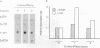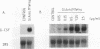Abstract
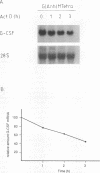
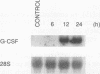
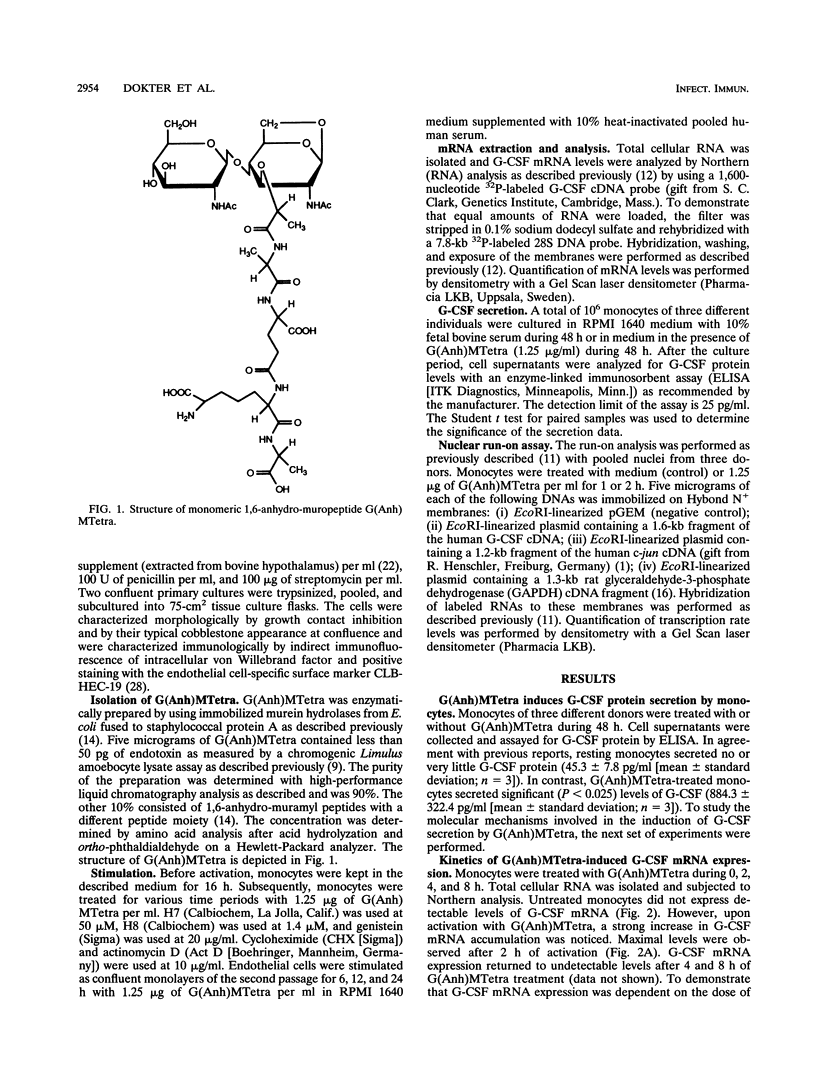
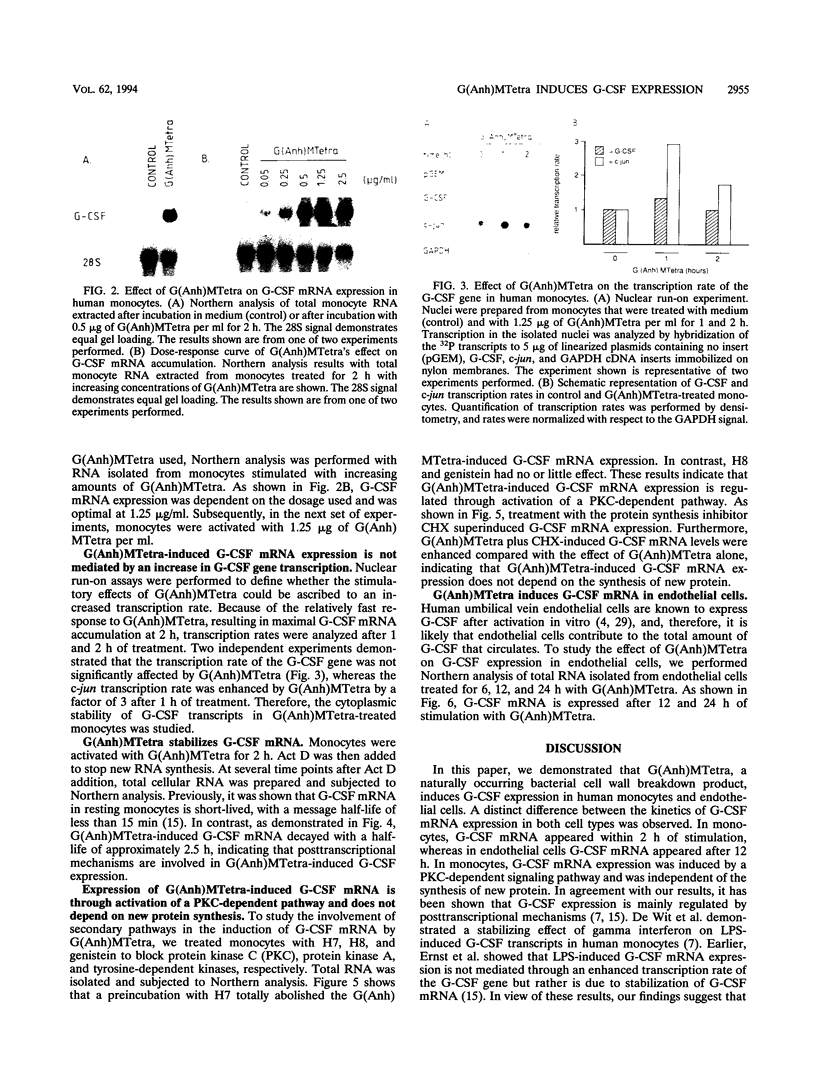
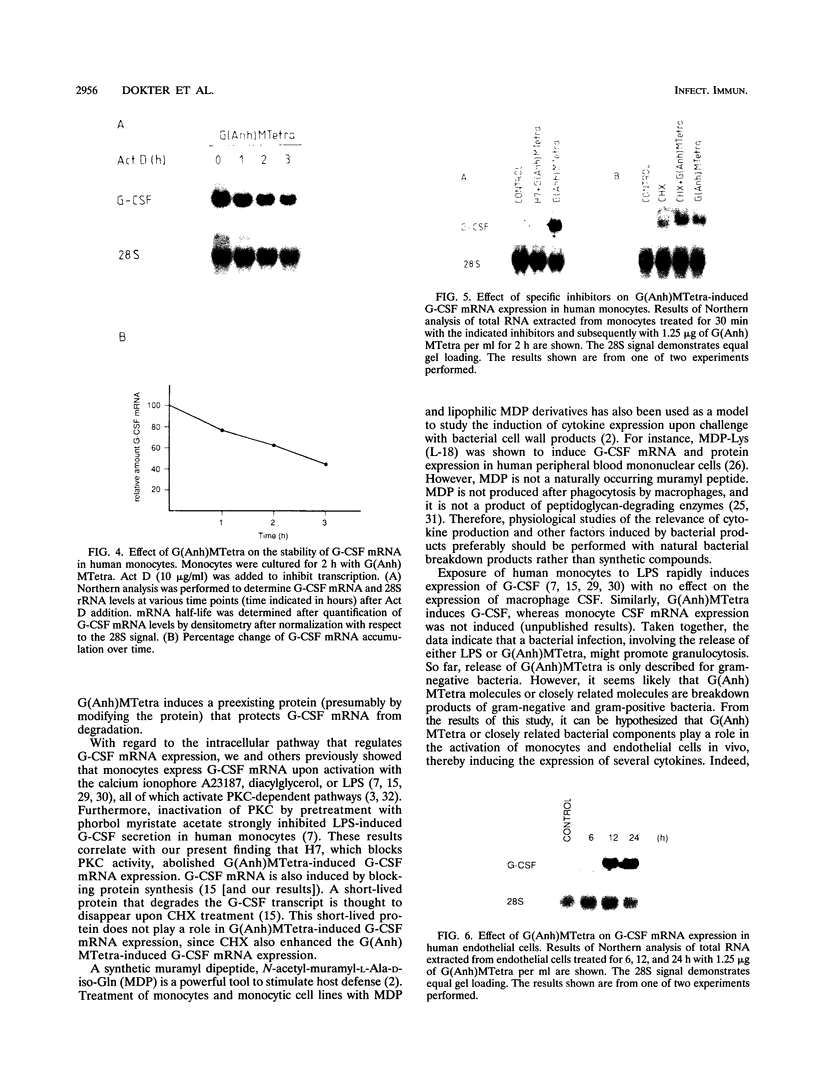
N-Acetylglucosaminyl-1,6-anhydro-N-acetylmuramyl-L-alanyl-D-isoglutam yl-m- diaminopimelyl-D-alanine [G (Anh)MTetra], a naturally occurring breakdown product of peptidoglycan from bacterial cell walls, was studied for its ability to induce granulocyte colony-stimulating factor (G-CSF) mRNA and protein expression in human adherent monocytes. Resting monocytes did not express G-CSF mRNA or secrete G-CSF protein. In contrast, monocytes exposed to G(Anh)MTetra showed a dose-dependent increase in G-CSF mRNA accumulation, which correlates with the secretion of G-CSF protein. Maximal levels of G-CSF mRNA were reached within 2 h of activation. Expression of G-CSF was mediated by an increase in the stability of G-CSF transcripts rather than by an increase in the transcription rate of the G-CSF gene. Experiments with the protein synthesis inhibitor cycloheximide revealed that G(Anh)MTetra-induced G-CSF mRNA expression was independent of new protein synthesis. Furthermore, it was shown that the effect of G(Anh)MTetra was regulated by a protein kinase C-dependent pathway, whereas protein kinase A and tyrosine kinases were not involved. Finally, it was shown that G(Anh)MTetra also induced G-CSF mRNA expression in human endothelial cells. The data indicate that, besides lipopolysaccharide, other naturally occurring bacterial cell wall components are able to induce G-CSF expression in different hematopoietic cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Allegretto E. A., Okino S. T., Hattori K., Boyle W. J., Hunter T., Karin M. Oncogene jun encodes a sequence-specific trans-activator similar to AP-1. Nature. 1988 Mar 10;332(6160):166–171. doi: 10.1038/332166a0. [DOI] [PubMed] [Google Scholar]
- Azuma I. Review: inducer of cytokines in vivo: overview of field and romurtide experience. Int J Immunopharmacol. 1992 Apr;14(3):487–496. doi: 10.1016/0192-0561(92)90180-s. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Harlan J. M., Adamson J. W. Interleukin 1 stimulates human endothelial cells to produce granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor. J Immunol. 1987 Jul 15;139(2):464–468. [PubMed] [Google Scholar]
- Cookson B. T., Tyler A. N., Goldman W. E. Primary structure of the peptidoglycan-derived tracheal cytotoxin of Bordetella pertussis. Biochemistry. 1989 Feb 21;28(4):1744–1749. doi: 10.1021/bi00430a048. [DOI] [PubMed] [Google Scholar]
- Demetri G. D., Griffin J. D. Granulocyte colony-stimulating factor and its receptor. Blood. 1991 Dec 1;78(11):2791–2808. [PubMed] [Google Scholar]
- Dinarello C. A., Krueger J. M. Induction of interleukin 1 by synthetic and naturally occurring muramyl peptides. Fed Proc. 1986 Oct;45(11):2545–2548. [PubMed] [Google Scholar]
- Dofferhoff A. S., Esselink M. T., de Vries-Hospers H. G., van Zanten A., Bom V. J., Weits J., Vellenga E. The release of endotoxin from antibiotic-treated Escherichia coli and the production of tumour necrosis factor by human monocytes. J Antimicrob Chemother. 1993 Mar;31(3):373–384. doi: 10.1093/jac/31.3.373. [DOI] [PubMed] [Google Scholar]
- Dokter W. H., Dijkstra A. J., Koopmans S. B., Stulp B. K., Keck W., Halie M. R., Vellenga E. G(Anh)MTetra, a natural bacterial cell wall breakdown product, induces interleukin-1 beta and interleukin-6 expression in human monocytes. A study of the molecular mechanisms involved in inflammatory cytokine expression. J Biol Chem. 1994 Feb 11;269(6):4201–4206. [PubMed] [Google Scholar]
- Dokter W. H., Esselink M. T., Halie M. R., Vellenga E. Interleukin-4 inhibits the lipopolysaccharide-induced expression of c-jun and c-fos messenger RNA and activator protein-1 binding activity in human monocytes. Blood. 1993 Jan 15;81(2):337–343. [PubMed] [Google Scholar]
- Dokter W. H., Sierdsema S. J., Esselink M. T., Halie M. R., Vellenga E. IL-7 enhances the expression of IL-3 and granulocyte-macrophage-CSF mRNA in activated human T cells by post-transcriptional mechanisms. J Immunol. 1993 Apr 1;150(7):2584–2590. [PubMed] [Google Scholar]
- Engel H., Smink A. J., van Wijngaarden L., Keck W. Murein-metabolizing enzymes from Escherichia coli: existence of a second lytic transglycosylase. J Bacteriol. 1992 Oct;174(20):6394–6403. doi: 10.1128/jb.174.20.6394-6403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel H., van Leeuwen A., Dijkstra A., Keck W. Enzymatic preparation of 1,6-anhydro-muropeptides by immobilized murein hydrolases from Escherichia coli fused to staphylococcal protein A. Appl Microbiol Biotechnol. 1992 Sep;37(6):772–783. doi: 10.1007/BF00174845. [DOI] [PubMed] [Google Scholar]
- Ernst T. J., Ritchie A. R., Demetri G. D., Griffin J. D. Regulation of granulocyte- and monocyte-colony stimulating factor mRNA levels in human blood monocytes is mediated primarily at a post-transcriptional level. J Biol Chem. 1989 Apr 5;264(10):5700–5703. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Tsutsumi H., Kumakawa T., Abe H., Hirai M., Kurosawa S., Mori M., Fukushima M. Levels of serum granulocyte colony-stimulating factor in patients with infections. Blood. 1990 Nov 15;76(10):1962–1964. [PubMed] [Google Scholar]
- Krueger J. M., Karnovsky M. L., Martin S. A., Pappenheimer J. R., Walter J., Biemann K. Peptidoglycans as promoters of slow-wave sleep. II. Somnogenic and pyrogenic activities of some naturally occurring muramyl peptides; correlations with mass spectrometric structure determination. J Biol Chem. 1984 Oct 25;259(20):12659–12662. [PubMed] [Google Scholar]
- Krueger J. M., Pappenheimer J. R., Karnovsky M. L. The composition of sleep-promoting factor isolated from human urine. J Biol Chem. 1982 Feb 25;257(4):1664–1669. [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Karnovsky M. L., Krueger J. M., Pappenheimer J. R., Biemann K. Peptidoglycans as promoters of slow-wave sleep. I. Structure of the sleep-promoting factor isolated from human urine. J Biol Chem. 1984 Oct 25;259(20):12652–12658. [PubMed] [Google Scholar]
- Martin S. A., Rosenthal R. S., Biemann K. Fast atom bombardment mass spectrometry and tandem mass spectrometry of biologically active peptidoglycan monomers from Neisseria gonorrhoeae. J Biol Chem. 1987 Jun 5;262(16):7514–7522. [PubMed] [Google Scholar]
- Polanski M., Gray G. R. Incorporation of bacterial peptidoglycan constituents into macrophage lipids during phagocytosis. J Immunol. 1989 Oct 15;143(8):2706–2713. [PubMed] [Google Scholar]
- Shimoda K., Okamura S., Kawasaki C., Omori F., Matsuguchi T., Niho Y. Muroctasin [MDP-Lys(18)] augments the production of granulocyte colony-stimulating factor (G-CSF) from human peripheral blood mononuclear cells in vitro. Int J Immunopharmacol. 1990;12(7):729–736. doi: 10.1016/0192-0561(90)90035-l. [DOI] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Release of soluble peptidoglycan from growing conococci: demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980 Sep;29(3):914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellenga E., Rambaldi A., Ernst T. J., Ostapovicz D., Griffin J. D. Independent regulation of M-CSF and G-CSF gene expression in human monocytes. Blood. 1988 Jun;71(6):1529–1532. [PubMed] [Google Scholar]
- Vellenga E., van der Vinne B., De Wolf J. T., Halie M. R. Simultaneous expression and regulation of G-CSF and IL-6 mRNA in adherent human monocytes and fibroblasts. Br J Haematol. 1991 May;78(1):14–18. doi: 10.1111/j.1365-2141.1991.tb04375.x. [DOI] [PubMed] [Google Scholar]
- Vermeulen M. W., Gray G. R. Processing of Bacillus subtilis peptidoglycan by a mouse macrophage cell line. Infect Immun. 1984 Nov;46(2):476–483. doi: 10.1128/iai.46.2.476-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman P. D., Raetz C. R. The activation of protein kinase C by biologically active lipid moieties of lipopolysaccharide. J Biol Chem. 1984 Aug 25;259(16):10048–10052. [PubMed] [Google Scholar]
- de Wit H., Dokter W. H., Esselink M. T., Halie M. R., Vellenga E. Interferon-gamma enhances the LPS-induced G-CSF gene expression in human adherent monocytes, which is regulated at transcriptional and posttranscriptional levels. Exp Hematol. 1993 Jun;21(6):785–790. [PubMed] [Google Scholar]
- van Mourik J. A., Leeksma O. C., Reinders J. H., de Groot P. G., Zandbergen-Spaargaren J. Vascular endothelial cells synthesize a plasma membrane protein indistinguishable from the platelet membrane glycoprotein IIa. J Biol Chem. 1985 Sep 15;260(20):11300–11306. [PubMed] [Google Scholar]