrix elaboration.
With this optimized hMSC concentration, we explored the impact of temporal exposure to the chondrogenic factors—TGF-β1 and dexamethasone—on extracellular matrix deposition in the sIPN scaffold. Specifically, we wanted to test whether both factors were required to initiate chondrogenesis, as documented in the pellet culture system,2,4–6 and whether sustained stimulation was required to maintain matrix protein production. These are important considerations for the use of MSCs in cartilage engineering applications since it is necessary to both facilitate differentiation and maximize matrix protein production. We first examined TGF-β, which has been documented to play an important role during both early28,29 and late phases30 of chondrogenesis by both initiating differentiation4–6,13 and increasing production of extracellular matrix proteins.7,31 As previously determined in pellet culture, we found that TGF-β1 was required for initiating differentiation of hMSCs in the PEGDA-based sIPN. Constructs cultured without TGF-β1 did not produce measurable quantities of either proteoglycan or collagen.
Despite the requirement for TGF-β1 to initiate chondrogenesis, sustained exposure had a differential effect on proteoglycan and collagen production. Proteoglycan content of the constructs was increased after 6 weeks of culture when TGF-β1 was withdrawn from the medium at any point after the first 7 days. However, DNA content was also significantly higher with TGF-β1 withdrawal, such that the proteoglycan production per cell was not significantly different between this and the control condition. Thus, one effect of TGF-β1 withdrawal appears to be either greater cell proliferation and/or cell survival. Additionally, sustained TGF-β1 supplementation does not appear to be essential for proteoglycan synthesis.
In contrast, collagen content per cell was significantly reduced by withdrawal of TGF-β1 at any time point compared with sustained exposure, suggesting that TGF-β has a more direct role in collagen biosynthesis. O'Driscoll et al. have also examined the effects of limiting chondroprogenitor cell exposure to TGF-β1.9,11 They reported that chondrogenesis of rabbit periosteal explants cultured in agarose was similar after either 2 or 14 days of TGF-β1 treatment. However, matrix quantification was not normalized to DNA content. Since our data indicate a higher number of cells after TGF-β1 withdrawal, this may explain the discrepancy between the studies.
When we altered the experimental conditions to examine the role of dexamethasone, we found that it was not required for differentiation of hMSCs in hydrogels. Gene and protein measurements indicated that key markers of cartilage, aggrecan and collagen II, were produced similarly in constructs cultured with or without dexamethasone. This result provides a clear contrast with the pellet culture system where we found chondrogenesis in only a small number of preparations cultured without dexamethasone, and that inclusion of dexamethasone substantially increased matrix production and provided chondrogenesis in all preparations.2,5,6
Dexamethasone is a synthetic glucocorticosteroid (GC) reported to promote differentiation in a number of systems, including the chondrogenic6 and osteogenic32 differentiation of MSCs. The precise mechanism through which dexamethasone promotes chondrogenesis is unknown, but Derfoul et al. have presented data showing dexamethasone acts indirectly through the major active form of the GC receptor, GCa, to induce cartilage matrix genes in pellet culture.7 It is presently unclear what factors replace the need for dexamethasone in hydrogel-based culture of hMSCs. Fundamental differences clearly exist between scaffold-free pellets and hydrogels. Pellet culture was designed to approximate the mesenchymal condensation process and it was postulated that direct cell–cell communication was very important for differentiation.5,6 However, MSC chondrogenesis has now been established in a variety of scaffold-based systems that significantly limit direct cell–cell contact,13,17,33,34 implying that intercellular contact is not required for chondrogenesis. The common element to both in vitro culture systems is the spherical or rounded cellular conformation of hMSCs. This is achieved without cell–cell connections in the hydrogels compared with pellets. That such cell–cell connections are of secondary importance to a chondrogenic progenitor cell is long established; Solursh et al. first noted that rounded solitary progenitor cells express cartilage matrix molecules,35 and they and others established that the induction of rounding of solitary progenitor cells induces chondrogenesis.35–37 They further demonstrated that it is not simply round cell shape that promotes differentiation, but the disruption of mechanotransduction mechanisms.38 This occurs in hydrogel encapsulation of hMSCs since the cells do not establish substratum connections with PEG. Thus, the rapid stabilization of the three-dimensional shape with disrupted mechanotransduction, and the increased access to TGF-β, which can readily diffuse throughout the hydrogel, may be important in facilitating chondrogenesis in the absence of dexamethasone in this system.
A secondary finding from culture without dexamethasone was an apparent change in the chondrogenic phenotype. SOX9 gene expression, a marker of early chondrogenesis, was more highly activated after 1 week of culture without dexamethasone than in control cultures containing dexamethasone. We also saw decreased deposition of both collagens I and X after 6 weeks of culture by immunohistochemistry. COL1A gene expression was also decreased throughout culture, whereas the decrease in COL10A1 was not significant. It is well established that MSCs undergoing chondrogenesis have a propensity to express collagens I and X.6,39 The presence of these nonhyaline collagens during in vitro differentiation has led to the notion that use of MSCs in vivo for repair of full-thickness cartilage lesions will result in the formation of a fibrocartilaginous construct40 or endochondral ossification.41,42 These are important considerations since the biomechanical properties of articular cartilage are the product of the unique, anisotropic assembly of collagen II and proteoglycans in the extracellular matrix.43,44 Chondrogenesis in vivo is classically studied through the process of endochondral ossification that occurs during long bone formation, relatively little is known about the developmental processes that produce either hyaline or fibrocartilage.45–47 It is possible that differentiation of the progenitor cells toward a more hyaline chondrocyte phenotype occurs in the absence of dexamethasone, whereas exposure to dexamethasone promotes fibrocartilage development.
To address the temporal effect of dexamethasone, withdrawal of the glucocorticoid after initial inclusion in the medium was also studied in the same manner as the experiments performed with TGF-β1. Proteoglycan content at 6 weeks was unaltered by dexamethasone withdrawal at any time and collagen content was similar to control levels with dexamethasone treatment for more than 7 days. However, there was a significant reduction in collagen content after a short (7 day) exposure to dexamethasone. These data are contrary to what one would predict given the similar matrix production from hMSCs never exposed to dexamethasone and further supports the notion that the initial exposure to dexamethasone can influence the phenotype of the resultant chondrocyte.
In conclusion, our experiments define an optimal cell concentration for hMSCs in a PEGDA-based sIPN and demonstrate a clear relationship between cell concentration and extracellular matrix deposition. After optimizing cell density we explored the relationship between chondrogenic factor exposure and chondrogenesis of hMSCs in vitro. Significantly, we found that dexamethasone is dispensable in the hydrogel-based culture of hMSCs. In contrast, TGF-β1 is required to initiate MSC differentiation and sustained exposure enhances collagen synthesis. However, TGF-β1 withdrawal also increases either cell proliferation or survival such that collagen production per cell is equivalent to control constructs if TGF-β1 supplementation is sustained for at least 21 days. This has a clear implication for cartilage engineering strategies. Collagens form a structural network for cartilage that provides the tensile strength essential for appropriate joint biomechanics. Sufficient production and distribution of collagen in neocartilage constructs will be essential to producing a mechanically suitable replacement tissue and this has proven to be a considerable challenge in the field of engineering functional cartilage.
Acknowledgments
The authors would like to thank Teresa Pizzuto (Case Western Reserve University, Cleveland) for her assistance in histology preparations and Dr. Jamie Fitzgerald (Oregon Health and Science University) for his review of the article. Studies in this article were supported in part by NIH RO1 AR048132 (B.J.).
Disclosure Statement
Drs. Johnstone and Yoo receive royalties for the license of the patented method for in vitro chondrogenesis to Osiris Therapeutics, Inc., Columbia, MD.
References
- 1.Johnstone B. Yoo J. Mesenchymal cell transfer for articular cartilage repair. Expert Opin Biol Ther. 2001;1:915. doi: 10.1517/14712598.1.6.915. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone B. Yoo J. Stewart M. Cell Sources for Cartilage Tissue Engineering. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- 3.Richardson S.M. Hoyland J.A. Mobasheri R. Csaki C. Shakibaei M. Mobasheri A. Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J Cell Physiol. 2010;222:23. doi: 10.1002/jcp.21915. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Yoo J.U. Barthel T.S. Nishimura K. Solchaga L. Caplan A.I. Goldberg V.M., et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 7.Derfoul A. Perkins G.L. Hall D.J. Tuan R.S. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24:1487. doi: 10.1634/stemcells.2005-0415. [DOI] [PubMed] [Google Scholar]
- 8.Awad H.A. Halvorsen Y.D. Gimble J.M. Guilak F. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 2003;9:1301. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 9.Miura Y. Fitzsimmons J.S. Commisso C.N. Gallay S.H. O'Driscoll S.W. Enhancement of periosteal chondrogenesis in vitro. Dose-response for transforming growth factor-beta 1 (TGF-beta 1) Clin Orthop Relat Res. 1994;301:271. [PubMed] [Google Scholar]
- 10.Mizuta H. Sanyal A. Fukumoto T. Fitzsimmons J.S. Matsui N. Bolander M.E., et al. The spatiotemporal expression of TGF-beta1 and its receptors during periosteal chondrogenesis in vitro. J Orthop Res. 2002;20:562. doi: 10.1016/S0736-0266(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 11.Miura Y. Parvizi J. Fitzsimmons J.S. O'Driscoll S.W. Brief exposure to high-dose transforming growth factor-beta1 enhances periosteal chondrogenesis in vitro: a preliminary report. J Bone Joint Surg Am. 2002;84-A:793. doi: 10.2106/00004623-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Diekman B.O. Rowland C.R. Caplan A.I. Lennon D. Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage derived matrix. Tissue Eng Part A. 2010;16:523. doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams C.G. Kim T.K. Taboas A. Malik A. Manson P. Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 14.Nicodemus G.D. Bryant S. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:149. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 16.Ifkovits J.L. Burdick J.A. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 17.Buxton A.N. Zhu J. Marchant R. West J.L. Yoo J.U. Johnstone B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007;13:2549. doi: 10.1089/ten.2007.0075. [DOI] [PubMed] [Google Scholar]
- 18.Mann B.K. Schmedlen R.H. West J.L. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 19.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 20.Sanford L.P. Ormsby I. Gittenberger-de Groot A.C. Sariola H. Friedman R. Boivin G.P., et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Targeting arthritis. Department of Health and Human Services. Atlanta, GA: 2003. [Google Scholar]
- 22.Murphy L. Schwartz T.A. Helmick C.G. Renner J.B. Tudor G. Koch G., et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stockwell R.A. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109:411. [PMC free article] [PubMed] [Google Scholar]
- 24.Mobasheri A. Bondy C.A. Moley K. Mendes A.F. Rosa S.C. Richardson S.M., et al. Facilitative Glucose Transporters in Articular Chondrocytes. New York, NY: Springer; 2008. [PubMed] [Google Scholar]
- 25.Mobasheri A. Vannucci S.J. Bondy C.A. Carter S.D. Innes J.F. Arteaga M.F., et al. Glucose transport and metabolism in chondrocytes: a key to understanding chondrogenesis, skeletal development and cartilage degradation in osteoarthritis. Histol Histopathol. 2002;17:1239. doi: 10.14670/HH-17.1239. [DOI] [PubMed] [Google Scholar]
- 26.Otte P. Basic cell metabolism of articular cartilage. Manometric studies. Z Rheumatol. 1991;50:304. [PubMed] [Google Scholar]
- 27.Kavalkovich K.W. Boynton R.E. Murphy J.M. Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 2002;38:457. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Chimal-Monroy J. Diaz de Leon L. Expression of N-cadherin, N-CAM, fibronectin and tenascin is stimulated by TGF-beta1, beta2, beta3 and beta5 during the formation of precartilage condensations. Int J Dev Biol. 1999;43:59. [PubMed] [Google Scholar]
- 29.Tuli R. Tuli S. Nandi S. Huang X. Manner P.A. Hozack W.J., et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 30.Serra R. Johnson M. Filvaroff E.H. LaBorde J. Sheehan D.M. Derynck R., et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe H. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-β-induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem. 2001;227:14466. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- 32.Cheng S.L. Yang J.W. Rifas L. Zhang S.F. Avioli L.V. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 33.Mauck R.L. Yuan X. Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Salinas C.N. Cole B.B. Kasko A.M. Anseth K.S. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 2007;13:1025. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- 35.Solursh M. Linsenmayer T.F. Jensen K.L. Chondrogenesis from single limb mesenchyme cells. Dev Biol. 1982;94:259. doi: 10.1016/0012-1606(82)90090-2. [DOI] [PubMed] [Google Scholar]
- 36.Solursh M. Reiter R.S. Determination of limb bud chondrocytes during a transient block of the cell cycle. Cell Differ. 1975;4:131. doi: 10.1016/0045-6039(75)90034-2. [DOI] [PubMed] [Google Scholar]
- 37.Archer C.W. Rooney P. Wolpert L. Cell shape and cartilage differentiation of early chick limb bud cells in culture. Cell Differ. 1982;11:245. doi: 10.1016/0045-6039(82)90072-0. [DOI] [PubMed] [Google Scholar]
- 38.Zanetti N.C. Solursh M. Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J Cell Biol. 1984;99:115. doi: 10.1083/jcb.99.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelttari K. Steck E. Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 40.Bedi A. Feeley B.T. Williams R.J., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 41.Dickhut A. Pelttari K. Janicki P. Wagner W. Eckstein V. Egermann M., et al. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 42.Pelttari K. Winter A. Steck E. Goetzke K. Hennig T. Ochs B.G., et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 43.Carter D.R. Beaupre G.S. Wong M. Smith R.L. Andriacchi T.P. Schurman D.J. The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res. 2004;S69 doi: 10.1097/01.blo.0000144970.05107.7e. [DOI] [PubMed] [Google Scholar]
- 44.Wong M. Carter D.R. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1. doi: 10.1016/s8756-3282(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 45.Khan I.M. Redman S.N. Williams R. Dowthwaite G.P. Oldfield S.F. Archer C.W. The development of synovial joints. Curr Top Dev Biol. 2007;79:1. doi: 10.1016/S0070-2153(06)79001-9. [DOI] [PubMed] [Google Scholar]
- 46.Ito M.M. Kida M.Y. Morphological and biochemical re-evaluation of the process of cavitation in the rat knee joint: cellular and cell strata alterations in the interzone. J Anat. 2000;197(Pt 4):659. doi: 10.1046/j.1469-7580.2000.19740659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyama E. Shibukawa Y. Nagayama M. Sugito H. Young B. Yuasa T., et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316:62. doi: 10.1016/j.ydbio.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]