Abstract
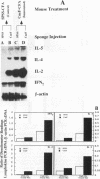
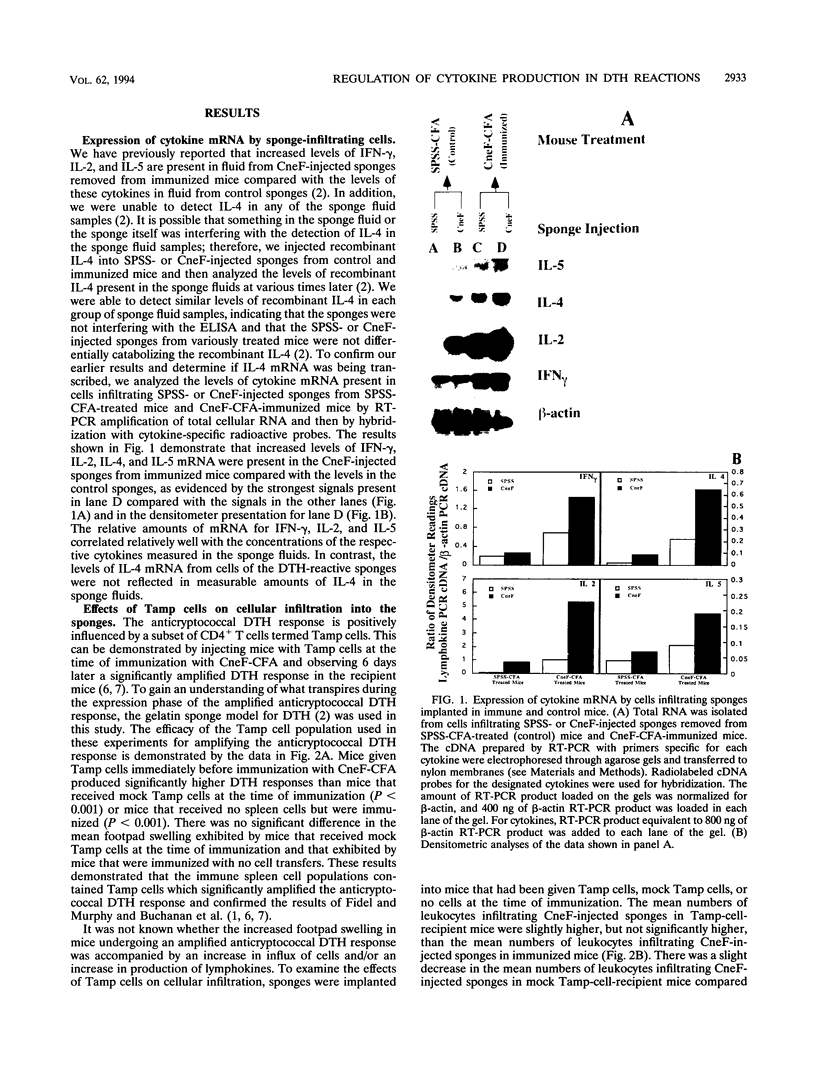
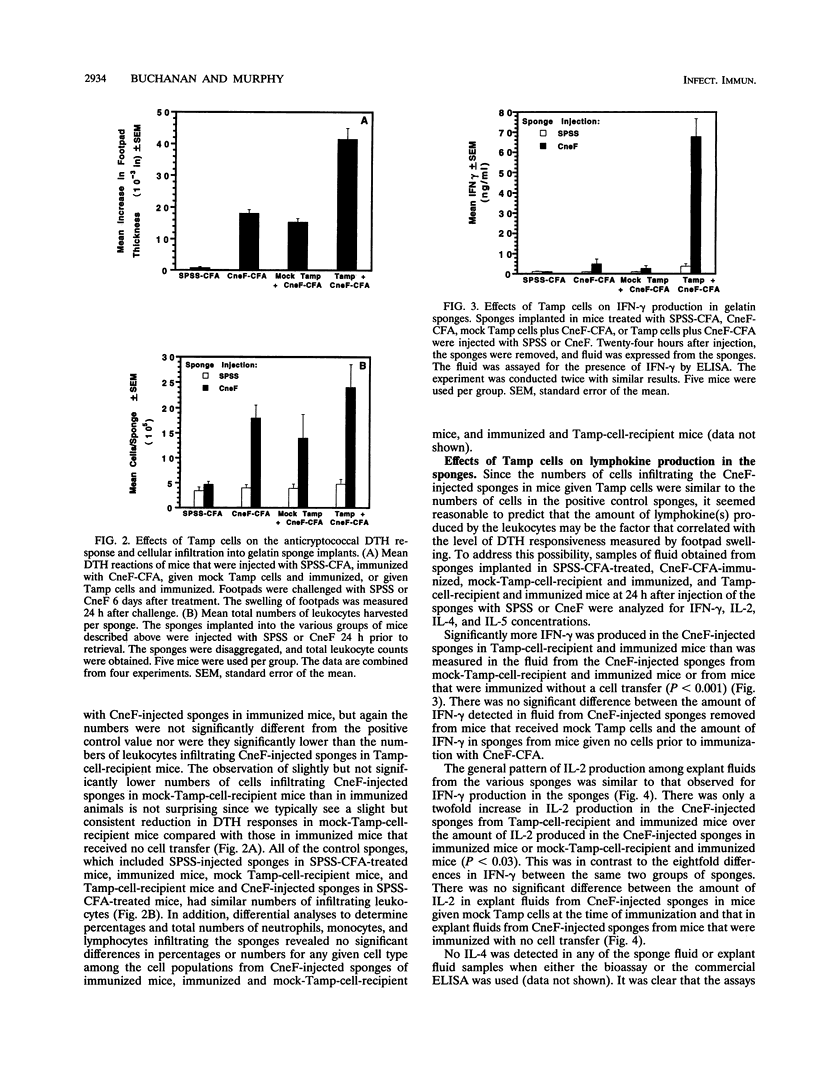
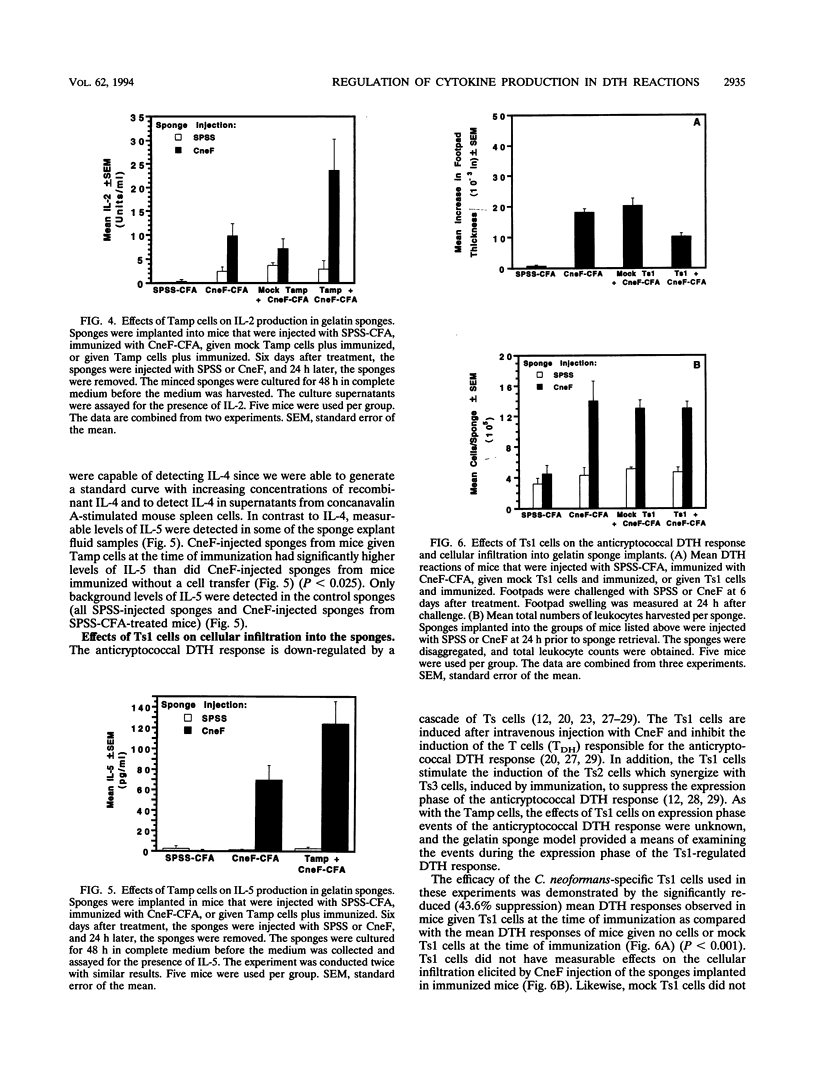
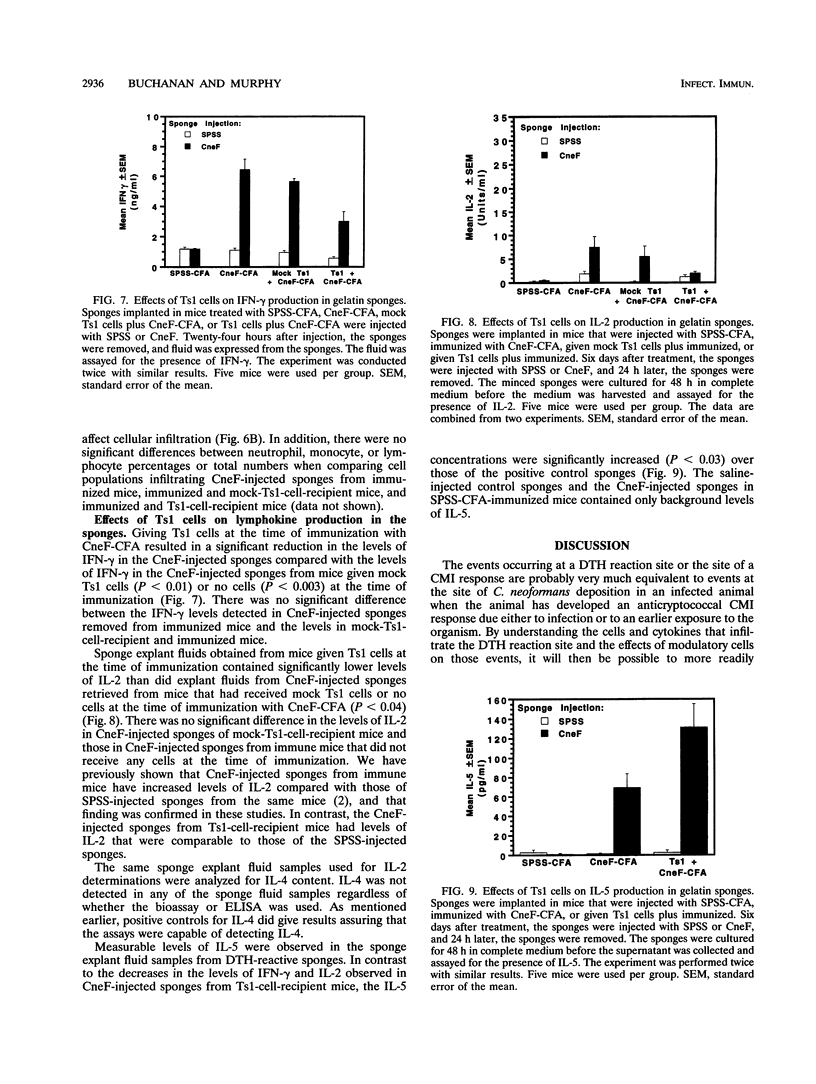
Effects of both positive and negative regulatory T cells on cellular infiltration and cytokine production during the expression phase of the anticryptococcal immune response were examined. Tamp cells, which are induced by cryptococcal antigen, significantly amplify the anticryptococcal delayed-type hypersensitivity response, whereas a cascade of T suppressor (Ts) cells inhibits the response and decreases the clearance of Cryptococcus neoformans during an infection. By using the gelatin sponge implantation model, we found that Tamp cells do not stimulate a significant increase in cellular infiltration into the sponges in response to cryptococcal antigen compared with that into delayed-type hypersensitivity-reactive sponges in immune control mice. However, Tamp cells do stimulate significant increases in the production of gamma interferon and interleukin-2 (IL-2) in the antigen-injected sponges over the level of the representative cytokine in antigen-injected sponges from the immune control mice. Likewise, Ts1 cells, induced with cryptococcal antigen, do not significantly affect antigen-induced cellular infiltration into sponges in immune mice. In contrast, decreased levels of gamma interferon and IL-2 are observed in antigen-injected sponges from Ts1-cell-recipient, immunized mice compared with those of the positive immune controls. The presence of either Tamp or Ts1 cells in immunized mice stimulates increased production of IL-5 but not IL-4 over that of the positive immune controls.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan K. L., Fidel P. L., Jr, Murphy J. W. Effects of Cryptococcus neoformans-specific suppressor T cells on the amplified anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1991 Jan;59(1):29–35. doi: 10.1128/iai.59.1.29-35.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K. L., Murphy J. W. Characterization of cellular infiltrates and cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1993 Jul;61(7):2854–2865. doi: 10.1128/iai.61.7.2854-2865.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Erba H. P., Gunning P., Kedes L. Nucleotide sequence of the human gamma cytoskeletal actin mRNA: anomalous evolution of vertebrate non-muscle actin genes. Nucleic Acids Res. 1986 Jul 11;14(13):5275–5294. doi: 10.1093/nar/14.13.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel P. L., Jr, Murphy J. W. Characterization of a cell population which amplifies the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1990 Feb;58(2):393–398. doi: 10.1128/iai.58.2.393-398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel P. L., Jr, Murphy J. W. Effects of cyclosporin A on the cells responsible for the anticryptococcal cell-mediated immune response and its regulation. Infect Immun. 1989 Apr;57(4):1158–1164. doi: 10.1128/iai.57.4.1158-1164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Schwamberger G., Kaufmann S. H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989 May 1;142(9):3219–3224. [PubMed] [Google Scholar]
- Gordon M. A., Vedder D. K. Serologic tests in diagnosis and prognosis of cryptococcosis. JAMA. 1966 Sep 19;197(12):961–967. [PubMed] [Google Scholar]
- Hidore M. R., Nabavi N., Sonleitner F., Murphy J. W. Murine natural killer cells are fungicidal to Cryptococcus neoformans. Infect Immun. 1991 May;59(5):1747–1754. doi: 10.1128/iai.59.5.1747-1754.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G. B., Yates J. L., Lipscomb M. F. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect Immun. 1991 Apr;59(4):1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpour F. R., Murphy J. W. Characterization of a third-order suppressor T cell (Ts3) induced by cryptococcal antigen(s). Infect Immun. 1987 Jul;55(7):1657–1662. doi: 10.1128/iai.55.7.1657-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi T., Harada N., Severinson E., Tanabe T., Sideras P., Konishi M., Azuma C., Tominaga A., Bergstedt-Lindqvist S., Takahashi M. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature. 1986 Nov 6;324(6092):70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J. Differential stimulation of murine resident peritoneal cells by selectively opsonized encapsulated and acapsular Cryptococcus neoformans. Infect Immun. 1988 Oct;56(10):2544–2551. doi: 10.1128/iai.56.10.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Dupont M. P. Phenotypic and functional characterization of human lymphocytes activated by interleukin-2 to directly inhibit growth of Cryptococcus neoformans in vitro. J Clin Invest. 1993 Apr;91(4):1490–1498. doi: 10.1172/JCI116354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W., Cauley L. K. Host-etiological agent interactions in intranasally and intraperitoneally induced Cryptococcosis in mice. Infect Immun. 1980 Aug;29(2):633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody C. H., Lipscomb M. F., Street N. E., Toews G. B. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J Immunol. 1990 Feb 15;144(4):1472–1477. [PubMed] [Google Scholar]
- Mody C. H., Tyler C. L., Sitrin R. G., Jackson C., Toews G. B. Interferon-gamma activates rat alveolar macrophages for anticryptococcal activity. Am J Respir Cell Mol Biol. 1991 Jul;5(1):19–26. doi: 10.1165/ajrcmb/5.1.19. [DOI] [PubMed] [Google Scholar]
- Murphy J. W. Clearance of Cryptococcus neoformans from immunologically suppressed mice. Infect Immun. 1989 Jul;57(7):1946–1952. doi: 10.1128/iai.57.7.1946-1952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cox R. A. Induction of antigen-specific suppression by circulating Cryptococcus neoformans antigen. Clin Exp Immunol. 1988 Aug;73(2):174–180. [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Cytokine profiles associated with induction of the anticryptococcal cell-mediated immune response. Infect Immun. 1993 Nov;61(11):4750–4759. doi: 10.1128/iai.61.11.4750-4759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Effects of first-order Cryptococcus-specific T-suppressor cells on induction of cells responsible for delayed-type hypersensitivity. Infect Immun. 1985 May;48(2):439–445. doi: 10.1128/iai.48.2.439-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Immunoregulation in cryptococcosis. Immunol Ser. 1989;47:319–345. [PubMed] [Google Scholar]
- Murphy J. W. Influence of cryptococcal antigens on cell-mediated immunity. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S432–S435. doi: 10.1093/cid/10.supplement_2.s432. [DOI] [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells (Ts1) and induction of second-order suppressor cells. J Immunol. 1983 Jun;130(6):2876–2881. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2). J Immunol. 1985 Jan;134(1):577–584. [PubMed] [Google Scholar]
- NEILL J. M., SUGG J. Y., McCAULEY D. W. Serologically reactive material in spinal fluid, blood, and urine from a human case of cryptococcosis (torulosis). Proc Soc Exp Biol Med. 1951 Aug;77(4):775–778. doi: 10.3181/00379727-77-18924. [DOI] [PubMed] [Google Scholar]
- Nowak T. P., Barondes S. H. Agglutinin from Limulus polyphemus. Purification with formalinized horse erythrocytes as the affinity adsorbent. Biochim Biophys Acta. 1975 May 30;393(1):115–123. [PubMed] [Google Scholar]
- Salkowski C. A., Balish E. A monoclonal antibody to gamma interferon blocks augmentation of natural killer cell activity induced during systemic cryptococcosis. Infect Immun. 1991 Feb;59(2):486–493. doi: 10.1128/iai.59.2.486-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]