Abstract
Melanoma incidences have increased over the last few decades and metastatic melanoma is one of the hardest malignancies to treat. Thus, novel approaches are needed for an effective management of melanoma. Interferon-α2b (IFN), an immunomodulatory cytokine commonly used in melanoma treatment, has shown marginal efficacy and often results in discontinuation of therapy due to toxicity. We earlier demonstrated that epigallocatechin-3-gallate (EGCG), the major polyphenolic constituent of green tea, caused cell cycle arrest and apoptosis of human melanoma cells via modulation in cki-cyclin-cdk machinery and Bcl-2 family proteins. This study was undertaken to determine if EGCG could enhance the anti-proliferative effects of IFN. In this study, we demonstrated that EGCG and/or IFN treatments to melanoma cells resulted in a marked i) decrease in cell proliferation and colony formation ability, and ii) induction of apoptosis. Interestingly, the combination was found to be more effective than either of the agents alone. Further, the anti-proliferative effects of EGCG and/or IFN were accompanied with an increase in FAS protein levels and a decrease in nuclear factor NF-κB/p65 in the nucleus as well as NF-κB promoter activity. EGCG and/or IFN also resulted in an increase in FAS-L mediated apoptosis. Further, EGCG and/or IFN treatments resulted in a decrease in melanoma tumor growth and protein levels of proliferation marker PCNA, in athymic nude mice implanted with melanoma tumors. The combination of the two modalities demonstrated a better response than either of them alone. Our data suggest that EGCG could impart therapeutic advantage if used in conjunction with IFN.
Introduction
Melanoma ranks highest in mortality among the various cutaneous malignancies and its incidence is increasing at an alarming pace. Melanomas are notorious for their resistance to apoptosis in response to wide variety of external stimuli 1, 2. They acquire selective growth advantages by reprogramming their proliferation and survival pathways during tumor progression and thus exert resistance to therapy. Melanoma, like any other cancer, is not caused by a mutation in a single gene but requires genetic modulations and environmental factors affecting several pathways. More than 60% of melanomas have mutated B-Raf oncogene, 20% bear mutations in N-Ras and about 10% of all familial cases have germ line mutation in the CDNK2A locus that encodes INK4A(p16), ARF(p14/p18) and CDK4 3.
Melanoma cells acquire resistance to apoptosis (ability to undergo programmed cell death) in response to a wide range of external stimuli; thereby imparting them a selective advantage for progression and metastasis as well as their notorious resistance to therapy 4, 5. Recent advances in melanoma biology have enabled the identification of diverse pathways by which melanoma cells manage to escape apoptosis 4, 5. Defects in several pathways at genomic, transcriptional and post-translational levels have been implicated in melanoma survival and growth. Studies have shown that melanoma cell carry impaired signaling at multiple pathways which could be related or unrelated; these include i) G-proteins and protein kinases (Ras, B-Raf), ii) transcription factor effectors (c-Jun, ATF2, Stat3 and NF-κB), and iii) receptors such as TNF, Fas and TRAIL 4, 5. All of these signaling pathways play critical roles in acquiring melanoma's resistance to apoptosis and offer avenues to design better strategies to combat this hard to treat neoplasm. The immunomodulator, IFN, was approved by the Food and Drug Administration in 1995 as a therapy for high-risk melanoma patients 6. However, unfortunately, this therapy is only marginally effective and often results in discontinuation of treatment due to associated toxicity 7. Moreover, there is no effective therapy for high risk patients to prevent relapse or additional tumors and it is a challenge to design novel strategies to improve the poor prognosis, especially with advanced stage disease 8. Recent studies have suggested that certain naturally occurring plant-derived chemopreventive agents could enhance the therapeutic efficacy of existing cancer treatments by sensitizing the cancer cells to the treatment thereby enabling a dose advantage and therefore reduced toxicity 9, 10.
The major polyphenolic constituent of green tea, (−) - epigallocatechin-3-gallate (EGCG), has been shown to impart anti-proliferative and chemopreventive effects against several cancers including skin cancer 11 12 13. We have earlier demonstrated that EGCG treatment of melanoma cells results in cell cycle blockade and induction of apoptosis via modulation of the cki-cyclin-cdk network and Bcl2 family proteins 14. This study was designed to determine if EGCG could enhance the anti-proliferative effects of IFN and it’s mechanism of action. Our choice of EGCG was also based on the promise that it is being increasingly appreciated that for an effective management of melanoma, approaches targeted at multiple pathways could be more useful and EGCG has been shown to inhibit multiple pro-proliferative pathways 15. Our data demonstrated that EGCG can indeed enhance the therapeutic response of IFN both in vitro as well as in vivo. Further, our data also suggest the involvement of Fas and NF-κB signaling in the anti-proliferative response of the combination of EGCG and IFN.
Results
Effects of EGCG and/or IFN on growth and proliferation of melanoma cells
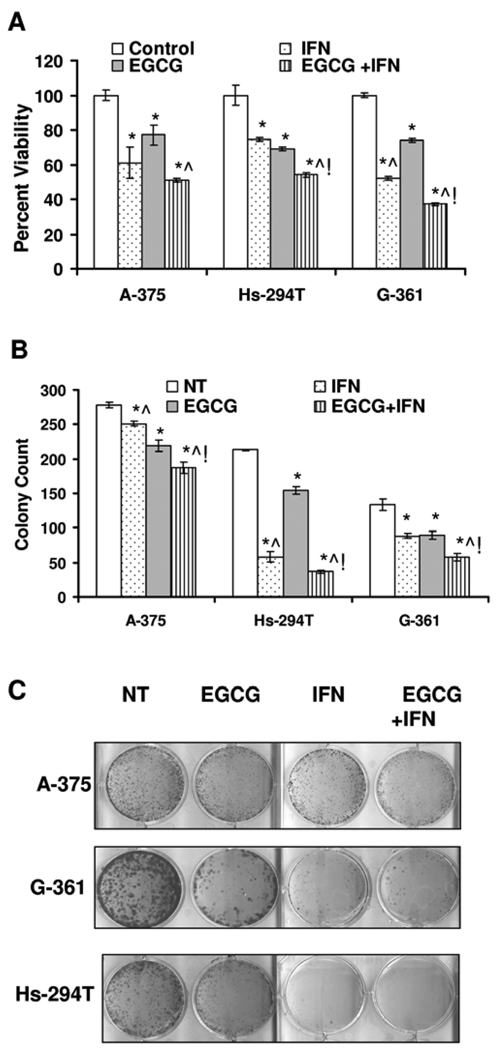
Our first aim was to determine if a combination of EGCG and IFN has a better anti-proliferative response against melanoma cells in vitro. Employing multiple human melanoma cell lines (A-375, G-361 and Hs-294T) representing different stages of disease progression, we evaluated the effects of EGCG and IFN (alone or in combination) on the proliferation of melanoma cells by Trypan Blue exclusion analysis. Our data demonstrated that EGCG as well as IFN alone treatment resulted in a significant decrease in the number of proliferating cells (Figure 1A). Interestingly, compared to the either agents alone, the combination of EGCG and IFN was found to have more pronounced and statistically significant cell growth inhibitory effect in Hs-294T and G-361 cells and showed a similar trend in the A-375 cells. The G-361 cells were most responsive to the combination treatment where it resulted in 56% reduction in the number of proliferating cells when compared to the control.
Figure 1. EGCG and/or IFN inhibit cellular proliferation in melanoma cells.
A. Effect of on cell viability. The melanoma cells were plated at the density of 1×105 in six well plates treated with 10µg/ml EGCG and 1000IU/ml IFN and their combination. The cells were harvested 48 hrs after the treatment, washed with PBS. The cell viability was assayed by Trypan blue dye exclusion. The data is shown as cell viability determined by Trypan blue exclusion assay, that is expressed as percent proliferation mean + S.E. from three experiments. The p-value <0.05 was considered statistically significant compared with no treatment control (*), 10µg/ml EGCG (^) and 1000IU/ml (!). The experiment was repeated three times in triplicate with similar results. B and C. Effect of treatment on colony formation ability of melanoma cells. A-375, Hs-294T and G-361 melanoma cells were plated in 6 well plates, in triplicate, at a very low density (1, 4, 5 × 103 cells/well), treated with single agents and their combination as stated in materials and methods. At the end of experiments colonies of the melanoma cells were washed with PBS and stained with crystal violet, counted and pictured. The experiment was repeated two or more times in triplicates with similar results. The p-value <0.05 was considered statistically significant compared with no treatment control (*), 10µg/ml EGCG (^) and 1000IU/ ml (!).
Next, we determined the effect of EGCG and/or IFN treatments on clonogenic survival of the melanoma cells employing a colony formation assay. As shown in Figures 1B and 1C, we found that EGCG as well as IFN alone resulted in a marked decrease in the number of colonies of melanoma cells and the combination of the two agents caused a much better and significant decrease in the number of colonies that either of the agents alone.
Effect of EGCG and/or IFN on rate of apoptosis in melanoma cells
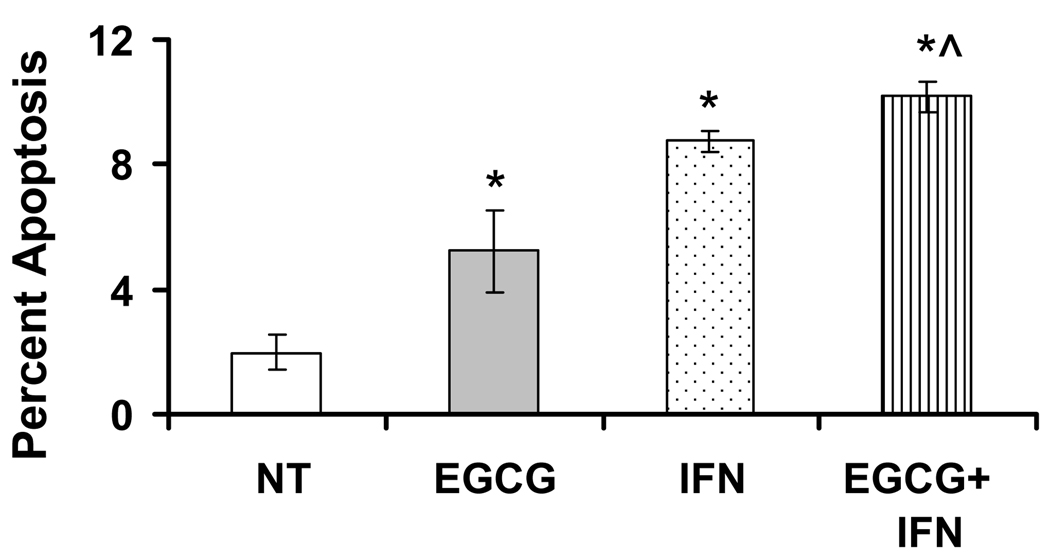
Earlier studies from our laboratory have demonstrated that EGCG causes an induction of apoptosis of melanoma cells 14. We therefore determined if the observed anti-proliferative effects of EGCG and/or IFN are mediated via apoptotic elimination of the melanoma cells. For this purpose and for all further in vitro as well as in vivo experiments, we selected Hs-294T cells because these cells grow at a consistent intermediate pace relative to A-375 and G-361 cells. As shown by a flow cytometric evaluation of annexin V-FITC binding, we found that a significant induction of apoptosis by EGCG and/or IFN treatments (Figure 2). The combination of the two agents demonstrated a better response than either of the agents alone; however, it was not statistically significant (Figure 2).
Figure 2. EGCG and/or IFN induce apoptosis in Hs-294T melanoma cells.
The EGCG and/or IFN treated Hs-294T melanoma cells were stained with FITC conjugated anti-Annexin V antibody and PI. Briefly, 48 hrs post treatment control and treated cells were collected, washed and stained with FITC conjugated Annexin V FITC and PI at room temperature for 15 mins and analyzed using BD Facscan. The analyses were performed using FlowJo software using proper negative and positive controls. The experiments were repeated 3 or more times with triplicate samples. The p-value <0.05 was considered statistically significant compared with no treatment no treatment control (*), 10µg/ml EGCG (^) and 1000IU/ml (!).
Effect of EGCG and/or IFN on Fas/CD95 expression and Fas ligand (FasL) mediated apoptosis in melanoma cells
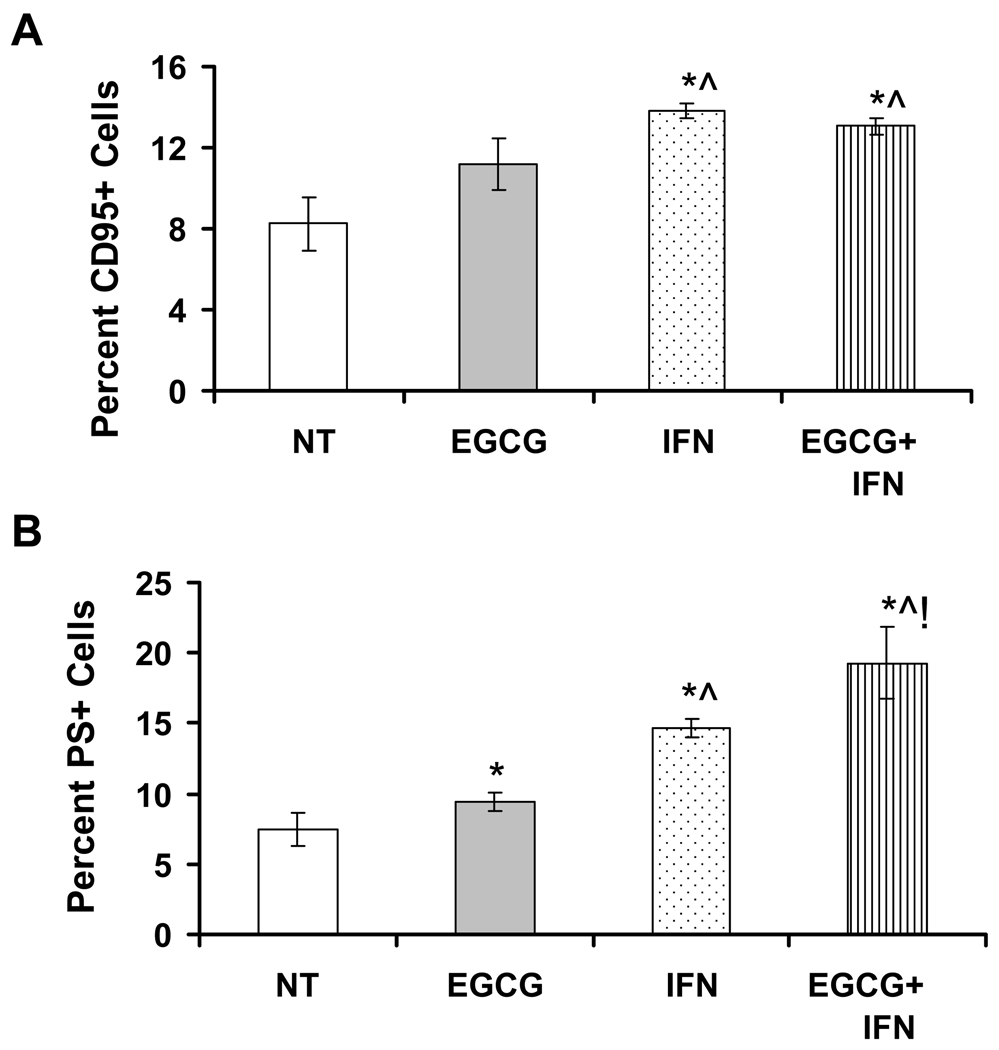
The Fas/FasL signaling system plays an important role in regulation of apoptosis in mammalian cells. We next assessed the involvement of Fas/CD95 during EGCG and/or IFN mediated induction of apoptosis of Hs-294T cells. Our results showed that EGCG and/or IFN treatment to Hs-294T melanoma cells resulted in a significant increase in Fas protein as assessed by the flow cytometric analysis of CD95 positive cells (Figure 3A). However, the combination treatment was not found to be significantly better than IFN treatment alone (Figure 3A). Further, we assessed the effect of EGCG and/or IFN on FasL mediated apoptosis. As shown in Figure 3B, EGCG as well as IFN treatments resulted in a significant increase in FasL mediated apoptosis as judged by phosphatidylserine (PS) positive cells, which is a direct measurement of PS at the outer leaflet of the plasma membrane and indicative of apoptosis. Our data also demonstrated that the combination of the two agents showed a better and statistically significant response compared to EGCG or IFN treatments alone (Figure 3B).
Figure 3. Effect of EGCG, IFN and their combination on Fas (CD95) protein and FasL mediated apoptosis in Hs-294T melanoma cells.
A. Effect on Fas protein. Hs-294T cells (1×104) were plated in 12 well plates, in triplicate, and treated with 10µg/ml EGCG, 1000 IU/ml and combination for 48 hrs. Cells were stained with anti-Fas antibody for 30 min at room temperature in the dark, washed, resuspended in FACS buffer and analyzed with a LSR II bench top flow cytometer. Data analysis was performed by FlowJo software. The experiments were repeated 3 or more times with triplicate samples. The p-value <0.05 was considered statistically significant compared with no treatment no treatment control (*), 10µg/ml EGCG (^) and 1000IU/ml (!). B. Effect on FasL mediated apoptosis. Hs-294T cells (2×104) were plated and treated with 10µg/ml EGCG, 1000IU/ml IFN and their combination for 48 hrs and then additional 16 hrs with FasL/Flag/anti-Flag for aggregation of Fas. Cells were collected, fixed and washed with PBS and stained with anti-phosphatidylserine (PS) at 40C for one hour. Cells were washed again and subjected to flow cytometry with LSR II bench top flow cytometer and analyzed by FlowJo software. The p-value <0.05 was considered statistically significant compared with no treatment no treatment control (*), 10µg/ml EGCG (^) and 1000IU/ml (!).
Effect of EGCG and/or IFN on NF-κB pathway in melanoma cells
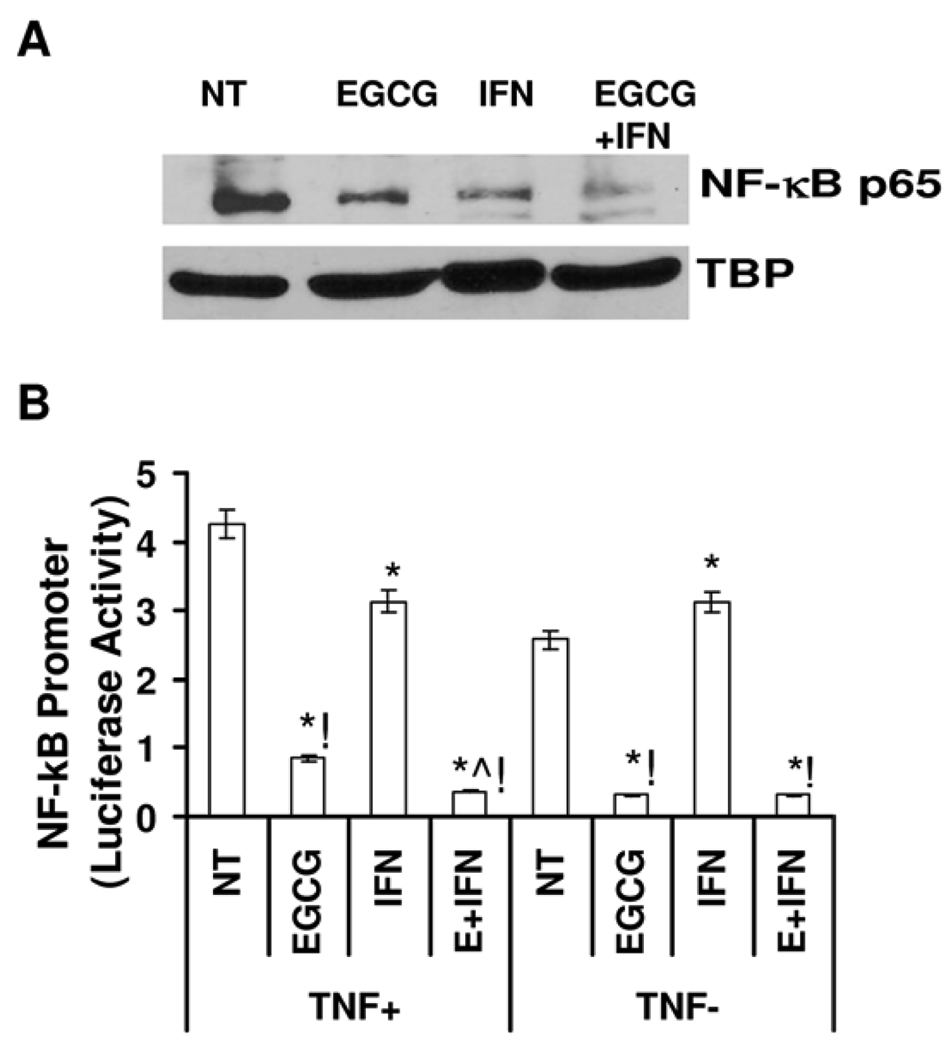
NF-κB is responsible for regulating many genes involved in immune response, cell adhesion, differentiation, proliferation, angiogenesis and apoptosis 16. Because i) NF-κB is constitutively active in melanoma 17, ii) EGCG has been shown to be a strong inhibitor of NF-κB, and iii) transcription of the genes for Fas and FasL have been shown to be regulated by NF-κB 18, we determined whether or not the observed anti-proliferative effects of EGCG and/or IFN are mediated via modulations in NF-κB signaling. As shown by the immunoblot analysis, we found that the EGCG and/or IFN treatments resulted in a marked decrease in NF-κB/p65 protein levels in the nucleus of Hs-294T cells (Figure 4A). The greatest reduction in the nuclear NF-κB/p65 was observed with the combination of EGCG and IFN. Further, employing a dual promoter luciferase activity assay, we also determined if the observed change in NF-κB protein is due to it’s transcriptional inhibition. For this purpose, following treatments, the cells were transiently transfected with the pNF-κB TA-LUC firefly luciferase vector (along with renilla luciferase for normalization) and the luciferase activity was measured. Our data demonstrated that EGCG and/or IFN treatments resulted in a significant decrease in the luciferase promoter activity of NF-κB, most in combination group. These results are consistent with the observed nuclear activation of NF-κB as shown in Figure 4A.
Figure 4. Effect of EGCG, IFN and their combination on NF-κB protein and promoter activity.
A. Effect of treatment on NF-κB protein levels. Following treatment NF-κB protein in nuclear lysates of Hs-294T cells was detected by Western blot. Equal loading was confirmed by re-probing the blot for tata binding protein. Data represents three experiments with similar results. Details of the experiments are given in “Materials and Methods”. B. Effect of treatment on NF-κB promoter activity. Luciferase reporter activity of NF-κB in control and treated Hs-294T cells in presence and absence of TNF and normalized by Renilla luciferase activity. The results are expressed as percentages of control and are the means ± S.E. of at least three different experiments. The statistically significant changes (p< 0.05) compared to vehicle treated control are marked as (*).
Effect of EGCG and/or IFN on growth of tumors in athymic nude mice implanted with Hs-294T melanoma cells
In our final set of experiments, we determined the in vivo relevance of our in vitro finding in a nude mouse xenografts model. The athymic nude mice were implanted with Hs-294T melanoma cells and the effect of EGCG and/or IFN treatments was determined on tumor growth in this model. For this purpose, we employed two different protocols representing chemopreventive and therapeutic efficacy of the treatments. In the first (chemoprevention) protocol, the treatments were started on the same when melanoma cells were implanted onto the athymic nude mice. In the second (therapy) protocol, tumors were first established and the treatment was started when the first tumor reached to the size of approximately 70 mm3.
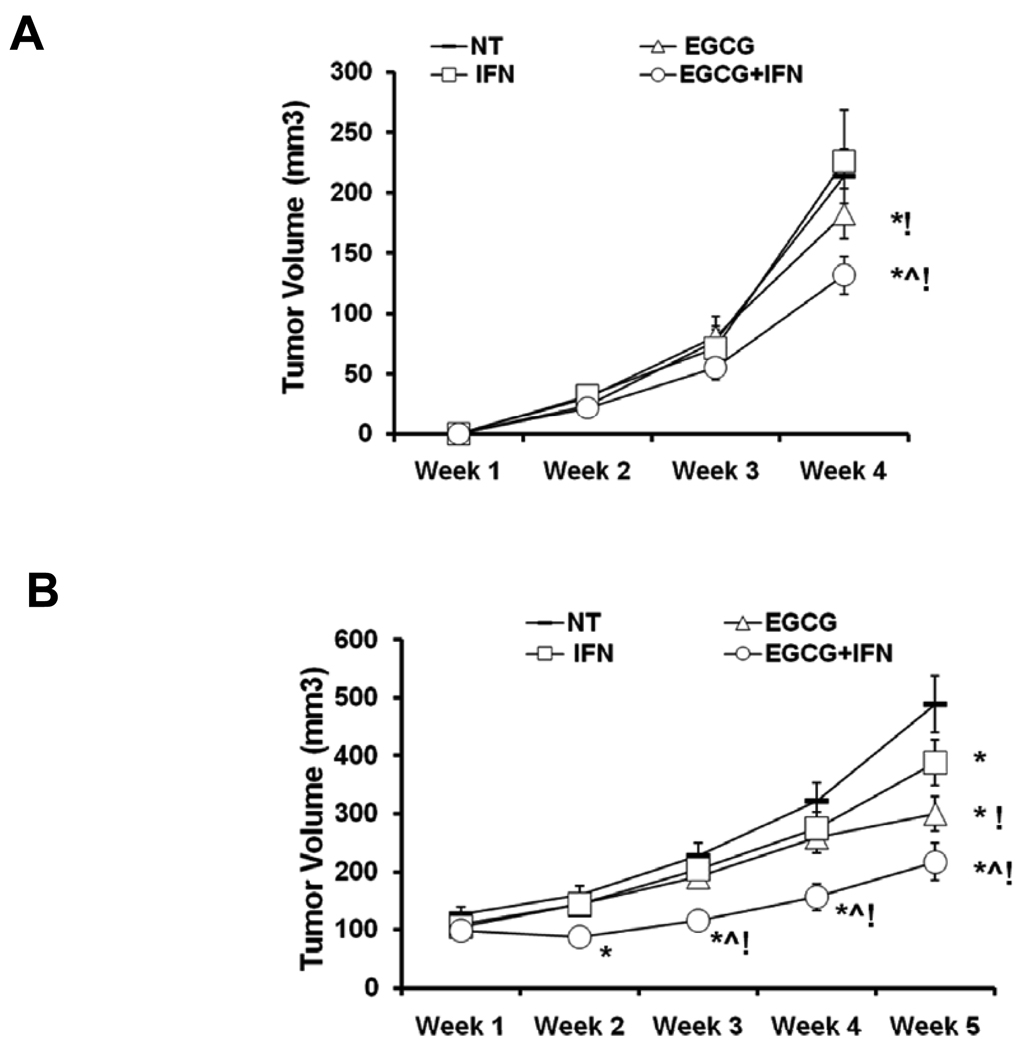
As shown by the data in figure 5, we found that in both experimental protocols, compared to control, EGCG and/or IFN treatments resulted in a marked decrease in tumor growth. The best tumor growth inhibitory response was observed with the combination of EGCG and IFN showed, although it was not statistically different from the EGCG treatment (Figures 5A and 5B). Further, in any treatment group, we did not observe any sign of toxicity such as loss of body weight or apparent morbidity, suggesting that treatments were well tolerated by the animals.
Figure 5. Effect of EGCG, IFN and their combination on tumor volume in athymic nude mice implanted with Hs-294T cells.
A. Effect on tumorigenesis in nude mice (pre-treatment). The animals were implanted with 0.5×106 Hs-294T melanoma cells with matrigel on the left flanks. Each animal received 1 mg EGCG dissolved in saline intraperitoneally daily and 1000 IU of IFN dissolved in saline subcutaneously or both for four weeks for five days/week on the day of implantation of tumor cells. Tumor volumes on the flanks in the same group were measured and expressed as mean ± S.E. Average tumor volume of vehicle- or single agents and combination treated animals was plotted over weeks as detailed in Materials and Methods. The statistically significant changes (p< 0.05) compared to vehicle treated control are marked as (*) and (!) when compared with IFN. B. Effect on tumorigenesis in nude mice (post-treatment). In this experiment, treatment started when tumors reached a volume of 70mm3. Average tumor volume of vehicle- or single agents and combination treated animals was plotted over five weeks. The statistical significant changes (p< 0.05) compared to vehicle treated control are marked as (*) and (!) when compared with IFN.
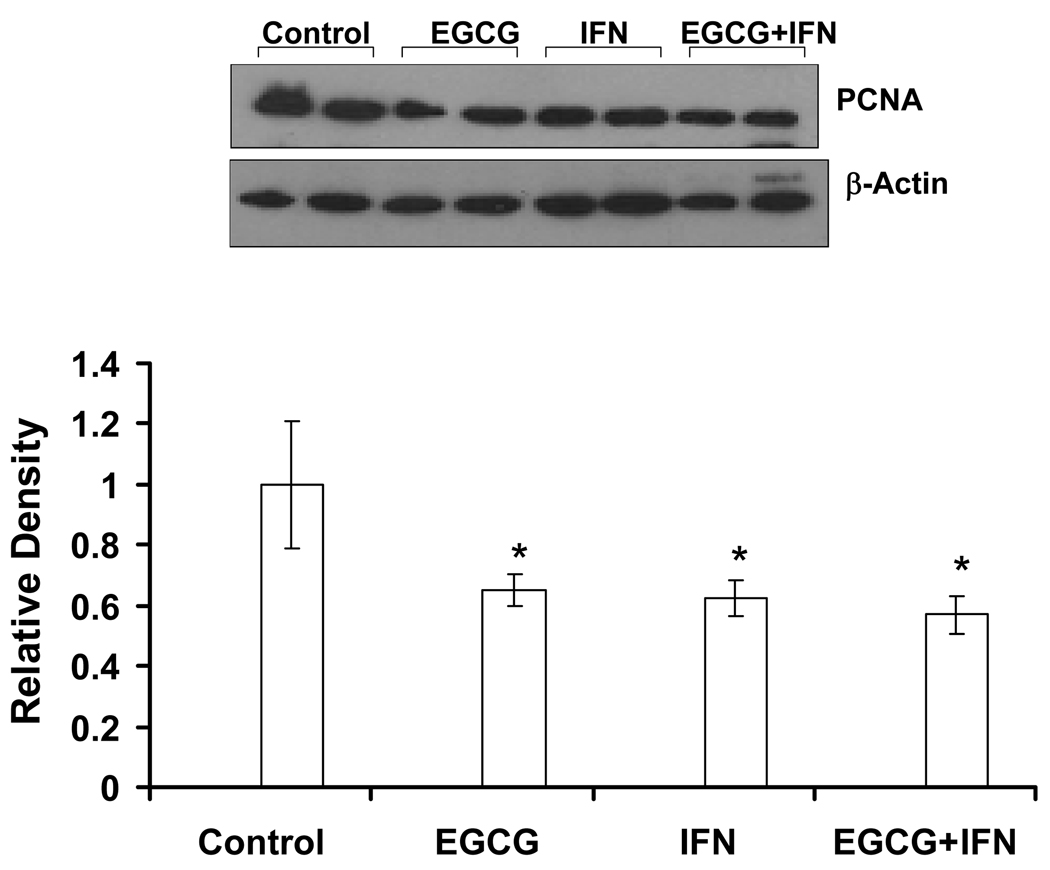
Because EGCG is a well known anti-proliferative agent, we assessed the effect of EGCG and/or IFN treatments on the ubiquitous proliferation marker PCNA, which is a requisite auxiliary protein for DNA polymerase-driven DNA synthesis and is cell cycle regulated 19. As shown by the western blot analysis, EGCG and/or IFN treatments showed an appreciable decrease in PCNA providing another correlate of reduced tumor proliferation (Figure 6).
Figure 6. Effect of EGCG, IFN and their combination on the proliferation marker PCNA in Hs-294T tumors in athymic nude mice.
Following sacrifice of animals, tumors were harvested and the levels of PCNA were determined by Western blot analysis as described under ‘Methods’. Equal loading was confirmed by stripping the blot and reprobing it for β-actin. Error bars represent 95% confidence level. The protein levels were quantitated by a densitometric analysis of protein bands.
Materials and Methods
Cell Culture and Treatment
The human melanoma lines A-375, G-361 and metastatic Hs-294T were obtained from The American Type Culture Collection (ATCC; Manassas, VA). The cells were maintained at standard tissue culture conditions as recommended by the vendor. EGCG (>98% purity) was obtained from Sigma (St. Louis, MO) and dissolved in PBS. Intron A (IFNα–2B), mentioned as IFN, was obtained from Schering Corporation (Kenilworth, NJ).
Cell Viability
The human melanoma cells were plated at the density of 1×105 (in six well plates in triplicates) and pretreated with 10 µg/ml (20 µM) EGCG and 1000 IU/ml IFN dissolved in PBS for 48 hrs. The cells were harvested after trypsinization, washed with PBS and the viability was evaluated by Trypan blue dye exclusion.
Colony formation assay
A-375, Hs-294T and G-361elanoma cells were plated in 6 well plates, in triplicate, at a very low density (1, 4, 5 × 103 cells/well respectively), treated with single agents and their combination, and allowed them to growth for 8–10 days (5 days for A-375). At the end of treatment, colonies were washed with PBS and stained with a crystal violet (a formulation of 1:2:2 of methanol: water: 0.1% crystal violet in acetic acid).
Apoptosis by Annexin V binding
The extent of change in apoptotic cells in vehicle control and treated samples was assessed with the Annexin V-FITC and PI staining (BD Biosciences, San Jose, CA). Hs-294T cells (1×104) were plated in 12 well plates, in triplicate, and treated with EGCG, IFN and EGCG and IFN for 48 hrs. Floater and adherent cells were collected, washed with PBS and cell pellet dissolved in 1× binding buffer and stained with propidium iodide and Annexin V-FITC for 15 mins in dark. The cells were analyzed using a FACScan bench top cytometer (Becton Dickinson Biosciences; San Jose, CA). The analyses were performed using FlowJo software (Tree Star, Inc. Ashland, OR). The experiments were done in triplicates with 2 or more times.
Surface Fas staining
Fas expression was determined by staining the vehicle control and treated with FITC conjugated anti-FAS monoclonal antibody (Becton Dickinson Biosciences, San Jose, CA). Briefly, cells were washed with PBS and blocked with 1:10 normal goat serum in PBS for 10 minutes. Cells were stained for 30 min at room temperature in dark, then washed twice with PBS, resuspended in FACS buffer and analyzed with a LSR II bench top flow cytometer. Data analysis was performed by FlowJo software (Tree Star, Inc. Ashland, OR).
FasL mediated apoptosis
Vehicle and drug treated cells in growth medium were incubated further for 16 hours with 50 ng/ml recombinant fusion protein of FasL (Alexis, San Diego, CA), aggregated with 1 µg/ml anti-Flag antibody (Sigma-Aldrich, St. Louis, MO). Cells were collected, fixed and washed. Apoptosis was determined by Alexa 488 conjugated anti-PS antibody from (Millipore, Billerica, MA). Isotype-matched monoclonal antibodies of irrelevant specificity were used as negative controls. Data analysis was performed by FlowJo software.
NF-κB Luciferase assay
Treated and untreated cells were transfected with NF-κB promoter reporter construct pNFκB-TA-Luc (1.0 µg) along with 0.25 µg renilla luciferase (Clontech Laboratories, Mountain View, CA). The promoterless firefly luciferase reporter vector pTA-Luc was used as a transfection control. Firefly and renilla luciferase activities were measured 48 hrs post transfection, transiently, using Dual Luciferase kit (Promega, Madison, WI) and expressed as relative luciferase activity.
Xenograft experiments
Female nu/nu athymic nude mice (6-weeks old) obtained from NxGen Biosciences, Inc. (San Diego, CA) were used in this study. During the entire experimental protocol, the animals were housed in the University of Wisconsin Animal Resource Facility, received autoclaved laboratory chow diet and drinking water ad libitum and were subjected to a 12 h light/12 h dark cycle. Each mouse was subcutaneously implanted with 0.5×106 Hs-294T melanoma cells with equal volume of matrigel (in 100 µl) on the left flanks. Each animal (n=8 for each group) received 1 mg EGCG dissolved in saline intraperitoneally daily and 1000 IU of IFN dissolved in saline subcutaneously or both for 4–5 to weeks for five days/week. The treatment regimen started either on the day of implantation of tumor cells or when palpated tumor reached about 70 mm3 by two blinded independent investigators on day 7 using a digital Vernier caliper to take two measurements at 90° to each other till 4 to 5 weeks, respectively. The control mice were treated with normal saline as vehicle only. The animals were monitored daily for any sign of toxic symptom and morbidity. Animals and tumors were measured weekly using the formula, V = 0.5328XLXBXH (mm3). At the end of experiment, the animals were sacrificed and tumors were harvested and processed for further analyses.
Immunoblot Analysis
For western blot analysis, the tumor samples, were lysed in 50 mM Tris-HCl lysis buffer (pH 7.4) containing 150 mM NaCl, 1mM EDTA, 1% NP-40, 1 mM Sodium Fluoride, 1 mM sodium orthovanadate and protease inhibitor cocktail. Protein sample were electrophoresed (25 µg protein) and transferred onto a nitrocellulose membrane. The membrane was blocked and probed with appropriate primary antibodies and secondary antibodies HRP conjugates, followed by chemiluminescent detection. For NF-kB immunoblots nuclear and cytoplasmic lysates were used.
Statistical Analyses
For in vitro cell studies, Student’s T-test was applied. The data show mean ± s.d. from triplicates of each experiment, and each experiment was done at least twice or more times independently.
Discussion
We demonstrated previously that the major polyphenolic constituent of green tea EGCG, which is a strong antioxidant, causes i) inhibition of growth and viability, ii) arrest of cell cycle, and iii) induction of apoptosis of melanoma cells in vitro 14. In this study, we determined if EGCG could enhance the anti-proliferative response of IFN. Though a standard treatment of melanoma, IFN is suboptimally effective and associated with clinical toxicity often resulting in discontinuation of treatment 7. Therefore, approaches aimed at enhancing the therapeutic response of IFN via its combination with non-toxic agents could have potential future application towards melanoma management.
In this study, our data suggested that indeed the combination of EGCG and IFN has a better anti-proliferative response against melanoma cells in vitro in culture system as well as in vivo in nude mouse xenografts model. We found that the observed anti-proliferative response of EGCG and/or IFN are mediated via an apoptotic elimination of melanoma cells. The apoptotic machinery is dysfunctional in melanoma and is one of the critical factors why melanomas are often resistant to many therapies 4, 20, 21. We have shown an enhanced apoptosis of melanoma cells by the combination treatment.
Further, our in vitro data has shown that the observed anti-proliferative effects of EGCG and/of IFN are accompanied with an activation of Fas signaling. The Fas/FasL signaling system has been shown to play an important role in chemotherapy-induced apoptosis in several different cell types 22. IFN-γ was found to increase Fas in oral malignant melanoma cell lines (MMN9, PMP, MAA, HMG) in vitro, and combination therapy using IFN-γ and anti-Fas antibody (CH-11) has shown a synergistic anti-proliferative effect in MMN9 cells 23. Ekmekcioglu et al. have recently shown that MDA7/IL-24 induces the secretion of endogenous IFN-β, leading to the arrest of melanoma cell growth and induction of apoptosis, in conjunction with expression of TRAIL and Fas-FasL 24. EGCG has also been demonstrated to upregulate Fas/FasL signaling during apoptosis of cancer cells 12 25. Thus, our data showing an upregulation of Fas and an increase in FasL mediated apoptosis by EGCG and/or IFN provides an important clue regarding the potential mechanism of action of the combination. Furthermore, we have also found that the observed anti-proliferative effects of this combination are accompanied with and inhibition of NF-κB pathway. NF-κB family maintains cellular homeostasis by enhancing the transcription of genes involved in inflammation, immune response, cell proliferation, and apoptosis 26. Melanoma cells often express inflammatory mediators through enhanced activation of NF-κB 27 and constitutive activation of inhibitor of kappaB kinase (IKK) confers melanoma resistance to apoptosis and chemotherapy 28. We found that our treatment approach has been effective in down modulating the constitutively active NF-κB expression at the protein level as well as NF-κB promoter luciferase activity in these cells with the combination and single treatments. In addition, our findings are important because both Fas as well as NF-κB pathway have been shown to play critical roles in melanoma genesis 4 29 30. Further, based on the recent studies, both of these pathways are being appreciated as targets for drug development against cancer 31.
Finally, we have shown that our in vitro data possesses relevance to the in vivo situation where EGCG and/or IFN treatments resulted in a decrease in melanoma tumor growth in athymic nude mice and the response of the combination was found to be better than either of the agents alone. We employed two distinct protocols and found that the combination of the two treatments achieved a better outcome in reducing tumor volume and the proliferation maker PCNA. We believe that our data suggest that EGCG could impart therapeutic advantage if used in conjunction with IFN. It is conceivable that the effective dose of IFN could be lowered if used in combination with EGCG. The adjuvant therapy with EGCG may greatly reduce the toxicity of IFN and increase the drug tolerability to provide significant clinical benefits.
Acknowledgements
This work was supported by Merit Review funding from the Department of Veterans Affairs (to GSW), a research grant from Dermatology Foundation (to MN) and R21 CA116163 grant from the NIH (to MN). We also thank Caleb Creswell for his help in this project.
References
- 1.Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- 2.Demierre MF. Epidemiology and prevention of cutaneous melanoma. Curr Treat Options Oncol. 2006;7:181–186. doi: 10.1007/s11864-006-0011-z. [DOI] [PubMed] [Google Scholar]
- 3.Sharpless E, Chin L. The INK4a/ARF locus and melanoma. Oncogene. 2003;22:3092–3098. doi: 10.1038/sj.onc.1206461. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–3161. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- 5.Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev. 2007;17:31–39. doi: 10.1016/j.gde.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Rieger PT. Interferon-alpha: a clinical update. Cancer Pract. 1995;3:356–365. [PubMed] [Google Scholar]
- 7.Sleijfer S, Bannink M, Van Gool AR, Kruit WH, Stoter G. Side effects of interferon-alpha therapy. Pharm World Sci. 2005;27:423–431. doi: 10.1007/s11096-005-1319-7. [DOI] [PubMed] [Google Scholar]
- 8.Meyskens FL, Jr, Farmer PJ, Yang S, Anton-Culver H. New perspectives on melanoma pathogenesis and chemoprevention. Recent Results Cancer Res. 2007;174:191–195. doi: 10.1007/978-3-540-37696-5_16. [DOI] [PubMed] [Google Scholar]
- 9.Adhami VM, Mukhtar H. Anti-oxidants from green tea and pomegranate for chemoprevention of prostate cancer. Mol Biotechnol. 2007;37:52–57. doi: 10.1007/s12033-007-0047-8. [DOI] [PubMed] [Google Scholar]
- 10.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 11.Valcic S, Timmermann BN, Alberts DS, Wachter GA, Krutzsch M, Wymer J, Guillen JM. Inhibitory effect of six green tea catechins and caffeine on the growth of four selected human tumor cell lines. Anticancer Drugs. 1996;7:461–468. doi: 10.1097/00001813-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kuo PL, Lin CC. Green tea constituent (−)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J Biomed Sci. 2003;10:219–227. doi: 10.1007/BF02256057. [DOI] [PubMed] [Google Scholar]
- 13.Mukhtar H, Agarwal R. Skin cancer chemoprevention; J Investig Dermatol Symp Proc; 1996. pp. 209–214. [PubMed] [Google Scholar]
- 14.Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (−)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–521. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 15.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 16.Amiri KI, Richmond A. Role of nuclear factor-kappa B in melanoma. Cancer Metastasis Rev. 2005;24:301–313. doi: 10.1007/s10555-005-1579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ianaro A, Tersigni M, Belardo G, Di Martino S, Napolitano M, Palmieri G, Sini M, De Maio A, Ombra M, Gentilcore G, Capone M, Ascierto M, Satriano RA, Farina B, Faraone-Mennella M, Ascierto PA, Ialenti A. NEMO-binding domain peptide inhibits proliferation of human melanoma cells. Cancer Lett. 2009;274:331–336. doi: 10.1016/j.canlet.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Ross MJ, Martinka S, D'Agati VD, Bruggeman LA. NF-kappaB regulates Fas-mediated apoptosis in HIV-associated nephropathy. J Am Soc Nephrol. 2005;16:2403–2411. doi: 10.1681/ASN.2004121101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warbrick E. The puzzle of PCNA's many partners. Bioessays. 2000;22:997–1006. doi: 10.1002/1521-1878(200011)22:11<997::AID-BIES6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Eberle J, Fecker LF, Hossini AM, Kurbanov BM, Fechner H. Apoptosis pathways and oncolytic adenoviral vectors: promising targets and tools to overcome therapy resistance of malignant melanoma. Exp Dermatol. 2008;17:1–11. doi: 10.1111/j.1600-0625.2007.00655.x. [DOI] [PubMed] [Google Scholar]
- 21.Hussein MR, Haemel AK, Wood GS. Apoptosis and melanoma: molecular mechanisms. J Pathol. 2003;199:275–288. doi: 10.1002/path.1300. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien DI, Nally K, Kelly RG, O'Connor TM, Shanahan F, O'Connell J. Targeting the Fas/Fas ligand pathway in cancer. Expert Opin Ther Targets. 2005;9:1031–1044. doi: 10.1517/14728222.9.5.1031. [DOI] [PubMed] [Google Scholar]
- 23.Kamei T, Inui M, Nakase M, Nakamura S, Okumura K, Hiramoto K, Tagawa T. Experimental therapy using interferon-gamma and anti-Fas antibody against oral malignant melanoma cells. Melanoma Res. 2005;15:393–400. doi: 10.1097/00008390-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Ekmekcioglu SMJ, Udtha M, Chada S, Grimm EA. Killing of human melanoma cells induced by activation of class I interferon-regulated signaling pathways via MDA-7/IL-24. Cytokine. 2008;43(1):34–44. doi: 10.1016/j.cyto.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayakawa S, Saeki K, Sazuka M, Suzuki Y, Shoji Y, Ohta T, Kaji K, Yuo A, Isemura M. Apoptosis induction by epigallocatechin gallate involves its binding to Fas. Biochem Biophys Res Commun. 2001;285:1102–1106. doi: 10.1006/bbrc.2001.5293. [DOI] [PubMed] [Google Scholar]
- 26.Sun XF, Zhang H. NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histol Histopathol. 2007;22:1387–1398. doi: 10.14670/HH-22.1387. [DOI] [PubMed] [Google Scholar]
- 27.Ueda Y, Su Y, Richmond A. CCAAT displacement protein regulates nuclear factor-kappa beta-mediated chemokine transcription in melanoma cells. Melanoma Res. 2007;17:91–103. doi: 10.1097/CMR.0b013e3280a60888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A. BMS-345541 targets inhibitor of kappaB kinase and induces apoptosis in melanoma: involvement of nuclear factor kappaB and mitochondria pathways. Clin Cancer Res. 2006;12:950–960. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erb P, Ji J, Kump E, Mielgo A, Wernli M. Apoptosis and pathogenesis of melanoma and nonmelanoma skin cancer. Adv Exp Med Biol. 2008;624:283–295. doi: 10.1007/978-0-387-77574-6_22. [DOI] [PubMed] [Google Scholar]
- 30.Ueda Y, Richmond A. NF-kappaB activation in melanoma. Pigment Cell Res. 2006;19:112–124. doi: 10.1111/j.1600-0749.2006.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shammas MA, Neri P, Koley H, Batchu RB, Bertheau RC, Munshi V, Prabhala R, Fulciniti M, Tai YT, Treon SP, Goyal RK, Anderson KC, Munshi NC. Specific killing of multiple myeloma cells by (−)-epigallocatechin-3-gallate extracted from green tea: biologic activity and therapeutic implications. Blood. 2006;108:2804–2810. doi: 10.1182/blood-2006-05-022814. [DOI] [PMC free article] [PubMed] [Google Scholar]