Abstract
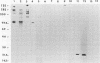
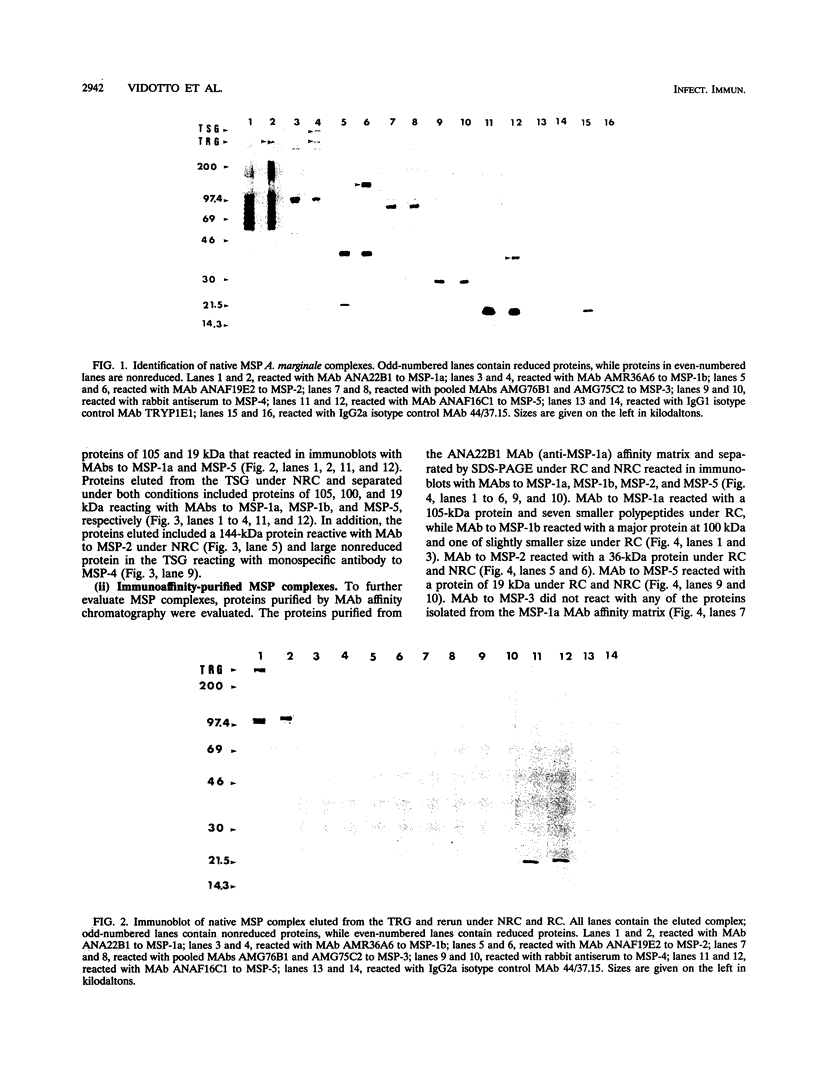
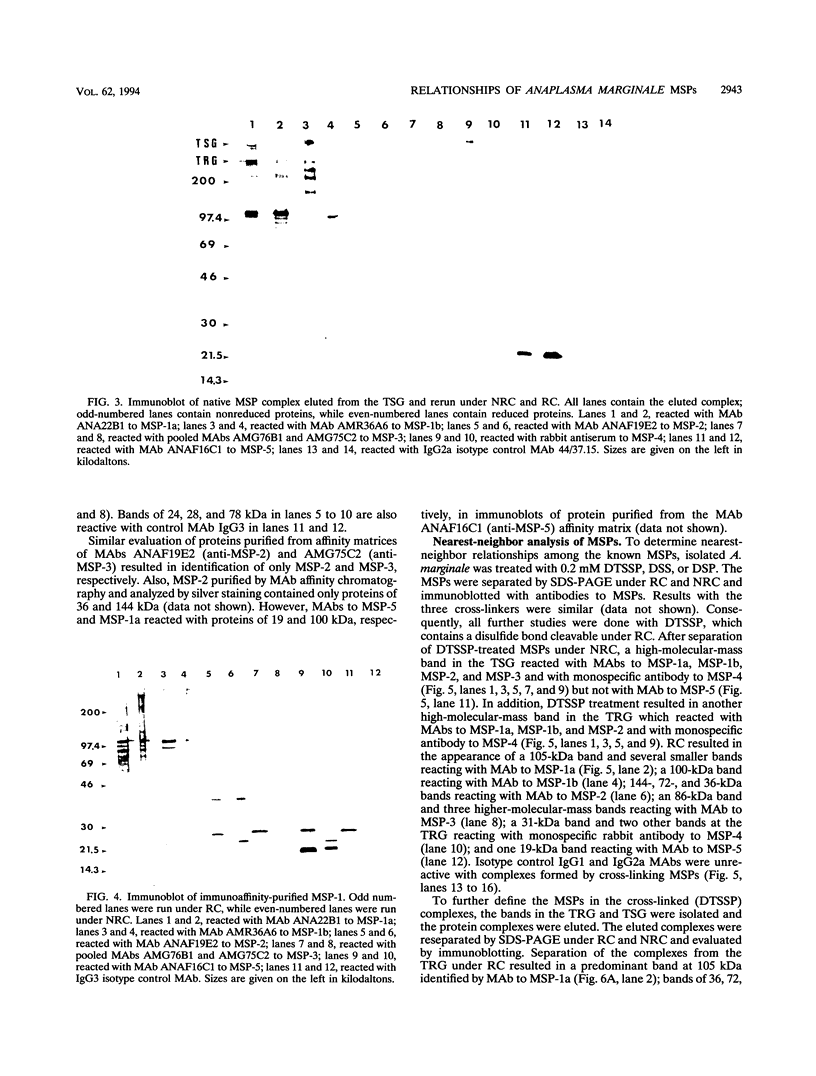
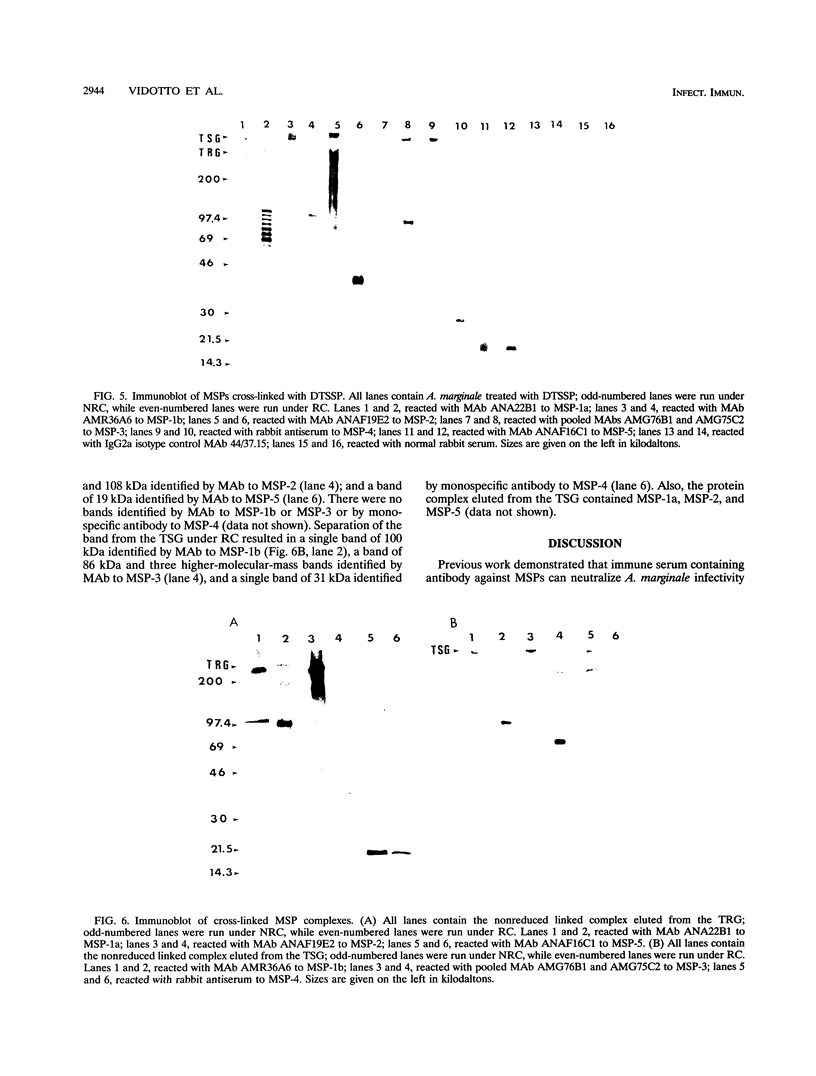
Immunization with Anaplasma marginale membranes containing major surface proteins (MSPs) induces protective immunity against clinical disease (N. Tebele, T. C. McGuire, and G. H. Palmer, Infect. Immun. 59:3199-3204, 1991). For use in design of a recombinant antigen subunit vaccine for A. marginale, intermolecular relationships of known A. marginale MSPs were analyzed. Under nonreducing conditions, MSP-2 and MSP-5 occur as multimers. A large (> 300-kDa-molecular-mass), nonreduced protein complex contained MSP-1a linked by disulfide bonds to MSP-1b and by noncovalent bonds to MSP-5. MSP-2 was also noncovalently bound to this complex. The nearest neighbor membrane proteins were identified by cross-linking reactions followed by immunoblotting with anti-MSP antibodies. A cross-linked aggregate retained in the stacking gel contained MSP-1a, MSP-1b, MSP-2, MSP-3, MSP-4, and MSP-5. Collectively, the data indicate that MSP-2 and MSP-5 occur as monomers and disulfide-bonded multimers. The MSP-1 complex occurs as both disulfide-bonded and noncovalently associated MSP-1 and MSP-1b, and MSP-2 and MSP-5 are noncovalently associated with MSP-1. Also, MSP-1, MSP-2, MSP-3, and MSP-4 are nearest neighbors, and MSP-5 is noncovalently associated with this cross-linked complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. R., McGuire T. C., Palmer G. H., Leib S. R., Harkins T. M., McElwain T. F., Barbet A. F. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3220–3224. doi: 10.1073/pnas.87.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., Allred D. R. The msp1 beta multigene family of Anaplasma marginale: nucleotide sequence analysis of an expressed copy. Infect Immun. 1991 Mar;59(3):971–976. doi: 10.1128/iai.59.3.971-976.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., Palmer G. H., Myler P. J., McGuire T. C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987 Oct;55(10):2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Ji T. H. Bifunctional reagents. Methods Enzymol. 1983;91:580–609. doi: 10.1016/s0076-6879(83)91053-4. [DOI] [PubMed] [Google Scholar]
- Ji T. H. The application of chemical crosslinking for studies on cell membranes and the identification of surface reporters. Biochim Biophys Acta. 1979 Apr 23;559(1):39–69. doi: 10.1016/0304-4157(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Kuttler K. L., Zaugg J. L., Johnson L. W. Serologic and clinical responses of premunized, vaccinated, and previously infected cattle to challenge exposure by two different Anaplasma marginale isolates. Am J Vet Res. 1984 Nov;45(11):2223–2226. [PubMed] [Google Scholar]
- Lerner R. A. Tapping the immunological repertoire to produce antibodies of predetermined specificity. Nature. 1982 Oct 14;299(5884):593–596. doi: 10.1038/299592a0. [DOI] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Lutter L. C., Bode U., Kurland C. G., Stöffler G. Ribosomal protein neighborhoods. 3. Cooperativity of assembly. Mol Gen Genet. 1974 Mar 14;129(2):167–176. doi: 10.1007/BF00268629. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Davis W. C., Brassfield A. L., McElwain T. F., Palmer G. H. Identification of Anaplasma marginale long-term carrier cattle by detection of serum antibody to isolated MSP-3. J Clin Microbiol. 1991 Apr;29(4):788–793. doi: 10.1128/jcm.29.4.788-793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro-James S., James M. A., Benitez M. T., Leon E., Baek B. K., Guillen A. T. Efficacy of purified Anaplasma marginale initial bodies as a vaccine against anaplasmosis. Parasitol Res. 1991;77(2):93–101. doi: 10.1007/BF00935421. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., Sawyer W. D., Haak R. A. Cross-linking analysis of the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):785–791. doi: 10.1128/iai.28.3.785-791.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle S. M., Palmer G. H., Barbet A. F. Expression and immune recognition of the conserved MSP4 outer membrane protein of Anaplasma marginale. Infect Immun. 1993 Dec;61(12):5245–5251. doi: 10.1128/iai.61.12.5245-5251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle S. M., Palmer G. H., Barbet A. F., McGuire T. C. Molecular size variations in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988 Jun;56(6):1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Cantor G. H., McGuire T. C. Immunization of cattle with the MSP-1 surface protein complex induces protection against a structurally variant Anaplasma marginale isolate. Infect Immun. 1989 Nov;57(11):3666–3669. doi: 10.1128/iai.57.11.3666-3669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Kuttler K. L., McGuire T. C. Detection of an Anaplasma marginale common surface protein present in all stages of infection. J Clin Microbiol. 1986 Jun;23(6):1078–1083. doi: 10.1128/jcm.23.6.1078-1083.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- Palmer G. H., Oberle S. M., Barbet A. F., Goff W. L., Davis W. C., McGuire T. C. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988 Jun;56(6):1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Cappiello D., Wilkowski C., Vogt V. M. Chemical crosslinking of proteins in avian sarcoma and leukemia viruses. Virology. 1980 Apr 15;102(1):205–210. doi: 10.1016/0042-6822(80)90081-1. [DOI] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Ristic M., Carson C. A. Methods of immunoprophylaxis against bovine anaplasmosis with emphasis on use of the attenuated Anaplasma marginale vaccine. Adv Exp Med Biol. 1977;93:151–188. doi: 10.1007/978-1-4615-8855-9_10. [DOI] [PubMed] [Google Scholar]
- Staros J. V. N-hydroxysulfosuccinimide active esters: bis(N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry. 1982 Aug 17;21(17):3950–3955. doi: 10.1021/bi00260a008. [DOI] [PubMed] [Google Scholar]
- Swift B. L., Thomas G. M. Bovine anaplasmosis: elimination of the carrier state with injectable long-acting oxytetracycline. J Am Vet Med Assoc. 1983 Jul 1;183(1):63–65. [PubMed] [Google Scholar]
- Tebele N., McGuire T. C., Palmer G. H. Induction of protective immunity by using Anaplasma marginale initial body membranes. Infect Immun. 1991 Sep;59(9):3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Visser E. S., McGuire T. C., Palmer G. H., Davis W. C., Shkap V., Pipano E., Knowles D. P., Jr The Anaplasma marginale msp5 gene encodes a 19-kilodalton protein conserved in all recognized Anaplasma species. Infect Immun. 1992 Dec;60(12):5139–5144. doi: 10.1128/iai.60.12.5139-5144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino O., Corrier D. E., Terry M. K., Carson C. A., Lee A. J., Kuttler K. L., Ristic M., Treviño G. S. Comparison of three methods of immunization against bovine anaplasmosis: evaluation of protection afforded against field challenge exposure. Am J Vet Res. 1980 Jul;41(7):1066–1068. [PubMed] [Google Scholar]